Abstract
Objective:
To report the electrodiagnostic features of immune checkpoint inhibitor (ICI)-related neuropathy.
Methods:
We retrospectively reviewed clinical presentations and electrodiagnostic features of 23 patients studied after receiving immune checkpoint inhibitors (ICIs). The presentations for electrodiagnostic evaluation included an acute neuropathy or neuromuscular junction disorder. We applied established electrodiagnostic criteria for polyneuropathy and acute demyelinating neuropathy.
Results:
We identified acute demyelinating neuropathy (13 cases), axonal sensory motor neuropathy (5), pure sensory neuropathy (4) and mononeuropathy (1). 13 patients had acute demyelinating neuropathy confirmed by demonstrating demyelination in 2 or more nerves; 3 additional patients had demyelination in only one nerve. Analysis of motor nerve conduction parameters revealed demyelination involving median and ulnar nerve distal motor latencies as well as median, ulnar and peroneal nerve conduction velocities. Conduction block was found in median, ulnar and peroneal nerves. The remaining one-third patients without demyelination had acute painful axonal neuropathy. Coexisting myopathic changes (6) and neuromuscular junction dysfunction (4) were also identified.
Conclusions:
Our findings suggest that, while immune-mediated motor nerve demyelination is the primary underlying mechanism of ICI-related neuropathy, axonal painful neuropathy can also be an important presentation. Early recognition and effective intervention may reduce morbidity and permanent disability.
Significance:
Electrophysiological studies might be useful in the evaluation of ICI-related neuropathy.
Keywords: Immune checkpoint inhibitor, Immune-mediated polyneuropathy, Acute demyelinating polyradiculoneuropathy, Electrophysiological study
Introduction
Several immune checkpoint inhibitors (ICIs), including ipilimumab (anti-CTLA4), nivolumab and pembrolizumab (anti-PD1) and atezolizumab (anti-PD-L1) antibodies, have been used successfully to treat various cancers such as melanoma, sarcoma, and malignancies of lung, bladder, kidney, and colon. The widespread prescription of ICIs is leading to an unprecedented occurrence of neurological immune-related adverse effects (irAEs) in cancer patients. These irAEs may involve every level of peripheral nervous system individually or in combination, including peripheral nerve, neuromuscular junction and muscle. Several case reports of these neuromuscular complications have documented acute demyelinating polyradiculoneuropathy (AIDP) including Miller Fisher syndrome, myositis and myasthenia gravis (Suzuki et al. , 2017, Baird-Gunning et al. , 2018, Touat et al. , 2018).
Peripheral nervous system complications of ICIs can be highly variable. They may have an acute onset similar to Guillain-Barré syndrome (GBS) (Wilgenhof et al. , 2011, Bompaire et al. , 2012, Gaudy-Marqueste et al. , 2013) or a more indolent and protracted development like chronic inflammatory demyelinating polyneuropathy (CIDP) (Liao et al. , 2014, Thaipisuttikul et al. , 2015). They also may occur at the start or even several months after completion of a course of ICIs. In a retrospective review of neurologic complications of ICIs at our institution, we identified 38 patients with peripheral nervous system complications and underwent electrodiagnostic evaluation. In this report we examine the electrophysiological features of these peripheral nervous system irAEs in 23 patients who underwent electrodiagnostic studies, in order to understand its underlying pathophysiologic mechanisms and to develop more sensitive and accurate electrodiagnostic parameters for confirming the clinical diagnosis.
Methods
Patient selection:
We performed a retrospective study of patients who were treated with ipilimumab (anti-CTLA4), nivolumab and pembrolizumab (anti-PD1), and atezolizumab (anti-PD-L1) either as monotherapy or in combination at Memorial Sloan Kettering Cancer Center (MSKCC) between October 2010 and June 2017. Only patients referred for electrodiagnostic evaluation for new neuromuscular symptoms that developed within 100 days of receiving ICIs were included in the study and all patients included underwent electrodiagnostic evaluation. One patient from this study has been reported elsewhere (Thaipisuttikul et al., 2015).
Electrodiagnostic studies:
Electrodiagnostic evaluation of compound muscle action potential (CMAP) in median, ulnar, peroneal and tibial nerves and sensory nerve action potential (SNAP) in median, ulnar, radial, superficial peroneal and sural nerves and needle electromyography (EMG) were performed based on the published protocols for evaluation of polyneuropathy(Donofrio et al. , 1990). All the patients underwent 2 to 3 limbs standard nerve conduction study. The studies were performed using Cadwell Sierra Wave EMG equipment (Cadwell industries, Inc., WA, USA) and skin temperature was maintained above 32°C for all electrodiagnostic studies.
The neuropathy severity was graded according to the previously published electrodiagnostic criteria for evaluation of chemotherapy-induced polyneuropathy at our institution (Chen et al. , 2013): no neuropathy when sural SNAP and other parameters are normal; mild when sural SNAP is less than 10 μV but present; moderate when sural SNAP is absent and SNAPs in upper extremities are also affected; and severe when radial SNAP is abnormal or absent and all the lower extremity CMAPs and SNAPs are absent.
Demyelinating neuropathy has four following electrodiagnostic features: (1) reduced motor conduction velocity; (2) motor conduction block or abnormal temporal dispersion; (3) prolonged motor distal latency; and (4) prolonged minimal F-wave latency or absent F waves. According to the published criteria for acute demyelinating neuropathy (Ho et al. , 1995), our criteria of acute demyelination were set at either lower than 85-90% of lower limit of normal conduction velocity, or higher than 110-120% of upper limit of normal latencies of our predetermined laboratory values. Possible conduction block was established when proximal to distal CMAP amplitude ratio was less than 70%.
Repetitive nerve stimulation of 3 Hz frequency was performed on ulnar, spinal accessory or facial nerves for evaluation of neuromuscular junction function as clinically indicated. More than 10% amplitude decrement of the responses in at least one of the nerves tested was determined as evidence of neuromuscular junction dysfunction. Concentric needle electromyography (EMG) was performed on deltoid, vastus, tibialis anterior, gastrocnemius and other selected muscles. Finding of diffuse or proximally predominant fibrillation and positive waves as well as myopathic motor unit action potentials were considered as evidence of myopathy.
Data collection:
The medical records were reviewed for clinical data, including cancer types, reasons for electrodiagnostic referral, history of ICI administration, prior neurotoxic chemotherapy, cerebrospinal fluid (CSF) results, and electrodiagnostic results including nerve conduction study, repetitive nerve stimulation and needle EMG findings. Clinically documented highest grade of neurologic toxicity based on common terminology criteria for adverse events (CTCAE), version 4.0 was recorded. This study has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and was approved by the Internal Review Board of MSKCC.
Results
Clinical:
A total of 4869 patients received ICIs as monotherapy or in combination at MSKCC during the study period. 38 patients had an electrodiagnostic study after developing new neuromuscular complaints including numbness, paresthesia, pain, weakness, muscle cramps, ptosis, diplopia, dysarthria and dyspnea (Table 1). Among them, 23 patients had an electrodiagnostic study performed within 100 days of last ICI administration (ranging from 2 to 94 days, median 20 days). The patients ranged in age from 44 to 92 years old; 18 were men and 5 women. 7 patients had melanoma, and other cancers included renal, non-small cell lung, prostate, bladder, colorectal, hepatic and Merkel cell cancers. 4 patients were diagnosed with chemotherapy-induced peripheral neuropathy based on clinical diagnosis prior to starting ICIs. Known neurotoxic agents were received by 8 patients prior to ICIs and included carboplatin (3), cisplatin (3), oxaliplatin (1), paclitaxel (1), vinorelbine (1) and docetaxel (1).
Table 1:
Patient Characteristics
| All patients (n=23) N (%) |
|
|---|---|
| Median age at time of treatment start, years (range) | 64 (44-92 years) |
| Gender | |
| Male | 18 (78%) |
| Female | 5 (22%) |
| Primary tumor type | |
| Melanoma | 7 (30%) |
| Non-small cell lung cancer | 4 (17%) |
| Renal cell cancer | 4 (17%) |
| Bladder | 3 (13%) |
| Prostate | 2 (9%) |
| Colorectal | 1 (4%) |
| Hepatocellular | 1 (4%) |
| Merkel cell cancer | 1 (4%) |
| Symptoms | |
| Numbness | 8 (35%) |
| Paresthesia | 13 (57%) |
| Pain | 14 (61%) |
| Weakness | 12 (52%) |
| Neuropathy on exam | 8 (35%) |
| Muscle cramps and pain | 2 (9%) |
| Ptosis and diplopia | 4 (17%) |
| Dysarthria | 1 (4%) |
| Dyspnea | 1 (4%) |
| Median time to onset of neurotoxicity, weeks (range) | 5 (1-43 weeks) |
| Doses of ICI received prior to onset, median (range) | 2 (1-20 doses) |
| Treatment | |
| Neuropathic pain medications | 12 (52%) |
| Corticosteroids | 19 (83%) |
| IVIG or plasmapheresis | 5 (22%) |
| Hospitalization | 12 (52%) |
ICI: immune checkpoint inhibitor; IVIG: intravenous immunoglobulin
Seven patients had cerebrospinal fluid (CSF) studied. Elevated CSF protein was found in 5 patients, ranging from 43 to 220 mg/dL. Pleocytosis ranging from 8 to 94 cells/μL was found in 5 patients with lymphocyte predominance in 4 cases and neutrophil predominance in 1 case.
Twelve patients required hospital admission at the onset of neuromuscular symptoms including muscle weakness (8 cases), pain (2), diplopia (1) and dysarthria (1). Corticosteroids including oral prednisone or intravenous methylprednisolone were used in 19 patients. 5 patients received additional immunomodulating therapy with intravenous immunoglobulin infusion and/or plasmapheresis. Among these 19 patients, 17 patients reported clinical improvement in neurological symptoms based on chart review, one patient had worsening symptoms and another patient died of respiratory failure. No patient received re-challenge with ICIs in our cohort.
Electrodiagnosis:
ICIs received include PD1, PD-L1, CTLA4 inhibitor as well as a combination of PD1 and CTLA4 inhibitors. The types of ICI-related neuropathy include demyelinating neuropathy, axonal sensorimotor polyneuropathy, pure sensory axonal neuropathy, and mononeuropathy multiplex based on electrophysiological studies. 6 patients had co-existent myopathy and other 4 cases showed neuromuscular junction dysfunction (Figure 1A).
Figure 1.
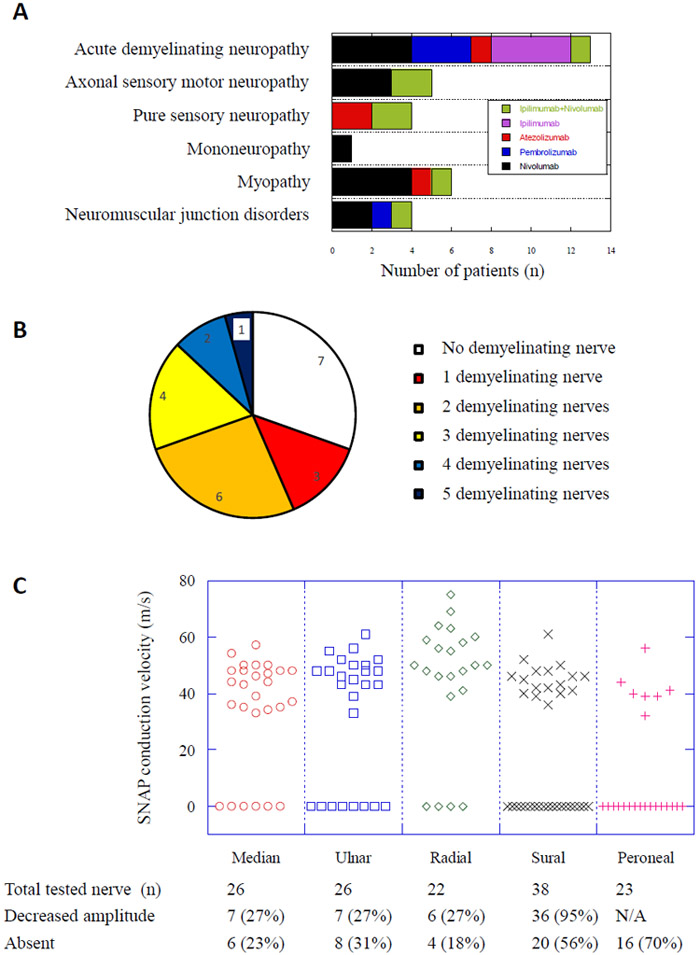
Panel A showed number of cases with various neuromuscular disorders as diagnosed with electrodiagnostic studies and the inset showed the related checkpoint inhibitors as represented with various colors. Panel B showed the proportions of cases showing various numbers of motor nerves with demyelination findings, ranging from 0 to 5 nerves as indicated by different colors. Panel C showed the results of sensory nerve conduction study in median, ulnar, radial, sural and superficial peroneal nerves. The distributions of SNAP conduction velocity were demonstrated in the graph. The nerves with absent SNAP response were represented as 0 in the graph. The numbers of nerves tested and nerves showing either decreased amplitudes or absent response were shown at the bottom.
Based on the established criteria for acute demyelinating neuropathy, 13 patients had 2 or more nerves with demyelination, 3 patients had only one nerve with demyelination and 7 patients do not have demyelination (Figure 1B). When more stringent criteria for chronic demyelinating polyneuropathy (Albers et al. , 1989) were used, only one patient fulfilled CIDP criteria while additional 8 patients had only a single nerve with abnormalities consistent with chronic demyelination as listed as some of the most abnormal values in the Table 2. Using the electrodiagnostic scale, neuropathy was graded as mild in 10 cases, moderate in 9 cases and severe in 4 cases. Their correlation with the clinical neurotoxicity scale was shown in Table 3.
Table 2.
Motor nerve conduction abnormalities suggestive of demyelination
| Parameters | Normal value |
M±SD (n) | Most abnormal value (%)* |
Number of tests |
Number of abnormality (%)^ |
Number of Demyelination (%)^ |
|---|---|---|---|---|---|---|
| Median N. DML | < 4 ms | 3.9±0.9 (23) | 5.5 (137.5%)# | 23 | 9 (39%) | 4 (17%) |
| Ulnar N. DML | < 3 ms | 3.0±0.6 (22) | 4.1 (136.7%)# | 22 | 10 (45%) | 9 (41%) |
| Peroneal N. DML | < 6 ms | 4.3±0.8 (35) | 6.1 (101.7%) | 38 | 3 (8%) | 0 (0%) |
| Tibial N. DML | < 7 ms | 5.5±1.0 (34) | 8.1 (115.7%) | 36 | 4 (11%) | 1 (3%) |
| Median N. CV | > 50 m/s | 49.3±6.1 (22) | 41(82%) | 22 | 11 (50%) | 6 (27%) |
| Ulnar N. CV | > 50 m/s | 52.1±8.0 (23) | 42 (84%) | 23 | 4 (17%) | 2 (9%) |
| Ulnar N. CV at elbow | > 50 m/s | 48.7±12.6 (20) | 30 (60%)# | 20 | 8 (40%) | 7 (35%) |
| Peroneal N. CV | > 40 m/s | 40.7±4.6 (20) | 25 (62.5%)# | 38 | 14 (29%) | 4 (11%) |
| Peroneal N. CV at knee | > 40 m/s | 45.2±9.4 (17) | 36 (90%) | 20 | 7 (35%) | 0 (0%) |
| Median F wave latency | < 32 m/s | 31.7±3.6 (19) | 35.7 (111.6%) | 20 | 9 (45%) | 1 (5%) |
| Ulnar F wave latency | < 32 m/s | 30.5±2.0 (14) | 33.7 (105.3%) | 19 | 9 (47%) | 0 (0%) |
| Peroneal F wave latency | < 55 m/s | 54.6±8.1 (19) | 80.5 (146.4%)# | 35 | 22 (63%) | 2 (6%) |
| Tibial F wave latency | < 55 m/s | 57.4±10.1 (24) | 68.9 (125.3%) | 35 | 26 (74%) | 2 (6%) |
| Median N. CB | 23 | 2 (9%) | ||||
| Ulnar N. CB | 23 | 4 (17%) | ||||
| Ulnar N. CB at elbow | 20 | 5 (25%) | ||||
| Peroneal N. CB | 38 | 12 (32%) | ||||
| Peroneal N. CB at knee | 21 | 3 (14%) |
Note: M±SD (n) showed mean and standard deviation of study values in the number (n) of patients whose responses were recordable.
The percentage changes from ULN or LLN were shown in parenthesis.
indicates the abnormal values consistent with chronic demyelination.
The percentage of abnormal test results was shown in the parenthesis.
ULN: upper limit of normal; LLN: lower limit of normal; N.: nerve; DML: distal motor latency; CV: conduction velocity; CB: conduction block.
Table 3.
Correlation of electrodiagnostic severity with CTCAE grade
| Severity based on NCS | CTCAE grade | Subtotal (%) | |||
|---|---|---|---|---|---|
| I | II | III | V | ||
| Mild | 4 | 5 | 1 | 10 (43%) | |
| Moderate | 6 | 3 | 9 (39%) | ||
| Severe | 2 | 2 | 4 (17%) | ||
CTCAE: common terminology criteria for adverse events;
NCS: nerve conduction study.
The abnormal motor conduction and demyelination were widely distributed in various segments of the peripheral nerves tested and revealed significant heterogeneity among individuals, even though the mean values of each parameter remained in the vicinity of normal limits (Table 2). The most common abnormalities are prolonged distal motor latencies of median (39%) and ulnar (45%) nerves as well as slowed conduction along the median (50%) and ulnar (17%) nerves in the forearm and peroneal nerve (29%) in the leg. Prolonged distal motor latency in the demyelination range was more common in median (17%) and ulnar (41%) nerves than tibial nerve (3%). Slow conduction consistent with demyelination and conduction block were found in peroneal, ulnar and median nerves where both distal and proximal motor nerve stimulations were performed, in both non-entrapment sites as well as entrapment sites such as ulnar nerve across the elbow. All patients had more significant neuropathy in lower extremities, consistent with polyneuropathy rather than multiple entrapment neuropathies limited to upper extremities.
Counterintuitively, very few F wave latencies showed prolongation consistent with demyelination in our series (Table 2). In further analysis, we noted more than 30% of either ulnar or tibial nerves and 46% of peroneal nerves failed to generate a recordable F response. 10 out of 11 tibial nerves without F response were classified as non-demyelination due to normal distal latencies. We suspect that the absence of tibial F wave in these cases might be due to proximal demyelination which was not further recognized since proximal tibial nerve stimulation was not performed in these cases.
Follow-up electrodiagnostic studies were performed in 8 patients at the intervals ranging from 8 to 404 days (median 164 days) after the first evaluation. 4 patients showed worsening neuropathy, 2 showed improvement and other 2 showed stable examination. Among the 4 patients with worsening of neuropathy, 3 patients had demyelination changes at the first evaluation. Neither the interval between last dose of ICIs and the first study nor the interval between two studies was noted to influence the above changes. The only fatal case (grade V in table 3) with GBS-like course had an electrodiagnostic study at the third day of symptom onset showing mild demyelinating neuropathy and myopathy and no follow-up study was performed due to rapid deterioration.
Seven patients did not have demyelination based on our criteria and were included in the groups of axonal sensorimotor polyneuropathy and pure sensory axonal neuropathy. All these patients had mild to moderate sensory neuropathy based on electrophysiological criteria and grade I to II based on CTCAE grading and none had severe neuropathy; 2 of these patients had coexistent myositis. Interestingly, while 50% of patients with demyelinating neuropathy did not report pain as a major symptom, all the patients with axonal neuropathy reported neuropathic pain as the reason for neurologic consultation. Patients with a history of prior exposure to neurotoxic chemotherapeutic agents or pre-existing chemotherapy-induced polyneuropathy was not found to be preponderantly related to a specific type of polyneuropathy.
Sensory nerve conduction study revealed a length-dependent axonal loss in 94% of patients with decreased amplitude or absent response from sural nerves. The conduction velocity of upper extremity SNAPs ranged from 33 to 75 m/s and lower extremity ranged from 32 to 61 m/s. In contrary to the typical pattern of GBS, the demyelination pattern or sural sparing pattern was not seen here possibly related to pre-existing chemotherapy-induced peripheral neuropathy in this group or due to immune-mediated sensory neuronopathy (Figure 1C).
As shown in Figure 1A, 6 patients had myopathic changes with myopathic motor unit action potentials and early recruitment in EMG of deltoid (2 cases), triceps (2), vastus (3), tibialis anterior (2) and gastrocnemius (3). Four of them showed significantly elevated serum creatine kinase (CK) (668 to 5231 units/L) at the time of study. Decremental response at 3 Hz repetitive nerve stimulation was found in 4 patients (2 cases at ulnar nerve, 1 each at facial and spinal accessory nerve). Further serological study found that 3 cases were positive for striated muscle antibody and 1 case was positive for P/Q-type calcium channel antibody, antineuronal nuclear antibody-1 (ANNA-1) and collapsing response mediator protein-5 (CRMP-5) antibodies. All 4 cases were negative for acetylcholine receptor and muscle specific kinase (MuSK) antibodies and clinically benefited from treatment of pyridostigmine and corticosteroids.
Discussion
ICIs (anti-CTLA4, anti-PD1, and anti-PD-L1 antibodies) kill malignant cells by restoring anti-cancer immunity. CTLA4 is an immune checkpoint molecule expressed on immune cells including both CD4+ and CD8+ T cell subsets as well as B cells. Ipilimumab, an anti-CTLA4 antibody, can restore priming and activation of T cells by tumor antigens and promote anti-tumor T cell response. PD-L1/PD-1 interaction is another checkpoint of anti-cancer immunity functioning at downstream of T cell priming and activation. While PD-1 is expressed on the surface of activated T cells, its specific ligand PD-L1 is present in multiple tissue types including many different cancer cells. The binding of PD-L1 to PD-1 inhibits T cell proliferation and activity, reduces cytokine production and hence helps malignant cells to evade the host immune response. Therapeutic antibodies engineered to target PD-L1/PD-1 interaction include those that inhibit PD-1 (nivolumab and pembrolizumab) or PD-L1 (atezolizumab). ICI therapy improves anti-tumor response and patient survival across multiple cancer types including melanoma, lung, bladder, renal, and colon cancers. The agents targeting the PD-L1/PD-1 pathway exhibit a more favorable toxicity profile than CTLA4 blockade and traditional chemotherapies (Lee et al. , 2018). While the most common irAEs associated with these ICIs are immune-related gastrointestinal, dermatologic, hepatic, and constitutional symptoms, immune-related neuromuscular complications are considered as rare events (Voskens et al. , 2013).
The autoimmune neuropathy can occur when immunologic tolerance to myelin or axonal antigens is lost. Clinically known autoimmune neuropathies include AIDP, CIDP, multifocal motor neuropathy (MMN), Anti-MAG/SPGP neuropathy, Anti-Hu sensory neuronopathy, vasculitic neuropathy, immune-mediated diabetic neuropathy, neuropathy with systemic autoimmune disorders and neuropathy due to immune reconstitution syndrome in treated HIV patients. Studies on clinical and experimental immune-mediated neuropathy have revealed that autoimmune responses toward peripheral nervous tissues are not limited to compact myelin along the main nerve trunk. They can also affect the neuronal body, axonal structures at the nodes of Ranvier and neuromuscular junction. As the rapid saltatory propagation of action potential along the motor axon relies on the integrity of compact myelin, immune-mediated demyelination may significantly slow conduction velocity, a phenomenon which is generally considered pathognomonic for autoimmune neuropathy. Based on this principle, various criteria for diagnosing inflammatory demyelinating neuropathy have been proposed but the existing criteria typically have low sensitivity for acute demyelination in the range of 39 to 89% (Van den Bergh et al. , 2004). Depending on the targets of autoimmunity, autoimmune neuropathy can also present as sensory neuronopathy such as anti-Hu (ANNA-1) neuropathy or axonal neuropathy such as acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN).
ICI-related neuropathy arises when anti-neuronal autoimmunity was unleashed due to therapeutic inhibition of checkpoint blockade. We reviewed 23 nerve conduction studies from patients with neuropathy after receiving ICIs. The relationship of neuropathy to administration of ICIs was supported by a close temporal association of symptomatic onset, a favorable response to corticosteroids or immunomodulating therapy and other clinical data obtained from chart review. The electrodiagnostic criteria suggestive of AIDP were met in 13 patients and additional 3 patients had one motor nerve demonstrating electrophysiological evidence of demyelination. These results are consistent with the hypothesis that compact myelin is likely to be the major target of ICI-related neuropathy. There were also 7 cases who presented with acute painful axonal neuropathy with no demyelination seen on electrophysiological study. In addition, sensory nerve conduction studies in all patients showed predominantly axonal changes. These results suggest that ICIs may also cause sensory predominant axonal degeneration and result in painful sensory neuropathy which cannot be distinguished from axonal neuropathy by electrophysiological methods. This finding is consistent with the previous clinical observations that ipilimumab can cause either painful paresthesia or neuralgiform neuropathy (Voskens et al. , 2013, Thaipisuttikul et al. , 2015). The other possible explanation is that a pre-existing neuropathy, whether it is due to entrapment, diabetes, radiation or chemotherapy-induced neuropathy, might increase the vulnerability to immune-mediated adverse effects and presents as worsening neuropathy after receiving ICIs.
We also identified coexistent 6 cases of myositis and 4 cases of neuromuscular junction disorders in our series, which appeared in difference from the report of 10 patients with ICI-related myositis with only one case having mild axonal sensory neuropathy (Touat et al. , 2018). The reason for the difference might be due to difference in the initial presentations, patient selection, prior history of chemotherapy and electrodiagnostic criteria applied.
The relatively poor correlation between the clinical severity and neurophysiologic abnormalities as shown in table 3 might result from the fact that the electrodiagnostic criteria we used are more suitable for chemotherapy induced axonal sensory neuropathy than the immune-mediated neuropathies as we described here. The other limitations of our study include its retrospective nature, rarity of ICI-related peripheral nerve injury and not complete exclusion of other possible superimposed etiologies of neuropathy. We also do not have any nerve biopsy samples to examine. However, our study confirmed that acute demyelination underlies most cases of ICI-related neuropathy, especially for severe and progressive cases. Our study also identified that one third of patients have only a painful axonal neuropathy which required further characterization. Since the actual frequency and natural history of ICI-related neuropathy are unclear, clinical vigilance, early discontinuation of ICIs and administration of corticosteroids and immune suppressants are required to prevent permanent disability or fatality. For these reasons, it is important that consulting neurologists can recognize these syndromes early and provide the best management for these complications. As demyelination polyneuropathy is not the typical presentation of chemotherapy-induced polyneuropathy (Chen et al. , 2013), our result suggests that electrophysiological evidence of acute demyelination in addition to clinical evidence for other autoimmune disorders including myositis and myasthenia gravis might have a high predictive value for ICI-related neuropathy in cancer patients.
HIGHLIGHTS.
Immune checkpoint inhibitors (ICIs) may induce immune-mediated polyneuropathy in rare cases.
ICI-related neuropathy presents as acute demyelinating polyneuropathy or axonal painful neuropathy.
Electrophysiological tests are useful in evaluation of ICI-related neuropathy.
Acknowledgements
This study is supported by National Institute of Health (NIH) Cancer Center Core Grant (P30-CA008748).
Abbreviations:
- ICI
immune checkpoint inhibitor
- PD1
programmed cell death protein 1
- PD-L1
programmed cell death protein 1 ligand
- CTLA4
Cytotoxic T-lymphocyte associated protein 4
- irAE
immune-related adverse effect
- AIDP
acute inflammatory demyelinating polyradiculoneuropathy
- GBS
Guillain-Barré syndrome
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CMAP
compound muscle action potential
- SNAP
sensory nerve action potential
- CTCAE
common terminology criteria for adverse events
Footnotes
Declaration of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers JW, Kelly JJ Jr. Acquired inflammatory demyelinating polyneuropathies: clinical and electrodiagnostic features. Muscle Nerve 1989;12:435–51. [DOI] [PubMed] [Google Scholar]
- Baird-Gunning JJD, Weerasinghe D, Silsby M, Gawarikar Y, Carlino MS, Smith JL, et al. Miller Fisher Syndrome Associated With Immunotherapy for Metastatic Melanoma. Neurohospitalist 2018;8:191–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bompaire F, Mateus C, Taillia H, De Greslan T, Lahutte M, Sallansonnet-Froment M, et al. Severe meningo-radiculo-neuritis associated with ipilimumab. Invest New Drugs 2012;30:2407–10. [DOI] [PubMed] [Google Scholar]
- Chen X, Stubblefield MD, Custodio CM, Hudis CA, Seidman AD, DeAngelis LM. Electrophysiological features of taxane-induced polyneuropathy in patients with breast cancer. J Clin Neurophysiol 2013;30:199–203. [DOI] [PubMed] [Google Scholar]
- Donofrio PD, Albers JW. AAEM minimonograph #34: polyneuropathy: classification by nerve conduction studies and electromyography. Muscle Nerve 1990;13:889–903. [DOI] [PubMed] [Google Scholar]
- Gaudy-Marqueste C, Monestier S, Franques J, Cantais E, Richard MA, Grob JJ. A severe case of ipilimumab-induced guillain-barre syndrome revealed by an occlusive enteric neuropathy: a differential diagnosis for ipilimumab-induced colitis. J Immunother 2013;36:77–8. [DOI] [PubMed] [Google Scholar]
- Ho TW, Mishu B, Li CY, Gao CY, Cornblath DR, Griffin JW, et al. Guillain-Barre syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain 1995;118:597–605. [DOI] [PubMed] [Google Scholar]
- Lee A, Sun S, Sandler A, Hoang T. Recent progress in therapeutic antibodies for cancer immunotherapy. Curr Opin Chem Biol 2018;44:56–65. [DOI] [PubMed] [Google Scholar]
- Liao B, Shroff S, Kamiya-Matsuoka C, Tummala S. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro-oncology. 2014;16:589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Ishikawa N, Konoeda F, Seki N, Fukushima S, Takahashi K, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017;89:1127–34. [DOI] [PubMed] [Google Scholar]
- Thaipisuttikul I, Chapman P, Avila EK. Peripheral neuropathy associated with ipilimumab: a report of 2 cases. J Immunother 2015;38:77–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touat M, Maisonobe T, Knauss S, Ben Hadj Salem O, Hervier B, Aure K, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–e94. [DOI] [PubMed] [Google Scholar]
- Van den Bergh PY, Pieret F. Electrodiagnostic criteria for acute and chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve 2004;29:565–74. [DOI] [PubMed] [Google Scholar]
- Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PloS One 2013;8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patient. Ann Oncol 2011;22:991–3. [DOI] [PubMed] [Google Scholar]


