Abstract
Vitamin A deficiency increases susceptibility to skin infection. However, the mechanisms by which vitamin A regulates skin immunity remain unclear. Here, we show that resistin-like molecule α (RELMα), a small secreted cysteine-rich protein, is expressed by epidermal keratinocytes and sebocytes and serves as an antimicrobial protein that is required for vitamin A-dependent resistance to skin infection. RELMα was induced by microbiota colonization of the murine skin, was bactericidal in vitro and protected against bacterial infection of the skin in vivo. RELMα expression required dietary vitamin A and was induced by the therapeutic vitamin A analog isotretinoin, which protected against skin infection in a RELMα-dependent manner. The RELM family member Resistin was expressed in human skin, was induced by vitamin A analogs, and killed skin bacteria, indicating a conserved function for RELM proteins in skin innate immunity. Our findings provide insight into how vitamin A promotes resistance to skin infection.
eTOC blurb
Vitamin A regulates skin immunity via unknown mechanisms. Harris et. al. show that RELMα is an antimicrobial protein expressed by epidermal cells that shapes resident skin bacterial communities and limits skin infection. RELMα is induced by dietary vitamin A and mediates vitamin A-dependent resistance to skin infection.
Graphical Abstract
Introduction
The skin is the human body’s largest organ and an essential barrier between the body’s internal tissues and the microbe-filled outer world. It is colonized by a diverse and complex community of commensal and pathogenic microorganisms that includes bacteria, viruses, fungi, and mites (Grice et al., 2008; Foulongne et al., 2012; Duerkop and Hooper, 2013; Oh et al., 2016; Findley et al., 2013; Grice et al., 2009).
The skin manages interactions with this diverse microbial community through multiple immune defense strategies. This includes the production of antimicrobial proteins that kill bacteria by targeting their essential cell wall or cell membrane structures (Gallo and Hooper, 2012). Several distinct antimicrobial protein families, including cathelicidins, S100 proteins, and β-defensins, have been identified in skin, and the cathelicidins have been shown to protect against skin infection (Gallo and Hooper, 2012). However, we still have a limited understanding of the diversity of antimicrobial proteins expressed by the skin, how antimicrobial proteins regulate skin microbial communities and protect against infection, and how skin antimicrobial proteins are regulated by environmental factors such as microorganisms and the host diet.
Vitamin A is a lipid-soluble nutrient that is essential for immunity to infection at many body sites. In the intestine, vitamin A is required for immunoglobulin A production by intestinal B cells (Mora et al., 2006) and T cell homing to the intestine (Iwata et al., 2004). These effects of vitamin A are mediated by its derivative, retinoic acid. Retinoic acid controls gene expression through retinoic acid receptors (RARs), transcription factors that control expression of specific target genes (Idres et al., 2002; Elder et al.; 1991). Vitamin A deficiency in humans results in markedly increased susceptibility to skin infection and inflammation (Russell and Suter, 2012), suggesting that vitamin A also promotes immune function in the skin. This idea is supported by the fact that therapeutic vitamin A analogs are frequently used to treat inflammatory skin diseases such as psoriasis and acne (Saurat, 1999; Orfanos et al., 1987; Ellis and Krach, 2001). However, little is known about how dietary vitamin A affects skin immunity.
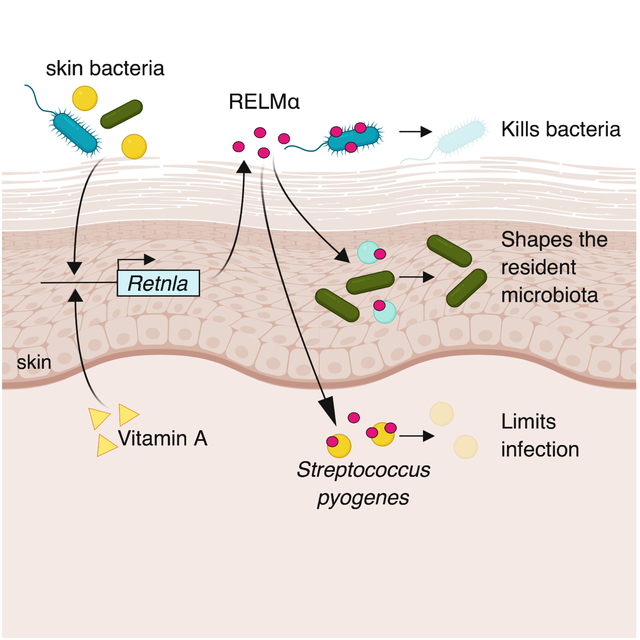
Here, we identify resistin-like molecule α (RELMα) as a skin antimicrobial protein that is essential for vitamin A-dependent resistance to skin infection. We find that bacterial colonization triggers expression of RELMα in mouse skin and that RELMα kills bacterial species that colonize the skin. We show that RELMα shapes the composition of resident skin bacterial communities and protects against pathogenic bacterial infection of the skin. Importantly, we find that dietary vitamin A is required for RELMα expression, and that the therapeutic vitamin A analog isotretinoin protects against skin infection in part through RELMα. Our findings thus illuminate a mechanism by which vitamin A promotes innate immunity and protects against skin infection.
Results
RELMα is expressed in the skin and expression is induced by the microbiota.
As a first step towards understanding how skin immunity is regulated by environmental factors, we sought to identify skin antimicrobial proteins whose expression is inducible by bacteria. We used whole transcriptome RNA-sequencing (RNAseq) to compare transcript abundances in the skin of germ-free mice to those in the skin of germ-free mice challenged topically with Staphylococcus aureus. This Gram-positive bacterial species resides in the nasopharynx of 30 percent of the human population and is a frequent cause of skin disease (Jenkins et al., 2015; Krismer et al., 2017; Kong et al., 2012; Kobayashi et al., 2015). Colonization with S. aureus had a broad impact on gene expression in the skin (Figure S1A and S1B). One of the most prominent responses to S. aureus challenge was an increase in the abundance of Retnla transcripts (Figure 1A and 1B). Colonization of germ-free mice with a microbiota derived from conventionally-raised mice also increased Retnla transcript abundance, and Retnla transcript abundance was higher in mice raised in a conventional facility as compared to germ-free mice (Figure 1C). These data establish that bacteria stimulate Retnla expression in the skin.
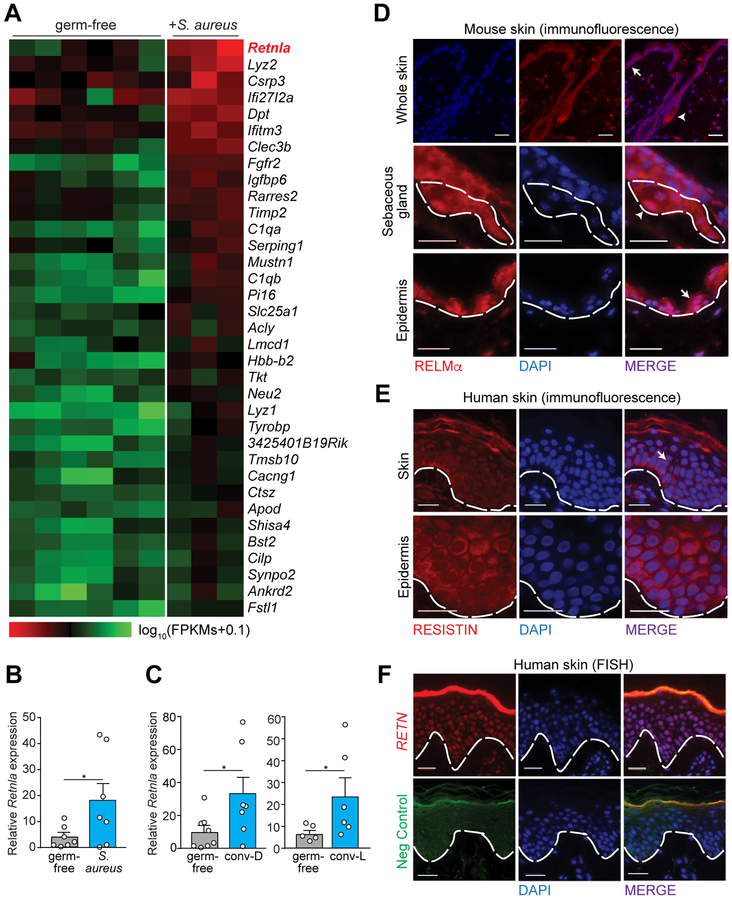
Figure 1: RELMα is expressed in the skin and expression is induced by the microbiota.
(A) Heatmap comparing transcript abundances in the skin of germ-free mice (n=6) and germ-free mice after topical exposure to Staphylococcus aureus (n=3). Transcript abundance was determined by RNAseq. The heatmap shows expression levels (log10(FPKMs+0.1)) ordered by transcript abundance. Retnla is highlighted in red.
(B) qRT-PCR analysis of skin Retnla expression in germ-free mice and germ-free mice after exposure to S. aureus for 3 days.
(C) qRT-PCR analysis of skin Retnla expression in germ-free mice, germ-free mice exposed to a conventional microbiota for 4 days (conv-D), or mice from a conventional facility (conv-L).
(D) Immunofluorescence detection of RELMα in mouse skin. Epidermis (arrow, above dashed line) and sebaceous gland (arrowhead, inside dashed line) are indicated.
(E) Immunofluorescence detection of RETN in human skin.
(F) Fluorescence in situ hybridization (FISH) detection of RETN in human skin. Defa5 staining as negative control. Nuclei are stained with DAPI. Scale bars, 25 μm. Epidermis above dashed line. SG= sebaceous gland. Epi=Epidermis. Means±SEM are plotted; *P<0.05 as determined by one-tailed Welch’s t-test.
Retnla encodes the protein resistin-like molecule α (RELMα), which belongs to the protein family that encompasses resistin and the resistin-like molecules (RELMs) (Banerjee and Lazar, 2001) (Figure S2A and S2B). Resistin and other RELMs have been characterized as hormones that modulate insulin production (Steppan et al., 2001; Rajala et al., 2003). However, we recently found that RELMβ is a directly bactericidal protein that kills Gram-negative bacteria at the surface of the colon and thus promotes host-bacterial mutualism in the intestine (Propheter et al., 2017). This finding led to the hypothesis that RELMα might be a bactericidal protein of the skin.
RELMα is known to be produced by monocytes, white adipose tissue, and lung epithelial cells (Steppan et al., 2001; Pine et al., 2018), but is undescribed in skin epithelium. Immunofluorescence analysis of mouse skin revealed that RELMα was expressed by keratinocytes and sebocytes within the epidermis (Figure 1D, Figure S2C–E). While the mouse genome encodes four RELM family members, the human genome encodes only two RELM proteins: Resistin-like molecule β (RELMβ), which is expressed in the intestine (Rajala et al., 2003), and Resistin (RETN), which is expressed in keratinocytes and sebaceous glands of the skin (Harrison et al., 2007). Immunofluorescence and fluorescence in situ hybridization (FISH) analysis confirmed that, like mouse RELMα (mRELMα), human RETN (hRETN) is expressed by epidermal keratinocytes (Figure 1E,1F, S2C). The location of RELMα expression in monocytes, adipocytes, keratinocytes and sebaceous glands is shared with other cutaneous antimicrobial peptides such as cathelicidin (Braff et al., 2005; Chronnell et al., 2001; Zhang et al., 2015; Zhang and Gallo, 2016) (Figure 1F), suggesting that mRELMα and hRETN might function in antimicrobial defense of the skin.
RELMα kills bacteria by disrupting their membranes
We next tested the ability of mRELMα and hRETN to kill bacteria. We produced recombinant mRELMα and hRETN in Escherichia coli and purified folded, monomeric protein (Figure S3A). We added the purified proteins to a panel of commensal and pathogenic bacteria that included both Gram-positive and Gram-negative species (Fig. 2A,B). Both mRELMα and hRETN caused a dose-dependent reduction in the viability of strains of the Gram-positive species Streptococcus pyogenes (Figure 2A) and the Gram-negative species Pseudomonas aeruginosa (>99% decline in viability after a 2 hour exposure to 2.5 μM of either protein) (Figure 2B). The viability of the intestinal Gram-negative bacterial species Citrobacter rodentium and Escherichia coli K12 also declined, but much less markedly (Figure 2B). Propionibacterium acnes, a Gram-positive commensal species linked to acne in human skin (Holland et al., 1998; Beylot et al., 2014; Coughlin et al., 2017), was highly susceptible to hRETN but less so to mRELMα (Figure 2B). Thus, mRELMα and hRETN are bactericidal for Gram-positive and Gram-negative bacteria at low micromolar concentrations, similar to other skin antimicrobial proteins (Figure S3B) (Ganz and Lehrer, 1995; Durr et al., 2006).
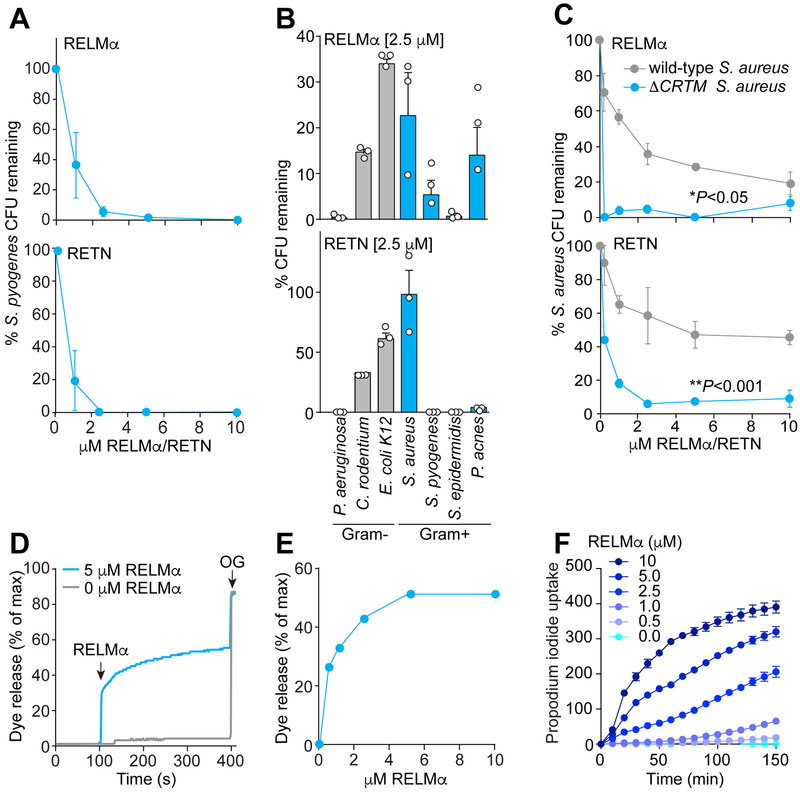
Figure 2: RELMα kills bacteria by disrupting their membranes.
(A) Purified recombinant mRELMα or hRETN was added to mid-logarithmic phase Streptococcus pyogenes for 2 hours and surviving bacteria were quantified by dilution plating. Colony forming units (CFUs) are expressed as a percentage of untreated bacteria.
(B) 2.5 μM of mRELMα or hRETN was added to mid-logarithmic phase bacteria for 2 hours and surviving bacteria were quantified by dilution plating. Means±SEM are plotted.
(C) Antibacterial efficacy of mRELMα and hRETN against wild-type S. aureus and an isogenic S. aureus mutant that lacks staphyloxanthin (ΔCRTM). Means±SEM are plotted. *P<0.05 **P<0.01 by paired Student’s t-test.
(D) Carboxyfluorescein (CF)-loaded liposomes were exposed to 5 μM mouse RELMα. Dye efflux was measured over time and is expressed as a percentage of maximal efflux in the presence of the detergent octylglucoside (OG).
(E) Percentage of dye release for varying doses of RELMα at the 500s time point.
(F) Propidium iodide uptake by S. pyogenes in the presence of increasing concentrations of mRELMα. Assays were performed in triplicate. All results are representative of at least two independent experiments.
Interestingly, while a strain of the Gram-positive skin commensal Staphylococcus epidermidis was susceptible to mRELMα and hRETN, a strain of the pathogen Staphylococcus aureus was resistant (Figure 2B). This suggested that S. aureus might have specific attributes that protect it from mRELMα-and hRETN-mediated killing. A unique feature of S. aureus is its ability to produce staphyloxanthin (STX), a yellow pigment that intercalates into the S. aureus membrane and protects it from attack by pore-forming antimicrobial proteins (Mishra et al., 2011; Liu et al., 2005). Indeed, S. aureus mutants lacking STX (DCRTM) were more susceptible to killing by mRELMα and hRETN (Figure 2C), indicating that STX is required for S. aureus resistance to RELM bactericidal activity.
The requirement of STX for S. aureus resistance to mRELMα suggested that mRELMα bactericidal activity might involve membrane permeabilization. This idea was also supported by our prior finding that mRELMβ killed Gram-negative bacteria by forming pores that permeabilized bacterial membranes (Propheter et al., 2017). To determine if mRELMα disrupts membranes, we performed liposome disruption assays on liposomes with a lipid composition similar to that of bacterial membranes (85% of the neutral lipid phosphatidylcholine and 15% of the negatively charged lipid phosphatidylserine). The liposomes encapsulated carboxyfluorescein (CF), a self-quenching dye that fluoresces upon dilution. mRELMα and hRETN both induced rapid dye release (Figure 2D, 2E, S3C), suggesting that these proteins permeabilize membranes. Additionally, mRELMα promoted dose-dependent uptake of the membrane impermeant dye propidium iodide by S. pyogenes (Figure 2F). Thus, mRELMα permeabilizes bacterial membranes, suggesting a mechanism for its bactericidal activity.
The skin surface has unique physical and chemical properties relative to other body sites, including an acidic pH (Zlotogorski, 1987) and the presence of a high proportion of cholesterol in keratinocyte cell membranes. We therefore assessed the sensitivity of mRELMα antibacterial activity to pH and membrane cholesterol. To carry out these assays, we used the acid-resilient bacterial species Listeria monocytogenes. mRELMα antibacterial activity was most potent at pH 5 and declined at pH 7 (Figure S3D and S3E), indicating that mRELMα is most active at physiological skin pH. Similarly, incorporation of 30% cholesterol into liposome membranes, reflecting the composition of keratinocyte membranes (Pappas, 2009), resulted in lowered RELMα membrane permeabilization activity (Figure S3F). This suggests that elevated membrane cholesterol might be one mechanism by which keratinocytes limit self-inflicted damage from the production of membrane-permeabilizing antimicrobial proteins.
Mice lacking RELMα have an altered skin microbiota
The antibacterial activity of RELMα suggested that it might regulate the composition of the resident skin microbiota in vivo. We therefore used CRISPR/Cas9-mediated gene targeting to delete the mouse Retnla gene locus (Figure S4A). We verified that RELMα was absent in Retnla−/− mouse skin, and that skin pH and the expression of other skin antimicrobial genes were not affected (Figure S4B–E). We then compared the composition of skin microbial communities of wild-type and Retnla−/− littermates using 16S rRNA gene sequencing. Principal coordinate analysis (PCoA) revealed that the wild-type and Retnla−/− mice had distinct skin microbiotas (Figure 3A–C and S5). Consistent with our in vitro findings (Figure 2B), the relative abundance of coagulase-negative staphylococci and streptococcus was increased in male and female Retnla−/− mice respectively (Figure 3C). RELMα is expressed at low levels in the colon (Figure S5B). Accordingly, Retnla−/− mice maintained similar fecal microbiomes even though they had divergent skin microbiomes (Figure S5C and S5D). Together, these data show that RELMα shapes the composition of the skin microbiota.
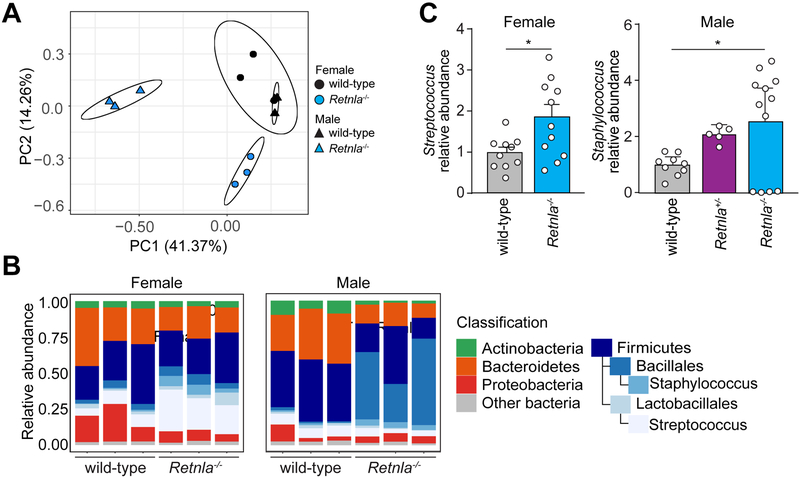
Figure 3: Mice lacking RELMα have an altered skin microbiota.
(A) Retnla−/− and wild-type littermates were separated at weaning into separate cages for at least 8 weeks, and skin samples were taken from the dorsal surface. Skin microbiota compositions were determined by 16S rRNA analysis and are plotted on a principle coordinate analysis (PCoA) plot.
(B) Relative abundances of bacterial genera in wild-type and Retnla−/− mice respectively.
(C) Relative abundance of coagulase negative Staphylococcus and Streptococcus species on the dorsal flank of wild type compared to Retnla−/− mice. Means±SEM are plotted *P<0.05 by Welch’s t-test.
Mice lacking RELMα are more susceptible to bacterial infection
We next assessed the susceptibility of Retnla−/− mice to bacterial infection. Retnla−/− mice superficially infected with S. pyogenes (i.e., without breaking the skin) showed elevated numbers of S. pyogenes when compared to wild type mice (Figure 4A). Retnla−/− mice were also more susceptible to infection with STX-deficient S. aureus (ΔCRTM). In contrast, infection of Retnla−/− and wild type mice with the parental wild-type S. aureus strain did not produce marked differences in S. aureus numbers (Figure 4B), consistent with the resistance of this strain to mRELMα bactericidal activity (Figure 2C). Retnla−/− mice also showed increased susceptibility to intradermal infection with S. pyogenes (Figure 4C) and increased erythema during infection (Figure S4F). Thus, RELMα protects against pathogenic bacterial infection of the skin in vivo.
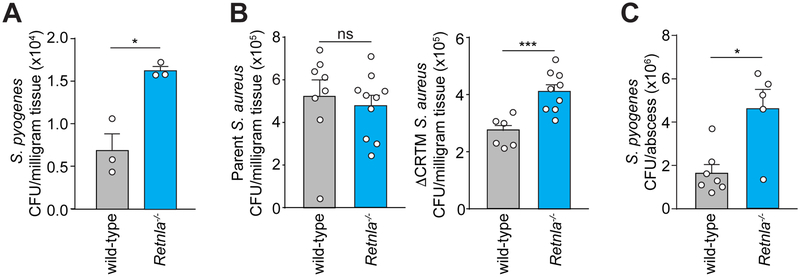
Figure 4: Mice lacking RELMα are more susceptible to bacterial infection.
(A,B) S. pyogenes (A) or wild-type or staphyloxanthin-deficient (ΔCRTM) Staphylococcus strains (B) were grown to logarithmic phase and applied on a gauze rectangle to the dorsal skin of Retnla−/− or wild-type mice under occlusion for 2 days. CFU were determined in sections of inoculated skin.
(C) S. pyogenes was injected intradermally into the skin of Retnla−/− or wild-type mice. Skin abscesses were removed and CFU were determined. Each symbol represents one mouse. Means±SEM are plotted *P<0.05; ***P<0.001; ns=not significant by Welch’s t-test (A,B) or unpaired t-test (C).
See also Figure S4.
Vitamin A is required for RELMα expression
Skin immunity is highly sensitive to the presence of dietary vitamin A (West et al., 1995, Everts, 2012). Vitamin A deficiency in humans results in markedly increased susceptibility to skin infection and inflammation (Russell and Suter, 2012), and oral administration of synthetic retinoids (compounds biochemically related to vitamin A) is a widely-used treatment for inflammatory skin diseases (Orfanos et al., 1987). Despite the clinical effectiveness of oral retinoid administration, little is known about the mechanisms by which vitamin A and synthetic retinoids regulate cutaneous immunity (Oeff et al., 2006).
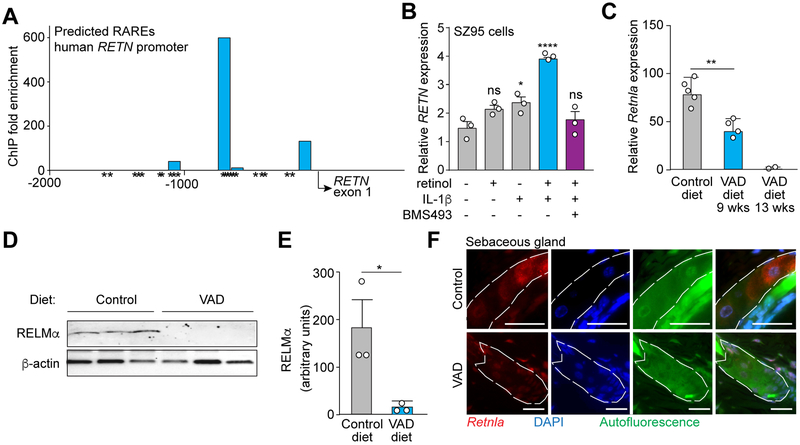
Vitamin A typically regulates gene transcription through its derivative, retinoic acid, which binds to RARs. RARs activate transcription of specific target genes by binding to retinoic acid response elements (RAREs) (Idres et al., 2002). To determine if the expression of RELM family members might be dependent on vitamin A, we first conducted an in silico analysis for RARE sites in the RETN promoter using NUBIScan software (Podvinec et al., 2002). NUBIScan predicted 21 putative RARE sites in the human RETN promoter (Figure S6). We then used chromatin immunoprecipitation (ChIP) assay to assess binding of RARs to the RETN promoter in SZ95 sebocytes (Zouboulis et al., 1999). Indeed, RARs bound at several predicted retinoic acid response elements (RAREs) in the human RETN promoter (Figure 5A). Next, to test if expression of RETN could be stimulated by vitamin A derivatives, we added retinol (a vitamin A derivative enzymatically upstream of retinoic acid) to cultured human sebocytes. Retinol enhanced expression of RETN transcripts in the presence of the proinflammatory cytokine IL-1β (Figure 5B), indicating that retinol acts synergistically with a proinflammatory stimulus to stimulate RETN expression in sebocytes. Finally, addition of BMS493, a pharmacological inhibitor of RARs, abrogated the increase in RETN expression (Figure 5B). Thus, RARs bind to the RETN promoter and mediate retinol-stimulated RETN expression.
Figure 5: Vitamin A is required for RELMα expression.
(A) RAR binding to the human RETN promoter was measured by chromatin immunoprecipitation (ChIP) with an anti-RAR antibody. Predicted retinoic acid response elements (RAREs) were identified by in silico analysis using NUBIScan (Podvinec et al., 2002) and are indicated by an asterisk (*). Predicted RARE sequences are shown in Figure S6. Data are representative of two independent experiments.
(B) qRT-PCR analysis of human RETN expression in the human sebocyte cell line SZ95. Cells were treated with retinol, IL-1β, the pan-RAR inhibitor BMS493, or a combination.
(C) Mice on a Vitamin A deprived diet express less Retnla transcript. qRT-PCR analysis of Retnla expression in the skin of mice on a control or vitamin A-deficient (VAD) diet administered for 9 weeks or 13 weeks.
(D,E) Mice on a Vitamin A deprived diet express less RELMα protein. Western blot detection of RELMα in skin of mice fed a control or VAD diet (D), with quantification (E).
(F) FISH detection of Retnla in mouse skin shows decreased Retnla transcripts in sebaceous glands (dashed line) of VAD diet-fed mice. Scale bar, 25 μm. Data are representative of at least two experiments. *P<0.05, **P<0.01,***P<0.001 by One-way ANOVA (B, C); unpaired t-test (E).
To determine if expression of mouse Retnla was similarly dependent on vitamin A, we conducted studies on mice fed a vitamin A-deficient diet. We found that Retnla transcripts were less abundant and RELMα protein levels were lower in the skin of mice fed a vitamin A-deficient diet (Figure 5C–E). Although sebaceous glands can degenerate with inadequate dietary vitamin A (Zouboulis et al., 1993; Wolbach and Howe, 1925), we did not observe sebaceous gland atresia in mice after vitamin A deprivation (Figure 4F). Indeed, FISH analysis showed a decreased abundance of Retnla transcripts in the sebaceous glands of vitamin A-deprived mice (Figure 4F). In contrast, the expression of genes encoding other known skin antimicrobial proteins was not markedly affected by vitamin A deprivation (Figure S6). Thus, mRELMα is unique among known skin antimicrobial proteins in that its expression requires vitamin A in the diet.
RELMα provides vitamin A-dependent antimicrobial protection in the skin
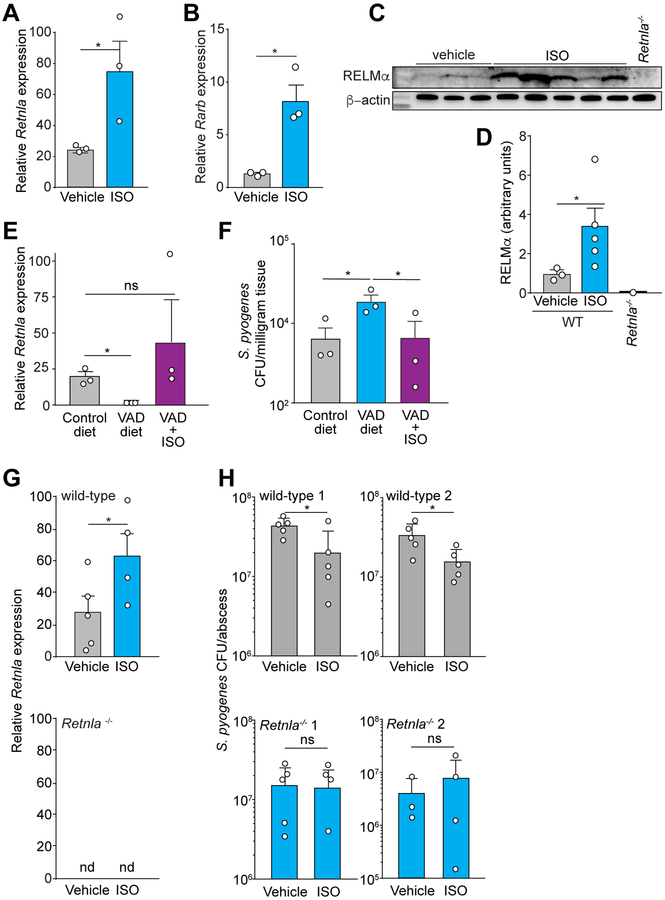
We next asked whether administration of exogenous therapeutic retinoids can stimulate RELMα expression in vivo. Mice treated orally with the therapeutic retinoid isotretinoin (13-cis retinoic acid) showed increased expression of Retnla in the skin compared to vehicle-treated mice (Figure 6A, 6C, 6D). This paralleled the increased expression of Rarb, encoding RARβ (Figure 6B), an established target of synthetic retinoids (Idres et al., 2002). Further, isotretinoin treatment rescued Retnla expression in mice on a vitamin A-deficient diet (Figure 6E). Mice fed a vitamin A-deficient diet were also more susceptible to skin infection by S. pyogenes than mice fed a vitamin A-replete control diet, and treatment with isotretinoin rescued this susceptibility (Figure 6F). Thus, retinoid-induced expression of Retnla correlated with a reduced susceptibility to skin infection.
Figure 6: RELMα provides vitamin A-dependent antimicrobial protection of the skin.
(A,B) Treatment of mice with the synthetic retinoid isotretinoin (ISO) for 3 days increases the abundance of transcripts encoding RELMα (A) and a known target of retinoids, retinoic acid receptor β (B).
(C,D) Western blot of mouse RELMα expression in the skin of wild-type mice treated orally with the synthetic retinoid isotretinoin (ISO) or vehicle (C) with quantification in (D).
(E) qRT-PCR analysis of mouse Retnla expression in the skin of mice on a control diet, a VAD diet, or a VAD diet supplemented with ISO.
(F) Mid-logarithmic phase S. pyogenes was injected intradermally into the skin of mice on a control diet, VAD diet, or VAD diet rescued with ISO. Skin abscesses were removed and CFU were determined.
(G) qRT-PCR analysis of mouse Retnla expression in the skin of wildtype or Retnla−/− mice treated orally by gavage with ISO or vehicle. nd, not detected.
(H) Mid-logarithmic phase S. pyogenes was injected intradermally into the skin of wild-type or Retnla−/− mice treated by gavage with ISO or vehicle. Each symbol represents one mouse. Means±SEM are plotted. (A-G) Data are representative of at least two experiments. (H) Each graph represents an independent experiment. One-way ANOVA (G); Welch’s t-test (C); unpaired t-test (A,B,H); Kruskal-Wallis test (E,F). *P<0.05, **P<0.01, ***P<0.001, ns=not significant.
To determine whether RELMα caused the reduced susceptibility to infection with isotretinoin treatment, we studied Retnla−/− mice. Isotretinoin treatment of wild-type mice fed a normal chow diet increased RELMα expression and increased resistance to S. pyogenes infection of the skin (Figure 6G–H). In contrast, isotretinoin treatment of Retnla−/− mice did not alter susceptibility to infection (Figure 6H). Altogether, our data show that RELMα expression requires dietary vitamin A, that therapeutic retinoids such as isotretinoin stimulate Retnla expression, and that the ability of retinoids to protect against skin infection depends on RELMα.
Discussion
The skin is in direct contact with the external environment and thus continuously interfaces with large numbers of microorganisms. The skin copes with this immense microbial challenge in part through the secretion of a variety of antimicrobial proteins (Gallo and Hooper, 2012). In this study we have discovered that RELM proteins constitute a previously unknown group of antibacterial proteins that shape resident skin bacterial communities and limit pathogenic bacterial infection of the skin. Our findings provide insight into how innate immunity regulates skin microbial ecology and resistance to infection.
RELMα expression is remarkably sensitive to environmental cues that include skin bacteria and the host diet. We found that complex communities of resident microorganisms as well as pathogenic S. aureus trigger RELMα expression when introduced onto germ-free mouse skin. This is consistent with our finding that mouse RELMα and human RETN kill a variety of bacterial species. However, the diversity of skin microbial communities is immense (Grice et al., 2009), and the skin is also colonized by fungi, such as species of Malasseezia, and species of bacteria, such as members of the genus Corynebacterium, that were not directly tested as possible targets of RELMα (Grice, 2014; Findley et al., 2013; Jo et al., 2016). Additional studies will be required for a more comprehensive understanding of the range of microorganisms that are targeted by mouse RELMα and human RETN, and to identify which bacterial species (in addition to S. aureus) can trigger RELMα and RETN expression.
An important remaining question is how skin bacteria trigger RELMα expression in the skin. Several possible mechanisms are suggested by prior studies of skin and gut antimicrobial proteins. One possibility is that RELMα expression is controlled by host pattern recognition receptors, such as Toll-like receptors (TLR), which are expressed on skin epithelial cells. This idea is suggested by the fact that epithelial cell TLR signaling controls the expression of several epithelial antimicrobial proteins, such as REGIIIγ and RELMβ in the gut (Vaishnava et al., 2011) and β-defensin on the skin (Sumikawa et al., 2006). Cathelicidin expression is also controlled by TLR signaling, but in an indirect manner. Activation of keratinocyte TLR2 induces expression of the CYP27B1 gene, which encodes 25-hydroxyvitamin D3–1-α-hydroxylase. This enzyme controls production of the active form of vitamin D, which binds to the vitamin D receptor (VDR) and promotes transcription of the gene encoding cathelicidin (Liu et al., 2006; Schauber et al., 2007). Our finding that the vitamin A derivative retinol drives RETN expression through RAR(s) suggests that skin bacteria could similarly regulate retinol or retinoic acid levels in keratinocytes and sebocytes and thus promote RAR-dependent transcription of RELM-encoding genes.
A second possible mechanism involves capture of bacterial signals by pattern recognition receptors on immune cells that patrol the tissues that underlie the skin surface, followed by signaling back to the epidermal layer through cytokines. This idea is suggested by studies of intestinal REGIIIγ, whose expression can be triggered by a cytokine signaling relay among dendritic cells, type 3 innate lymphoid cells (ILC), and intestinal epithelial cells (Sanos et al., 2009). Similarly, a rich network of skin-resident dendritic cells and ILC resides in the subcutaneous tissues (Belkaid and Segre, 2014; Kobayashi et al., 2019), and could convey regulatory signals to keratinocytes and sebocytes to regulate RELMα expression.
A third possibility is that skin bacteria induce RELM protein expression through their metabolic products. In the gut, microbial fermentation of dietary fiber produces short chain fatty acids (SCFA), such as butyrate, which can alter epithelial cell gene expression (Ganapathy et al., 2013). Although the skin surface is normally aerobic, lipid-rich anaerobic environments can arise under certain conditions, such as occlusion of sebaceous follicles (Sanford et al., 2016). Such conditions allow for the production of SCFAs by skin bacteria such as P. acnes, which in turn can alter keratinocyte gene expression (Sanford et al., 2019). This suggests that SCFAs or other metabolic products of skin bacteria could regulate RELM protein expression.
The host diet is another important environmental factor, in addition to skin bacteria, that regulates RELMα expression. Our studies of mice fed a vitamin A-deficient diet uncovered an unexpected requirement for dietary vitamin A in skin expression of RELMα. We also found that expression of the human RETN gene in sebocytes is enhanced by the vitamin A derivative retinol through direct binding of RARs to the RETN promoter. RELMα and RETN represent unique instances of antimicrobial proteins whose expression is regulated by vitamin A or its derivatives, thus revealing a role for vitamin A in skin innate immunity. This vitamin A requirement could help to explain why people with inadequate vitamin A in their diets tend to have more skin infections (Russell and Suter, 2012).
The finding that vitamin A is required for RELMα expression could also provide insight into why vitamin A analogs are effective treatments for certain skin diseases. Analogs such as isotretinoin are routinely used to treat skin inflammatory diseases such as psoriasis and acne, yet the molecular basis for the success of these treatments is poorly understood (Saurat, 1999; Orfanos et al., 1987; Ellis and Krach, 2001). We found that isotretinoin induced RELMα expression and moreover, protected mice from skin infection only when RELMα was present. This indicates that isotretinoin enhances antibacterial immunity of mouse skin in part by regulating RELMα, thus suggesting a mechanism by which therapeutic vitamin A analogs could promote skin innate immunity.
Fat-soluble vitamins appear to be of particular importance for the expression of skin antimicrobial proteins. While RELMα is a unique example of an antimicrobial protein regulated by vitamin A, prior work identified a role for vitamin D in the expression of cathelicidin (Liu et al., 2006; Schauber et al., 2006). This occurs through vitamin D binding to the VDR, which in turn binds to the cathelicidin gene promoter to regulate its transcription (Liu et al., 2006; Schauber et al., 2007). Interestingly, vitamins A and D are both lipophilic, suggesting that fat-soluble vitamins are critical for skin innate immunity. Because of their lipophilic chemistries, vitamin A and D both require adequate dietary fat intake for their absorption (Moyersoen et al., 2017; Albahrani and Greaves, 2016). This implies that low-fat diets could impact the development of skin disease and suggests an interesting topic for future study.
Our studies of human RETN indicate a conserved function for RELM proteins in vitamin A-dependent innate immunity of the skin. A role for RETN in the immune defense of human skin was suggested by a large study that analyzed polymorphisms in the RETN gene promoter of acne patients and healthy controls. This study uncovered a strong association between RETN gene polymorphisms and acne, particularly in female patients (Younis et al., 2016). Alongside our findings, these data suggest a role for RETN in skin conditions, such as acne vulgaris and hidradenitis suppurativa, that are thought to arise in part from changes in the microbiome (Ring et al., 2017; O’Neill and Gallo, 2018).
The antimicrobial activity of RETN could also underlie microbiome shifts that occur on the skin after isotretinoin treatment. Three studies have analyzed the skin microbiome of patients treated with isotretinoin, finding that that treatment is associated with a decreased abundance of P. acnes (new nomenclature Cutibacterium acnes) (Kelhala et al., 2018; Ryan-Kewley et al., 2017; McCoy et al., 2018). Given our finding that human RETN can kill P. acnes, these data suggest that increased RETN expression in patients on isotretinoin might underlie the microbiome shifts seen in treated patients. However, additional studies are needed to determine whether isotretinoin stimulates RETN expression in human patients.
In summary, we have discovered that RELMα is a previously unknown antimicrobial protein of the skin, and that vitamin A plays an unexpected role in skin innate immunity by regulating RELMα expression. Our studies of human RETN indicate a conserved function for RELM family proteins in vitamin A-dependent defense of the skin. Altogether, our findings provide insight into how vitamin A promotes resistance to skin infection and help to illuminate how diet regulates skin innate immunity.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Lora V. Hooper (Lora.Hooper@UTSouthwestern.edu). The SZ95 sebocyte cell line was obtained from Christos C. Zouboulis and cannot be distributed to other groups without permission from Dr. Zouboulis.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All animal studies were approved by the Institutional Animal Care and Use Committees of the UT Southwestern Medical Center (Protocol # 2015–101212 and 2015–101064) and conducted in compliance with regulatory guidelines. Age and sex matched male and female mice 8–14 weeks old were used for all experiments. All mice used in the study were maintained in 12 hr light-dark cycle. The following strains of mice were used for the study; C57BL/6, RELMα knockout (Retnla−/−) on C57BL/6 background. Mice used in the study were monitored daily for signs of any obvious physical stress and behavioral changes and euthanized per protocol if found in distress. C57BL/6 wild-type mice were bred and maintained in the specific pathogen free (SPF) barrier facility at the University of Texas (UT) Southwestern Medical Center on standard chow. Germ-free C57BL/6 mice were bred and maintained in flexible film vinyl isolators in the gnotobiotic mouse facility at UT Southwestern where they were housed in open top cages with autoclaved bedding and given autoclaved diet (Hooper Lab Diet 6F5KAI, Lab Diet, St. Louis, MO) and autoclaved nanopore water. Mice feed and bedding were changed every week and earlier if needed. GF status was confirmed by culture of fecal pellet, feed, and bedding on brain heart infusion (BHI), Sabouraud dextrose, and nutrient media under both aerobic and anaerobic condition as well as PCR of 16S rRNA gene in fecal DNA using universal primers. For S. aureus colonization experiments, germ free mice were swabbed daily for three days with 1 × 109 CFUs of mid-log phase S. aureus (ATCC 25923). S. aureus was cultured in Tryptic Soy Broth, spun down and resuspended in PBS. Selective plating was done to confirm colonization of the skin. Germ free mice were conventionalized by exposing mice to the bedding, food, and fecal material from the non-barrier facility at UT Southwestern for 4 days. S. aureus and S. pyogenes were not present in the microbiota, as confirmed by selective plating.
Retnla−/− mice were generated using CRISPR/Cas9 genome editing with a guide RNA targeting regions upstream and downstream of the Retnla locus (Figure S4). Guide RNAs were injected into fertilized C57BL/6J embryos along with in vitro transcribed Cas9 mRNA by the UT Southwestern Transgenic Core facility. Healthy blastocysts were implanted into pseudopregnant mice. The resulting litters were screened by genomic sequencing to detect the deletion of Retnla, and mice harboring the deleted allele were bred to homozygosity.
Bacterial strains
Bacteria were grown in species-specific growth media: Escherichia coli (ATCC; ATC-PTA-7555), Pseudomonas aeruginosa (ATCC27853), and Citrobacter rodentium (ATCC51459) were grown in Luria Broth. Listeria monocytogenes (EGD-e; BD Biosciences #237500), Streptococcus pyogenes (ATCC12384), Staphylococcus aureus and Staphylococcus aureus DCRTM (both from George Liu) were grown in Brain Heart Infusion Broth (BD Biosciences). Staphylococcus aureus (ATCC25923) and Streptococcus epidermidis (Hooper lab clinical isolate) were grown in Tryptic Soy Broth. Propionibacterium acnes (ATCC6919) was grown in Modified Clostridial Broth.
Sebocyte cell culture
SZ95 cells are an immortalized human sebocyte cell line generated from a 87 year old female person and transformed with Simian virus 40 (SV40). SZ95 were obtained with the permission of Christos Zouboulis. Cell identity was confirmed by the characteristic appearance of sebocytes in culture with intracellular lipid droplets. SZ95 have also been shown to express human Resistin, as was confirmed in our study. The SZ95 cells were negative for mycoplasma contamination by PCR (Sigma-Aldrich Venor GeM Mycoplasma Detection Kit).
METHOD DETAIL
SImmunofluorescence
Skin samples were fixed in formalin and embedded in paraffin by the UT Southwestern histology core. Samples were deparaffined with xylene followed by rehydration with decreasing concentrations of ethanol. Boiling 0.1 M trisodium citrate buffer 0.2% tween was used for antigen retrieval. Slides were blocked with 2% BSA in PBS, then with 1%BSA/0.3%Triton X-100/PBS, and then incubated with primary antibodies against RELMα, RETN, or an IgG control (Figure S2C–E) at 4°C overnight at 1:200 dilutions (LS-Bio, LS-C104472, LS-C131843 or Abcam ab39626). Secondary antibodies Alexa Fluor® 594 (Thermo Fisher) were diluted 1:350 and applied to slides for 1 hour at room temperature in the dark. Slides were then washed and mounted with DAPI Fluoromount-G® (Southern Biotechnology 0100–20). Images were captured using a Zeiss AxioImager M1 microscope or a Discover Echo REVOVLE 4 microscope. Human skin was obtained from patients recruited from elective plastic surgery cases performed at UT Southwestern Medical Center. This study was approved by the UT Southwestern Institutional Review Board and complied with Declaration of Helsinki Principles. Sebaceous glands were identified by their characteristic autofluorescence pattern (Croce and Bottiroli, 2014; Kong et al., 2013), location adjacent to the hair follicle, and review by a board certified dermatopathologist, Travis Vandergriff, M.D.
Fluorescence in situ hybridization (FISH)
Fluorescent probes spanning the second and third exons, with 5’ and 3’ Alexa modifications, were generated against the mouse transcripts Retnla and Defa5, and the human transcript RETN (Integrated DNA Technologies). Skin samples were fixed in formalin and embedded in paraffin. Sections were deparaffinized with 2 changes of xylene. Sections were rehydrated with successive ethanol washes and then with double distilled water. Probes were diluted to 1 μM in warm hybridization buffer (0.9M NaCl, 20 mM Tris-HCL, pH 7.2, 0.1% SDS), applied directly to slides, and incubated in a humidified chamber overnight at 50° C. Slides were washed with Wash Buffer (0.9 M NaCl/20 mM Tris-HCl, pH7.2). Slides were then washed and mounted with DAPI Fluoromount-G® (Southern Biotechnology 0100–20). Images were captured using a Zeiss AxioImager M1 microscope or a Discover Echo REVOVLE 4 microscope.
FISH probes:
RETN-FISH: spanning exon 2 and exon 3 of the human Retn mRNA.
5’-/5Alexa594N/GCT TAT TGC CCT AAA TAT TAG GGA GCC GGC GAC CTC C/3Alexa594n/-3’
Defa5-FISH:
5’-/5Alexa488N/CTG GTC CTC TTC CCC TGG CTG CTC CTC AGT ATT AGT/3Alexa488n/-3’
Retnla-FISH: spanning exon 2 and exon 3 of the mouse Retnla mRNA
5’-/5Alexa594N/CAG TGG AGG GAT AGT TAG CTG GAT TGG CAA GAA GTT CC/3Alexa594n/-3’
Quantitative real-time PCR
RNA was isolated from whole mouse skin using the RNAeasy Plus universal kit (Qiagen 73404). 1 μg of RNA was converted to cDNA (Thermofisher 4368814 High Capacity cDNA reverse transcription kit). qRT-PCR was performed using TaqMan® Gene Expression Assays (see Key Resources Table) and the QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems). Relative expression values were determined using the comparative Ct (ΔΔCt) method, and transcript abundances were normalized to Gapdh or 18S transcript abundance.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-RELMα | Life Span | Cat# LS-C104472; RRID:AB_2180030 |
| anti-RETN | Life Span | Cat# LS-C131843–100; RRID:AB_10834395 |
| anti-RELMα | Abcam | Cat# ab39626, RRID:AB_777652 |
| Alexa Fluor® 594 secondary antibody | Thermo Fisher Scientific | Cat# R37119, RRID:AB_2556547 |
| Anti-Actin | Sigma-Aldrich | Cat# A5060, RRID:AB_476738 |
| Bacterial and Virus Strains | ||
| Staphylococcus aureus | ATCC | ATCC 25923 |
| Escherichia coli K-12 | ATCC | ATC-PTA-7555 |
| Pseudomonas aeruginosa | ATCC | ATCC 27853 |
| Listeria monocytogenes, EGD-e | BD Biosciences | BD# 237500 |
| Staphylococcus epidermidis, clinical isolate | Hooper Lab | N/A |
| Streptococcus pyogenes | ATCC | ATCC12384 |
| Propionibacterium acnes | ATCC | ATCC 6919 |
| Staphylococcus aureus (parent strain) | Dr. George Liu | N/A |
| Staphylococcus aureus (ΔCRTM) | Dr. George Liu | N/A |
| Citrobacter rodentium, DBS100 | ATCC | ATCC 51459 |
| Biological Samples | ||
| Human skin tissue-Control samples | UT Southwestern Tissue Bank/PI: Ben Chong M.D. | STU 082010–241 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RIPA buffer | Thermo Fisher Scientific | Cat# 89900 |
| DAPI Fluoromount-G® | Southern Biotechnology | Cat# 0100–2 |
| cOmplete™ Protease Inhibitor Cocktail | Sigma-Aldrich | Cat# 11697498001 |
| Sebomed® Basal Medium | Merck | Cat# F8205 |
| Fetal Bovine Serum | Gibco | Cat# 10082147 |
| Retinol | Sigma-Aldrich | Cat# R7632 |
| IL-1β | Thermo Fisher Scientific | Cat# 10139HNAE |
| BMS493 | TOCRIS | Cat# 3509 |
| Magna protein A beads | Millipore | Cat#16–661 |
| Vitamin A Deficient Diet | ENVIGO | TD.09838 |
| Control diet (to Vitamin A) | ENVIGO | TD.09839 |
| 13-cis retinoic acid | Sigma-Aldrich | Cat# R3255 |
| Corn oil | Sigma-Aldrich | Cat# C8267 |
| One Shot TOP10 competent cells | Thermo Fisher Scientific | Cat# C404010 |
| BL21 DE3RIL cells | Agilent | Cat#230245 |
| chicken lysozyme | LS-Bio | LS-G132095–1 |
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine or POPC | AVANTI | Cat #850457C |
| 1,2-dioleoyl-sn-glycero-3-phospho-L-serine or DOPS | AVANTI | Cat #840035C |
| cholesterol (ovine) | AVANTI | Cat # 700000 |
| PD-10 Column | Sigma-Aldrich | GE17-0851-01 |
| 5(6)-carboxyfluorescein | Sigma-Aldrich | Cat # 21877 |
| n-octylglucoside | Anatrace | Cat # O311 |
| propidium iodide (PI) | Thermo Fisher Scientific | Cat# P3566 |
| 96-well Costar plates | Fisher | Cat # 07-200-567 |
| Ni-NTA column | Qiagen | Cat#30210 |
| Nair Depilatory cream | Nair | N/A |
| S. aureus selective plates | Hardy Diagnostics | Cat#A70 |
| CHROMagar™ Staph aureus | BBL™ | Cat# 214982 |
| Agencourt AmpureXP beads | Beckman Counter Genomics | Cat# A63881 |
| 15-blade scalpel | Fine Scientific Tools | Cat#10115–10 |
| Tegaderm | 3M 9505W | Cat# 3M 9505W |
| Band-Aid Sheer Strips | BAND-AID | N/A |
| Andis ProClip | ProClip | N/A |
| human epidermal growth factor | Thermo Fisher Scientific | Cat# PHG0313 |
| Critical Commercial Assays | ||
| TruSeq ChIP Sample Prep Kit | Illumina | Cat# IP-202–1012 |
| RNAeasy Plus universal kit | Qiagen | Cat# 73404 |
| LA Taq Hot Start polymerase kit | Takara | Cat# RR042B |
| mini-extruder kit | Avanti Polar Lipids | Cat #610000 |
| Truseq RNA sample preparation kit v2 | Illumina | Cat#-RS-122–2001 |
| High Capacity cDNA reverse transcription kit | Thermo Fisher Scientific | Cat# 4368814 |
| KOD Hot Start Polymerase kit | Millipore | Cat #71086 |
| QIAprep Spin Miniprep kit | Qiagen | Cat#27106 |
| Agencourt Ampure XP kit | Beckman Counter Genomics | Cat# A63881 |
| QuantIT dsDNA High-Sensitivity Assay kit | Thermo Fisher Scientific | Cat#Q33120 |
| MinElute PCR Purification Kit | Qiagen | Cat#28006 |
| PE300 (Paired end 300 bp) v3 kit | Illumina | Cat# MS-102–3001 |
| Quick PCR purification kit | Qiagen | Cat# 28104 |
| Deposited Data | ||
| RNA-seq data | This paper | GEO: GSE108718 https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE108718 |
| 16S rRNA skin data | This paper | (SRA) BioProject ID PRJNA527213 https://www.ncbi.nlm.nih.gov/bioproject |
| 16S rRNA fecal data | This paper | (SRA) BioProject ID PRJNA528925 http://www.ncbi.nlm.nih.gov/bioproject/528925 |
| Experimental Models: Cell Lines | ||
| SZ95 Cell Line | Christos Zouboulis | RRID:CVCL_9803 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | UT Southwestern | RRID: IMSR_JAX:008471 |
| Mouse: Retnla−/− | This paper | N/A |
| Oligonucleotides | ||
| Real-time quantitative PCR primers | Thermo Fisher Scientific | Table S1 |
| Chromatin immunoprecipitation (ChIP) assay primers | Integrated DNA Technologies | Table S2 |
| mRELMα expression cloning Forward Primer GGATACCATATGGATGAAACGATCGAAATCATCG |
Integrated DNA Technologies | Custom Primer |
| mRELMα expression cloning Reverse Primer GATGATGGATCCTTAAGACAGTTGACAGCAGCGGGC |
Integrated DNA Technologies | Custom Primer |
| hRETN expression cloning Forward Primer GGATACCATATGCGCTCTAAAACCCTGTGTTCC |
Integrated DNA Technologies | Custom Primer |
| hRETN expression cloning Reverse Primer GATGATGGATCCTTACGGTTGAACACGACAGCAACG |
Integrated DNA Technologies | Custom Primer |
|
RETN-FISH: spanning exon 2 and exon 3 of the human Retn mRNA. 5’-/5Alexa594N/GCT TAT TGC CCT AAA TAT TAG GGA GCC GGC GAC CTC C/3Alexa594n/-3’ |
Integrated DNA Technologies | Custom Oligonucleotide |
|
Defa5-FISH: 5’-/5Alexa488N/CTG GTC CTC TTC CCC TGG CTG CTC CTC AGT ATT AGT/3Alexa488n/-3’ |
Integrated DNA Technologies | Custom Oligonucleotide |
|
Retnla-FISH: spanning exon 2 and exon 3 of the mouse Retnla mRNA 5’-/5Alexa594N/CAG TGG AGG GAT AGT TAG CTG GAT TGG CAA GAA GTT CC/3Alexa94n/-3’ |
Integrated DNA Technologies | Custom Oligonucleotide |
| 16S V1 (primer 27 F; 5′-AGAGTTTGATCCTGGCTCAG-3′) | (Grice et al., 2008) | N/A |
| 16S V3 (primer 534 R; 5′-ATTACCGCGGCTGCTGG-3′) | (Grice et al., 2008) | N/A |
| Recombinant DNA | ||
| pET28a expression vector | Millipore | Cat#69864 |
| Software and Algorithms | ||
| Fiji (ImageJ) | http://fiji.sc | RRID:SCR_002285 |
| GraphPad PRISM | GraphPad Software | Version 7.0; RRID:SCR_002798 |
| TopHat | Top Hat | Version 2.1.1; RRID:SCR_013035 |
| Mothur | Mothur | RRID:SCR_011947 |
| UCHIME | UCHIME | RRID:SCR_008057 |
| Illumina Nextera protocol | Illumina | Part # 15044223 Rev. B |
| CLC Bio Microbial Genomics Module | Qiagen |
https://www.qiagenbioinformatics.com/plugins/clc-microbial-genomics-module/). |
| Image Lab Software | BioRad | RRID:SCR_014210 |
| Other | ||
| Bio-Rad ChemiDoc™ Touch system | BioRad | Cat# 1708370 |
| Zeiss Axio Imager M1 microscope | Zeis | N/A |
| Discover Echo REVOVLE 4 | Discover Echo | N/A |
| QuantStudio 7 Flex Real-Time PCR System | Applied Biosystems | Cat# 4485701 |
| Agilent 2100 Bioanalyzer | Agilent | G2939A |
| Ultrasonic Cell Disruptor | Misonix | N/A |
| Amicon Ultra centrifugal filters | Millipore | Cat#UFC900324 |
| Superdex 75/300 | GE Healthcare | Cat #17-5174-01 |
| PTI-QuantaMaster 300 fluorometer | Horiba | N/A |
| Spectramax plate reader | Molecular Devices | N/A |
| Illumina MiSeq platform | Illumina | RRID:SCR_016379 |
| Illumina HiSeq 2500 | Illumina | RRID:SCR_016383 |
Whole transcriptome sequencing and data analysis
RNA was extracted from whole mouse skin using the RNAeasy Plus universal kit (Qiagen 73404). RNA quality was assessed by Agilent 2100 Bioanalyzer. Truseq RNA sample preparation kit v2 (Illumina) was used for the preparation of sequencing libraries. Sequencing was performed on an Illumina HiSeq 2500 for signal end 50 bp length reads. Sequence data were mapped against the mm10 genome using TopHat and FPKMs were generated using Cuffdiff with parameters FPKM >10, fold change >2, and adjusted p value <0.01.
Western blot
Mouse skin was homogenized in RIPA buffer (ThermoFisher 89900) containing cOmplete™ Protease Inhibitor Cocktail (Sigma 11697498001). Equal amounts of protein were loaded onto a 4–20% gradient SDS-PAGE and transferred to a PVDF membrane. After blocking with 5% milk in TBS-T, the membranes were incubated at 4°C overnight with anti-RELMα antibody (Abcam ab39626) or anti-Actin (Sigma A5060). Membranes were then incubated with anti-rabbit secondary antibodies conjugated with HRP. Membranes were visualized and bands quantified using a Bio-Rad ChemiDoc™ Touch system.
Sebocyte cell culture
SZ95 sebocytes were cultured in Sebomed® Basal Medium (Biochrom F8205) supplemented with 0.1 ng/ml human epidermal growth factor (ThermoFisher PHG0313) and 10% fetal bovine serum (Gibco 10082147). Cells were maintained at 5% CO2 at 37° C. Prior to stimulation, SZ95 sebocytes were adapted to serum-free medium for 48 hours. Cells were stimulated with retinol (100 nM) (Sigma R7632) and IL-1b (50 pg/ml) (ThermoFisher 10139HNAE). Retinoic acid receptor activity was inhibited with BMS493 (TOCRIS 3509) for 3 hours prior to stimulation. 24 hours post-stimulation cells were harvested and human RETN and GAPDH transcripts were analyzed as described above. Amber lighting was used throughout to minimize retinoid degradation.
Chromatin immunoprecipitation (ChIP) assays.
SZ95 cells were crosslinked in cell culture media with 1% formaldehyde for 8 minutes at room temperature, followed by 125 μM glycine at 4°C for 10 min to quench the reaction. Shearing of nuclear DNA was completed per manufacturer’s protocol (Diagenode). For each immunoprecipitation reaction 20 μl of Magna protein A beads (Millipore) and chromatin from 1 × 107 cells was combined with 5 μg of goat anti-RAR (Santa Cruz) or total goat IgG (Millipore). Complexed RETN promoter sequences were quantified using SYBR Green-based real-time PCR. Primer sequence and primers are listed in Key Resources Table. The ratio of specific antibody pull-down to input DNA was used to calculate relative enrichment of the RETN promoter.
Vitamin A deprivation
Vitamin A-deficient (TD.09838) and control (approximately 20,000 IU vitamin A/kg; TD.09839) diets were purchased from ENVIGO. At day 10 of gestation, pregnant females were placed on either the vitamin A-deficient or -replete diet. Mothers and pups were maintained on the diets until weaning, and pups stayed on the diet for 2–3 months before sacrifice.
Treatment with therapeutically-administered retinoids
Isotretinoin (13-cis retinoic acid; Sigma R3255) was dissolved in DMSO and further diluted in corn oil (Sigma C8267). Mice were treated by oral gavage for 3 consecutive days with 1 mg of isotretinoin in 10% DMSO/corn oil or 10% DMSO/corn oil (vehicle). Mice were sacrificed and the skin was analyzed.
Protein expression and refolding
Sequences encoding mRELMα, and hRETN were PCR-amplified from codon-optimized genes (GenScript (Piscataway, NJ) sequences listed below) using the primers listed in the Key Resources Table and the KOD Hot Start Polymerase kit (EMD Millipore #71086). PCR amplified products were purified using the Quick PCR purification kit (Qiagen; 28104), cloned via NdeI and BamHI sites (New England Biolabs) into the pET28a expression vector (EMD Millipore #69864), transformed into One Shot TOP10 competent cells (ThermoFisher; C404010), and plated for ampicillin-resistant clones. Plasmid DNA was purified using the QIAprep Spin Miniprep kit (Qiagen; 27106) and sequenced by the UT Southwestern Sequencing Core Facility. To generate recombinant protein, expression plasmids were transformed into chemically competent BL21 DE3RIL (Agilent #230245) cells and plated on LB agar (Sigma; L7533) with chloramphenicol (Cam) (30 μg/ml) and kanamycin sulfate (Kan)(50 μg/mL). A 10-mL overnight culture was used to inoculate 1 liter of LB (Sigma; L7658) containing Cam and Kan. The culture was grown to midlogarithmic phase (OD600 ~0.5–0.7), and protein expression was induced with 0.4 mM isopropyl-β-D-thiogalactoside (GoldBio Technology #I2481) and grown for 16–20 hours at 20°C with shaking. Bacterial cells were pelleted, resuspended in 75 ml of lysis buffer (20 mM Tris, pH 7.5, 1% Triton X-100, and 2 mM PMSF), and lysed by sonication (Misonix Ultrasonic Cell Disruptor). Lysed cells were centrifuged, and the pellets were resuspended in 40 ml of inclusion body buffer (100 mM Tris, pH 9.0, 7 M guanidine hydrochloride) followed by the addition of 100 mM sodium sulfite and 20 mM sodium tetrathionate. This mixture was stirred overnight at room temperature. The solubilized inclusion bodies were then passed over a Ni-NTA column (Qiagen; 30210) equilibrated with wash buffer (25 mM Tris, pH 7.5, 20 mM glycine, and 6 M urea). The column was then washed, and bound protein was eluted with wash buffer containing 250 mM imidazole. Fractions containing protein were pooled and diluted to 0.1 mg/ml in prechilled refolding buffer (100 mM Tris, pH 8.5, 20 mM glycine, 300 mM NaCl, 5 mM EDTA, and 2 M urea) at 4°C. After the solution became homogeneous, cysteine (5 mM final concentration) was added and mixed until dissolved. Once dissolved, the solution was removed from stirrer and left at 4°C for 72 h. Protein solutions containing mRELMα or hRETN were then dialyzed [at least 20:1 (vol/vol)] against a 30 mM sodium acetate pH 4.5 with three buffer changes, each for 8–14 hours at 4°C. Proteins were concentrated in Amicon Ultra centrifugal filters (Millipore #UFC900324) and separated by size-exclusion chromatography on Superdex 75/300 (GE Healthcare #17-5174-01), and fractions containing monomeric protein were pooled, concentrated, flash frozen in liquid N2, and stored at −80°C. To confirm protein identity, we excised mRELMα and hRETN from SDS-PAGE gels, digested with proteases, and analyzed by liquid chromatography tandem mass spectrometry for peptides and sequence coverage. mRELMα and hRETN were present 100% and 99.9% abundance, respectively.
Codon-optimized genes:
Mouse Retnla:
GATGAAACGATCGAAATCATCGTGGAAAACAAAGTTAAAGAACTGCTGGCGAACCCGGCCAATTATCCGTCAACCGTCACGAAAACCCTGAGCTGTACGTCTGTGAAAACATGAATCGTTGGGCATCGTGTCCGGCTGGTATGACCGCCACCGGTTGCGCCTGTGGTTTTGCATGTGGCAGTTGGGAAATCCAGTCCGGCGATACCTGCAACTGTCTGTGCCTGCTGGTTGACTGGACCACGGCCCGCTGCTGTCAACTGTCT
Human RETN:
AGCTCTAAAACCCTGTGTTCCATGGAAGAAGCAATTAACGAACGTATCCAGGAAGTCGCGGGTAGCCTGATTTTTCGCGCCATTAGTTCCATCGGCCTGGAATGTCAATCAGTGACCTCGCGTGGTGATCTGGCAACCTGTCCGCGCGGCTTCGCTGTGACCGGTTGCACGTGTGGTAGCGCATGCGGCTCTTGGGATGTTCGTGCTGAAACCACGTGCCATTGTCAGTGTGCCGGTATGGACTGGACCGGTGCACGTTGCTGTCGTGTTCAACCG
Bacterial killing assays
Bacteria were grown in species-specific growth media: E. coli (ATC-PTA-7555), P. aeruginosa (ATCC27853), and C. rodentium (ATCC51459): Luria Broth; L. monocytogenes (BD#237500), S. pyogenes (ATCC12384), and S. aureus and S. aureus DCRTM (both from George Liu): Brain Heart Infusion Broth (BD Biosciences); S. aureus (ATCC25923) and S. epidermidis (Hooper lab clinical isolate): Tryptic Soy Broth; P. acnes (ATCC6919): Modified Clostridial Broth. Bacterial cultures were grown to mid-logarithmic phase and then pelleted and washed twice in assay buffer (10 mM MES, pH 6.0, 25 mM NaCl). Initial bacterial concentrations ranged from 105 to 106 CFU/ml. Bacteria were then incubated at 37°C for 2 hours in assay buffer with varying concentrations of recombinant protein or chicken lysozyme (LS-Bio LS-G132095–1). Colony forming units were quantified by dilution plating onto agar plates made from the species-specific growth media listed above.
Liposome disruption assay
Unilamellar lipisomes were prepared using Lipids from Avanti. 85% 1-palmitoyl-2-oleoyl-snglycero-3-phosphocholine or POPC (AVANTI PC #850457C) and 15% 1,2-dioleoyl-sn-glycero-3-phospho-L-serine or DOPS (AVANTI PS #840035C) dissolved in chloroform were mixed in defined molar ratios in glass tubes and then dried under a stream of N2, followed by drying under vacuum overnight to ensure complete removal of organic solvents. Dried lipids were then combined with 5(6)-carboxyfluorescein (Sigma #21877), vortexed for 5 minutes. Lipids were then transferred to 2-mL cryotubes and subjected to five freeze–thaw cycles in liquid N2 and stored at −80 °C. Lipids were then thawed and passed through a 100-nm pore membrane using a mini-extruder kit (Avanti Polar Lipids #610000) and purified on a PD-10 column to remove excess dye. For cholesterol liposomes, 55% POPC, 15%DOPS, and 30% cholesterol (ovine) (AVANTI #700000) were used to generate liposomes. Prepared liposomes were diluted in assay buffer (10 mM MES, pH 5.5, 25 mM NaCl) to a working concentration of 100 μM. QuantaMaster 300 fluorometer (Photon Technology International) was used to monitor fluorescence. The protein of interest was added to the system at varying concentrations. At the end time point, 1% v/v n-octylglucoside detergent (OG, Anatrace #O311) was added to completely disrupt the liposomes. Fluorescence was measured over time in seconds and as a percentage of total dye release by the detergent OG.
Dye uptake assay
Streptococcus pyogenes (ATCC12384) was grown to mid-logarithmic phase in Brain Heart Infusion Broth (BD Biosciences), washed with assay buffer (10 mM MES, 25mM NaCl) at pH 5.0 or pH 7.0 containing 5.5 μg/ml propidium iodide (PI) (Thermo Fisher; P3566). S. pyogenes samples (90 μl each) were then added to black 96-well Costar plates (Fisher; 07-200-567) and placed into a Spectramax plate reader (Molecular Devices) that was preequilibrated to 37°C. After an initial reading (T0, 0 s), 10 μl of Recombinant purified RELMα at varying concentrations or BSA were added and fluorescence output [excitation (Ex), 535 nm; emission (Em), 617 nm] was measured every 10 minutes for 2h.
Skin infections
The dorsal back hair was removed from C57BL6 Retnla−/− or wild-type male mice by shaving (Andis ProClip), followed by depilatory cream (Nair™). After 24 hours, the dorsal skin was superficially abraded in a crosshatch pattern by a 15-blade scalpel (Fine Scientific Tools 10115–10). S. pyogenes (ATCC12384) or S. aureus (from George Liu) were grown to log phase in Brain Heart Infusion Broth (BD Biosciences) and placed on a gauze rectangle. The gauze was applied to the dorsal skin of Retnla−/− or wild-type mice and held in place with 2 tegaderm (3M 9505W) and a Band-Aid Sheer Strips (BAND-AID) for 48 hours. The rectangular section of inoculated skin was removed and homogenized in sterile PBS. Colony forming units (CFUs) were analyzed by dilution plating on Streptococcus or S. aureus selective plates (Hardy Diagnostics A70 Selective strep agar) (214982 BBL™ CHROMagar™ Staph aureus). For intradermal infection, mice were injected with 100 μl of log phase S. pyogenes in PBS after removal of dorsal back hair. After 48 hours, the intradermal abscess was removed from the skin and homogenized in sterile PBS. CFUs were calculated by dilution plating on Streptococcus selective plates. For skin infection studies, S. pyogenes was cultured in Todd Hewitt Broth with 0.2% yeast.
Skin pH
The skin pH of C57BL/6 wild-type and Retnla−/− mice was measured using the Orion Star A211 pH Meter (ThermoFisher) with the Orion 8102BNUWP probe. One drop of deionized water was placed on the back skin before applying the probe. Data shown are the average of two readings for each mouse.
16S rRNA sequencing and analysis of skin microbiomes
C57BL/6 wild-type and Retnla−/− littermates were separated at weaning into separate cages in order to assess for gene-dependent changes in the microbiota. Samples were taken and processed as described previously (Grice et al., 2009; Byrd et al., 2017). Briefly, DNA was obtained from the ear and back skin of mice and amplified using the LA Taq Hot Start polymerase kit (Takara) with universal primers flanking 16S rRNA gene variable regions V1 (primer 27 F; 5′-AGAGTTTGATCCTGGCTCAG-3′) and V3 (primer 534 R; 5′-ATTACCGCGGCTGCTGG-3′). For each sample, the universal primers were tagged with unique sequences (‘barcodes’) to allow for multiplexing/demultiplexing (Lennon et al., 2010) and with Illumina adapters. PCR products were purified using the Agencourt Ampure XP kit (Beckman Counter Genomics) and quantified using the QuantIT dsDNA High-Sensitivity Assay kit (Invitrogen Life Technologies). Approximately equivalent amounts of each PCR product were then pooled and purified on a column from the MinElute PCR Purification Kit (Qiagen) into 30 μl TE buffer before sequencing at the NIH Intramural Sequencing Center on an Illumina MiSeq platform with 2X300bp read length. As previously described (Conlan et al., 2012), this sequencing strategy allows resolution to the species level for Staphylococcus.
Mothur-based analysis pipeline was used for sequence analysis (Schloss et al., 2009). Briefly, sequences were pre-processed to remove primer and barcode sequence, and paired-end reads were merged using FLASh tool (Magoc and Salzberg, 2011). Assembled reads were quality filtered (qaverage=35), subsampled (5,000 reads/sample), and chimeras identified and removed with UCHIME (Edgar et al., 2011). Next, reads were aligned and classified to genus level using a ribosomal database project naïve Bayesian classifier (Wang et al., 2007). Operational taxonomic units (OTUs) were defined at 97% similarity using average neighborhood clustering. Principal coordinate analysis (PCoA) was performed based upon the Theta distance between samples measuring OTU abundance (Yue and Clayton, 2005).
16S rRNA of fecal sequencing and analysis of fecal microbiomes
The hypervariable regions V3 and V4 of the bacterial 16S rRNA gene were captured using the Illumina Nextera protocol (Part # 15044223 Rev. B). A single amplicon of ~460 bp was amplified using the 16S Forward and Reverse Primers as described in the Illumina protocol. The PCR product was cleaned up using Agencourt AmpureXP beads from Beckman Counter Genomics. Illumina adapter and barcode sequences were ligated to the amplicon in order to attach them to the MiSeqDx flow cell and for multiplexing. Quality and quantity of each sequencing library was assessed using Bioanlayzer and picogreen measurements, respectively. Approximately 6 pM of pooled libraries was loaded onto a MiSeqDX flow cell and sequenced using PE300 (Paired end 300 bp) v3 kit. Raw fastq files were demultiplexed based on unique barcodes and assessed for quality. Samples with more than 50K QC pass sequencing reads were used for downstream 16S OTU analysis.
Taxonomic classification and Operational taxonomic units (OTUs) abundance analysis was done using the CLC Bio Microbial Genomics Module (https://www.qiagenbioinformatics.com/plugins/clc-microbial-genomics-module/). Individual sample reads were annotated with the Greengene database and taxonomic features were determined. Alpha and beta diversity were calculated to understand the within and between sample diversity, respectively.
DATA AVAILABILITY
RNAseq data (Figures 1A, S1, and S6) have been submitted to the Gene Expression Omnibus with an accession number: GSE108718. 16S rRNA gene sequencing data (Figures 3 and S5) have been submitted to the Sequence Read Archive (SRA) with BioProject IDs: PRJNA527213 and PRJNA528925.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical details of experiments can be found in the figure legends, including how significance was defined and the statistical methods used. Data represent mean ± standard error of the mean. Numbers of experiments noted in figure legends reflect independent experiments that were performed on different days. No method was used to predetermine sample size. Blinding was not performed for these experiments. Formal randomization techniques were not used; however, mice were allocated to experiments randomly and samples processed in an arbitrary order. Skin samples that were determined to be in the anagen portion of the hair cycle were excluded. All statistical analyses were performed with GraphPad Prism software. To assess the statistical significance of a difference between two treatments, we used two-tailed Student’s t-tests (when a parametric test was appropriate) or Welch’s tests (when the data were normally distributed but variances were unequal among treatments). One-tailed student’s t-test was used when the null hypothesis was that treatment did not stimulate Retlna expression in mouse skin. To assess the statistical significance of differences between more than two treatments, we used one-way ANOVAs. The Kruskal-Wallis test was used when the data in any treatment group significantly deviated from normality or variances were unequal among treatments. The only mice excluded from experiments were mice that died during the course of experimentation. A single mouse in Figure 6F from the VAD group died on day 1 of infection and was excluded.
Supplementary Material
Highlights.
Skin microbiota induces epidermal RELMα, which kills bacteria via membrane disruption
RELMα-deficient mice have altered skin microbiota and are more susceptible to infection
Dietary vitamin A is required for RELMα expression
RELMα is required for vitamin A-dependent resistance to skin infection
Acknowledgements:
We thank C. L. Behrendt-Boyd, T. Leal, and B. Hassell for assistance with mouse experiments, S. Muhkerjee for her assistance with protein isolation and the liposome experiments, B. Chong for human skin samples, G. Lui for the staphyloxanthin-deficient Staphylococcus aureus strain, and T. Vandergriff for review of the skin histology. This work was supported by NIH grant R01 DK070855 (L.V.H.), a Burroughs Wellcome Foundation Investigators in the Pathogenesis of Infectious Diseases Award (L.V.H.), a Welch Foundation (Grant I-1874 to L.V.H.), the Walter M. and Helen D. Bader Center for Research on Arthritis and Autoimmune Diseases (L.V.H.), and the Howard Hughes Medical Institute (L.V.H.). S.G. was supported by NIH Grant T32 AI007520, D.C.P. was supported by NIH Grants T32 AI007520 and F32 DK100074, Z.K. was supported by NIH Grant T32 AI005284, and S. B. was supported by a Gruss-Lipper postdoctoral fellowship. J.-H.J. was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI15C1095) and the Intramural Research Program of the National Cancer Institute. H.H.K was supported by the Intramural Research Programs of the National Cancer Institute, National Institute of Arthritis and Musculoskeletal and Skin Diseases. J.A.S was supported by the Intramural Research Programs of the National Human Genome Research Institute. T.A.H. was supported by a Dermatology Foundation Career Development Award, a UT Southwestern Disease Oriented Clinical Scholars Program, a Burroughs Wellcome Fund Career Award for Medical Scientists, and a Burroughs Wellcome Fund Postdoctoral Enrichment Program Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests. Correspondence and requests for materials should be addressed to L.V.H (Lora.Hooper@UTSouthwestern.edu) or T.A.H (Tamia.Harris-Tryon@UTSouthwestern.edu).
References
- Albahrani AA, and Greaves RF (2016). Fat-soluble vitamins: clinical indications and current challenges for chromatographic measurement. Clin. Biochem. Rev 37, 27–47. [PMC free article] [PubMed] [Google Scholar]
- Banerjee RR, and Lazar MA (2001). Dimerization of resistin and resistin-like molecules is determined by a single cysteine. J. Biol. Chem 276, 25970–25973. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, and Segre JA (2014). Dialogue between skin microbiota and immunity. Science 346, 954–959. [DOI] [PubMed] [Google Scholar]
- Beylot C, Auffret N, Poli F, Claudel JP, Leccia MT, Del Giudice P, and Dreno B (2014). Propionibacterium acnes: an update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol 28, 271–278. [DOI] [PubMed] [Google Scholar]
- Braff MH, Di Nardo A, and Gallo RL (2005). Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J. Investig. Dermatol 124, 394–400. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Deming C, Cassidy SKB, Harrison OJ, Ng WI, Conlan S, Program, N.C.S., Belkaid Y, Segre JA, and Kong HH (2017). Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med 9, eaal4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronnell CM, Ghali LR, Ali RS, Quinn AG, Holland DB, Bull JJ, Cunliffe WJ, McKay IA, Philpott MP, and Muller-Rover S (2001). Human β defensin-1 and −2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J. Investig. Dermatol 117, 1120–1125. [DOI] [PubMed] [Google Scholar]
- Conlan S, Kong HH, and Segre JA (2012). Species-level analysis of DNA sequence data from the NIH Human Microbiome Project. PloS One 7, e47075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin CC, Swink SM, Horwinski J, Sfyroera G, Bugayev J, Grice EA, and Yan AC (2017). The preadolescent acne microbiome: A prospective, randomized, pilot study investigating characterization and effects of acne therapy. Pediatr. Dermatol 34, 661–664. [DOI] [PubMed] [Google Scholar]
- Croce AC, and Bottiroli G (2014). Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur. J. Histochem 58, 2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerkop BA, and Hooper LV (2013). Resident viruses and their interactions with the immune system. Nat. Immunol 14, 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr UH, Sudheendra US, and Ramamoorthy A (2006). LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758, 1408–1425. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, and Knight R (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JT, Fisher GJ, Zhang QY, Eisen D, Krust A, Kastner P, Chambon P, and Voorhees JJ (1991). Retinoic acid receptor gene expression in human skin. J. Investig. Dermatol 96, 425–433. [DOI] [PubMed] [Google Scholar]
- Ellis CN, and Krach KJ (2001). Uses and complications of isotretinoin therapy. J. Am. Acad. Dermatol 45, S150–157. [DOI] [PubMed] [Google Scholar]
- Everts HB (2012). Endogenous retinoids in the hair follicle and sebaceous gland. Biochim. Biophys. Acta 1821, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, Schoenfeld D, Nomicos E, Park M, Program, N.I.H.I.S.C.C.S., et al. (2013). Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulongne V, Sauvage V, Hebert C, Dereure O, Cheval J, Gouilh MA, Pariente K, Segondy M, Burguiere A, Manuguerra JC, et al. (2012). Human skin microbiota: high diversity of DNA viruses identified on the human skin by high throughput sequencing. PloS One 7, e38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RL and Hooper LV (2012). Epithelial antimicrobial defence of the skin and intestine. Nat. Rev. Immunol 12, 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad PD, Martin PM, and Singh N (2013). Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol 13, 869–874. [DOI] [PubMed] [Google Scholar]
- Ganz T, and Lehrer RI (1995). Defensins. Pharmacology & Therapeutics 66, 191–205. [DOI] [PubMed] [Google Scholar]
- Grice EA (2014). The skin microbiome: potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin. Cutan. Med. Surg 33, 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Renaud G, Young AC, Program, N.C.S., Bouffard GG, Blakesley RW, Wolfsberg TG, Turner ML, and Segre JA (2008). A diversity profile of the human skin microbiota. Genome Res. 18, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Program, N.C.S., Bouffard GG, Blakesley RW, Murray PR, et al. (2009). Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison WJ, Bull JJ, Seltmann H, Zouboulis CC, and Philpott MP (2007). Expression of lipogenic factors galectin-12, resistin, SREBP-1, and SCD in human sebaceous glands and cultured sebocytes. J. Investig. Dermatol 127, 1309–1317. [DOI] [PubMed] [Google Scholar]
- Holland KT, Aldana O, Bojar RA, Cunliffe WJ, Eady EA, Holland DB, Ingham E, McGeown C, Till A, and Walters C (1998). Propionibacterium acnes and acne. Dermatology 196, 67–68. [DOI] [PubMed] [Google Scholar]
- Idres N, Marill J, Flexor MA, and Chabot GG (2002). Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J. Biol. Chem 277, 31491–31498. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, and Song SY (2004). Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538. [DOI] [PubMed] [Google Scholar]
- Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, Stover CK, and Sellman BR (2015). Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. MBio 6, e02272–02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo JH, Kennedy EA, and Kong HH (2016). Topographical and physiological differences of the skin mycobiome in health and disease. Virulence 8, 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelhala HL, Aho VTE, Fyhrquist N, Pereira PAB, Kubin ME, Paulin L, Palatsi R, Auvinen P, Tasanen K, and Lauerma A (2018). Isotretinoin and lymecycline treatments modify the skin microbiota in acne. Exp. Dermatol 27, 30–36. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, Kong HH, Amagai M, and Nagao K (2015). Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity 42, 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Voisin B, Kim DY, Kennedy EA, Jo JH, Shih HY, Truong A, Doebel T, Sakamoto K, Cui CY, et al. (2019). Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell 176, 982–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Program, N.C.S., et al. (2012). Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 22, 850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong K, Rowlands CJ, Varma S, Perkins W, Leach IH, Koloydenko AA, Williams HC, and Notingher I (2013). Diagnosis of tumors during tissue-conserving surgery with integrated autofluorescence and Raman scattering microscopy. Proc. Natl. Acad. Sci. USA 110, 15189–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krismer B, Weidenmaier C, Zipperer A, and Peschel A (2017). The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol 15, 675–687. [DOI] [PubMed] [Google Scholar]
- Lennon NJ, Lintner RE, Anderson S, Alvarez P, Barry A, Brockman W, Daza R, Erlich RL, Giannoukos G, Green L, et al. (2010). A scalable, fully automated process for construction of sequence-ready barcoded libraries for 454. Genome Biol. 11, R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, and Nizet V (2005). Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med 202, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, and Modlin RL (2006). Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773. [DOI] [PubMed] [Google Scholar]
- Magoc T, and Salzberg SL (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy WH, Otchere E, Rosa BA, Martin J, Mann CM, and Mitreva M (2018). Skin ecology during sebaceous drought—how skin microbes respond to isotretinoin. J. Investig. Dermatol 139, 732–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NN, Liu GY, Yeaman MR, Nast CC, Proctor RA, McKinnell J, and Bayer AS (2011). Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 55, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. (2006). Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314, 1157–1160. [DOI] [PubMed] [Google Scholar]
- Moyersoen I, Demarest S, De Rider K, Tafforeau J, Lachat C, and van Camp J (2017). Fat-soluble vitamin intake from the consumption of food, fortified food and supplements: design and methods of the Belgian VITADEK study. Arch. Public Health 75, 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill AM, and Gallo RL (2018). Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome 6, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeff MK, Seltmann H, Hiroi N, Nastos A, Makrantonaki E, Bornstein SR, and Zouboulis CC (2006). Differential regulation of Toll-like receptor and CD14 pathways by retinoids and corticosteroids in human sebocytes. Dermatology 213, 266. [DOI] [PubMed] [Google Scholar]
- Oh J, Byrd AL, Park M; NISC Comparative Sequencing Program, Kong HH, and Segre JA (2016). Temporal stability of the human skin microbiome. Cell 165, 854–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanos CE, Ehlert R, and Gollnick H (1987). The retinoids. A review of their clinical pharmacology and therapeutic use. Drugs 34, 459–503. [DOI] [PubMed] [Google Scholar]
- Pappas A (2009). Epidermal surface lipids. Dermatoendocrinol 1, 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine GM, Batugedara HM, and Nair MG (2018) Here, there and everywhere: Resistin-like molecules in infection, inflammation, and metabolic disorders. Cytokine 110, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podvinec M, Kaufmann MR, Handschin C, and Meyer UA (2002). NUBIScan, an in silico approach for prediction of nuclear receptor response elements. Mol. Endocrinol 16, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Propheter DC, Chara AL, Harris TA, Ruhn KA, and Hooper LV (2017). Resistin-like molecule β is a bactericidal protein that promotes spatial segregation of the microbiota and the colonic epithelium. Proc. Natl. Acad. Sci. USA 114, 11027–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala MW, Obici S, Scherer PE, and Rossetti L (2003). Adipose-derived resistin and gut-derived resistin-like molecule-b selectively impair insulin action on glucose production. J. Clin. Invest 111, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring HC, Thorsen J, Saunte DM, Lilje B, Bay L, Riis PT, Larsen N, Andersen LO, Nielsen HV, Miller IM, et al. (2017). The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 153, 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RM, and Suter PM (2012). Vitamin and trace mineral deficiency and excess In Harrison’s Principles of Internal Medicine, Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, and Loscalzo J, eds. (New York, NY: McGraw-Hill; ). [Google Scholar]
- Ryan-kewley AE, Williams ER, Hepburn N & Dixon RA (2017). Non-antibiotic isotretinoin treatment differentially controls Propionibacterium acnes on skin of acne patients. Front. Microbiol 8, 1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JA, Zhang LJ, Williams MR, Gangoiti JA, Huang CM, and Gallo RL (2016). Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol 1, eaah4609. [DOI] [PubMed] [Google Scholar]
- Sanford JA, O’Neill AM, Zouboulis CC, and Gallo RL (2019). Short-chain fatty acids from Cutibacterium acnes activate both a canonical and epigenetic inflammatory response in human sebocytes. J. Immunol 202, 1767–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, and Diefenbach A (2009). RORgt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol 10, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurat JH (1999). Retinoids and psoriasis: novel issues in retinoid pharmacology and implications for psoriasis treatment. J. Am. Acad. Dermatol 41, S2–6. [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Yamasaki K, Brouha B, and Gallo RL (2006). Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 118, 509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, et al. (2007). Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest 117, 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Brown EJ, Wright CM, Bhat S, Banerjee RR, Dai CY, Enders GH, Silberg DG, Wen X, Wu GD, et al. (2001). A family of tissue-specific resistin-like molecules. Proc. Natl. Acad. Sci. USA 98, 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa Y, Asada H, Hoshino K, Azukizawa H, Katayama I, Akira S, and Itami S (2006). Induction of β-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2. Microbes Infect. 8, 1513–1521. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, and Hooper LV (2011). The antibacterial lectin RegIIIg promotes the spatial segregation of microbiota and host in the intestine. Science 14, 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, and Cole JR (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol 73, 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KP Jr., Katz J, Shrestha SR, LeClerq SC, Khatry SK, Pradhan EK, Adhikari R, Wu LS, Pokhrel RP, and Sommer A (1995). Mortality of infants < 6 mo of age supplemented with vitamin A: a randomized, double-masked trial in Nepal. Am. J. Clin. Nutr 62, 143–148. [DOI] [PubMed] [Google Scholar]
- Wolbach SB, and Howe PR (1925). Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med 42, 753–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis S, Blumenberg M, and Javed Q (2016). Resistin gene polymorphisms are associated with acne and serum lipid levels, providing a potential nexus between lipid metabolism and inflammation. Arch Dermatol Res 308, 229–37. [DOI] [PubMed] [Google Scholar]
- Yue JC, and Clayton MK (2005). A similarity measure based on species proportions. Commun. Stat-Theor. M 34, 2123–2131. [Google Scholar]
- Zhang LJ, and Gallo RL (2016). Antimicrobial peptides. Curr. Biol 26, R14–19. [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, and Gallo RL (2015). Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347, 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotogorski A (1987). Distribution of skin surface pH on the forehead and cheek of adults. Arch. Dermatol. Res 279, 398–401. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Korge BP, Mischke D, and Orfanos CE (1993). Altered proliferation, synthetic activity, and differentiation of cultured human sebocytes in the absence of vitamin A and their modulation by synthetic retinoids. J. Investig. Dermatol 101, 628–633. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Seltmann H, Neitzel H, and Orfanos CE (1999). Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95). J. Investig. Dermatol 113, 1011–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNAseq data (Figures 1A, S1, and S6) have been submitted to the Gene Expression Omnibus with an accession number: GSE108718. 16S rRNA gene sequencing data (Figures 3 and S5) have been submitted to the Sequence Read Archive (SRA) with BioProject IDs: PRJNA527213 and PRJNA528925.