Abstract
Background
It is known that secreted protein acidic and cysteine rich (osteonectin), cwcv and kazal-like domains proteoglycan 2 (SPOCK2) plays a significant role in the development and progression of several human cancers; however, the role of SPOCK2 in prostate cancer (PCa) remains unclear. This study aimed to find the role and mechanism of SPOCK2 in the development and progression of PCa.
Methods
The messenger ribonucleic acid (mRNA) expression of SPOCK2 in PCa tissue was detected by real-time polymerase chain reaction (PCR). Upregulation of the SPOCK2 gene was achieved using the DU145 and LNCaP cells by transfecting the cells with SPOCK2 recombinant fragment. Cell invasion and migration ability were detected by transwell chamber and wound healing assay. The expression of membrane-type 1 matrix metalloproteinase (MT1-MMP) and matrix metalloproteinase 2 (MMP2) in the cells was detected by Western Blot and zymography gel assay.
Results
The mRNA level of SPOCK2 was significantly lower in the PCa tissue compared to benign prostate hyperplasia. Upregulation of SPOCK2 inhibited cell invasion and migration in DU145 and LNCaP cells, inhibited the expression of MT1-MMP and MMP2 and, inhibited activation of MMP2 in DU145 and LNCaP cells.
Conclusion
SPOCK2 is associated with the progression of PCa. Upregulation of SPOCK2 can inhibit PCa cell invasion and metastasis by decreasing MT1-MMP and MMP2 gene expression and decreasing MMP2 protein activation.
Keywords: SPOCK2, Prostate cancer, Migration, Invasion, MT1-MMP, MMP2
Introduction
Prostate cancer (PCa) is the most common cancer diagnosed among men in developed countries, and it has become the most common malignant tumor of the urinary system and the fastest growing male malignancy in China (Zheng et al., 2016; Chen et al., 2015; Chen et al., 2016b). Though the use of prostate-specific antigen screening has improved the detection rate of PCa (Welch & Albertsen, 2009) and reduced the mortality risk of PCa, it did not improve all-cause mortality rates (Fenton et al., 2018; Schröder et al., 2012). The 5-year survival rate for most patients with local or regional PCa is 90%, while for men diagnosed with advanced PCa, the 5-year survival rate is only 30%, and metastasis and recurrence of PCa the leading causes of the death (Shah et al., 2016; Antonarakis et al., 2010). Studying the mechanism of invasion and metastasis of PCa is therefore of great significance for improving the prognosis of PCa.
The secreted protein acidic and cysteine rich gene in combination with osteonectin, cwcv and kazal like domains proteoglycan 2 (SPOCK2), also known as testincan-2, is a member of the SPOCK family. This gene encodes a protein that binds to glycosaminoglycans to form part of the extracellular matrix (ECM), which plays an important role in cell invasion and metastasis (https://www.ncbi.nlm.nih.gov/gene/9806). It was found that SPOCK2 protein is expressed in the ECM of alveolar cells and that polymorphism of this gene was correlated with human alveolar progression, and it was consequently suggested that SPOCK2 might be a gene of interest in alveolar dysplasia (Hadchouel et al., 2011). Another study further found that oligonucleotide polymorphism of the SPOCK2 gene was also related to the deletion of 16q chromosome in breast cancer (Nordgard et al., 2008), which was the first report to describe the correlation between the SPOCK2 gene and cancer. In addition, Chung et al. (2008) found that abnormal methylation of the SPOCK2 gene was closely associated with PCa, breast cancer, and colon cancer by screening differential methylation genes, and it was deduced that screening for epigenetic inactivation of SPOCK2 could be used as a diagnostic marker for PCa (Verma, Patel & Verma, 2011). This study was the first to highlight the correlation between SPOCK2 and PCa. In a study of glioma, it was found that SPOCK1 and SPOCK3 can inhibit the invasiveness of glioma cells by suppressing membrane-type 1 matrix metalloproteinase (MT1-MMP)-mediated matrix metalloproteinase 2 (MMP2) activation, and that high level of SPOCK2 expression could abrogate the inhibition of MMP2 by SPOCK1 and SPOCK3 and increase the invasiveness of glioma cells (Nakada et al., 2001, 2003). In contrast, in a study of PCa, SPOCK1 promoted tumor growth and metastasis (Chen et al., 2016a), however, the role of the SPOCK2 gene in PCa remains to be reported. The current study aimed to investigate the role and mechanism of action of SPOCK2 in the development and progression of PCa.
Materials and Methods
Tissue sample collection
All specimens were obtained from patients hospitalized in the Department of Urology of Shengjing Hospital (Shenyang, China). Specimens were collected from 30 surgical patients with PCa and 20 surgical patients with benign prostate hyperplasia (BPH). Informed consent was obtained from all patients, and agreement was obtained from the Ethics Committee of China Medical University before collecting the samples (2018PS302K). None of the patients had other surgical, endocrine, immunological or metabolic diseases, or had taken any other hormonal medication in the three months prior to surgery.
Cell culture and transfection
The DU145 and LNCaP cells were purchased from the Shanghai Institute of Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), in a sterile incubator at 37 °C under 5% CO2.
The SPOCK2 recombinant fragment and the adenoviral vector were purchased from Gene Pharma (Shanghai, China). The transfection was performed according to the manufacturer’s instructions. Mock transfected cells were used as negative controls; cells transfected with empty vector were used as vector controls. At 48 h after transfection, the supernatant was discarded and replaced with complete medium and the cells cultured for another 24 h at 37 °C under 5% CO2 for further study.
Detection of SPOCK2 mRNA expression in tissue and cells by real-time PCR
Total RNA was obtained from tissue and cells using TRIzol reagent (BioTeke, Beijing, China). All procedures were performed under RNAase free conditions according to the manufacturer’s instructions. After quantification of RNA, the expression of SPOCK2 messenger ribonucleic acid (mRNA) was detected by real-time polymerase chain reaction (PCR). Primer information is shown in Table 1. The reaction conditions were as follows: incubation at 94 °C for 5 min, 40 cycles of incubation at 94 °C for 10 s, 60 °C for 20 s and 72 °C for 30 s. Data were analyzed using the 2−ΔΔCT method. Each sample was assayed in triplicate.
Table 1. The primers of SPOCK2 for Real-time PCR.
| Name | Sequence | Tm | Product length |
|---|---|---|---|
| SPOCK2 F | ATGGAGGACGAGCAATGGCT | 61.8 | 93 |
| SPOCK2 R | TGGGTCGGACGAGGGAAC | 60.8 | |
| β-actin F | CTTAGTTGCGTTACACCCTTTCTTG | 62 | 156 |
| β-actin R | CTGTCACCTTCACCGTTCCAGTTT | 64.4 |
Detection of SPOCK2, MT1-MMP and MMP2 protein expression in cells by WB
Cells were harvested for 48 h after transfection. Radioimmunoprecipitation assay buffer was added to the samples and the samples placed on ice for 5 min. The samples were then centrifuged at 12,000 rpm, 4 °C for 10 min and the supernatant removed to obtain total protein. After total protein was quantified, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was carried out at 80V for 2.5 h. After being blocked for 1 h with 5% skim milk, the membranes were incubated with specific primary antibodies overnight at 4 °C followed by incubation with the secondary antibodies for 2 h at room temperature. The anti-SPOCK2 polyclonal antibody (1:200) was purchased from Santa Cruz, CA, USA, the anti-MT1-MMP (1:1,000) was purchased from Proteintech, Wuhan, China, and the anti-MMP2 (1:500) was purchased from Wanleibio, Shenyang, China. Secondary antibody (1:5,000) was purchased from Wanleibio, Shenyang, China. The film was scanned, and the optical density value of the target strip analyzed using a gel image processing system (Gel-Pro-Analyzer software). All assays were performed in triplicate.
Detection of cell invasion by transwell chamber
Matrigel was thawed on ice and diluted with serum-free medium. The transwell chamber was coated with 40 μl pre-diluted Matrigel and placed in a 37 °C incubator for 2 h to solidify. The Matrigel-coated transwell chamber was placed in a 24-well plate and 800 μl of medium containing 30% FBS was added to the lower chamber while 200 μl of the cell suspension (2 × 104) was added to the upper chamber. The 24-well plates were incubated at 37 °C under 5% CO2 for 26 h at which time cells could be seen in the lower chamber. Cells remaining on the top of the transwell chamber were scraped off with a cotton swab. Invasive cells were fixed with 4% paraformaldehyde for 20 min and stained with 0.5% crystal violet for 5 min; cells in the lower layer of the membrane were counted under an inverted microscope (200×).
Detection of cell migration ability by wound healing assay
Cells were transfected in six-well plates and cultured until reaching 90% confluence. A wound was created with a pipette tip, the cells washed twice and cultured in serum-free medium. The wound was observed and photographed at 0 h and 24 h using an inverted microscope (Nikon, Tokyo, Japan). Cell migration ability was described as the number of cells that migrated into the wound. All assays were performed in triplicate.
Detection of MMP2 expression in cells by zymography gel assay
The cell supernatant was obtained after centrifugation. After measuring the concentration, 60 μg protein was used for SDS-PAGE analysis with constant voltage overnight. The gel was then stained in the staining solution for 3 h. After being decolorized, the gel was photographed by a gel imaging system.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD) with SPSS version 13.0 software used for the statistical analyses. A Student’s t-test was used to determine the significance of two group differences. A one-way analysis of variance analysis was used for multiple comparisons. A P-value of P < 0.05 (two-tailed) was considered significant.
Results
SPOCK2 mRNA expression in tissue
There was no significant difference between the age of the PCa group (69.27 ± 9.15) and the BPH group (66.97 ± 5.95) (P > 0.05). Real-time PCR showed a significantly lower expression of SPOCK2 in the PCa tissues (2.25 ± 0.38) compared to the BPH tissues (6.12 ± 0.19) (P < 0.05).
SPOCK2 gene expression after transfection
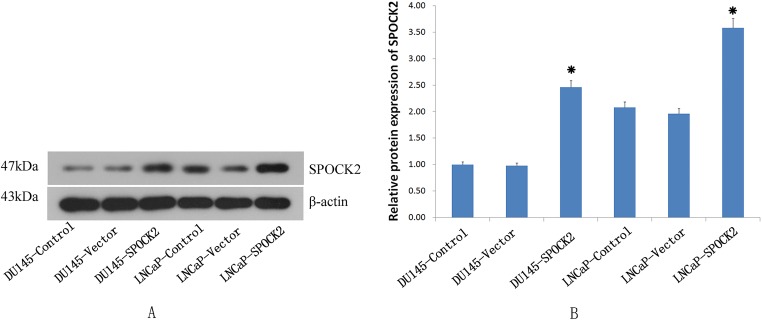
The recombinant SPOCK2 fragment was transfected into the DU145 and LNCaP cells by adenovirus, and the SPOCK2 mRNA level and protein expression in the cells after transfection detected by real-time PCR (Fig. 1) and Western Blot (WB) (Fig. 2), respectively. The results showed that SPOCK2 mRNA level and protein expression in DU145 and LNCaP cells after transfection were significantly increased (P < 0.05).
Figure 1. The SPOCK2 mRNA expression was measured using RT-PCR.
(A) The mRNA expression of SPOCK2 in PCa tissues was significantly lower than that in BPH tissues (*P < 0.05). (B) The mRNA expression of SPOCK2 in DU145 and LNCaP cells significantly increased after transfection (*P < 0.05).
Figure 2. The SPOCK2 protein expression in cells.
(A) The SPOCK2 protein expression in DU145 and LNCaP cells was detected via WB. (B) The protein expression of SPOCK2 in cells significantly increased after transfection (*P < 0.05).
Effect of SPOCK2 upregulation on cell invasion
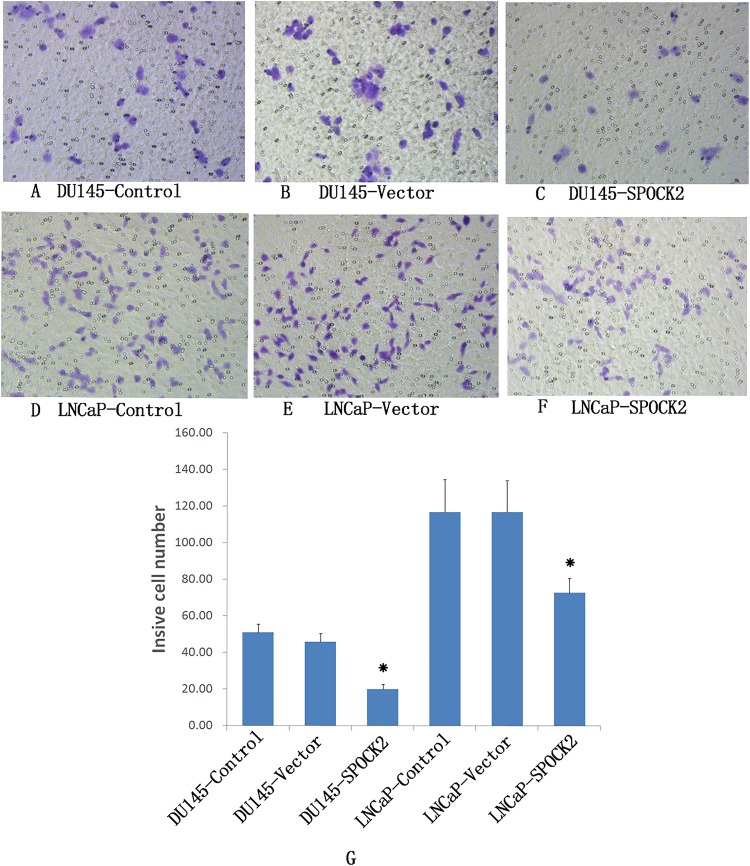
Transwell invasion chamber was used to detect the invasion of DU145 and LNCaP cells after transfection (Fig. 3). The results showed that the invasive ability of the transfected group was significantly lower than that of the negative control group and the vector control group (P < 0.05).
Figure 3. Upregulation of SPOCK2 on cell invasive ability.
(A–F) Upregulation of SPOCK2 on cell invasive ability in DU145 and LNCaP cells following transfection with SPOCK2 was detected by transwell invasion chamber. (G) The number of DU145 and LNCaP cells migrating into the lower chamber of the transwell invasion chamber was significantly reduced after transfection with SPOCK2 (*P < 0.05).
Effect of SPOCK2 upregulation on cell migration
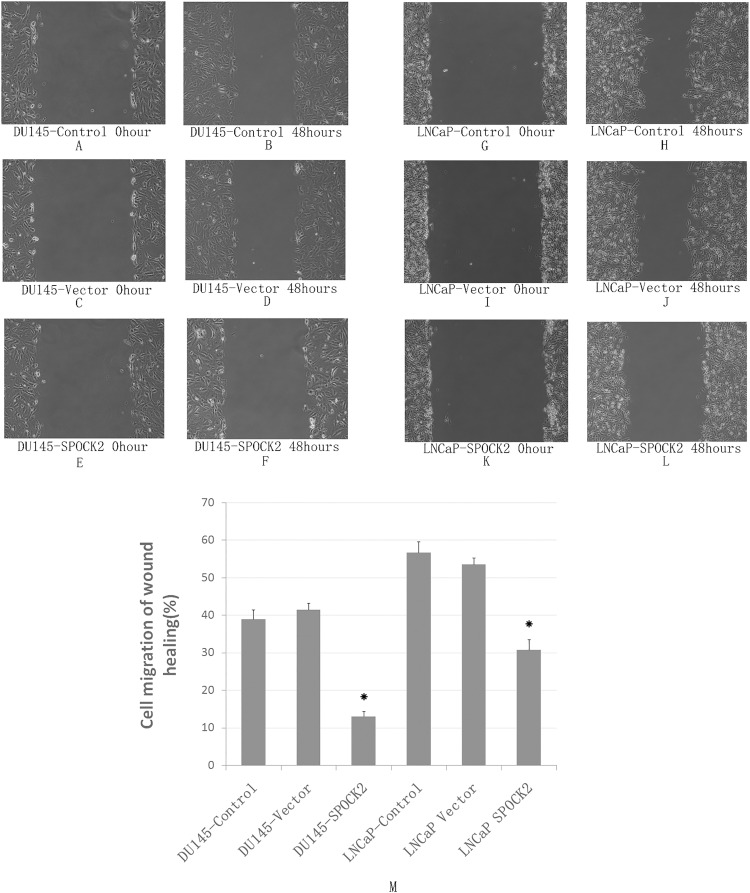
The wound healing assay was used to detect the migration ability of DU145 and LNCaP cells after transfection (Fig. 4). The results showed that the migration of both DU145 and LNCaP cells was significantly decreased after transfection compared with the negative control group and the vector control group (P < 0.05).
Figure 4. Upregulation of SPOCK2 on cell migration.
(A–L) Upregulation of SPOCK2 on cell migration following transfection of DU145 and LNCaP cells with SPOCK2 was detected by the wound healing assay. (B) (M) The wound healing assay showed that cell migration was significantly reduced after transfection with SPOCK2 (*P < 0.05).
Effect of SPOCK2 upregulation on MT1-MMP2 and MMP2 expression
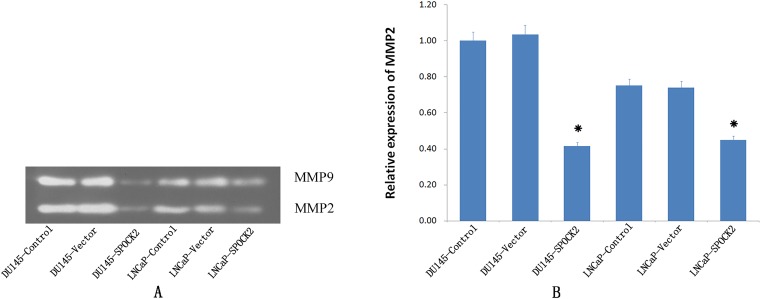
The expression of MT1-MMP2 and MMP2 protein in DU145 and LNCaP cells was detected by WB (Fig. 5). The expression of MT1-MMP2 and MMP2 in cells in the transfected group were significantly lower than those in the negative control group and the vector control group (P < 0.05).
Figure 5. Upregulation of SPOCK2 on the expression of MT1-MMP and MMP2.
(A, C) Protein expression of MT1-MMP2 in DU145 and LNCaP cells transfected with SPOCK2, via WB, showing a significant decrease in MT1-MMP protein expression following transfection (*P < 0.05). (B, D) Expression of MMP2 in DU145 and LNCaP cells after transfection with SPOCK2 was significantly decreased (*P < 0.05) as detected via WB.
Effect of SPOCK2 upregulation on MMP2 activation
The expression of activated MMP2 was detected using gelatin zymography after transfection (Fig. 6). Expression of activated MMP2 protein after transfection was significantly lower than in the negative control group or the vector control group (P < 0.05).
Figure 6. Upregulation of SPOCK2 on the activaion of MMP2.
(A) Upregulation of SPOCK2 on the activaion of MMP2 was detected by gelatin zymography. (B) Hostogram showed a significant decrease in MMP2 activation in DU145 and LNCaP cells following transfection of these cells with SPOCK2 (*P < 0.05).
Discussion
The SPOCK family includes three members, SPOCK1, SPOCK2 and SPOCK3. In 2008, Chung et al. first reported the relationship between SPOCK2 and PCa, colon cancer and breast cancer, by gene screening. The relationship between colon cancer and SPOCK2 was further verified by Sambuudash, Kim & Cho (2017), whereby it was found that the incidence of SPOCK2 methylation in colon cancer was significantly higher than that in adjacent normal mucosa tissues. Another study (Ren, Wang & Li, 2011) reported that absence of SPOCK2 expression caused by hypermethylation of the SPOCK2 gene, may be related to the development and progression of ovarian cancer. The relationship between PCa and the SPOCK2 gene was not further investigated. In the current study, using real-time PCR, the level of expression of the SPOCK2 gene was significantly lower in PCa tissues compared to that in BPH tissue, indicating that lower expression of the SPOCK2 gene may be associated with the tumorigenesis and progression of PCa. To further clarify the role of SPOCK2 in PCa cells, PCa cells were transfected with an adenovirus vector to increase the expression of the SPOCK2 gene. It was found that upregulation of this gene in PCa cells can inhibit cell invasion and migration. The results indicated the SPOCK2 may play an important role in the progression of PCa by affecting the migration and invasion ability of the PCa cells. As SPOCK2 is often inactivated by hypermethylation, a reversible epigenetic change (Przybilla et al., 2017), reversal of the expression of the SPOCK2 gene in PCa cells clinically, may improve the prognosis of patients with PCa.
The collagenase, MMP2, can decompose plasma fibronectin and laminin of the basement membrane, and is involved in various physiological and pathological processes in the human body (Song et al., 2015; Yi et al., 2014). The activation of MMP2 is regulated specifically by MT1-MMP, which itself is also capable of degrading a variety of ECM components (Koshikawa et al., 2000). Both MT1-MMP and MMP2 play an important role in the invasion and migration of PCa (Wang et al., 2009; Murray et al., 2012). To explore the mechanism by which SPOCK2 influences the invasion and migration of PCa cells, the expression of MT1-MMP and MMP2, and the activation of MMP2 in PCa cells was investigated using PCa cells with an upregulation of SPOCK2. The results indicated that upregulation of SPOCK2 in PCa cells could inhibit the expression of both MT1-MMP and MMP2 and decrease MMP2 activation. Together, the findings of the present study suggest that SPOCK2 may inhibit the invasion and migration of PCa cells by affecting MT1-MMP/MMP2 expression and activation. In a study of glioma, it was found that SPOCK1 and SPOCK3 could inhibit MT1-MMP-mediated MMP2 activation and inhibit glioma cell invasion by binding to MT1-MMP (Nakada et al., 2001). In contrast, high levels of SPOCK2 could abrogate this inhibition of MMP2 by SPOCK3 and increase the invasiveness of glioma cells (Nakada et al., 2003). In addition, in a study of gastric cancer, SPOCK1 was found to promote the invasion and metastasis of gastric cancer through Slug-induced epithelial-mesenchymal transition (Chen et al., 2018), and knockdown of SPOCK1 in colorectal cancer could inhibit proliferation and invasion of cancer cells by suppressing the PI3K/Akt Pathway (Zhao et al., 2016). These studies indicate that the effect of SPOCK2 on MT1-MMP/MMP2 in PCa cells may not work by directly binding to the MT1-MMP as the competitive inhibitor of SPOCK1 or SPOCK3. It is very possible that there may be other mechanisms by which SPOCK2 can affect the MT1-MMP/MMP pathway, which requires further investigation.
Conclusions
In conclusion, the present study demonstrates that SPOCK2 is associated with the progression of PCa, and that upregulation of SPOCK2 can inhibit PCa cell invasion and metastasis. This is achieved ultimately by its ability to decrease MT1-MMP and MMP2 gene expression and decrease activation of MMP2 protein.
Supplemental Information
Raw data of Real-time PCR for data analyses and preparation for Fig. 1
Raw data of Western Blot for data analyses and preparation for Fig. 2 and Fig. 5
The upper line: SPOCK2 expression for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: β-actin expression for DU145 Control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
The upper line: MT1-MMP expression for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: β-actin expression for DU145 Control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
The upper line: MMP2 expression for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: β-actin expression for DU145 Control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
The upper line: MMP2 expression in cells by zymography gel assay for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: MMP9 expression in cells by zymography gel assay for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Gang Liu performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper.
Fang Ren performed the experiments.
Yongsheng Song conceived and designed the experiments, approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The University of China Medical University approval to carry out the study within its facilities (Ethical Application Ref: 2018PS302K).
Data Availability
The following information was supplied regarding data availability:
All the raw measurements are available in the figures and in the Supplemental Files.
References
- Antonarakis et al. (2010).Antonarakis ES, Chen Y, Elsamanoudi SI, Brassell SA, Rocha MVD, Eisenberger MA, McLeod DG. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the center for prostate disease research national database. BJU International. 2010;108(3):378–385. doi: 10.1111/j.1464-410X.2010.09878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2016a).Chen Q, Yao YT, Xu H, Chen YB, Gu M, Cai ZK, Wang Z. SPOCK1 promotes tumor growth and metastasis in human prostate cancer. Drug Design, Development and Therapy. 2016a;10:2311–2321. doi: 10.2147/DDDT.S91321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2016b).Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016b;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chinese Journal of Cancer Research. 2015;27(1):2–12. doi: 10.3978/j.issn.1000-9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen D, Zhou H, Liu G, Zhao Y, Cao G, Liu Q. SPOCK1 promotes the invasion and metastasis of gastric cancer through Slug-induced epithelial-mesenchymal transition. Journal of Cellular and Molecular Medicine. 2018;22(2):797–807. doi: 10.1111/jcmm.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung et al. (2008).Chung W, Kwabi-Addo B, Ittmann M, Jelinek J, Shen L, Yu Y, Issa J-PJ. Identification of novel tumor markers in prostate, colon and breast cancer by unbiased methylation profiling. PLOS ONE. 2008;3(4):e2079. doi: 10.1371/journal.pone.0002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton et al. (2018).Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US preventive services task force. JAMA. 2018;319(18):1914–1931. doi: 10.1001/jama.2018.3712. [DOI] [PubMed] [Google Scholar]
- Hadchouel et al. (2011).Hadchouel A, Durrmeyer X, Bouzigon E, Incitti R, Huusko J, Jarreau P-H, Lenclen R, Demenais F, Franco-Montoya M-L, Layouni I, Patkai J, Bourbon J, Hallman M, Danan C, Delacourt C. Identification of SPOCK2 as a susceptibility gene for bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine. 2011;184(10):1164–1170. doi: 10.1164/rccm.201103-0548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshikawa et al. (2000).Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. Journal of Cell Biology. 2000;148(3):615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray et al. (2012).Murray NP, Reyes E, Tapia P, Badínez L, Orellana N. Differential expression of matrix metalloproteinase-2 expression in disseminated tumor cells and micrometastasis in bone marrow of patients with nonmetastatic and metastatic prostate cancer: theoretical considerations and clinical implications-an immunocytochemical study. Bone Marrow Research. 2012;2012:259351. doi: 10.1155/2012/259351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada et al. (2003).Nakada M, Miyamori H, Yamashita J, Sato H. Testican 2 abrogates inhibition of membrane-type matrix metalloproteinases by other testican family proteins. Cancer Research. 2003;63(12):3364–3369. [PubMed] [Google Scholar]
- Nakada et al. (2001).Nakada M, Yamada A, Takino T, Miyamori H, Takahashi T, Yamashita J, Sato H. Suppression of membrane-type 1 matrix metalloproteinase (MMP)-mediated MMP-2 activation and tumor invasion by testican 3 and its splicing variant gene product, N-Tes. Cancer Research. 2001;61(24):8896–8902. [PubMed] [Google Scholar]
- Nordgard et al. (2008).Nordgard SH, Johansen FE, Alnaes GIG, Bucher E, Syvänen A-C, Naume B, Børresen-Dale A-L, Kristensen VN. Genome-wide analysis identifies 16q deletion associated with survival, molecular subtypes, mRNA expression, and germline haplotypes in breast cancer patients. Genes, Chromosomes and Cancer. 2008;47(8):680–696. doi: 10.1002/gcc.20569. [DOI] [PubMed] [Google Scholar]
- Przybilla et al. (2017).Przybilla J, Hopp L, Lübbert M, Loeffler M, Galle J. Targeting DNA hypermethylation: computational modeling of DNA demethylation treatment of acute myeloid leukemia. Epigenetics. 2017;12(10):886–896. doi: 10.1080/15592294.2017.1361090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Wang & Li (2011).Ren F, Wang DB, Li T. Epigenetic inactivation of SPOCK2 in the malignant transformation of ovarian endometriosis. Zhonghua Fu Chan Ke Za Zhi. 2011;46(11):822–825. [PubMed] [Google Scholar]
- Sambuudash, Kim & Cho (2017).Sambuudash O, Kim H-S, Cho MY. Lack of aberrant methylation in an adjacent area of left-sided colorectal cancer. Yonsei Medical Journal. 2017;58(4):749–755. doi: 10.3349/ymj.2017.58.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder et al. (2012).Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Páez A, Määttänen L, Bangma CH, Aus G, Carlsson S, Villers A, Rebillard X, Van Der Kwast T, Kujala PM, Blijenberg BG, Stenman U-H, Huber A, Taari K, Hakama M, Moss SM, De Koning HJ, Auvinen A. Prostate-cancer mortality at 11 years of follow-up. New England Journal of Medicine. 2012;366(11):981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah et al. (2016).Shah ET, Upadhyaya A, Philp LK, Tang T, Skalamera D, Gunter J, Nelson CC, Williams ED, Hollier BG. Repositioning “old” drugs for new causes: identifying new inhibitors of prostate cancer cell migration and invasion. Clinical & Experimental Metastasis. 2016;33(4):385–399. doi: 10.1007/s10585-016-9785-y. [DOI] [PubMed] [Google Scholar]
- Song et al. (2015).Song H, Pan D, Sun W, Gu C, Zhang Y, Zhao P, Qi Z, Zhao S. SiRNA directed against annexin II receptor inhibits angiogenesis via suppressing MMP2 and MMP9 expression. Cellular Physiology and Biochemistry. 2015;35(3):875–884. doi: 10.1159/000369745. [DOI] [PubMed] [Google Scholar]
- Verma, Patel & Verma (2011).Verma M, Patel P, Verma M. Biomarkers in prostate cancer epidemiology. Cancers. 2011;3(4):3773–3798. doi: 10.3390/cancers3043773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2009).Wang X, Wilson MJ, Slaton JW, Sinha AA, Ewing SL, Pei D. Increased aggressiveness of human prostate PC-3 tumor cells expressing cell surface localized membrane type-1 matrix metalloproteinase (MT1-MMP) Journal of Andrology. 2009;30(3):259–274. doi: 10.2164/jandrol.108.006494. [DOI] [PubMed] [Google Scholar]
- Welch & Albertsen (2009).Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. Journal of the National Cancer Institute. 2009;101(19):1325–1329. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi et al. (2014).Yi SA, Ryu HW, Lee DH, Han JW, Kwon SH. HP1β suppresses metastasis of human cancer cells by decreasing the expression and activation of MMP2. International Journal of Oncology. 2014;45(6):2541–2548. doi: 10.3892/ijo.2014.2646. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2016).Zhao P, Guan H-T, Dai Z-J, Ma Y-G, Liu X-X, Wang X-J. Knockdown of SPOCK1 inhibits the proliferation and invasion in colorectal cancer cells by suppressing the PI3K/Akt pathway. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. 2016;24(6):437–445. doi: 10.3727/096504016X14685034103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2016).Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China, 2011. Cancer Letters. 2016;370(1):33–38. doi: 10.1016/j.canlet.2015.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data of Real-time PCR for data analyses and preparation for Fig. 1
Raw data of Western Blot for data analyses and preparation for Fig. 2 and Fig. 5
The upper line: SPOCK2 expression for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: β-actin expression for DU145 Control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
The upper line: MT1-MMP expression for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: β-actin expression for DU145 Control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
The upper line: MMP2 expression for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: β-actin expression for DU145 Control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
The upper line: MMP2 expression in cells by zymography gel assay for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively. The lower line: MMP9 expression in cells by zymography gel assay for DU145 control, DU145 vector control, DU145 SPOCK2, LNCaP control, LNCaP vector, and LNCaP SPOCK2, respectively.
Data Availability Statement
The following information was supplied regarding data availability:
All the raw measurements are available in the figures and in the Supplemental Files.