Abstract
Background: Urinary tract infection (UTI) is one of the most common bacterial infectious diseases encountered in clinical practice, and accounts for significant morbidity and high medical costs. To reduce its public health burden, there is the need for local research data to address aspects of prevention and management of UTI. The aim of this study was to investigate community-acquired UTI among adults in Accra, Ghana, including the risk factors, etiological agents, and antibiotic resistance.
Methods: This was a cross-sectional study involving 307 patients clinically diagnosed with UTI at the Korle Bu and Mamprobi polyclinics in Accra. Urine specimens were collected from the study participants and analyzed by culture, microscopy, and dipstick. The bacterial isolates were identified using standard microbiological methods and tested against a spectrum of antibiotics by the Kirby Bauer method. Multidrug resistant Enterobacteriaceae isolates were screened for Extended Spectrum β-lactamase (ESBL) production by the double disc method, and isolates that tested positive were analyzed by Polymerase Chain Reaction for ESBL genes. Demographic information and clinical history of study participants were collected.
Results: Based on the criteria for laboratory confirmed UTI, 31 (10.1%) of the 307 specimens were positive and the main risk factor of UTI among the study participants was pregnancy (P=0.02, OR=2.43). The most common uropathogen isolated was Escherichia coli (48.9%), followed by Klebseilla sp. (16.1%). Prevalence of resistance was highest for Piperacillin (87.1%) and Amoxicillin+Clavulanic Acid (87.1%) and lowest for Amikacin (12.9%). Prevalence of multidrug resistance among the uropathogens was 80.1% (25) and the most common ESBL gene detected was CTX-M-15.
Conclusion: Pregnant women constitute the key risk population of UTI in Accra, while Amikacin remains a suitable drug for the treatment of febrile UTI. The high prevalence of multidrug resistance among the uropathogens highlights the need for surveillance of antimicrobial resistance among these pathogens.
Keywords: extended spectrum β-lactamases, multidrug resistance, urinary tract infection
Introduction
Urinary tract infection (UTI) refers to microbial invasion of the urinary tract by one or more uropathogenic bacteria species, leading to significant bacteriuria and the presence of symptoms such as dysuria.1 It is one of the commonest diseases diagnosed in outpatients and affects approximately 150 million people yearly.2 The associated cost of healthcare is enormous, accounting for $659 million in direct costs for treating and $936 million in indirect costs, totaling $1.6 billion annually.3 Two main types of UTIs are known based on how the infection is acquired: hospital acquired UTI (Nosocomial UTI) and community acquired UTI.4 Hospital acquired UTI is defined as the onset of UTI in patients, 48 hours after admission, while community acquired UTI refers to the development of infections before admission to the hospital and not within 10 days after the patient has been discharged.4,5 Escherichia coli is the commonest cause of both community and hospital acquired UTI.6–8 Other common uropathogens encountered in community acquired UTI include Staphylococcus saprophyticus, Klebsiella pneumoniae, and Citrobacter spp.9–11 The etiology of hospital acquired UTI is, however, more varied and includes a wide range of organisms such as Pseudomonas aeruginosa and Proteus sp., which are hardly encountered in community-acquired UTI.12,13 Community-acquired UTI are usually uncomplicated, and the risk factors commonly include female sex, being sexually active, and use of spermicidal contraceptives.14,15 On the other hand, hospital acquired UTI is usually complicated and is associated with risk factors such as catheterization and recent antibiotic use.16,17
The use of antimicrobial therapy has contributed a great deal to the management of UTI. However, accumulated evidence shows that treatment of these infections is increasingly becoming difficult due to the rapid emergence of antimicrobial resistance in hospitals and the community, a phenomenon attributed to overuse and misuse of antibiotics.18,19 Although a global problem, antibiotic resistance of uropathogens carries more significance in the developing world, where treatment options are limited. In Ghana, antimicrobial treatment of UTI is mainly empirical due to a relative lack of appropriate laboratory facilities for culture and susceptibility testing of bacteria in several health facilities. Unfortunately, few studies have reported on antimicrobial resistance patterns of uropathogens in Ghana,20,21 and without recent surveillance data of antimicrobial susceptibility, empirical treatment of UTI could be ineffective and expensive. To help address this problem and contribute to effective management and prevention of UTI, this study was carried out. The aim of the study was to investigate community-acquired UTI in Accra, Ghana, including the risk factors, etiological agents, and antibiotic resistance.
Materials and methods
Study site and sampling
The study was conducted at two clinics in Accra, namely Korle Bu Polyclinic and Mamprobi Polyclinic. Accra is the capital city of Ghana and has a population of about 1.8 million people.22 The two polyclinics provide primary healthcare to a large section of the adult population in Accra due to their strategic location in the city center. Korle Bu Polyclinic is a 42-bed facility, while Mamprobi Polyclinic is a 54-bed facility (Prince Horlortu, personal communication, 2016). In addition to general medical services, both clinics provide healthcare in areas of minor surgeries, medical laboratory service, radiography, and assorted body imaging services. This study was cross-sectional in design and was conducted between April and September 2016. Using a 95% confidence level, a 22.5% estimated UTI prevalence reported previously23 and a 5% allowable error, 307 patients who were clinically suspected of having a UTI were randomly recruited from both polyclinics. Patients recruited met the inclusion criteria of not been admitted into the hospital for not more than 48 hours and satisfying the age requirement of 13 years and above. Patients who had been on antibiotics 2 weeks or earlier prior to the study were excluded. Information on demographic and clinical features of the study participants was extracted from their clinical records, and these included age, gender, body mass index (BMI), previous UTI, presence of diabetes, frequency of sex, and pregnancy (in the case of females). A mid-steam urine sample was obtained from each of the study participants for analysis in the bacteriology laboratory of the School of Biomedical and Allied Health Sciences, University of Ghana, which is less than a kilometer from each of the study clinics.
Laboratory investigations
Analysis of urine samples
The urine samples were cultured on Cysteine Lactose Electrolyte Deficient (Oxoid Ltd., Basingstoke, UK) media and incubated at 37°C for 18–24 hours.24 Following incubation, colonies of bacteria were counted and counts of ≥105 (cfu/mL) were regarded as significant bacteriuria.25 Isolated bacteria were identified based on their colonial morphology, Gram-stain reactions and biochemical tests including oxidase test, triple sugar iron (TSI) fermentation tests, indole test, citrate utilization test, urea utilization test, catalase test, coagulase test, and motility.
After the culture process, 5mL of the remaining urine samples were aseptically transferred into 15 mL falcon tubes for centrifugation. The macroscopic properties (color and appearance) of the urine samples were observed and recorded for each sample. Dipstick analysis of each sample was performed by immersing the urine test strip entirely into the urine samples for a brief period (2 seconds). The strips were removed, then examined for biochemical parameters including glucose, proteins, pH, specific gravity, ketones, nitrites, bilirubin, urobilinogen, and the presence of cells such as leucocytes and erythrocytes. Attention was paid to the nitrite and leucocyte, indicating portions of the strip as they serve as highlights for possible bacterial infection. The urine transferred into the clean transparent falcon tubes were centrifuged at 2,500 rpm for 3 minutes, after which the supernatant was decanted and the residue used to prepare a wet mount and observed under the dry high power objective of the light microscope. Evidence of pus cells, red blood cells, parasites, yeasts cells, epithelial cells, crystals, casts, and bacteria were sought for and recorded. Urinary tract infections in the study participants were determined by significant bacteriuria with the presence of pyuria.26
Antibiotic susceptibility testing
Susceptibility testing of isolates was performed using the Kirby Bauer disc diffusion method with strict adherence to the 2016 Clinical Laboratory Standards Institute (CLSI) guidelines. The antibiotics tested included: Nalidixic acid (30 µg), Ciprofloxacin (5 µg), Norfloxacin (20 µg), Levofloxacin (5 µg), Amikacin (30 µg), Gentamicin (10 µg), Nitrofurantoin (300 µg), Piperacillin (20 µg), Oxacillin (1 µg), Amoxicillin+Clavulanic Acid (Augmentin) (30 µg), Cefuroxime (30 µg), Ceftazidime (20 µg), and Tetracycline (30 µg). Standardized inoculums were prepared in sterile saline to turbidity comparable to 0.5 McFarland standard solution of barium sulfate. Muller-Hinton agar plates were swabbed with the standardized inoculums and antimicrobial discs aseptically placed on it after drying the plate for 3–5 minutes. The plates were then incubated at 37°C for 18 hours.27,28 The zone sizes were measured and interpreted using the 2016 CLSI guidelines (www.clsi.org).28
Screening for Extended Spectrum β-lactamases (ESBLs) was performed on multi-drug resistant Enterobacteriacae isolates using the double disc method.28 Antibiotic discs of Ceftazidime (30 µg), and Ceftazidime plus Clavulanic acid (30/10 µg) were placed on Mueller Hinton agar and incubated at 37°C for 18–24 hours. An organism was considered as an ESBL producer if there was a ≥5 mm increase in the zone diameter of Ceftazidime/Clavulanate disc and that of Ceftazidime disc alone.28 E. coli ATCC 25922 and a known in house ESBL producer were used as negative and positive controls, respectively. Positive ESBL isolates were further characterized using Polymerase Chain Reaction (PCR) (Qiagen Multiplex PCR kit) to determine the specific genes responsible for conferring resistance to the isolates.
For detection of ESBL genes, SHV, TEM, OXA-2, OXA-10, and the CTX-M group 1, 2, and 9 primers were used. All PCR protocols included an initial denaturation of 94°C for 15 minutes and a final extension at 72°C for 10 minutes. The PCR products were analyzed for nucleotide sequencing at Inqaba biotech™, South Africa, and compared with sequences in the NCBI database (http://www.ncbi.nlm.nih.gov/BLAST).
Data analysis
Data collected in the study were entered into Microsoft Excel version 2010 and analyzed using STATA version 12. Descriptive analyses were carried out on the study variables, which included computation of arithmetic means, percentages, and frequencies. Uni-variable associations were performed between UTI and all the other variables recorded: Analysis of variance was used for numeric variables, whereas Chi-square test was used for categorical variables. Variables significantly associated with urinary tract infection in the invariable analysis were used as independent variables in a logistic regression analysis to identify determinants of urinary tract infection. The statistical significance of the independent variables was evaluated by confidence intervals, and odds ratios including P-values; variables with P<0.05 were regarded as significant.
Ethical clearance
Ethical approval for the study was obtained from the ethical and protocol review committees of the Ghana Health Service and College of Health Sciences, University of Ghana (CHS-Et/M.4-P4.11/2015–2016). Written informed consent was also obtained from the study participants according to the Declaration of Helsinki.
Results
Demographic and clinical features of study participants
Demographic and clinical features of the 307 study participants are reported in Table 1. The mean age of the study participants was 37.2 (±17.6) years, and the majority (75%) of them were less than 50 years old. A high proportion of 79.8% (245) of the study participants were females, of which 27.4% (84) were pregnant. Diabetes occurred in 6.8% (21/307) of the study participants and 22.5% (69/307) had experience of previous UTI. About half of the study participants (56%) had a normal body mass index, 13.7% were obese, 27% were overweight and 3.3% were underweight. A proportion of 26.1% (80/307) did not practice sex; 33.2% (102/307) practiced sex twice or less a month, while 40.7% (125/307) practiced it more than twice a month.
Table 1.
Demographic and clinical features of the study participants
| Feature | N | % |
|---|---|---|
| Gender | ||
| Male | 62 | 20.2 |
| Female | 245 | 79.8 |
| Age (Mean=37.2±17.6) | ||
| <50 | 231 | 75.2 |
| ≥50 | 76 | 24.8 |
| Frequency of sex | ||
| No sexual activity | 80 | 26.1 |
| Twice or less a month | 102 | 33.2 |
| More than twice a month | 125 | 40.7 |
| Diabetes | ||
| Diabetic | 21 | 6.8 |
| Non-diabetic | 286 | 93.2 |
| Pregnancy | ||
| Yes | 84 | 27.4 |
| No | 223 | 72.6 |
| Body Mass Index | ||
| Underweight (<18) | 10 | 3.3 |
| Normal (18–24.9) | 172 | 56 |
| Overweight (25.0–29.9) | 83 | 27 |
| Obese (>30) | 42 | 13.7 |
| Previous UTI | ||
| Yes | 69 | 22.5 |
| No | 238 | 77.5 |
Abbreviations: N, number of study participants; UTI, Urinary Tract Infection.
UTI and associated risk factors
Thirty-one (10%) of the 307 patients enrolled in the study had UTI, all of which were uncomplicated. Urinary tract infections were more common in females (93.6%, 31/29) than males (6.4%, 31/2) (P=0.06). In the multivariate analysis, pregnancy was the only factor that was significantly associated with UTI among the study participants (Table 2). Age, BMI, gender, diabetes, frequency of sex, and previous UTI did not show a significant association with UTI.
Table 2.
Risk factors of urinary tract infection identified through logistic regression
| Feature | OR | 95% CI | P-value |
|---|---|---|---|
| Gender | 4.03 | 0.9–17.4 | 0.06 |
| Age (Mean=37.2±17.6) | 0.97 | 0.9–1.0 | 0.07 |
| Frequency of sex | 1.55 | 0.9–2.5 | 0.08 |
| Diabetes | 0.43 | 0.05–3.3 | 0.41 |
| Pregnancy | 2.42 | 1.1–5.2 | 0.02 |
| Body Mass Index | 1.01 | 0.6–1.6 | 0.97 |
| Previous UTI | 0.81 | 0.3–2.1 | 0.66 |
Abbreviations: OR, odds ratio; CI, Confidence Interval; UTI, Urinary Tract Infection.
Causative organisms of UTI and antibiotic resistance
Eight different bacterial species were isolated from urine specimens of the study participants; the most prevalent was Escherichia coli (48.4%) followed by Klebsiella sp. (16.1%) and Staphylococcus aureus (Table 3).
Table 3.
Bacteria isolated from urine specimens
| BACTERIA | N | % |
|---|---|---|
| Escherichia coli | 15 | 48.4 |
| Klebsiella sp. | 5 | 16.1 |
| Staphylococcus aureus | 4 | 12.9 |
| Citrobacter sp. | 3 | 9.7 |
| Pseudomonas aeruginosa | 1 | 3.2 |
| Proteus mirabilis | 1 | 3.2 |
| Klebsiella pneumoniae | 1 | 3.2 |
| Enterobacter sp. | 1 | 3.2 |
Abbreviation: N, number of positive urine specimens.
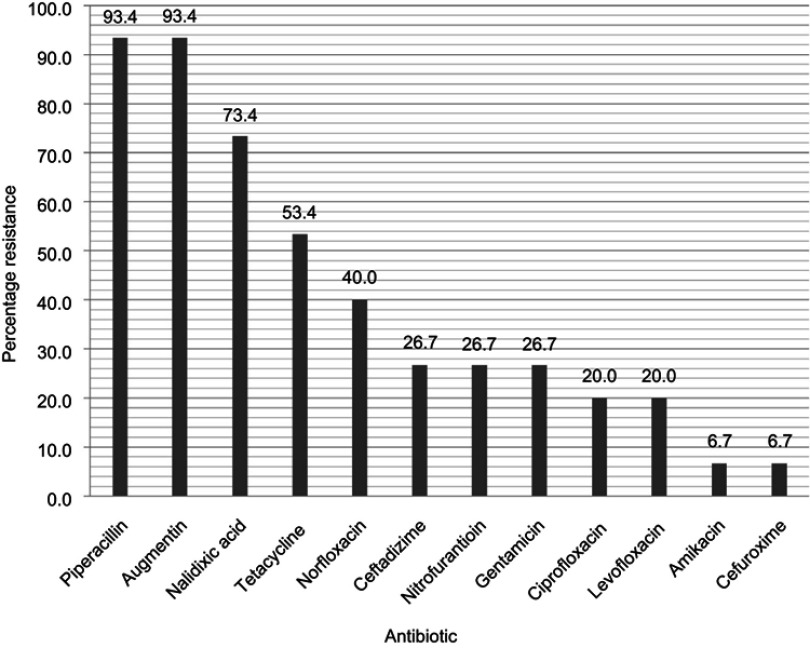
Overall prevalence of antibiotic resistance among the bacterial isolates ranged from Amikacin (12%) to Piperacillin (87%) (Table 4). Antibiotic resistance was particularly high for β-lactam antibiotics (Table 4). As shown in Figure 1 for E. coli (most common uropathogen isolated), the prevalence of antibiotic resistance decreased across Piperacillin/Amoxicillin+Clavulanic Acid (93.4%), Nalidixic acid (73.4%), Tetracycline (53.4%) Norfloxacin (40.0%), Ceftadizime (26.7%), Nitrofurantoin (26.7%), Gentamicin (26.7%), Ciprofloxacin (20.0%), Levofloxacin (20.0%), Amikacin (6.7%), and Cefuroxime (6.7%). The overall prevalence of MDR among the bacterial isolates was 77.4% (24/31); Multi-drug resistance (MDR) prevalence among E. coli isolates was 66.7% (10/15). The predominant ESBL gene detected among the sequenced isolates was CTX-M-15 type followed by TEM-3 (Table 5).
Table 4.
Overall prevalence of resistance among the antibiotics tested
| Class of antibiotics | Antibiotic tested | N | (%) |
|---|---|---|---|
| Quinolones Fluoroquinolones |
Nalidixic acid-Nal (30 µg), Ciprofloxacin-Cip (5 µg), Norfloxacin-Nor (20 µg), Levofloxacin-Lev (5 µg) | 22, 8, 13, 7 | 70.97, 25.81, 41.94, 22.58 |
| Aminoglycosides | Amikacin-Amk (30 µg), Gentamicin-Gen (10 µg) | 4, 10 | 12.90, 32.26 |
| Furadantins | Nitrofurantoin-Nit (300 µg) | 13 | 41.94 |
| β-lactam- b lactamase inhibitors | Piperacillin-Pip (20 µg), Oxacillin-Ox (1 µg), amoxicillin+clavulanic acid-Aug (30 µg) | 27, 3, 27 | 87.10, 75.00, 87.10 |
| Cephalosporin 2nd generation, Cephalosporin 3rd generation | Cefuroxime-Cef (30 µg), Ceftazidime-Cft (20 µg) | 7, 15 | 22.58, 48.39 |
| Tetracyclines | Tetracycline-Tet (30 µg) | 18 | 58.06 |
Abbreviation: N, Number of resistant isolates.
Figure 1.
Antibiogram of Escherichia coli isolated from urine specimens.
Table 5.
Distribution of ESBL genotypes and genes among multi-drug resistant Enterobacteriacae isolates from urine
| Specimen number | Bacteria | ESBL genotype | ESBL sequence type |
|---|---|---|---|
| 62 | Escherichia coli | TEM | TEM-1 |
| 108 | Escherichia coli | TEM, CTX-M | TEM-116, CTX-M-15 |
| 134 | Citrobacter sp. | CTX-M | CTX-M-15 |
| 136 | Klebsiella sp. | TEM, CTX-M | TEM-116, CTX-M-15 |
| 149 | Citrobacter sp. | TEM, CTX-M | TEM-1, CTX-M-15 |
| 284 | Klebsiella sp. | TEM, SHV | TEM-3, SHV-1 |
| 189 | Proteus sp. | CTX-M | CTX-M-15 |
Abbreviation: ESBL, extended spectrum β-lactamases.
Discussion
In this study, we investigated community-acquired UTI among adults in Accra with the goal of providing the necessary epidemiological information to reduce the burden of these infections. In the analysis of risk factors, only pregnancy emerged as a risk factor of UTI. The association of pregnancy with UTI concurs with several studies,29–31 and is probably due to the series of structural and functional urinary tract changes that occur during the course of pregnancy. During pregnancy, there is a usually high level of circulating progesterone, which causes urethral sphincter relaxation and a reduction in smooth muscle tone with slowing of ureteral peristalsis. Simultaneously, the enlarged uterus compresses the urinary bladder, thus increasing the intravesical pressure, which may result in vesico-ureteral reflux and urine retention in the bladder after micturition.31 Urinary stasis and impairment of the physiological anti-reflux mechanism create conditions favorable for bacterial growth and ascending infection.
Uropathogens isolated from the study participants were mainly members of the Enterobacteriaceae which concurs with previously published data.17,32–36 E. coli, which is the leading causative organism of both community and hospital,6,8,37 was the most predominant organism isolated from the study participants. In Ghana, E. coli was the most common uropathogen isolated from stroke patients,21 sickle cell disease patients,38 and pregnant women.20 In the pathogenesis of UTI, E. coli employs factors such iron acquisition systems, fimbriae that mediate attachment to host tissues, toxins (hemolysins and autotransporter toxins), flagella, and special proteins that help to weaken and aid evasion of the host innate and adaptive immune system.39 Furthermore, E. coli is known to form bacterial communities within the tissue of the bladder wall to enable it to proliferate without the influence of antibacterial molecules and host inflammatory cells and also away from the flow of urine.39–41 Two pathogenic mechanisms allow microorganisms to reach the urinary tract: the ascending and the hematogenous routes. In the ascending route, which is more common, microorganisms of the intestinal microbiota such as E. coli colonize the periurethral space and ascend through the urethra to the bladder and eventually the kidneys. The hematogenous route occurs in patients with bacteremia or endocarditis, mostly caused by S. aureus, which spreads to the kidneys.39
We observed very high percentage resistance for Piperacillin (87.1%), Amoxicillin+Clavulanic Acid (87.1%), and Nalidixic Acid (71%), which concur with previous studies on antibiotic resistance of uropathogens in Ghana.21,38,42 This raises concerns about the suitability of these antibiotics for empirical treatment of UTI in Ghana. In line with the trend of increasing MDR, especially in the developing world,43–48 we observed a prevalence of 77.4% overall and 66.7% for E. coli. This, coupled with ESBLs detected in Enterobacteriacae isolates, poses a major challenge to the treatment of UTI in Ghana. ESBL producing uropathogenic E. coli appear to be associated with more serious UTI resulting in septicemia.49 CTX-M, the most common ESBL harbored by the Enterobacteriaecae in this study, is a new family of plasmid-mediated ESBLs that preferentially hydrolyzes cefotaxime. These ESBLs, particularly CTX-M-15 and CTX-M-14, are known to be associated with community-acquired infections, which concurs with this study. The problem of ESBLs is the fact that they could be transferred from one organism to another through plasmids and by conjugation among bacteria. Plasmid profiling of MDR E. coli by Baral et al.43 identified the presence of plasmids with varying sizes ranging from 2–51 kilobases coupled with a high frequency of conjugation. Some studies have attributed the emergence and spread of MDR to clonal groups of E. coli which have common antimicrobial sensitivity patterns and virulence.50 Our data depicts the situation of alarming high levels of antibiotic resistance in Ghana, which is due to several factors, including the prescription of antibiotics by poorly trained health workers and also weak government regulations and law enforcement. These allow for the sale of sub-standard drugs, which are mostly from unlicensed outlets resulting in self-medication practices that are now a common trend in Ghana.18 Additionally, it is common for unqualified health practitioners to offer antibiotics in small quantities to ignorant individuals with the motive of cutting cost and quickly treating suspected infections, which results in sub-inhibitory concentrations within the body’s tissues, thereby facilitating selection of drug resistant strains.19 In Ghana, antibiotics can be obtained without prescription from many pharmacies,18 and this now seems to be the mainstay of treating common infections in the community, including uncomplicated UTI. With the high incidence of antibiotic resistance of uropathogens observed in the current study, this mode of treating UTI should be discouraged as it is likely to result in treatment failures. There is the need for patients to report to licensed clinics where proper clinical investigations including antibiotic susceptibility testing of uropathogens can be done to ensure effective treatment.
In contrast to Piperacillin, Amoxicillin+Clavulanic acid, and Nalidixic Acid, there was a generally high susceptibility of the urinary isolates to Amikacin (>85%), which makes it a suitable option for treatment of UTI in Ghana. A recent study in Ghana also reported that a high percentage (93.6%) of urinary isolates were susceptible to Amikacin.42 It is important to note that Amikacin is used for treating cases of febrile UTI and is regarded as an efficient option before carbapenem treatment, especially in patients with infections caused by ESBL-producing uropathogens that are extensively antibiotic resistant. Amikacin is a relatively expensive antibiotic in Ghana, and the high cost is likely to protect the efficacy of this drug in the country for a long period of time.
Although all the study subjects were clinically diagnosed with UTI, the prevalence of culture-positive urine specimens was as low as 10%. By comparison, similar studies in India and the US reported that 45% and 50%, respectively, of patients clinically diagnosed with UTI had culture-positive urine.51,52 The disparity between clinical and laboratory (culture) diagnosis of UTI can be partly attributed to non-specific clinical symptoms, which is a common problem in the diagnosis of the infection. It could also be due to medication with antibiotics among the patients prior to specimen collection, although we excluded patients who had taken antibiotics 2 weeks or earlier prior to the study. Our data probably shows a situation of high prevalence of over-diagnosis of UTI, which requires attention and further investigation. Inconsistency between clinical and laboratory diagnosis of UTI could lead to inappropriate use of antibiotics resulting in antimicrobial resistance, higher healthcare costs, increased antibiotic exposure, a greater number of adverse reactions, and other unintended outcomes, such as Clostridium difficile infection.
The main limitation of the study is that we obtained few urine culture positive samples, which limited the numbers of isolates for antimicrobial susceptibility testing. This could have also affected identification of some risk factors of UTI in the study.
Conclusion
E. coli is the most common etiological agent of community acquired UTI in the study area, while pregnancy is the main risk factor. While antibiotics such as Amoxicillin+Clavulanic Acid and Piperacillin are unsuitable for empirical treatment of any form of UTI in the study area, Amikacin remains a suitable drug for the treatment of febrile UTI. The high prevalence of MDR and occurrence of ESBLs among the uropathogens highlights the need for surveillance of antimicrobial resistance among these pathogens. In this regard, there could be a national system of pooling isolates of uropathogens from various hospitals. The study has exposed a serious problem with UTI diagnosis in Ghana which needs to be addressed. There is the need for further studies.
Acknowledgments
The authors thank the technical staff of the Department of Medical Microbiology, University of Ghana for their technical support. We also acknowledge the HAI (Healthcare Associated Infection) Ghana project for the financial support towards payment of the article processing charges for this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Puca E. Urinary Tract Infection in Adults. Clin Microbiol Open Access. 2014;3(6). doi: 10.4172/2327-5073.1000e120 [DOI] [Google Scholar]
- 2.Gales AC, Jones RN, Gordon KA, et al. Activity and spectrum of 22 antimicrobial agents tested against urinary tract infection pathogens in hospitalized patients in Latin America: report from the second year of the SENTRY antimicrobial surveillance program (1998). J Antimicrob Chemother. 2000;45(3):295–303. doi: 10.1093/jac/45.3.295 [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1):5–13. doi: 10.1016/S0002-9343(02)01054-9 [DOI] [PubMed] [Google Scholar]
- 4.Shalini Maya A, Prabhakar K, Lakshmi Sarayu Y. A Study on prevalence and evaluation of clinical isolates from community acquired infections using different media in semiurban areas. World J Med Sci. 2010;5(2):49–53. [Google Scholar]
- 5.Wagenlehner FME, Naber KG. Treatment of bacterial urinary tract infections: presence and future. Eur Urol. 2006;49(2):235–244. doi: 10.1016/j.eururo.2005.12.017 [DOI] [PubMed] [Google Scholar]
- 6.Dias Neto JA, Martins ACP, Da Silva LDM, et al. Community acquired urinary tract infection: etiology and bacterial susceptibility. Acta Cir Bras. 2003;18:33–36. doi: 10.1590/S0102-86502003001200012 [DOI] [Google Scholar]
- 7.Barros ICDAR, Ribeiro ADU, Costa ACVD, et al. Microorganisms prevalent in urinary tract infections and antimicrobial sensitivity profile: analysis of patients attended at the military police hospital of the State of Goiás, Brazil, in the period from 1998 to 2008. J Health Sci Inst. 2011;29(4):243–247. [Google Scholar]
- 8.Nerurkar A, Solanky P, Naik SS. Bacterial pathogens in urinary tract infection and antibiotic susceptibility pattern. J Pharm Biomed Sci. 2012;21(21):2010–2012. [Google Scholar]
- 9.Lutterodt MG, Afriyie D, Asare G, Amponsah SK, Abutiate H, Darko D. Antimicrobial use and susceptibility pattern of uropathogens associated with urinary tract infections at the Ghana Police Hospital. Glob J Pharmacol. 2014;8(3):306–315. [Google Scholar]
- 10.Afriyie DK, Gyansa-Lutterodt M, Amponsah SK, et al. Susceptibility pattern of uropathogens to ciprofloxacin at the Ghana police hospital. Pan Afr Med J. 2015;22(1). doi: 10.11604/pamj.2015.22.87.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahadin J, Teo SSH, Mathew S. Aetiology of community-acquired urinary tract infection and antimicrobial susceptibility patterns of uropathogens isolated. Singapore Med J. 2011;52(6):415–420. [PubMed] [Google Scholar]
- 12.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101–111. doi: 10.1016/j.jiph.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Ronald A. The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med. 2012;113(1):14–19. doi: 10.1016/S0002-9343(02)01055-0 [DOI] [PubMed] [Google Scholar]
- 14.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28(1):1–13. doi: 10.1016/j.idc.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 15.Hooton TM. Uncomplicated urinary tract infection. N Engl J Med. 2012;366(11):1028–1037. doi: 10.1056/NEJMoa1114705 [DOI] [PubMed] [Google Scholar]
- 16.Iacovelli V, Gaziev G, Topazio L, Bove P, Vespasiani G, Agrò EF. Nosocomial urinary tract infections: a review. Urol J. 2014;81(4):222–227. doi: 10.5301/uro.5000092 [DOI] [PubMed] [Google Scholar]
- 17.Chin TL, McNulty C, Beck C, MacGowan A. Antimicrobial resistance surveillance in urinary tract infections in primary care. J Antimicrob Chemother. 2016;71(10):2723–2728. doi: 10.1093/jac/dkw223 [DOI] [PubMed] [Google Scholar]
- 18.Donkor ES, Tetteh-Quarcoo PB, Nartey P, Agyeman IO. Self-medication practices with antibiotics among tertiary level students in Accra, Ghana: a cross-sectional study. Int J Environ Res Public Health. 2012;9(10):3519–3529. doi: 10.3390/ijerph9020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5(1):18–27. doi: 10.3201/eid0501.990103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boye A, Siakwa PM, Boampong JN, et al. Asymptomatic urinary tract infections in pregnant women attending antenatal clinic in Cape Coast, Ghana. E3 J Med Res. 2012;1(6):74–83. [Google Scholar]
- 21.Donkor E, Darkwah S, Akpalu A. Post-stroke bacteriuria: a longitudinal study among stroke outpatients and inpatients at the Korle-Bu teaching hospital in Ghana. Med Sci. 2017;5(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghana Statistical Service. Population and housing census. Ghana Stat Serv. 2010;2012:1–117. [Google Scholar]
- 23.Fofana BK Isolation of uropathogenic bacteria and their antimicrobial susceptibility pattern in urine samples of patients with suspected urinary tract infection in Eastern regional hospital, Koforidua; 2016. Available from: http://ugspace.ug.edu.gh/handle/123456789/21510. Accessed January12, 2018.
- 24.Aspevall O, Hallander H, Gant V, Kouri T. European guidelines for urinalysis: a collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin Microbiol Infect. 2001;7(4):173–178. [DOI] [PubMed] [Google Scholar]
- 25.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious diseases society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654. doi: 10.1086/427507 [DOI] [PubMed] [Google Scholar]
- 26.Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am. 2014;28(1):75–89. doi: 10.1016/j.idc.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- 28.CLSI. M100S-S26 performance standards for antimicrobial susceptibility testing, 26th informational supplement, 2016. [Google Scholar]
- 29.Yan L, Jin Y, Hang H, Yan B. The association between urinary tract infection during pregnancy and preeclampsia: a meta-analysis. Medicine (Baltimore). 2018;97(36):e12192. doi: 10.1097/MD.0000000000012192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whalley P. Bacteriuria of pregnancy. Am J Obstet Gynecol. 1967;97(5):723–738. doi: 10.1016/0002-9378(67)90458-9 [DOI] [PubMed] [Google Scholar]
- 31.Vasudevan R. Urinary tract infection: an overview of the Infection and the associated risk factors. J Microbiol Exp. 2014;1(2):00008. doi: 10.15406/jmen.2014.01.00008 [DOI] [Google Scholar]
- 32.Abubakar EM. Antimicrobial susceptibility pattern of pathogenic bacteria causing urinary tract infections at the specialist hospital, Yola, Adamawa state, Nigeria. J Clin Med Res. 2009;1(1):1–8.22505957 [Google Scholar]
- 33.Akoachere J-FTK, Yvonne S, Akum NH, Seraphine EN. Etiologic profile and antimicrobial susceptibility of community-acquired urinary tract infection in two Cameroonian towns. BMC Res Notes. 2012;5(1):219. doi: 10.1186/1756-0500-5-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habte TM, Dube S, Ismail N, Hoosen AA. Hospital and community isolates of uropathogens at a tertiary hospital in South Africa. S Afr Med J. 2009;99(8):584–587. [PubMed] [Google Scholar]
- 35.Iregbu KC, Nwajiobi-Princewill PI. Urinary tract infections in a tertiary hospital in Abuja, Nigeria. Afr J Clin Exp Microbiol. 2013;14(3):169–173. [Google Scholar]
- 36.Sire JM, Nabeth P, Perrier-Gros-Claude JD, et al. Antimicrobial resistance in outpatient Escherichia coli urinary isolates in Dakar, Senegal. J Infect Dev Ctries. 2007;1(3):263–268. doi: 10.3855/jidc.362 [DOI] [PubMed] [Google Scholar]
- 37.Cristina I, Rodrigues DA, Ribeiro ADU, et al. Microorganisms prevalent in urinary tract infections and antimicrobial sensitivity profile : analysis of patients attended at the Military police hospital of the State of Goiás, Brazil, in the period from 1998 to 2008. J Health Sci Inst. 2011;29(4):243–247. [Google Scholar]
- 38.Donkor ES, Osei JA, Anim-Baidoo I, Darkwah S. Risk of asymptomatic bacteriuria among people with sickle cell disease in Accra, Ghana. Diseases. 2017;5(1):4. doi: 10.3390/diseases5010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Micro. 2015;13(5):269–284. doi: 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barber AE, Norton JP, Spivak AM, Mulvey MA. Urinary tract infections: current and emerging management strategies. Clin Infect Dis. 2013;57(5):719–724. doi: 10.1093/cid/cit284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nat Rev Urol. 2010;7:430–441. doi: 10.1038/nrurol.2010.184 [DOI] [PubMed] [Google Scholar]
- 42.Asafo-Adjei K, Mensah J, Labi A-K, Dayie NTKD, Donkor ES. Urinary tract infections among bladder outlet obstruction patients in Accra, Ghana: aetiology, antibiotic resistance, and risk factors. Diseases. 2018;6(3):65. doi: 10.3390/diseases6030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baral P, Neupane S, Marasini B, Ghimire K, Lekhak B, Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes. 2012;5(38). doi: 10.1186/1756-0500-5-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christabel M. Characterization of antibiotic resistance in environmental enteric pathogens from Kibera slum in Nairobi-Kenya. J Bacteriol Res. 2012;4(4):46–54. doi: 10.5897/JBR [DOI] [Google Scholar]
- 45.Tanwar J, Das S, Fatima Z, Hameed S. Multidrug resistance: an emerging crisis. Interdiscip Perspect Infect Dis. 2014;2014:1–7. doi: 10.1155/2014/541340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obeng-Nkrumah N, Twum-Danso K, Krogfelt K, Newman MJ. High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistance. Am J Trop Med Hyg. 2013;89(5):960–964. doi: 10.4269/ajtmh.12-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Codjoe FS, Donkor ES. Carbapenem resistance: a review. Med Sci. 2017;6(1):pii: E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tetteh-Quarcoo PB, Donkor ES, Attah SK, et al. Microbial carriage of cockroaches in a tertiary hospital in Ghana: public health implications. Environ Health Insights. 2013;7:59–66. doi: 10.4137/EHI.S12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linhares I, Raposo T, Rodrigues A, Almeida A. Incidence and diversity of antimicrobial multidrug resistance profiles of uropathogenic bacteria. Biomed Res Int. 2015;2015:354084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manges AR, Johnson JR, Foxman B, O’Bryan TT, Fullerton KE, Riley LW. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N Engl J Med. 2001;345(14):1007–1013. doi: 10.1056/NEJMoa011265 [DOI] [PubMed] [Google Scholar]
- 51.Huppert JS, Biro F, Lan D, Mortensen JE, Reed J, Slap GB. Urinary symptoms in adolescent females: STI or UTI? J Adolesc Health. 2007;40:418–424. doi: 10.1016/j.jadohealth.2006.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasan ASK, Kumar TN, Kishan NR, Neetha K. Laboratory diagnosis of urinary tract infections using diagnostics tests in adult patients. Int J Res Med Sci. 2014;2(2):415–421. doi: 10.5455/2320-6012.ijrms20140508 [DOI] [Google Scholar]