Introduction
Patients undergoing major vascular surgery are at high risk for postoperative cardiac complications, as well as perioperative morbidity and mortality 1–4. In addition to the elevated surgical risk associated with major vascular procedures, the increased perioperative morbidity and mortality has been attributed to a higher prevalence of cardiac disease in the vascular patient population5. For example, congestive heart failure (“CHF”) is an important predictor for postoperative cardiac events in patients undergoing vascular surgery6. While prior studies have investigated the association between left ventricular function and postoperative adverse cardiac outcomes in this surgical cohort, evaluation of the prognostic value of right ventricular function has been lacking7,8.
Right ventricular (“RV”) function may be an overlooked contributor to perioperative morbidity and mortality. While left ventricular (“LV”) function is critical, global cardiac function results from the intimate relationship between both the left and the right ventricles. Indeed, this intimate relationship is demonstrated by observing RV dysfunction as a result of left-sided heart failure even when ejection fraction (“EF”) is preserved. In fact, 33–50% of patients with left-sided heart failure have been reported to have evidence of RV dysfunction9. Similarly, through interventricular dependence, RV dysfunction by itself can compromise left ventricular function and has been shown to have important prognostic value10,11. Indeed, in patients undergoing open heart surgery, the presence of RV dysfunction has been found to independently predict worse short- and long-term outcomes12–14.
This study aimed to detect the prevalence of preexisting RV dysfunction in patients undergoing major vascular surgery and evaluate whether preexisting RV dysfunction, diagnosed by preoperative echocardiogram (“echo”), was independently associated with a higher incidence of postoperative major adverse cardiovascular events (“MACE”). In addition, the study investigated whether preexisting RV dysfunction was associated with longer hospital stays and higher in-hospital mortality relating to either cardiac events or as a composite all-cause event. Our hypothesis was that preexisting RV dysfunction would be associated with an increased risk of MACE and longer length of stay (“LOS”). In addition, we predicted that presence of RV dysfunction would be associated with higher incidence of in-hospital mortality relating to both cardiac events or as a composite all-cause event in patients undergoing non-emergent major vascular surgery.
Materials and Methods
Study Population
This study was approved by the Institutional Review Board at the University of California Irvine Medical Center (UCI IRB HS# 2017–4099). A retrospective single-centered chart review of all patients undergoing major vascular surgery between 2010 and 2017 was conducted. Major vascular procedures included all surgeries involving the aorta (both open and endovascular), open inferior vena cava thrombectomy with nephrectomy, and any vascular bypass surgery for peripheral vascular or arterial occlusive disease. Carotid endarterectomies and procedures relating to arteriovenous fistula were not included as these surgeries are considered intermediate- and low-risk respectively. In addition, any procedure that required cardiopulmonary bypass and any emergency surgery were excluded. Only adult patients between the age of 18 and 89, who were identified as American Society of Anesthesiologist (ASA) Physical Status Classification of III and above, were considered for inclusion. Finally, only those patients with preoperative echocardiogram performed within one-year of the indexed surgery and for which the study had image quality sufficient to retrospectively determine RV function were enrolled into the study. In patients who had multiple echo studies within one-year of the indexed surgery, study that was closest to the indexed surgery was selected for review.
Data Collection
Patients’ demographic and perioperative data were collected via manual chart review of the hospital’s electronic record. Intraoperative variables were collected from Surgical Information Systems (Surgical Information Systems Corp, Alpharetta GA), while demographic and perioperative variables were obtained from the main hospital chart, Quest (Allscripts Corporation, Alpharetta GA). For each patient meeting inclusion criteria, age and gender were collected, as well as presence or absence of history of congestive heart failure (“CHF”), coronary artery disease (“CAD”), hypertension (“HTN”), cerebrovascular accident (“CVA”), diabetes (“DM”), chronic obstructive pulmonary disease (“COPD”), obstructive sleep apnea (“OSA”), and pulmonary hypertension. In addition, intra- and post-operative variables including type of anesthesia (general anesthesia versus monitored anesthesia care), length of surgery, need for intraoperative transfusion of allogeneic blood products, need for intraoperative infusion of inotropic or vasopressor agents, post-operative development of respiratory complications and acute kidney injury, as well as, post-operative need for subsequent surgeries during the same admission and post-operative infections were collected so they could be evaluated as confounding factors. Post-operative respiratory complication was defined as prolonged intubation for more than 24 hours or need for re-intubation or tracheostomy. Post-operative acute kidney injury was defined as patients with a post-operative rise of creatinine greater than 60% from the baseline19. Post-operative need for subsequent surgeries included all procedures that required anesthesia care. Post-operative infection was defined as a composite event including wound or surgical site infection, urinary tract infection, pulmonary infection, and systemic infection.
Evaluation of Cardiac Function
The preoperative echocardiogram obtained within one year of the index surgery was used to identify patients with right ventricular dysfunction. All of the echo studies were originally performed by the cardiology service at the study institution. Almost all of the echo studies were transthoracic echocardiogram with only two studies being transesophageal echocardiogram. Both images and results of the studies were reviewed in the institution’s cardiovascular imaging database (Syngo Dynamics – Siemens Healthcare, Tarrytown, NY); online software that permitted image review and processing, as well as retrieval of the original study reports. Study reports were reviewed and the following collected: left ventricular ejection fraction (“EF”), right ventricular systolic pressure (“RVSP”), any valvular pathology categorized as severe, presence of left ventricular diastolic dysfunction, and right ventricular function.
Right ventricular (“RV”) function was reported as a binary variable (normal versus abnormal). RV function collected from the official report was determined based on visual estimation by the cardiologists. A second experienced cardiologist was asked to review all echo studies and visually estimate right ventricular function independently from the results of the official report. The second experienced cardiologist was blinded to all clinical information. Visual estimation of the right ventricular function was determined based on multiple acoustic windows including apical 4-chamber (lateral wall of the RV and RV apex), parasternal short-axis (anterior, lateral, and inferior wall of the RV), parasternal RV inflow (anterior and inferior wall of the RV), and subcostal 4-chamber (inferior wall of the RV). For echo studies in which the second cardiologist’s interpretation of the RV function was different from that of the original report, tricuspid annular plane systolic excursion (TAPSE) was obtained. A TAPSE <1.6cm was used as the cutoff to differentiate abnormal from normal RV function when the disagreements occurred15. TAPSE was obtained by the principle investigator who is echo certified by the National Board of Echocardiography. The principle investigator was also blinded to all clinical information at the time in which TAPSE was obtained.
Outcomes and Definitions
Primary outcome was incidence of post-operative major adverse cardiovascular events (“MACE”). Post-operative MACE was defined broadly as composite events including non-fatal cardiac arrest, myocardial infarction, development of congestive heart failure, cerebrovascular event such as stroke, and cardiovascular mortality defined as death attributable to any- or a combination of the adverse cardiovascular events just described16–18. Secondary outcomes included length of hospital stay (LOS), as well as, in-hospital cardiac- and all-cause mortality.
Statistical Analysis
All statistical analysis was performed using SPSS for windows version 24 (SPSS Inc, Chicago, IL). Dichotomous variables are reported as counts and percentages while continuous variables are described as mean and standard deviation (if normally distributed) or median with interquartile range. For continuous variables, normal distribution was tested using Shapiro-Wilk test. Differences between the groups were performed using Fisher’s exact test for dichotomous variables, student t-test or Mann-Whitney U test for continuous variables with normal and non-normal distribution respectively. Logistic regression analysis was performed to estimate odds ratio (OR) and 95% confidence interval (CI) for effect of RV dysfunction on binary outcomes. Length of stay (LOS) was treated as count data; as such, LOS was analyzed via Poisson’s regression to estimate incident rate ratio (IRR) and 95% CI. Of note, all patients who had died during the hospital stay were not included in the analyses for LOS. Multivariate regression was performed for both logistic and Poisson’s regression to account for covariates. Only covariates found to be individually associated with outcome of interest with p<0.10 were included in the multivariate regression. Where 2 covariates are expected to have high association resulting in multicollinearity, only the continuous variable is included in the final multivariable model. For goodness-of-fit, Hosmer and Lemeshow test was used to test the logistic regression model while the Omnibus test was used for Poisson’s regression model. For all tests, a p-value <0.05 was considered statistically significant.
Results
Group Characteristics
108 patients were found that met criteria and were included for data analysis. The majority of the patients were male (75%) with a median age of 72[60, 78]. 9.3% of the patients had RV dysfunction. A comparison of demographic data in patients with and without RV dysfunction showed that there was no difference in sex and age between the groups (p= 0.264 and p=0.542 respectively). Comparing other preoperative covariates between the two groups, 70% of the patients with RV dysfunction had a history of CHF compare to 27% in patients without RV dysfunction (p=0.009). There was a higher percentage of patients with history of pulmonary hypertension in those with RV dysfunction (40% vs. 11%; p=0.049). Examining left ventricular ejection fraction (LVEF), the median LVEF was significantly lower in patients with RV dysfunction (p<0.001) (Figure 1). Comparing right ventricular systolic pressure (RVSP) between the two groups, those with RV dysfunction had a higher mean (p=0.009). Out of 108 patients, only six had severe valvular pathology. Among these six patients, only one patient belonged to the group with right ventricular dysfunction. As such, valvular pathology was not compared between the groups and not included in the subsequent analysis. In addition, a large percentage of patients had indeterminant diastolic dysfunction based on the final echo report. Therefore, diastolic dysfunction was not compared between the groups and excluded in the remaining analysis. For the remaining preoperative variables, there was no significant difference found between the two groups (Table 1).
Figure 1: Boxplot: Median LVEF.
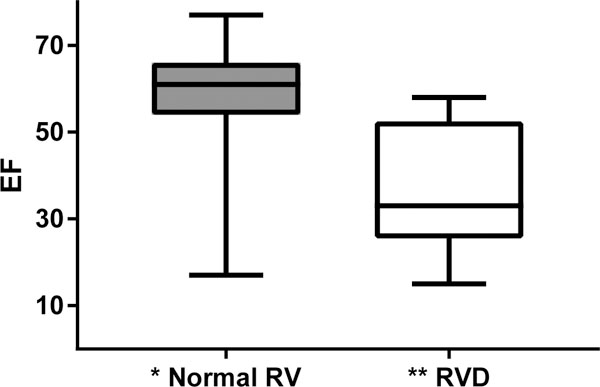
Abbreviation: EF, ejection fraction; RV, right ventricle; RVD, right ventricular dysfunction
*The median EF for patients with normal RV function is 61% (25th = 54%, 75th = 66%)
**The median EF for patients with RVD is 37% (25th = 27%, 75th = 52%)
Table 1–
Baseline Demographic and Comorbidity Data Between Groups
| Normal Right Ventricular Function N= 98 |
Right Ventricular Dysfunction N = 10 |
P value | |
|---|---|---|---|
| Male, n (%) | 75 (77) | 6 (60) | 0.264 |
| Age | 72 (62–79) | 70 (47–80) | 0.542 |
| CAD, n (%) | 49 (50) | 8 (80) | 0.098 |
| CHF, n (%) | 26 (27) | 7 (70) | 0.009 |
| CVA, n (%) | 15 (15) | 4 (40) | 0.072 |
| DM, n (%) | 33 (34) | 2 (20) | 0.494 |
| COPD, n (%) | 22 (22) | 2 (20) | 1.000 |
| OSA, n (%) | 5 (5) | 0 (0) | 1.000 |
| Pulmonary HTN, n (%) | 11 (11) | 4 (40) | 0.049 |
| HTN, n (%) | 84 (86) | 9 (90) | 1.000 |
| RVSP | 35.0 (13.3) | 47.4 (11.8) | 0.009 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; CVA, cerebrovascular accident; DM, diabetes; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea, Pulmonary HTN, pulmonary hypertension; HTN, hypertension; RVSP, right ventricular systolic pressure.
Data are presented as mean ± standard deviation for RVSP, median (25th, 75th) for age, and n (%) for categorical variables. Groups are compared using Student’s t-test for RVSP, Mann-Whitney U test for age and Fisher’s exact test for categorical variables
For intraoperative variables, there was no difference between the groups in regards to length of surgery, types of anesthesia, use of infusion of inotropic or vasopressor agents, and need for transfusion of allogenic blood products (Table 4). Finally, for postoperative variables, no difference was found between the groups in regards to incidence of postoperative respiratory complications and acute kidney injury (Table 4).
Table 4–
Other Intra- and Post-operative Variables & Remaining Secondary Outcomes
| Normal Right Ventricular Function N= 98 |
Right Ventricular Dysfunction N = 10 |
P value | |
|---|---|---|---|
| Other Intraoperative- & Post-operative Variables | |||
| Intraoperative Infusion of Inotropic Medication |
24 (25) | 3 (30) | 0.708 |
| Intraoperative Need for Transfusion |
46 (47) | 4 (40) | 0.749 |
| Postoperative- Respiratory Complications |
18 (18) | 3 (30) | 0.379 |
| Postoperative AKI | 16 (16) | 2 (20) | 0.672 |
| Length of Surgery | 392 (300–522) | 362 (201–433) | 0.146 |
| General Anesthesia | 91 (93) | 9 (90) | 0.553 |
| Remaining Secondary Outcome | |||
| In-hospital Cardiac Death |
4 (4) | 1 (10) | 0.391 |
| In-hospital All Cause Death |
6 (6) | 2 (20) | 0.160 |
Abbreviation: AKI, acute kidney injury. All values are reported as count (%).
Length of surgery is reported in minutes as median (25%, 75%).
Primary Outcome
Univariate Analyses
A total of 10 patients suffered post-operative MACE making an overall incidence of 9.3%. 7 of the patients had at least one cardiac arrest event, one patient had an acute decompensated heart failure, one patient suffered a myocardial infarction, and one patient suffered a stroke. The incidence of MACE for those with RV dysfunction was 40% compared to 6.1% in those without RV dysfunction (p=0.006). In the univariate analyses, the odds ratio (OR) for those with MACE was 10.1 in patients with RV dysfunction compare to those without RV dysfunction (95% CI, 2.2–45.8; p=0.003). Using LVEF as a single predictor, an increase in EF predicted a lower risk of MACE with an OR = 0.9 (95% CI, 0.9–1.0; p=0.012). In the subgroup analysis, for patients with moderately reduced EF defined as EF <45%, the OR for MACE was 7.8 compared to those with normal EF (95% CI, 1.9–32.9; p=0.005). For patients with low EF defined as EF <35%, the OR for MACE was 11.7 (95% CI, 2.5–55.5; p=0.002) compared to patients with EF > 35%. In the remaining univariate analyses, history of CHF, CVA, and pulmonary hypertension, as well as right ventricular systolic pressure (RVSP), were not associated with post-operative MACE (Table 2).
Table 2–
Models for Postoperative Major Cardiac Events
| OR | 95% CI | P-value | |
|---|---|---|---|
| Univariable Models | |||
| RVD | 10.1 | 2.2, 45.8 | 0.003 |
| LVEF | 0.9 | 0.9, 1.0 | 0.012 |
| LVEF <45% | 7.8 | 1.9, 32.9 | 0.005 |
| LVEF <35% | 11.7 | 2.5, 55.5 | 0.002 |
| CHF | 1.0 | 0.2, 4.0 | 0.952 |
| CVA | 1.2 | 0.2, 6.0 | 0.846 |
| Pulmonary HTN | 1.9 | 0.3, 10.3 | 0.471 |
| RVSP | 1.0 | 1.0, 1.1 | 0.318 |
| Multivariable Model | |||
| RVD | 6.3 | 1.0, 38.5 | 0.046 |
| LVEF | 1.0 | 0.9, 1.0 | 0.212 |
Abbreviations: RVD, right ventricular dysfunction; LVEF, left ventricular ejection fraction; CHF, congestive heart failure; CVA, cerebrovascular accident; pulmonary HTN, pulmonary hypertension; RVSP, right ventricular systolic pressure; OR, odds ratio; CI, confidence interval.
Logistic regression was performed to estimate OR, 95% CI, and p-value. Only covariate that is individually associated with outcome with a p value < 0.1 was included in the multivariable model. Only LVEF was included in the multivariable model because of multi-collinearity.
Multivariate analyses
Since raw LVEF, EF <45%, and EF <35% were expected to exhibit collinearity, only LVEF was selected as a covariate in the multivariate analyses. After controlling for LVEF, RV dysfunction was independently associated with MACE with an OR = 6.3 (95% CI, 1.0–38.5; p=0.046), whereas LVEF was not independently associated with MACE (OR [95% CI], 1.0 [0.92–1.02]) (Table 2).
Secondary Outcomes
Patients with RV dysfunction had 50% longer length of stay (LOS) than those without RV dysfunction (IRR [95% CI], 1.5 [1.2–1.8]; p<0.001). Other covariates that were significantly associated with longer LOS in univariate analysis included the need for multiple surgeries during the hospital stay (IRR [95% CI], 2.0 [1.7 – 2.2]; p<0.001), presence of postoperative infection (IRR [95% CI], 1.3 [1.1 – 1.5]; p=0.001), and RVSP (IRR [95% CI], 1.0 [1.0–1.0]; p<0.001). In contrast, LVEF did not predict LOS (IRR [95% CI], 1.0 [1.0–1.0]). In multivariable analysis for LOS, RVD, RVSP, and postoperative need for multiple surgeries were all independently associated with longer LOS in the final model (p=0.001, p<0.001, and p<0.001 respectively) (Table 3-Model 5). Accounting for RVSP and postoperative need for multiple surgeries, RVD continued to be an independent risk factor for LOS (IRR [95% CI], 3.8 [1.8 – 8.32]; p=0.001).
Table 3–
Models for Length of Stay
| IRR | 95% CI | P-value | |
|---|---|---|---|
| Univariable Model | |||
| RVD | 1.50 | 1.2–1.8 | <0.001 |
| Multiple surgeries | 1.96 | 1.7–2.2 | < 0.001 |
| Post-infection | 1.28 | 1.1–1.5 | 0.001 |
| LVEF | 1.00 | 1.0–1.0 | 0.427 |
| EF<45% | 1.02 | 0.9–1.2 | 0.846 |
| EF<35% | 1.19 | 1.0–1.5 | 0.095 |
| CHF | 0.91 | 0.8–1.0 | 0.137 |
| CAD | 1.09 | 1.0–1.2 | 0.146 |
| CVA | 1.01 | 0.9–1.2 | 0.892 |
| Pulmonary HTN | 1.16 | 1.0–1.4 | 0.074 |
| RVSP | 1.01 | 1.0–1.0 | < 0.001 |
| Multivariable Model 1 | |||
| RVD | 1.57 | 1.2–2.0 | <0.001 |
| LVEF<35% | 0.90 | 0.7–1.2 | 0.379 |
| Multivariable Model 2 | |||
| RVD | 1.46 | 1.2–1.8 | <0.001 |
| Pulmonary HTN | 1.09 | 0.9–1.3 | 0.316 |
| Multivariable Model 3 | |||
| RVD | 1.51 | 1.2–1.8 | <0.001 |
| Postop Infection | 1.37 | 1.2–1.6 | <0.001 |
| Multivariable Model 4 | |||
| RVD | 1.57 | 1.3–1.9 | <0.001 |
| Postop Infection | 1.16 | 1.0–1.4 | 0.103 |
| Postop Need for Multiple Surgeries | 1.62 | 1.4–1.9 | <0.001 |
| Pulmonary HTN | 0.89 | 0.7–1.1 | 0.205 |
| Multivariable Model 5 | |||
| RVD | 3.82 | 1.8–8.3 | 0.001 |
| RVSP | 1.01 | 1.0–1.0 | < 0.001 |
| RVSPxRVD | 0.98 | 1.0–1.0 | 0.012 |
| Postop Need for Multiple Surgeries | 1.89 | 1.9–2.5 | < 0.001 |
Abbreviations: RVD, right ventricular dysfunction; LVEF, left ventricular ejection fraction; CHF congestive heart failure; CVA, cerebrovascular accident; Pulmonary HTN, pulmonary hypertension; RVSP, right ventricular systolic pressure; IRR, incident rate ratio; CI, confidence interval.
Poisson’s regression was performed to estimate IRR, 95% CI, and p-value. Only covariate that is individually associated with outcome with a p value < 0.1 was included in the multivariable model. LVEF <35% was removed in model 4 due to non-significant Omnibus test. Pulmonary HTN was removed in model 5 due to multicollinearity. All CI noted to be 1.0–1.0 are results of rounding.
For in-hospital mortality, there were a total of 8 deaths (7.4%). One patient died from massive intraoperative bleeding, one died from septic shock, two died from post-operative cardiac arrest, and the remaining died from protracted post-operative course relating to surgical complications. There was no difference in incidence of all-cause mortality between patients with and without RV dysfunction (p = 0.391). Similarly, for cardiac-related in-hospital mortality, there was no difference in incidence between patients with and without RV dysfunction (p=0.160) (Table 4).
Discussion
Key Findings
In this study, the prognostic significance of preexisting RV dysfunction in high-risk patients undergoing non-emergent major vascular surgery was evaluated. RV dysfunction was found to be independently associated with higher incidence of post-operative major cardiovascular events (MACE) and longer length of hospital stay. Indeed, the results showed that after accounting for left ventricular function, patients with RV dysfunction remained to have more than 6-fold risk for postoperative MACE compared to patients with normal RV function. In addition, patients with RV dysfunction had 50% longer hospital stay compare to those with normal RV function even after adjusting for factors known to contribute to longer LOS.
Implications
These results have significant clinical implications. First, right ventricular function has important prognostic value in high-risk patients undergoing major vascular surgery. Preoperative assessment of right ventricular function in patients with comorbidities known to be associated with right ventricular dysfunction should be considered especially when surgeries are performed electively. In addition, presence of right ventricular dysfunction should be considered as having added perioperative risk during stages of surgical planning and risk-discussion with patients. Second, perioperative management should involve strategies that aim to mitigate factors that can worsen right ventricular function in patients with pre-existing dysfunction. These strategies may include avoiding factors that can worsen pulmonary arterial pressure, instituting mechanical ventilatory strategies that promote low plateau and low peep pressure20, optimal blood pressure control to maintain coronary perfusion to the already compromised right ventricle, and consideration for invasive monitoring such as intraoperative transesophageal echocardiogram to guide both fluid and inotropic management.
Other Findings
The finding that left ventricular ejection fraction did not reach a statistical significance for association with postoperative MACE was interesting. While this may be due do the small sample size being underpowered, the results from this present study agreed with findings from prior studies. In Sprung et al. and Karkos et. al., a lower or abnormal ejection fraction was found not to be associated with postoperative myocardial infarction in vascular surgical cohorts6,7. Similarly, Martyal et. al. did not find left ventricular ejection fraction to be independently associated with postoperative cardiac complications in patients undergoing vascular surgery21. Finally, in a meta-analysis by Karkos et. al., resting LVEF was not a reliable predictor of perioperative cardiac events in vascular surgical cohort22. As suggested by Karkos et, al., resting LVEF does not always accurately predict cardiovascular response to perioperative stress. Together with the advancement in endovascular technology with decreased morbidity23, the reliability of LVEF as a predictor for perioperative cardiac events in patients undergoing vascular surgery is still questionable.
In the multivariable analysis for LOS, an interaction term between RVSP and RV dysfunction (RVSPxRVD) was included based on the clinical knowledge that RVSP reflects pressure generated by the right ventricle during systole. As such, RVSP is affected by right ventricular function itself and may be lower in a patient with poor right ventricular systolic function compare to one with normal function in presence of pulmonary hypertension. This reasoning is supported by our observation that while the mean RVSP is higher in those with RV dysfunction, patients with highest RVSP (2 standard deviations above the mean) all had normal RV function.
Of interest, we found that the interaction term of RVSPxRVD had a coefficient of 0.98 (p=0.012) for LOS. However, this does not represent a protective effect. Rather, this reflects the interaction between RVSP and RV function. To shed light on this observation, we performed a post-hoc analysis on the association between RVSP and LOS in relation to the interaction between RVSP and RV function. Dividing patients into 2 groups, those with RVSP less than and equal to 1 standard deviation (STD) from the mean and those with RVSP greater than 1 STD, we found a positive correlation between RVSP and LOS in the first group but a negative correlation in the latter group (Figure 3 & 4). That is, the direction of the association between RVSP and LOS appears to be opposite between groups with lower versus higher RVSP, and high RVSP appears to be associated with shorter LOS. However, evaluating the distribution of patients with and without RV dysfunction among those with RVSP greater than 1 STDs from the mean, we found that patients with an RVSP greater than 2 STDs (RVSP>63.8mmHg) all had normal RV function (Figure 4). As such, it is not that high RVSP is associated with shorter LOS; rather, those with high RVSP with normal RV function did not have prolonged LOS. From this, we can conclude that there is an interaction between RVSP and RV function, and that RV function is the mediator underlying the discrepant association between RVSP and LOS in patients with low versus high RVSP.
Figure 3: Scatterplot: Relationship between Length of Stay and RVSP in Subjects with RVSP ≤ 1 STD.
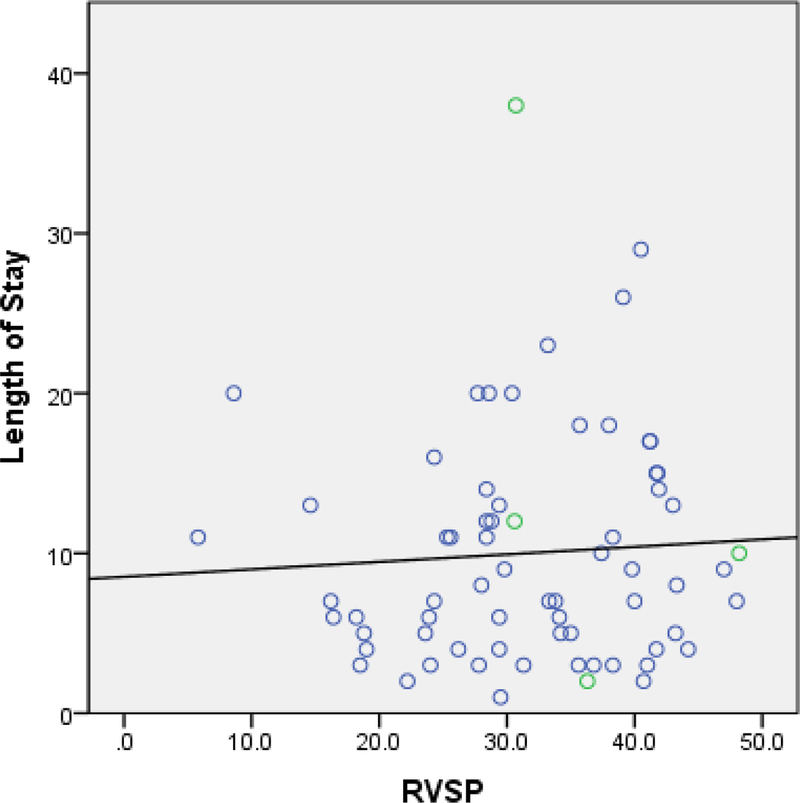
Abbreviation: RVSP, right ventricular systolic pressure; STD, standard deviation
Blue open circle = subjects with normal right ventricular function
Green open circle = subjects with right ventricular dysfunction
Figure 4: Scatterplot: Relationship between Length of Stay and RVSP in Subjects with RVSP > 1 STD.
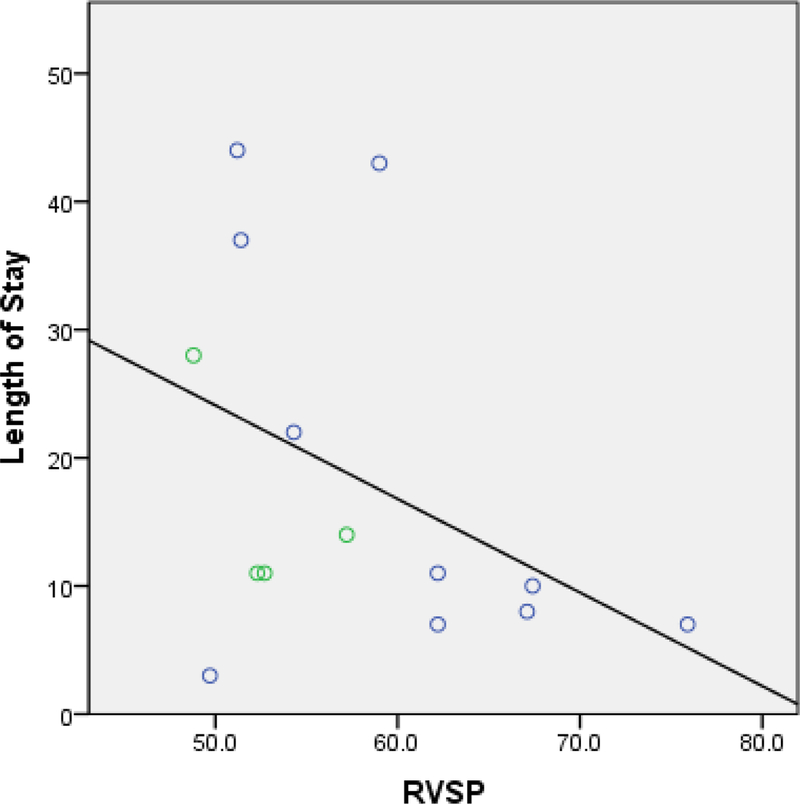
Abbreviation: RVSP, right ventricular systolic pressure; STD, standard deviation
Blue open circle = subjects with normal right ventricular function
Green open circle = subjects with right ventricular dysfunction
Limitations
While the study found an 9% prevalence for right ventricular dysfunction in this surgical cohort, more study is needed to validate this finding due to selection bias. By including only patients with preoperative echo, it is possible that patients with cardiac disease were preferentially selected. As such, the prevalence for RV dysfunction reported in this study may be overestimated. For assessment of right ventricular function, using one-year cutoff for preoperative echo is another limitation since cardiac function may change or even become progressively worse. However, the majority of the patients in this cohort (86%) had preoperative echo within one month of their designated surgery. There was only 1 patient with preoperative echo being closer to the one-year cutoff. For the remaining patients, echoes were performed within 2–6 months.
The relative overall small sample size resulted in the wide confidence intervals observed in this study. In addition, the small sample size and low event rates likely resulted in the study being underpowered to detect an association between RV dysfunction and in-hospital cardiac mortality; there were only 2 deaths that were attributed to cardiac complications in the cohort.
Other study limitations include data being collected from a single tertiary care center and the study being retrospective in nature. Since this study was performed only at one single center, generalization of the findings may be limited. Another major limitation is the retrospective nature of the study. Because only covariates that were well and consistently documented could be evaluated, it is possible that other unexplored variables are also associated with outcomes and may lead to confounding. In addition, events such as myocardial infarction was identified based purely on progress notes or discharge summary rather than on laboratory testing such as troponin, the findings of the study are dependent on accuracy of the medical charting. Of note, there is also limitation in using right ventricular systolic pressure (RVSP) as a surrogate marker for pulmonary arterial pressure since RVSP is dependent on quality of the tricuspid regurgitation spectral doppler signal and how estimation of right atrial pressure was derived. However, we believe the choice of RVSP is a reasonable one since preoperative right heart catheterization was not performed in this cohort.
Lastly, determining RV function based on visual estimation is inferior compare to an objective approach. However, we were able to obtain a Cohen’s kappa coefficient of 0.496 suggesting a moderate agreement between cardiologists who had read and interpreted the echo images. For the 10 cases (9%) in which there was disagreement in regards to RV function, we were able to obtain TAPSE as an objective parameter to determine RV function. Of note, we were not able to use TAPSE for all patients in this cohort because about one third of the echo studies had images that did not allow TAPSE measurement.
While the results of this study have important clinical implications, additional studies are needed to validate current findings. Future study approach including the use of a multi-centered dataset and a larger sample size will be of particular importance. A multi-centered study will not only increase the study power but also allow generalization of the findings. In addition, including other a priori covariates may shed lights on their effects on postoperative cardiac complications and interactions with presence of RV dysfunction. Notably, covariate such as diastolic dysfunction would be an important potential confounding factor since it is known that prevalence of diastolic dysfunction is high among vascular surgical cohort17. Finally, the use of newer echo parameters such as speckle tracking strain-and strain rate and 3D echo to assess RV function may have the advantages of having higher sensitivity and may add invaluable insight to current findings.
Conclusion
In this retrospective study of high-risk patients undergoing non-emergent major vascular surgery, preexisting right ventricular dysfunction was independently associated with higher incidence of postoperative major cardiovascular events and longer length of hospital stay. In contrast, left ventricular ejection fraction was not independently associated with these outcomes. Based on these findings, the prognostic value of RV dysfunction extends beyond the cardiac surgical cohort. Therefore, knowledge in the role of RV dysfunction, its pathophysiology, and its management in the perioperative setting should be understood by all anesthesiologists and physicians providing care in the perioperative setting.
Figure 2: Multiple Poisson Model on Length of Stay (Table 3 Model 5).
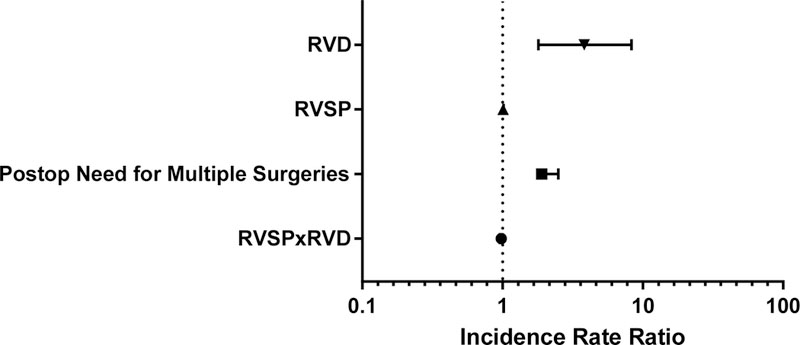
Abbreviation: RVD, right ventricular dysfunction; RVSP, right ventricular systolic pressure;
RVSPxRVD represents interactive term between RVSP and RVD
All covariates included in this final model are statistically significant with p<0.05
Acknowledgments
This work was supported by Medicine Faculty Research Award, School of Medicine – University of California Irvine.
References
- 1.Andrea L, Smeili A, Lotufo PA: Original Article Incidence and Predictors of Cardiovascular Complications and Death after Vascular Surgery. :510–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flu W, Schouten O, Van Kuijk J, et al. : Perioperative Cardiac Damage in Vascular Surgery Patients. [Internet]Eur. J. Vasc. Endovasc. Surg. [Internet] Elsevier Ltd, 40:1–8, 2010. Retrieved from: 10.1016/j.ejvs.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 3.Parmar CD, Torella F: Prediction of Major Adverse Cardiac Events in Vascular Surgery: Are Cardiac Risk Scores of Any Practical Value? Vasc. Endovascular Surg.2010 [DOI] [PubMed] [Google Scholar]
- 4.Kertai MD, Klein J, Van Urk H, et al. : Cardiac complications after elective major vascular surgery. Acta Anaesthesiol. Scand. 47:643–54, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Galland RB, Michaels JA, Toms A, et al. : A comparison of clinical index and ejection fractions in predicting cardiac complications following infrarenal aortic reconstruction. Eur. J. Vasc. Endovasc. Surg.1995 [DOI] [PubMed] [Google Scholar]
- 6.Karkos CD, Thomson GJL, Hughes R, et al. : Prediction of cardiac risk before abdominal aortic reconstruction: Comparison of a revised Goldman Cardiac Risk Index and radioisotope ejection fraction. J. Vasc. Surg.2002 [DOI] [PubMed] [Google Scholar]
- 7.Sprung J, Abdelmalak B, Gottlieb A, et al. : Analysis of risk factors for myocardial infarction and cardiac mortality after major vascular surgery. Anesthesiology 93:129–40, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Bertges DJ, Goodney PP, Zhao Y, et al. : The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J. Vasc. Surg.2010 [DOI] [PubMed] [Google Scholar]
- 9.Gorter TM, van Veldhuisen DJ, Bauersachs J, et al. : Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail.2018 [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group.: KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl.2012 [Google Scholar]
- 11.Santamore WP, Dell’Italia LJ: Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Prog. Cardiovasc. Dis. 40:289–308, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Kevin LG, Barnard M: Right ventricular failure. [Internet]Contin. Educ. Anaesthesia, Crit. Care Pain [Internet] 7:89–94, 2007. Retrieved from: https://academic.oup.com/bjaed/article-lookup/doi/10.1093/bjaceaccp/mkm016 [Google Scholar]
- 13.Maslow AD, Regan MM, Panzica P, et al. : Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth. Analg.2002 [DOI] [PubMed] [Google Scholar]
- 14.Lella LK, Sales VL, Goldsmith Y, et al. : Reduced right ventricular function predicts long-term cardiac re-hospitalization after cardiac surgery. PLoS One 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ternacle J, Berry M, Cognet T, et al. : Prognostic value of right ventricular two-dimensional global strain in patients referred for cardiac surgery. J. Am. Soc. Echocardiogr.2013 [DOI] [PubMed] [Google Scholar]
- 16.Kip KE, Hollabaugh K, Marroquin OC, et al. : The Problem With Composite End Points in Cardiovascular Studies. The Story of Major Adverse Cardiac Events and Percutaneous Coronary Intervention. J. Am. Coll. Cardiol.2008 [DOI] [PubMed] [Google Scholar]
- 17.de Jong M, van der Worp HB, van der Graaf Y, et al. : Pioglitazone and the secondary prevention of cardiovascular disease. A meta-analysis of randomized-controlled trials. Cardiovasc. Diabetol.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heianza Y, Ma W, Manson JE, et al. : Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta‐Analysis of Prospective Studies. J. Am. Heart Assoc.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudski L, Lai W, Afilalo J, et al. : Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr.2010 [DOI] [PubMed] [Google Scholar]
- 20.Nanjangud P, Nileshwar A: Strategies in Patients with Right Ventricular Failure on Mechanical Ventilation. Indian J. Respir. Care 6:791–2, 2018 [Google Scholar]
- 21.Matyal R, Hess PE, Subramaniam B, et al. : Perioperative diastolic dysfunction during vascular surgery and its association with postoperative outcome. J. Vasc. Surg.2009 [DOI] [PubMed] [Google Scholar]
- 22.Karkos CD, Baguneid MS, Triposkiadis F, et al. : Routine measurement of radioisotope left ventricular ejection fraction prior to vascular surgery: It is worthwhile? Eur. J. Vasc. Endovasc. Surg. 27:227–38, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Arko FR, Hill BB, Olcott C, et al. : Endovascular repair reduces early and late morbidity compared to open surgery for abdominal aortic aneurysm. J. Endovasc. Ther.2002 [DOI] [PubMed] [Google Scholar]


