Abstract
Polybrominated diphenyl ethers (PBDEs) are brominated flame retardants. Technical mixtures PentaBDE and OctaBDE were phased out in 2004 through voluntary and regulatory actions with DecaBDE remaining in limited use until 2013. Biomonitoring studies have shown widespread presence of PBDEs in the US and worldwide population. While some studies suggest that human serum concentrations are declining over time, it is unclear whether this trend will continue. Our objective was to examine temporal trends of PentaBDEs and their hydroxylated metabolites (OH-PBDEs) between 2008 and 2014 in populations of ethnically diverse, pregnant women residing in Northern California (n=111). Serum samples were collected and analyzed by high resolution mass spectrometry for five PentaBDE congeners and two OH-PBDEs. We found widespread exposures in participants from all three time points (2008/09, 2011/12, 2014). Temporal patterns varied substantially by congener. BDE −47, −99 and the OH-PBDEs decreased between 2008/09-2011/12 but plateaued between 2011/12-2014. In contrast, BDE-100 decreased across all years, BDE-153 decreased in the latter years, and BDE-28 decreased initially and then increased. These findings indicate that while policies to remove PBDEs from the marketplace have successfully lead to declines in exposures to some PBDE congeners, human exposures to these legacy pollutants could plateau and remain ubiquitous in human populations.
Keywords: Polybrominated diphenyl ethers (PBDEs), OH-PBDEs, biomonitoring, temporal trend, California, pregnant women
1.0. Introduction
Polybrominated diphenyl ethers (PBDEs) are brominated flame retardants that were used in household furnishings and electronics. They comprise three technical mixtures: PentaBDE, octaBDE, and decaBDE. Due to concerns about increasing exposures in the population and potential developmental effects, they were phased out in 2004 through voluntary and regulatory actions. DecaBDE (used in textiles, plastics etc.) remained in limited use until 2013. Biomonitoring studies have shown widespread presence of PBDEs in the US and worldwide population (Fromme et al., 2015). Most, but not all, biomonitoring studies suggest that concentrations have been decreasing due to the national and international phase out of these chemicals from commerce (Zota et al., 2013, Lignell et al. 2015).
Despite documented declines, studies worldwide of temporal observations of PBDE levels suggest there has been a leveling off of concentrations in recent years. In China, Li et al. reported a sizeable decrease of PBDEs (excluding BDE-209, the predominant congener observed in that study) between 2007 and 2011, but a smaller one between 2011 and 2013 (Li et al., 2017). A moderate annual decrease was observed in Sweden between 1996-2014 for lower brominated congeners BDE-47, −99, and −100 (Lignell et al., 2015). In contrast, a study of middle-age California women found that between 2011 and 2015 there was a modest annual percentage increase for several of the congeners of the PentaBDE mixture: BDE-47 (+5.6%), BDE-100 (+12.0%), and BDE-153 (+7.1%) (Hurley et al., 2017). These studies suggest that PBDEs could be following the trend of other legacy pollutants such as polychlorinated biphenyls (PCBs), where sharp temporal declines were followed by an exposure plateau. This plateau could be because of the possible shifting of dominant routes of exposure from household dust to diet (Harrad and Diamond, 2006, Cequier et al., 2015, Jiang et al., 2014, Wu et al. 2015).
Prenatal PBDEs can adversely affects child neurodevelopment making exposures in pregnant women particularly important (Lam, et al., 2017; National Academies of Sciences, Engineering, and Medicine., 2017). Prior studies have found that California residents have significantly higher PBDE exposures compared to the rest of the US population, most likely due to the historic furniture flammability standards in California (Zota et al., 2008). Biomonitoring of PBDE concentrations in California could provide information on PBDE trends now that these chemicals have been fully phased out of use, although they are still present in legacy items (e.g., old household furnishings, etc.). Our previous manuscript reported a decline in serum PentaBDE concentrations between two demographically similar cohorts of pregnant women seeking care in San Francisco, the first recruited in 2008/09 and second recruited in 2011/12. We found significant declines in BDE-28, BDE-47, BDE-99, BDE-100 and the OH-PBDEs, and a smaller, non-significant decline in BDE-153 (Zota et al., 2013).
In this paper, we present data from the third cohort recruited in 2014 to evaluate whether levels of PentaBDEs and their phenolic analogues in three demographically similar cohorts in Northern California have continued to decrease following the initial drop subsequent to the phase out. We focus on the PBDEs related to the PentaBDE commercial mixture (PBDE-28, −47, −99, - 100, & −153) and the most commonly detected hydroxyl metabolites.
2.0. Materials and Methods
2.1. Study Population
For all cohorts, we recruited and consented English- and Spanish-speaking patients between 15 and 24 weeks of pregnancy seeking medical care from the University of California, San Francisco (UCSF) Women’s Options Center at San Francisco General Hospital in San Francisco, California. The Women’s Options Center is an outpatient clinic providing pregnancy terminations and serving an ethnically diverse and predominantly lower income population from the San Francisco Bay area and other parts of Northern and Central California. Eligible study participants were identified by reviewing the patient’s medical record only after they had 1) consulted with a trained counselor for an elective second trimester termination procedure and 2) consented to the procedure as documentation of intent to proceed with the elective pregnancy termination. Our overall cohort consisted of 111 women, 25 nonsmoking women in 2008/09, 36 (smoking and nonsmoking) women in 2011/12, and 50 former or non-smoking women in 2014. Smokers were excluded a priori from the first cohort because the primary aim of that study was to evaluate effects of PBDE exposure on thyroid function and cigarette smoking can alter thyroid function. For all cohorts, we excluded participants if they were using street drugs or seeking a termination because of fetal anomalies. All study protocols were approved by the UCSF Committee on Human Research.
2.2. Sample Collection
Maternal blood was collected from each participant prior to medical procedures in red top Vacutainer tubes. After collection, the blood samples were centrifuged at 3000 RPM for 10 min at 4 °C. Serum was aliquoted into pre-screened sterilized amber vials using glass pipettes and stored at −80°C until analysis.
2.3. Analytical Methods
Full names and formulas of discussed PBDEs and phenolic analogues are in available in Table 1, a completed list of measured PBDEs is available in Appendix A, Table S1. We analyzed PBDE congeners and OH-PBDEs in serum at the Department of Toxic Substances Control (DTSC) (Berkeley, CA, USA) within its clean laboratory facility, where only human specimens are processed. The serum extraction and separation method are described in detail elsewhere (Zota et al., 2011). Specifics for the first, 2008/09, and second, 2011/12, cohorts are available in (Zota et al., 2013). For the third cohort, 2014, PBDEs and OH-PBDEs were measured using gas chromatography/high resolution double-focusing sector mass spectrometry (GC-HRMS, DFS, Thermo Fisher, Bremen, Germany) using a DB-5 MS column (15 m × 0.25 mm I.D., 0.10 μm film thickness, J & W Scientific, Folsom, CA). Hydroxylated compounds (OH-PBDEs) were measured after derivatization with diazomethane.
Table 1:
PBDE congener names, abbreviations and formulas measured in 2nd trimester pregnant women from Northern California
| Analyte Full Name | Abbreviation | Molecular Formula |
|---|---|---|
| 2,4,4’-Tribromodiphenyl ether | BDE-28 | C12H7Br3O |
| 2,2’,4,4’-Tetrabromodiphenyl ether | BDE-47 | C12H6Br4O |
| 2,2’,4,4’,5-Pentabromodiphenyl ether | BDE-99 | C12H5Br5O |
| 2,2’,4,4’,6-Pentabromodiphenyl ether | BDE-100 | C12H5Br5O |
| 2,2’,4,4’,5,5’-Hexabromodiphenyl ether | BDE-153 | C12H4Br6O |
| 6-Hydroxy-2,2’,4,4’-tetrabromodiphenyl ether | 6-OH-BDE47 | C12H6Br4O2 |
| 5-hydroxy-2,2’,4,4’-tetrabromodiphenyl ether | 5-OH-BDE47 | C12H6Br4O2 |
The instrument detection threshold (IDT) was defined according to the peak height/area. The method detection limit (MDL) was calculated as 3 times the standard deviation of the blank concentrations. MDLs are reported in Table 3, and are generally within a factor of 2, with BDE-47, 6-OH-BDE47, and 5-OH-BDE47 being notably higher in the 2008 cohort. For the 2014 cohort, precision and accuracy of PBDEs from surrogate spikes, reference material (SRM 1957, National Institute of Standards and Technology), and in-house control samples are reported in Appendix A, Table S2. Total cholesterol and triglyceride levels in maternal serum were measured by Quest Diagnostics in the first cohort and by Boston Children’s Hospital for the second and third. Total lipid content was calculated from measurements of total cholesterol and triglycerides using Phillip’s formula (Phillips et al., 1989).
2.4. Regression Analysis
We reported data for 5 PBDE congeners (BDE-28, BDE-47, BDE-99, BDE-100, BDE-153) and 2 hydroxylated metabolites (6-OH-BDE47, 5-OH-BDE47). Additional congeners were measured but not reported because our a priori focus was PentaBDEs. Other hydroxylated metabolites had low detection frequencies (e.g., 4’-OH-BDE49 detection frequency in 2011/12 and 2014, 6% and 18% respectively) and were not reported. Values below the MDL were imputed based on a log normal probability distribution whose parameters were calculated through a maximum likelihood estimation assuming that the undetected values fell between zero and as described in the previous paper (Zota et al., 2013).
We expressed all PBDE congeners as lipid normalized values (ng/g lipid) and OH-PBDEs were presented by weight wet (ng/mL). Concentrations were natural log normalized to account for their non-normal distribution. The demographic variables of age, maternal weight (BMI for a subset), race/ethnicity, insurance type (a marker of socioeconomic status), country of origin, parity, smoking, gestational age, and lipids were all considered for inclusion in the linear regression model. The Kruskal-Wallis, a non-parametric test, was applied to evaluate categorical demographic differences among the three cohorts, while one-way ANOVA was used for continuous parameters. Table 2 shows the results using the available data (i.e., missing data was not included in the totals). We used Spearman correlations as a conservative estimation of correlation. Separate regression models were constructed for each congener, the summation (ΣPBDE5), and individual and summed OH-PBDEs. Cohort (i.e., sample collection time period) was included as a three category variable (i.e., 2008/09, 2011/12, 2014) to represent a proxy for time. We used regression analysis to evaluate the difference in PBDE concentrations by cohort after adjusting for important demographic covariates. We used backwards stepwise regression to assess regression variables and each was included based on p-value (>0.2), maximization of data inclusion, and presence/absence in all models. The final models were all adjusted for the same set of covariates to make data comparable. We defined outliers as observations with an absolute studentized residual greater than 3.
Table 2:
Demographic comparisons across the three time periods of our study population of 2nd trimester pregnant women from Northern California (n=111)
| 2008-2009 (n=25)a N (%) |
2011-2012 (n=36)b N (%) |
2013-2014 (n=50)c N (%) |
p | |
|---|---|---|---|---|
| Age, years | 23.5±7.3 | 24.9±5.5 | 25.0±6 | 0.54 |
| 16-19 | 9 (36%) | 4 (11%) | 10 (20%) | |
| 20-29 | 12 (48%) | 25 (69%) | 31 (62%) | |
| 30-42 | 4 (16%) | 7 (19%) | 9 (18%) | |
| Ethnicity | 0.15 | |||
| Latina | 9 (36%) | 8 (23%) | 20 (40%) | |
| Black | 10 (40%) | 10 (29%) | 12 (24%) | |
| White | 3 (12%) | 12 (34%) | 11 (22%) | |
| Asian/Pacific Islander | 3 (12%) | 3 (8%) | 5 (10%) | |
| Other | 0 (0%) | 2 (5%) | 2 (4%) | |
| Insurance | 0.45 | |||
| Public | 20 (80%) | 28 (80%) | 41 (82%) | |
| Private/self-pay | 5 (20%) | 7 (20%) | 5 (10%) | |
| Country of origin | 0.39 | |||
| U.S. | 22 (88%) | 33 (94%) | 38 (76%) | |
| Non-U.S. | 3 (12%) | 2 (6%) | 7 (14%) | |
| Parity | 0.59 | |||
| Nulliparous | 8 (32%) | 11 (31%) | 21 (42%) | |
| Parity≥1 | 17 (68%) | 25 (69%) | 29 (58%) | |
| Current smokersd | <0.001 | |||
| No | 25 (100%) | 23 (64%) | 50 (100%) | |
| Yes | 0 (0%) | 13 (36%) | 0 (0%) | |
| Gestational age | 20.5±1.2 | 20.0±2.1 | 20.2±2.11 | 0.59 |
| Lipids (mg/mL) | 6.9±1.0 | 6.5±0.8 | 6.7±1.2 | 0.40 |
missing data: marital status n=1; weight n=1
missing data: weight n=1; insurance n=1; marital status n=6, country of origin n=1
missing data: marital status n=39; country of origin n=5, insurance n=5, weight=1
smokers were included in the 2nd cohort because of different study objectives.
The final model included adjustments for maternal weight and age, parity, insurance type, and race/ethnicity. Initially, there were 111 women across 3 cohorts, but after removal of participants with missing demographic data (2 for weight, and 5 for insurance type), 104 participants were used in the final model. Percent difference (eqn. 1) and confidence interval (CI, eqn. 2) were determined using standard
| equation 1 |
| equation 2 |
error (SE) and the estimated regression coefficient (β) for the cohort. The regression models were executed using 2008 as the referent population. Percent differences represent the difference between the specified cohort and 2008. Statistical significance was considered at α=0.05.
We conducted sensitivity analyses by testing different scenarios that evaluated the effects of study design, outliers and data collation. The facility used for lipid analysis and the presence/absence of smokers varied across cohorts. Accordingly, we evaluated both for their effects on the magnitude and significance of conclusions. Regression models were run with concentrations in wet weight and lipids as a factor and with/without smokers. Robustness of results to outliers was assessed by their removal. We examined normal probability plots of final model residuals which indicated that for the most part transformed data was normal. Residuals were graphed versus fitted values and homoscedasticity was observed. All statistical analysis was done in RStudio (0.98.501). We conducted exploratory calculations of PBDE decay in the study population by comparing our observations to estimates of concentrations assuming exponential decay. Calculations used the half-life values from the rat based estimations in Geyer et al. (2004), as values based on intake estimates may be underestimated (Wong et al., 2013). We also assumed a starting year of 2008.
3.0. Result and Discussion
3.1. Unadjusted Results
All three study cohorts were ethnically diverse women with similar ages across the three cohorts (Table 2). Women were primarily U.S. born and 80% were on public insurance (Table 2) We compared population characteristics across the three cohorts (Table 2), and apart from smoking, there were no significant differences observed across cohorts. The detection frequencies of all PBDE congeners were 100% in 2008. The detection frequencies of all congeners, except for BDE-47, decreased in later years. BDE-47 was consistently 100% across all years. BDE-28 and BDE-99 detection decreased since 2008, but flattened between 2011/12 and 2014. BDE-100 and BDE-153 saw notable decreases (Table 3) going from 97% detection frequency to around 66% and 62% respectively between 2011/12 and 2014. OH-PBDEs detection dropped since 2008/09. Between 2011/12 and 2014 both hydroxylated compounds had relatively steady detection frequencies with 5-OH-BDE47 dropping slightly, and there was high variance in the OH-PBDEs geometric means.
Table 3:
Temporal comparison of serum PBDE and OH-PBDE concentrations across the three cohorts of Northern California womena
| 2008-2009 (n=25) | 2011-2012 (n=36) | 2014 (n=50) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| congener | MDLb | % >MDL |
GMb (GSD) |
MDLb | % >MDL |
GMb (GSD) |
MDLb | % >MDL |
GMb (GSD) |
| BDE-28 | 0.66 | 100% | 2.32 (1.68) |
0.77 | 78% | 1.18 (2.26) |
1.19 | 80% | 2.72 (2.31) |
| BDE-47 | 11.7 | 100% | 43.1 (1.7) |
5.85 | 100% | 25.8 (2.03) |
4.78 | 100% | 24.6 (2.02) |
| BDE-99 | 3.63 | 100% | 11.5 (1.82) |
4.92 | 61% | 5.04 (2.78) |
2.61 | 66% | 4.89 (2.78) |
| BDE-100 | 0.91 | 100% | 9.00 (1.86) |
1.08 | 97% | 4.6 (2.27) |
1.19 | 66% | 2.41 (3.55) |
| BDE-153 | 0.93 | 100% | 15.5 (1.82) |
1.54 | 97% | 10.9 (2.17) |
2.39 | 62% | 4.75 (3.81) |
| ∑5PBDEs | 85.8 (1.24) |
51.6 (1.29) |
43.6 (1.28) |
||||||
| 6-OH-BDE47 | 0.010 | 92% | 0.025 (2.4) |
0.002 | 61% | 0.004 (3.91) |
0.002 | 66% | 0.004 (4.04) |
| 5-OH-BDE47 | 0.012 | 84% | 0.028 (3.5) |
0.002 | 64% | 0.004 (5.00) |
0.002 | 50% | 0.0021 (5.50) |
abbreviations are as follows, MDL= method detection limit; GM=geometric mean, GSD=geometric standard deviation
units for BDE congeners are ng/g, while hydroxylated metabolites are ng/mL
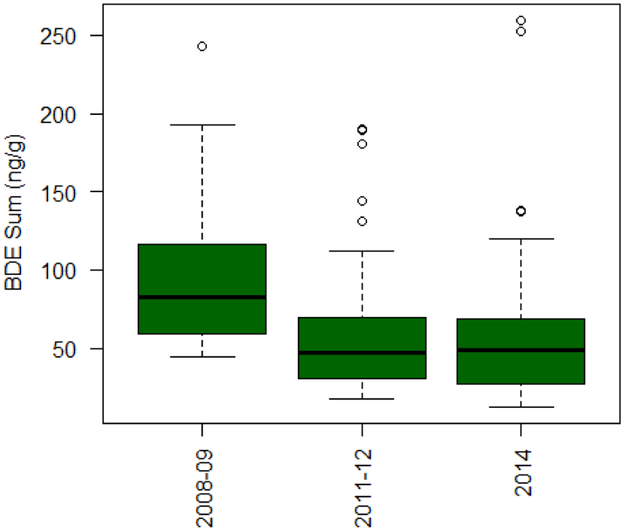
The unadjusted ∑PBDE5 (Figure 1) shows a concentration decline from the 2008/09 to 2011/12, but no change between 2011/12 and 2014. The dominant congener, BDE-47, showed approximately a 50% decrease from 2008/09 to 2011/12; whereas, concentrations were fairly similar between 2011/12 and 2014 (Table 3). Among all three cohorts, BDE-100 and 153 GMs decreased as did BDE-99 to a lesser degree (Table 3). BDE-28 was unique in decreasing from 2008/09 to 2011/12 but increasing again in 2014 (Table 3).
Figure 1:
Box plots of the sum of 5 PBDE congeners (ng/g) measuring in 2nd trimester pregnant women by study year.
BDE-47, BDE-99, and BDE-100 had the strongest inter-congener correlations of the PentaBDE congeners (Appendix A, Figure S1). BDE-28 and BDE-153 showed weak correlations with the other congeners. For example, the correlation between BDE-28 and BDE-47 was only r=0.53, and BDE-47 and BDE-153 was r=0.46. 5-OH-BDE47 and 6-OH-BDE47 did not correlate with any of the PBDEs or with each other. Lack of correlation was robust to the presence or absence of imputed values.
3.2. Adjusted Results
In the ∑PBDE5, there was a significant decline observed between 2008/09 and the latter years. However, we found approximately the same decline for both 2011/12 and 2014 (~45%) compared to the 2008/09 (referent group), indicating little change between the 2011/12 and 2014 (Table 4). We also observed this trend for BDE-47 and BDE-99. We did not observe this trend for BDE-28, −100, and −153, which will be discussed in further detail below.
Table 4:
Least squares geometric mean (LSGM) and % difference among cohorts of 2nd trimester pregnant women from Northern California.
| 2008-2009a | 2011-2012 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| congener | LSGMb | LSGMb | % differencec | p | LSGMb | % differencec | p |
| BDE-28 | 2.42 (1.18) | 1.22 (1.24) | −56 (−71, −32) | 0.00 | 2.56 (1.2) | 2.7 (−31, 52) | 0.89 |
| BDE-47 | 43.7 (1.2) | 25.8 (1.25) | −47 (−62, −24) | 0.00 | 23.5 (1.31) | −47 (−62, −27) | 0.00 |
| BDE-99 | 11.2 (1.3) | 5.07 (1.5) | −60 (−75, −35) | 0.00 | 4.61 (1.47) | −63 (−76, −42) | 0.00 |
| BDE-100 | 9.01 (1.4) | 4.61 (1.5) | −52 (−71, −19) | 0.01 | 2.09 (1.46) | −75 (−85, −60) | 0.00 |
| BDE-153 | 15.4 (1.67) | 11.4 (1.65) | −32 (−60, 15) | 0.16 | 4.69 (1.67) | −67 (−80, −47) | 0.00 |
| ∑5PBDEs | 85.9 (1.21) | 51.6 (1.24) | −45 (−61, −23) | 0.00 | 43.6 (1.29) | −49 (−63, −31) | 0.00 |
| 6-OH-BDE47 | 0.026 (1.7) | 0.004 (1.7) | −85 (−92, −71) | 0.00 | 0.004 (1.9) | −83 (−91, −68) | 0.00 |
| 5-OH-BDE47 | 0.028 (1.54) | 0.004 (1.7) | −88 (−95, −73) | 0.00 | 0.002 (1.6) | −93 (−97, −85) | 0.00 |
2008-2009 is the reference group.
Least squares geometric mean adjusted for maternal weigh and age, parity, insurance status and ethnicity. Units are ng/g lipid for BDEs, and ng/mL for metabolites. () show the geometric standard deviation.
% difference compared to referent cohort (2008). () indicate the 95% confidence interval.
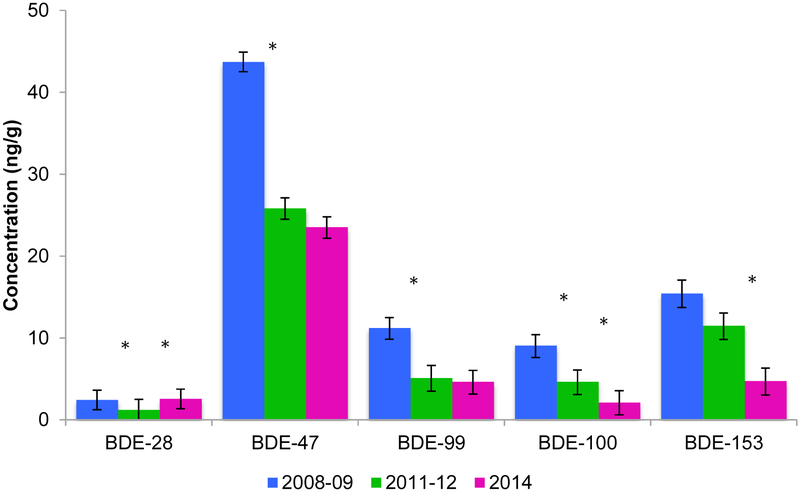
We did not find a statistical difference between BDE-28 measured in 2008/09 compared to 2014, while it was significantly lower in 2011/12 cohort indicating that BDE-28 increased between 2011/12 and 2014 (Figure 2). In contrast to BDE-28, the LSGMs of BDE-100 and BDE-153 showed steeper declines between 2011/12 and 2014 than between 2008/09 and 2011/12. ∑PBDE5 reflected the trend of the predominant congener BDE-47, with a significant decrease between 2008/09 and 2011/12 (45%) and a smaller difference (7.8%, p=0.61) difference between the latter two cohorts. For the OH-PBDEs, there was also a stark decline between the 2008/09 cohort and the more recent groups, but minimal difference between 2011/12 and 2014 (Table 4).
Figure 2:
Least Squares Geometric Means of PBDEs measured in 2nd trimester maternal serum across time adjusted for maternal weight and age, parity, insurance status, and ethnicity. Error bar represents the geometric standard deviation. An * indicates there was a significant difference (p<0.05) between the two adjacent cohorts.
Among the demographic characteristics examined, race/ethnicity was the only significant predictor of PBDE congeners (Appendix A, Table S2). We found the strongest associations with ethnicity for BDE-153 and 6-OH-BDE47. Caucasians and African-Americans had a 136-175% higher concentration of BDE-153 than Latinas. African-Americans and Asian/Pacific Islanders had a 102-182% higher concentration of 6-OH-BDE47 than Latinas.
When we compared our estimated trends based on half-lives to the levels measured in our cohorts, we found that BDE-47 and BDE-153 both deviated from the predictions. The 2014 BDE-47 levels appear greater than what would be expected, while BDE-153 concentrations were smaller. BDE-99 and 100 were consistent with the decay curves.
The final model was robust to sensitivity analyses. Wet weight normalized data with lipids as a separate covariate and presence and absence of smokers only had minor effect on the magnitude of difference and no effect on statistical conclusions (Appendix A, Table S3 & S4). In the 2014 cohort, two participants are on the upper end of the range (Figure 1). Of these two, one was removed because of missing demographic data (insurance status) and the other met outlier criteria in the final model, having both a high congener sum and BDE-153. The final model was evaluated without this participant and results showed the same conclusions, although the magnitude and precision of the effects varied by at most 2.6%. To keep models consistent across congeners, it was decided to include the outlier. The remaining discussion will refer to that modeling effort.
3.4. Study Limitations
There are some limitations to our study design. Primarily, we compared “snapshots” of exposure in three demographically similar populations of pregnant women, which is less ideal than a longitudinal examination of exposures in the same population of women. Our sample size was modest for an exposure study, but since most women were of lower socioeconomic status, there may be less potential for confounding. Our results may have limited generalizability due to the high PBDE exposures in the region (Zota, et al., 2008) and the unique demographic profiles of our study participants. However, the results are still valuable given that socially vulnerable populations are less frequently examined in environmental health studies. Furthermore, these results can provide insight into how chemical policy changes can influence chemical exposures.
3.5. Discussion
In this population of pregnant women from Northern California, a decline in the ∑PBDE5 concentration was observed between 2008/09 and 2011/12, but appeared to plateau between 2011/12 and 2014. The overall trend of the ∑PBDE5, BDE-47 and BDE-99 was consistent with observations for PCBs, and is likely explained by the changing sources of exposures. Initially, consumer products treated with PentaBDEs were a main source of exposure to humans. After PBDEs use was discontinued in these items, that source of exposure slowly diminished which could have contributed to our observed 45% decline between 2008/09 and 2011/12. However, due to persistence and bioaccumulation, PBDEs remain in reservoir sources, such as dust (Yu, et al., 2013) and food (Fraser et al., 2009), which could lead to persistent and ongoing exposures similar to PCBs. Additionally, that phase out has happened relatively recently (2004 was assumed to be peak exposure year in both models) and we are likely still in a transition phase (Gyalpo et al., 2015).
One surprising congener trend observed was the increase of BDE-28 from 3% to 6% of the congener sum from 2011/12 to 2014, as well as showing a 2014 concentration equal to that of 2008/09. BDE-28 is not a consistently reported component of the PentaBDE mixture, but was present in the PentaBDE formulation as a precursor to the formation of the higher brominated congeners (Alaee et al., 2003). In this study, BDE-28 was only weakly correlated with the PentaBDE congeners, which could suggest a different exposure source. One hypothesis is that BDE-28 may be the result of degradation of the higher brominated congeners, which could be a potential new source of both external and in vivo exposure. This is supported by a study from Hong Kong which reported a high proportion of BDE-28, 30% of ∑PBDE5 (Wang et al., 2013). They also observed that the BDE-28 percentage of total BDEs in blood plasma was higher than the percentage observed in fish muscle and indoor dust. They suggested that this could be due to BDE-28 levels being a result of degradation. In their study, BDE-28 only showed a high correlation with BDE-153 (Wang et al., 2013), different from the lack of correlation observed in here. Another study reported the collection of samples from Chinese women near an e-waste recycling facility showed BDE-28 as 16% of the ∑PBDE5 with BDE-153 having the highest concentration average. Unfortunately, no inter-congener correlations were reported (Zhao et al., 2013). De-bromination of higher congeners can occur through biotic (Zhang et al., 2014) or abiotic pathways (Wei et al., 2013), and further research is needed for a more full understanding of its effect on the congener profile in humans.
The high variance in the OH-PBDEs means could be explained by two factors. First, their lower detection frequency in the latter cohorts required the imputation of more (lower) values creating a large range (high variance) in the geometric means. This is slightly corrected in Table 4, where all the values have been adjusted for cohort number. Second, the latter two cohorts had concentrations near the analytical detection limit.
The major components of PentaBDE generally followed the decay trend predicted by their estimated half-lives. We saw greater decreases in BDE-153, t1/2 = 11.7 yrs. (Geyer et al., 2004) in the latter years than initially. BDE-47, with an estimated shorter half-life (t1/2=3 yrs., Geyer et al., 2004), showed greater reduction early in the time period. Recent attempts to use pharmo-kinetics models to equate exposure values and PBDE biomonitoring data have concluded that exposure sources have been underestimated in comparison to biomonitoring data (Gyalpo et al., 2015; Wong et al., 2013). Our estimated decay calculations compared with the three time points from this study indicate BDE-47 is not declining as fast as predicted by the half-life reported by Geyer et al. (2004).” The observed BDE-47 trend suggests continued external exposures some of which may be poorly characterized.
Continual exposure to BDE-47 is not surprising, given that California has reported some of the highest PentaBDE exposures in the world (Zota et al., 2008). However, the magnitude of exposure may be underestimated. Al-Omran and Harrad, (2017) demonstrated that using household dust collected from household vacuums underestimates the dust concentrations of the Penta-BDEs. Dust collected with specialized care by researchers provided higher estimates of the PBDEs studied in dust (Al-Omran and Harrad, 2017). A recent study showed that certain sub-populations may suffer continual exposure from waste products by proximity to landfills in California (Liu et al., 2016). While this may not be an important exposure route for everyone in our study population, it highlights a previously unconsidered source that could be contributing to ongoing human exposures. Another possibility is de-bromination of higher congeners. Many studies report BDE-209 as the predominant congener present in house dust (Bramwell et al., 2016), but its presence in human serum is more episodic and has been observed to have regional differences (Bramwell et al., 2016). While our analysis did not include BDE-209 because it is not part of the Penta mixture, we could not evaluate its contribution independently to exposures because it was not measured in the first two cohorts and it had a low frequency of detection (2%, data not shown) in the 2014 cohort. Research has also indicated that it is less bioaccessible than lower brominated congeners (Yu, et al., 2013), but its degradation could lead to higher concentrations of lower brominated congeners in dust (Yu, et al., 2013). Future studies should continue to monitor BDE-209 in dust and serum.
We observed that trends in BDE-99 and 100 were reasonably well predicted by their estimated half-lives, while BDE-153 estimations were larger than the observed concentrations. BDE-153 was only a minor component of the PentaBDE mixture, but comprised a larger percentage in the OctaBDE mixture. It is estimated to have the longest residence in the human body, being effectively stored in fat tissue (Bramwell et al., 2016). A longer residence time is supported by the data from the first two time periods in this study. Between 2008/09 and 2011/12 no significant decline was observed in BDE-153, while all other PentaBDE congeners decreased. Other temporal trend studies also support a longer half-life. A Swedish study showed that while BDE-47, −99, and −100 decreased between 1997 and 2010, BDE-153 actually increased (Darnerud et al., 2015). Makey et al. observed in their longitudinal study that while BDE-28, −47, −99, −100 were lower in 2010 than 2011, −153 stayed the same (Makey et al., 2014). Between 2011/12 and 2014, we did observe a significant decrease in BDE-153 (109%, p=0.002) in this population. This result is consistent with the longer half-life estimation of BDE-153, however, the magnitude of the decrease was greater than would be expected based on our rough calculation.
BDE-153 has been linked to different population characteristics and behaviors that perhaps were not adequately captured with our demographic information. Factors that have been shown to potentially influence exposures are diet, smoking and body composition. For example, Fraser et al. found that BDE-100 and −153 were associated with red meat fat (Fraser et al., 2009) – and we did not collect information on this population’s diet. We did include maternal weight in our demographic information and found that it was not associated with BDE-153 concentrations (β= −0.0033, p=0.15) in the full model. BMI information was only available for the 2011/12 and 2014 populations. In our analysis of the two later time periods with BMI, we found minimal difference between adjusting for BMI versus weight. The association of BDE-153 concentration with either weight or BMI was not significant (p= 0.27 and p=0.19 respectively). The small difference is likely because weight and BMI were highly correlated (spearman r=0.86). A study of children in Georgia found BDE-153 levels was positively associated with household smoking and negatively associated with BMI (Darrow et al., 2016). When using smoking as a factor in the BDE-153 model, it did not appear to be an important factor (p=0.29). Additional studies are needed to evaluate important factors that influence BDE-153 levels.
The strengths of our study are that the chemical analysis was performed in the same lab facilities, with the same method, although analysts did change. The regression model results appear robust to sensitivity analyses including lipid normalization versus wet weight, smoker removal and the removal of outliers. While our samples were not longitudinal of individuals, but rather cross-sectional with each cohort represented by different participants, we did recruit similar participants from the same clinic.
4.0. Conclusions
In a demographically similar population, we observed a decline in ∑PBDE5, BDE-47 and BDE-99 measured in pregnant women from Northern California between 2008/09 and 2011/12, with leveling out between 2011/12 and 2014. Most of the individual PentaBDE congeners followed a similar pattern (BDE −47, −99 and the OH-PBDEs), though one consistently decreased across both sample collection time periods (BDE-100), one decreased only in the latter years (BDE-153) and one decreased initially and then increased (BDE-28). Further investigations of exposure pathways and de-bromination are warranted to fully understand PBDEs in the human environment.
Supplementary Material
Highlights.
We measured PBDEs and OH-PBDEs in a demographically consistent population of pregnant women over six years.
Between 2008/09 and 2011/12, ∑PBDE5 concentrations decreased 45%, but there were minimal differences between 2011/12 and 2014.
BDE-47 was found in 100% of pregnant women in all years studied.
The decline in BDE-153 was greater between 2011/12 and 2014 than between the prior cohorts.
Acknowledgements
This study was funded by the National Institute of Environmental Health Sciences (P01ES022841, R21ES022422, R01ES010026, R00ES019881) and the U.S. EPA Science to Achieve Results (STAR) RD (83543301). We thank Dr. Linda Linderholm and Dr. Suhash Harwani for their assistance with the chemical analysis; Gregory Yeh and Wendy Duong for assistance with sample extraction; and Susanna Mitro for assistance with database management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
References
- Alaee M, Arias P, Sjödin A, Bergman Åke, 2003. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int 29, 683–689. doi: 10.1016/S0160-4120(03)00121-1 [DOI] [PubMed] [Google Scholar]
- Al-Omran LS, Harrad S, 2017. Influence of sampling approach on concentrations of legacy and “novel” brominated flame retardants in indoor dust. Chemosphere. 178, 51–58. doi: 10.1016/j.chemosphere.2017.02.096 [DOI] [PubMed] [Google Scholar]
- Bramwell L, Glinianaia SV, Rankin J, Rose M, Fernandes A, Harrad S, Pless-Mulolli T, 2016. Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review. Environ. Int 92–93, 680–694. doi: 10.1016/j.envint.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Cequier E, Marcé RM, Becher G, Thomsen C, 2015. Comparing human exposure to emerging and legacy lame retardants from the indoor environment and diet with concentrations measured in serum. Environ. Int 74, 54–59. doi: 10.1016/j.envint.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Lignell S, Aune M, Isaksson M, Cantillana T, Redeby J, Glynn A, 2015. Time trends of polybrominated diphenylether (PBDE) congeners in serum of Swedish mothers and comparisons to breast milk data. Environ. Res 138, 352–360. doi: 10.1016/j.envres.2015.02.031 [DOI] [PubMed] [Google Scholar]
- Darrow LA, Jacobson MH, Preston EV, Lee GE, Panuwet P, Hunter RE, Marder ME, Marcus M, Barr DB, 2016. Predictors of Serum Polybrominated Diphenyl Ether (PBDE) Concentrations among Children Aged 1–5 Years. Environ. Sci. Technol acs.est.6b04696. doi: 10.1021/acs.est.6b04696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, McClean MD, 2009. Diet contributes significantly to the body burden of PBDEs in the general U.S. population. Environ. Health Perspect 117, 1520–1525. doi: 10.1289/ehp.0900817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Becher G, Hilger B, Völkel W, 2015. Brominated flame retardants – Exposure and risk assessment for the general population. Int. J. Hyg. Environ. Health 219, 1–23. doi: 10.1016/j.ijheh.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Geyer H, Schramm K, Darnerud PO, Aune M, Feicht E, KW F, 2004. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compd. 66, 3820–3825. [Google Scholar]
- Gyalpo T, Toms LM, Mueller JF, Harden FA, Scheringer M, Hungerbühler K, 2015. Insights into PBDE uptake, body burden, and elimination gained from Australian age-Concentration trends observed shortly after peak exposure. Environ. Health Perspect 123, 978–984. doi: 10.1289/ehp.1408960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrad S, Diamond M, 2006. New Directions: Exposure to polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs): Current and future scenarios. Atmos. Environ 40, 1187–1188. doi: 10.1016/j.atmosenv.2005.10.006 [DOI] [Google Scholar]
- Hurley S, Goldberg D, Nelson DO, Guo W, Wang Y, Baek H-G, Park J-S, Petreas M, Berstein L, Anton-Culver H, Reynolds P, 2017. Temporal Evaluation of Polybrominated Diphenyl Ether (PBDE) Serum Levels in Middle-aged and Older California Women, 2011-2015. Environ. Sci. Technol 51, 4697–4708. doi: 10.1021/acs.est.7b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lin Z, Wu Y, Chen X, Hu Y, Li Y, Huang C, Dong Q, 2014. Daily intake of polybrominated diphenyl ethers via dust and diet from an e-waste recycling area in China. J. Hazard. Mater 276, 35–42. doi: 10.1016/j.jhazmat.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Lam J, Lanphear BP, Bellinger D, Axelrad DA, McPartland J, Sutton P, Davidson L, Daniels N, Sen S, Woodruff TJ, 2017. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-Analysis. Environ. Health Perspect 125, 086001. doi: 10.1289/ehp1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Jin J, Wang Y, Hu J, Xu M, Sun Y, Ma Y, 2017. Concentrations of organophosphorus, polybromobenzene, and polybrominated diphenyl ether flame retardants in human serum, and relationships between concentrations and donor ages. Chemosphere 171, 654–660. doi: 10.1016/j.chemosphere.2016.12.126 [DOI] [PubMed] [Google Scholar]
- Lignell S, Aune M, Glynn A, Cantilla T 2015. Levels of PBDEs and HBCD in blood serum from first time mothers in Uppsala - temporal trends 1996-2014. Swedish Environmental Protection Agency; (Online) Available from: http://www.imm.ki.se/Datavard/rapporter/Levels%20of%20PBDEs%20and%20HBCD%20in%20blood%20serum%20from%20first%20time%20mothers%20in%20Uppsala_temporal%20trends%201996_2014.pdf [Google Scholar]
- Liu R, Nelson DO, Hurley S, Petreas M, Park J-S, Wang Y, Guo W, Bernstein L, Hertz A, Reynolds P, 2016. Association between Serum Polybrominated Diphenyl Ether Levels and Residential Proximity to Solid-Waste Facilities. Environ. Sci. Technol 50, 3945–3953. doi: 10.1021/acs.est.5b04715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makey CM, McClean MD, Sjödin A, Weinberg J, Carignan CC, Webster TF, 2014. Temporal variability of polybrominated diphenyl ether (PBDE) serum concentrations over one year. Environ. Sci. Technol 48, 14642–14649. doi: 10.1021/es5026118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2017. Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals. Washington, DC: The National Academies Press. doi: 10.17226/24758. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert John T., Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol 18, 495–500. [DOI] [PubMed] [Google Scholar]
- Wang HS, Jiang GM, Chen ZJ, Du J, Man YB, Giesy JP, Wong CKC, Wong MH, 2013. Concentrations and congener profiles of polybrominated diphenyl ethers (PBDEs) in blood plasma from Hong Kong: Implications for sources and exposure route. J. Hazard. Mater 261, 253–259. doi: 10.1016/j.jhazmat.2013.07.033 [DOI] [PubMed] [Google Scholar]
- Wei H, Zou Y, Li A, Christensen ER, Rockne KJ, 2013. Photolytic debromination pathway of polybrominated diphenyl ethers in hexane by sunlight. Environ. Pollut 174, 194–200. doi: 10.1016/j.envpol.2012.11.035 [DOI] [PubMed] [Google Scholar]
- Wong F, Cousins IT, MacLeod M, 2013. Bounding uncertainties in intrinsic human elimination half-lives and intake of polybrominated diphenyl ethers in the North American population. Environ. Int 59, 168–174. doi: 10.1016/j.envint.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Wu X, Bennett DH, Moran RE, Sjödin A, Jones RS, Tancredi DJ, Tulve NS, Clifton MS, Colón M, Weather W, Hertz-Picciotto I, 2015. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environ. Health 14, 23. doi: 10.1186/s12940-015-0002-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yang D, Wang X, Huang N, Zhang X, Zhang D, Fu J, 2013. Factors influencing on the bioaccessibility of polybrominated diphenyl ethers in size-specific dust from air conditioner filters. Chemosphere, 93, 2603–2611. doi: 10.1016/j.chemosphere.2013.09.085 [DOI] [PubMed] [Google Scholar]
- Zhang F, Lu G, Liu J, Yan Z, Zhang Z, 2014. Bioaccumulation, distribution and metabolism of BDE-153 in the freshwater fish Carassius auratus after dietary exposure. Ecotoxicol. Environ. Saf 108, 16–22. doi: 10.1016/j.ecoenv.2014.06.030 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ruan X, Li Y, Yan M, Qin Z, 2013. Polybrominated diphenyl ethers (PBDEs) in aborted human fetuses and placental transfer during the first trimester of pregnancy. Environ. Sci. Technol 47, 5939–46. doi: 10.1021/es305349x [DOI] [PubMed] [Google Scholar]
- Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, Zoeller RT, Woodruff TJ, 2013. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol 47, 11776–11784. doi: 10.1021/es402204y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ, 2011. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ. Sci. Technol 45, 7896–7905. doi: 10.1021/es200422b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Rudel RA, Morello-Frosch RA, Brody JG, 2008. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ. Sci. Technol 42, 8158–8164. doi: 10.1021/es801792z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.