Abstract
Purpose
Integrase inhibitor (INI)-containing regimens are increasingly replacing protease inhibitor(PI)-containing regimens in clinical practice. The aim of this study was to evaluate the determinants of the durability of INI-containing regimens after the switch.
Patients and methods
We retrospectively analysed all of the people with HIV infection attending the University of Milan’s Infectious Diseases Unit at Luigi Sacco Hospital who were switched from a PI- to an INI-containing regimen between April 2008 and March 2017. The probability of remaining on an INI-containing regimen was estimated using Kaplan-Meier curves, and the baseline clinical predictors of INI-containing regimen durability were assessed using a multivariable Cox proportional hazard regression model.
Results
Three hundred and twelve patients were included in the analysis. The median time of observation was 21 months (interquartile range 10–36 months). The main reasons for switching from a PI-containing regimen to an INI-containing regimen were toxicities (31.4%) and simplification (31.1%). Univariate analysis revealed no difference in the probability of INI discontinuation between the patients treated with raltegravir, dolutegravir or elvitegravir (p=0.060), but the multivariable Cox regression model showed that the patients treated with dolutegravir were at less risk of discontinuation than those treated with raltegravir (adjusted hazard ratio 0.49, 95% confidence interval 0.26–0.95; p=0.034).
Conclusion
Switching from a PI- to an INI-containing regimen may be an option for patients under virological control. The patients switched to dolutegravir were less likely to discontinue the INI than those switched to raltegravir. Our findings support this therapeutic strategy and highlight the durability and efficacy of dolutegravir containing-regimens after switching from a PI-containing regimen.
Keywords: HIV, protease inhibitors, integrase inhibitors, dolutegravir, lipids, Framingham
Introduction
Combined antiretroviral therapy (cART) has dramatically changed the life expectancy of people living with HIV by allowing the control of viral replication and thus leading to a significant reduction in HIV infection-related morbidity and mortality.1 However, toxicities associated with the use of the first antiretroviral agents considerably limited the universal and early use of cART for a number of years and encouraged investigations into differences in the role of the various classes of antiretroviral compounds.2,3
Metabolic toxicity was detected as an untoward effect of first-generation antiretroviral agents very early in the use of cART. Lipodystrophic syndrome and metabolic abnormalities were found to be related to cumulative exposure to thymidine analogues and protease inhibitors (PI),4,5 and the findings of recent observational studies have highlighted potential associations between integrase inhibitors (particular dolutegravir [DTG]) and weight gain and altered fat distribution.6–8 Boosted PIs have long been the cornerstone of cART therapy, and still form the basis of a substantial number of cART regimens. Before the advent of integrase inhibitors (INIs), various strategies were used to avoid ritonavir exposure in the subjects who used unboosted atazanavir (ubATV) in order to reduce metabolic toxicities,9 and new compounds (particularly INIs) are now progressively replacing boosted PIs in both cART-naïve and cART-experienced patients.10
Initial trials highlighted an increased risk of cART discontinuation due to virological failure in patients switching from boosted lopinavir (LPV) to raltegravir (RAL),11 but subsequent studies have shown that switching from boosted PIs to INI-containing regimens is virologically effective and leads to improved metabolic profiles.12,13 It has also been demonstrated that switching from a boosted PI regimen to a single tablet regimen (STR) containing boosted elvitegravir (EVG) can be effective and well tolerated,14 and that simplifying PI-containing to DTG-containing regimens is safe and effective, particularly in patients aged >50 years and in those at high cardiovascular risk.15,16 The STRIIVING study showed no clear benefit in terms of lipid profiles in patients switched to DTG, but this may be partially explained by the low cardiovascular risk of the study patients and their different previous cART regimens, which included PIs or non-nucleoside reverse transcriptase inhibitors (NNRTIs).15 Furthermore, the NEAT022 study revealed a clear benefit in patients at high cardiovascular risk who were switched to DTG from boosted PIs.16
Consequently, simplification strategies involving a switch from boosted PI-containing cART regimens to an INI-containing regimen have now been introduced in routine clinical practice with the aim of reducing the long-term toxicities caused by lifelong cART exposure.11–18
The aim of this study was to assess the durability and metabolic impact of INI-containing regimens in a single-centre cohort of drug-experienced HIV-infected patients who had previously been treated with a PI-containing regimen.
Materials and methods
We retrospectively analysed all of the HIV-infected patients attending the University of Milan’s Infectious Diseases Unit at Luigi Sacco Hospital who were switched from a PI- to an INI-containing regimen between April 2008 and March 2017. Patients aged <18 years were excluded from the analysis.
The demographic, epidemiologic and clinical data in the patients’ clinical case files at the time of the switch from a PI- to an INI-containing regimen and every six months thereafter were anonymously entered in an ad hoc electronic database.
The discontinuations of INI-containing regimens were categorised as being due to virological failure (defined as two consecutive determinations of >50 HIV-RNA copies/mL or one detection of >1000 HIV-RNA copies/mL), toxicities, drug-drug interactions, simplifications, drop-outs, deaths or other reasons. A change from one to another INI-containing regimen was considered a failure in the durability analysis, but the patients who only changed the drug formulation were not considered as failures.
The primary study end-point was the discontinuation of the different INI-(RAL vs DTG)-containing regimens after switching from a PI-containing regimen. The secondary end-points were the durability of EVG vs RAL or DTG, the probability of virological failure on the different INI-containing regimens, the metabolic effects of switching from a PI- to an INI-containing regimen, and the change in 10-year cardiovascular risk as assessed by the Framingham algorithm 12 months after the switch.
Sample size was calculated on the basis of the primary end-point. It was estimated that a total of 272 participants (136 per group) would provide a power of at least 80% to exclude a non-inferiority margin of a 12.5% difference in the proportion of participants reaching the primary endpoint, assuming that treatment success was 90% among the patients in the DTG group and a two-sided α of 0.05.12,16,17
The collected data were grouped into dichotomous and continuous variables, and the patients were grouped on the basis of their previous PI (boosted ATV, ubATV, boosted darunavir [DRV], LPV, or boosted fosamprenavir [fAPV]) and its replacement INI (DTG, RAL or EVG).
The descriptive and inferential statistical analyses assumed that an α of 0.05 was statistically significant.
The probabilities of the discontinuation and virological failure of the different INI-containing regimens after switching from a PI-containing regimen were analysed by means of Kaplan-Meier curves. The baseline clinical predictors of the durability and virological failure of the INI-containing regimens were assessed using a multivariable Cox proportional hazard regression model, in which the co-variates were age, gender, the number of previous cART regimens, previous virological failure, initial PI, backbone, >50 HIV-RNA copies/mL at the time of the switch, CD4 cell count at the time of the switch, nadir CD4 cell count, and HCV co-infection.
The variations in total cholesterol (TC), HDL cholesterol and triglyceride (TG) levels were assessed using a multivariable linear mixed effects regression model, and the SAS PROC MIXED procedure with a random intercept was used to correlate repeated measures. Intra-individual variance was analysed using an autoregressive correlation structure of the first order, with TC, HDL cholesterol and TG as the dependent variables, and age, gender, baseline lipid values, statin use, concomitant tenofovir discontinuation, and initial PI as independent variables.
Ten-year cardiovascular risk was assessed using the Framingham algorithm at the time of the switch and 12 months after the switch from a PI- to INI-containing regimen.19 The patients whose data were insufficient for the Framingham risk calculation and those who modified their concomitant antiretroviral or non-antiretroviral treatment (eg by introducing a statin) during the first 12 months after the switch were excluded from the analysis. A multivariable linear mixed effect regression model was used to assess the predictors of the change in 10-year cardiovascular risk 12 months after the switch. The co-variates included in the final model were age, gender, baseline Framingham risk, and initial PI.
SAS version 9.4 software was used to make the statistical analyses, and a p-value of <0.05 was considered statistically significant.
The study protocol was reviewed and approved by the Comitato Etico Interaziendale Milano Area 1 (Ethics Committee), and an informed consent form was signed by all of the subjects who decided to participate in this study.
Results
Table 1 shows the characteristics of the 312 patients included in the analysis at the time of the switch from a PI- to an INI-containing regimen. The patients treated with RAL-containing regimens had been treated with a higher median number of previous cART regimens than those treated with DTG- or EVG-containing regimens (6, interquartile range [IQR] 2–11 vs respectively 4, IQR 2–7 and three, IQR 1–6; p=0.002), and included a greater proportion of patients who had experienced a previous virological failure (46, 34.1% vs respectively 22, 18% and 11, 20%; p=0.008) and patients switching with an HIV-RNA count of >50 copies/mL (34, 25.2% vs respectively 13, 10.7% and 11, 20%; p=0.011). Moreover, in comparison with those treated with DTG- or EVG-containing regimens, the patients treated with RAL-containing regimens were more frequently intravenous drug users (50, 37% vs 22, 18% and 3, 5.4%; p<0.0001) and had HCV co-infection (57, 42.2% vs 31, 25.4% and 9, 16.4%; p<0.001).
Table 1.
Patients characteristics at the time of switch from a PI- to an INI-containing regimen
| Patients characteristics | Total n=312 | RAL n=135 (43.3%) | DTG n=122 (39.1%) | EVG n=55 (17.6%) | p-value* |
|---|---|---|---|---|---|
| Female, n (%) | 85 (27.2) | 41 (30.4) | 30 (24.6) | 14 (25.5) | 0.552 |
| Age (yrs), median (IQR) | 49.3 (42.3–54.5) | 49.6 (42.8–54.0) | 50.0 (43.5–56.0) | 45.9 (40.4–53.7) | 0.146 |
| Risk group, n (%) | <0.0001 | ||||
| Heterosex | 125 (40.1) | 48 (35.6) | 49 (40.2) | 28 (50.9) | |
| MSM | 102 (32.7) | 32 (23.7) | 46 (37.7) | 24 (43.6) | |
| IVDUs | 75 (24.0) | 50 (37.0) | 22 (18.0) | 3 (5.4) | |
| Other | 10 (3.2) | 5 (3.7) | 5 (4.1) | 0 (0.0) | |
| Previous AIDS, n (%) | 86 (27.5) | 44 (32.6) | 30 (24.6) | 12 (21.8) | 0.206 |
| Previous VF, n (%) | 79 (25.3) | 46 (34.1) | 22 (18.0) | 11 (20.0) | 0.008 |
| Number of previous cART regimens, median (IQR) | 4 (2–9) | 6 (2–11) | 4 (2–7) | 3 (1–6) | 0.002 |
| Previous therapy duration (yrs), median (IQR) | 9.9 (3.9–16.7) | 11.6 (3.9–15.9) | 9.6 (5.2–17.3) | 6.8 (1.8–16.0) | 0.188 |
| CD4+ nadir (cells/mmc), median (IQR) | 179 (47–303) | 164 (40–262) | 188 (58–325) | 240 (104–333) | 0.017 |
| CD4+ nadir (cells/mmc) <200, n (%) | 163 (53.8) | 79 (58.5) | 65 (53.3) | 24 (43.6) | 0.173 |
| CD4+ (cells/mmc), median (IQR) | 599 (359–829) | 475 (286–732) | 735 (487–985) | 633 (389–837) | <0.0001 |
| CD4+ (cells/mmc) >500, n (%) | 184 (59.0) | 62 (45.9) | 88 (72.1) | 34 (61.8) | <0.001 |
| HIV-RNA >50 cp/mL, n (%) | 58 (18.6) | 34 (25.2) | 13 (10.7) | 11 (20.0) | 0.011 |
| Triglycerides (mg/dL), median (IQR) | 154 (106–243) | 155 (103–242) | 147 (104–214) | 128 (89–194) | 0.152 |
| Total Cholesterol (mg/dL), median (IQR) | 186 (157–223) | 183 (155–221) | 202 (173–234) | 188 (170–210) | 0.010 |
| HDL Cholesterol (mg/dL), median (IQR) | 41 (34–50) | 42 (35–55) | 43 (36–51) | 39 (33–47) | 0.335 |
| LDL Cholesterol (mg/dL), median (IQR) | 115 (94–134) | 103 (80–130) | 116 (95–143) | 107 (93–138) | 0.016 |
| Backbone, n (%) | <0.0001 | ||||
| TDF/FTC or TAF/FTC | 144 (46.1) | 66 (48.9) | 23 (18.8) | 55 (100.0) | |
| ABC/3TC | 120 (38.5) | 35 (25.9) | 85 (69.7) | 0 (0.0) | |
| Other | 48 (15.4) | 34 (25.2) | 14 (11.5) | 0 (0.0) | |
| STR, n (%) | 123 (39.4) | 0 (0.0) | 68 (55.7) | 55 (100.0) | <0.0001 |
| Dual regimens, n (%) | 24 (7.7) | 10 (7.4) | 14 (11.5) | 0 (0.0) | 0.016 |
| Previous PI, n (%) | <0.0001 | ||||
| ATV | 87 (27.9) | 32 (23.9) | 39 (32.0) | 16 (29.1) | |
| ATVub | 57 (18.3) | 24 (17.8) | 23 (18.8) | 10 (18.2) | |
| DRV | 72 (23.1) | 17 (12.6) | 34 (27.9) | 21 (38.2) | |
| LPV | 60 (19.2) | 43 (31.8) | 12 (9.8) | 5 (9.1) | |
| fAPV | 36 (11.5) | 19 (14.1) | 14 (11.5) | 3 (5.5) | |
| Concomitant TDF interruption, n (%) | 46 (14.7) | 10 (7.4) | 26 (21.3) | 10 (18.2) | 0.005 |
| HCV, n (%) | 97 (31.1) | 57 (42.2) | 31 (25.4) | 9 (16.4) | <0.001 |
| HBV, n (%) | 23 (7.4) | 10 (7.4) | 8 (6.6) | 5 (9.1) | 0.776 |
| Patients on statins, n (%) | 68 (21.8) | 29 (21.5) | 25 (20.5) | 14 (25.5) | 0.755 |
Note: *p-values are for χ2 or Fisher’s exact test and Kruskal-Wallis test.
Abbreviations: RAL, raltegravir; DTG, dolutegravir; EVG, elvitegravir; IQR, inter quartile range; yrs, years; n, number; MSM, men who have sex with men; IVDU, intra venous drug users; cART, combined antiretroviral therapy; cp, copies; eGFR, estimate glomerular filtration rate; STR, single tablet regimens; PI, protease inhibitor; ATV, boosted atazanavir; ATVub, unboosted Atazanavir; DRV, boosted darunavir; LPV, boosted lopinavir; fAPV, boosted fosamprenavir; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide.
The median time of observation was 21 months (IQR 10–36). The reasons for switching from a PI-containing regimen to an INI-containing regimen were toxicities (31.4%) followed by simplification (31.1%), virological failure (18.6%), drug-drug interactions (12.8%) and other reasons (6.1%). Two hundred and fifty-four patients switched to an INI-containing regimen when their HIV-RNA count was <50 copies/mL: 171 (67.3%) only changed the PI and 83 (32.7%) changed at least one other component of the regimen. Fifty-eight patients switched when their HIV-RNA count was >50 copies/mL: 29 (50.0%) only changed the PI and the other 29 (50.0%) also changed another component of the regimen. Fourteen (24.1%) of the patients with detectable viremia at the time of the switch did not undergo an HIV resistance test or the sample could not be amplified because of the low viremia level; the results of the test in the remaining 44 patients (75.9%) were wild-type in 19 (43.2%), one major mutation in one drug class (NRTI/NNRTI/PI) in 10 (22.7%), at least one major mutation in two classes in 10 (22.7%), and at least one major mutation in three classes in five (11.4%). After combining the choice of INI-containing regimens and the results of previous genotyping tests, 27 patients (61.4%) were treated with a regimen containing three fully active drugs, and 17 (38.6%) were treated with a regimen containing two fully active drugs.
Discontinuation rate
During the period of observation, 117 (37.5%) patients discontinued their INI-containing regimen. The reasons leading to discontinuation were simplification (average 28.2%: 33.7% of RAL, 25% of DTG and 5.9% of EVG discontinuations), virological failure (average 26.4%: 30% of RAL, 5.0% of DTG and 35.3% of EVG discontinuations), toxicities (average 15.4%: 8.7% of RAL, 35% of DTG and 23.5% of EVG discontinuations), drop-outs (average 9.4%: 7.5% of RAL, 15% of DTG and 11.8% of EVG discontinuations), deaths (average 7.7%: 5.1% of RAL, 10% of DTG and 5.9% of EVG discontinuations), drug-drug interactions (average 2.6%: 17.6% of EVG discontinuations), and other reasons (average 10.3%: 11% of RAL and 10% of DTG discontinuations). Only 4.3% of the INI discontinuations were due to neurological toxicity (two in the RAL group and three in the DTG group).
Durability of INI-containing regimens
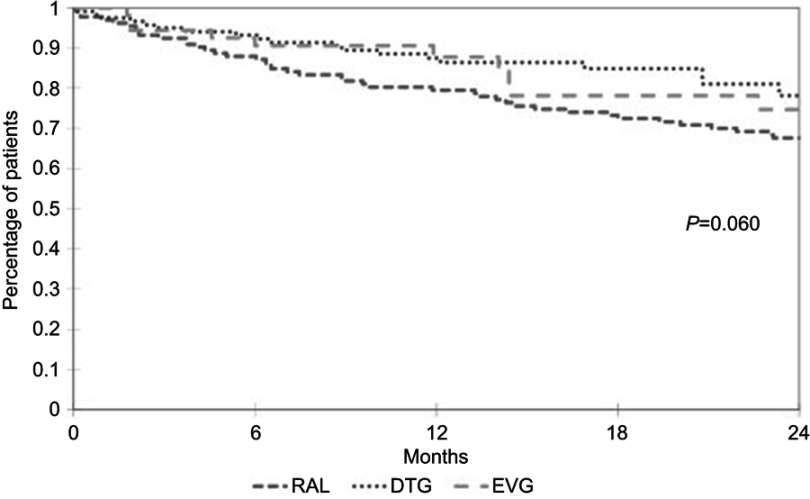
Figure 1 shows the durability of the different INI-containing regimens. Univariate analysis revealed no difference in the probability of INI discontinuation between the patients treated with RAL, DTG or EVG (p=0.060), but the risk of discontinuation was lower in the case of STRs than in the case of non-STRs (p=0.011). The probability of still being on an INI-containing regimen after 12 months was 79.5% (95% confidence interval [CI] 72.6–86.4) in the case of RAL, 87.5% (95% CI 81.3–93.7) in the case of DTG. and 87.8% (95% CI 78.5–95.1) in the case of EVG.
Figure 1.
Time dependent probability of INI-containing regimens discontinuation after switching from PI.
Note: No differences were observed in the three different INI (p=0.06).
Abbreviations: RAL, raltegravir; DTG, dolutegravir; EVG, elvitegravir.
The multivariable Cox regression model (Table 2) showed that the patients treated with DTG were at less risk of discontinuation than those treated with RAL (adjusted hazard ratio [aHR] 0.49, 95% CI 0.26–0.95; p=0.038) as were the patients switching from ubATV in comparison with those switching from boosted ATV (aHR 0.5, 95% CI 0.25–0.99; p=0.048)
Table 2.
Multivariate Cox regression model of the continuance of INI-containing regimens after switching from PI
| aHR | IC 95% | p value | |
|---|---|---|---|
| Age (x 1 yrs more) | 1.00 | 0.98–1.03 | 0.708 |
| Previous cART regimens (x 1 more) | 1.03 | 0.97–1.09 | 0.298 |
| Previous VF | 0.72 | 0.42–1.23 | 0.228 |
| Male vs Female | 1.17 | 0.71–1.95 | 0.533 |
| ATVub vs ATV | 0.50 | 0.25–0.99 | 0.048 |
| DRV vs ATV | 1.17 | 0.61–2.25 | 0.645 |
| LPV vs ATV | 0.73 | 0.41–1.32 | 0.302 |
| fAPV vs ATV | 0.87 | 0.43–1.75 | 0.701 |
| DTG vs RAL | 0.49 | 0.26–0.95 | 0.038 |
| EVG vs RAL | 0.57 | 0.27–1.20 | 0.140 |
| TDF/FTC vs ABC/3TC | 0.95 | 0.53–1.71 | 0.856 |
| Other vs ABC/3TC | 0.97 | 0.50–1.87 | 0.920 |
| HIV-RNA > 50 cps/mL | 1.02 | 0.60–1.74 | 0.941 |
| CD4 > 500 cells/mmc | 0.94 | 0.56–1.58 | 0.808 |
| CD4 nadir < 200 cells/mmc | 0.81 | 0.49–1.34 | 0.410 |
| HCV coinfection | 1.06 | 0.65–1.70 | 0.822 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; yrs, years; cART, combined antiretroviral therapy; cps, copies; ATV, boosted atazanavir; ATVub, unboosted Atazanavir; DRV, boosted darunavir; LPV, boosted lopinavir; fAPV, boosted fosamprenavir; DTG, dolutegravir; RAL, raltegravir; EVG, elvitegravir; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; ABC/3TC, abacavir/lamivudine.
Probability of virological failure on the INI-containing regimens
The 12-month probability of not experiencing virological failure on an INI-containing regimen was 92.2% (95% CI 87.2–97.1) in the case of RAL, 99% (95% CI 97.1–1.00) in the case of DTG, and 97.1% (95% CI 92.8–1.00) in the case of EVG.
Univariate analysis revealed a statistically significant difference in the risk of virological failure between the three INIs (p=0.01) that was not confirmed by the multivariable Cox regression model (Table 3). However, the factors associated with an increased risk of virological failure were a greater number of previous antiretroviral regimens (aHR 1.12, 95% CI 1.00–1.24; p=0.046) and an HIV-RNA count >50 copies/mL at the time of the switch from a PI- to an INI-containing regimen (aHR 2.80, 95% CI 1.22–6.45; p=0.015). A trend towards an increased risk of virological failure was also observed in the case of male gender (aHR 2.84; 95% CI 0.98–8.17; p=0.053).
Table 3.
Multivariate Cox regression model of the probability of virological failure of INI-containing regimens after switching from PI
| aHR | IC 95% | p value | |
|---|---|---|---|
| Age (x 1 yrs more) | 1.01 | 0.97–1.05 | 0.725 |
| Previous cART regimens (x 1 more) | 1.12 | 1.00–1.24 | 0.046 |
| Previous VF | 1.89 | 0.74–4,78 | 0.181 |
| Male vs Female | 2.84 | 0.98–8,17 | 0.053 |
| ATVub vs ATV | 0.63 | 0.18–2.24 | 0.473 |
| DRV vs ATV | 2.71 | 0.77–9.57 | 0.121 |
| LPV vs ATV | 1.17 | 0.39–3.52 | 0.783 |
| fAPV vs ATV | 1.12 | 0.32–3.96 | 0.859 |
| DTG vs RAL | 0.17 | 0.02–1.52 | 0.113 |
| EVG vs RAL | 0.92 | 0.28–3.06 | 0.898 |
| TDF/FTC vs ABC/3TC | 2.87 | 0.62–13.25 | 0.176 |
| Other vs ABC/3TC | 1.85 | 0.41–8.46 | 0.425 |
| HIV-RNA > 50 cp/mL | 2.80 | 1.22–6.45 | 0.015 |
| CD4 > 500 cells/mmc | 0.90 | 0.32–2.57 | 0.851 |
| CD4 nadir < 200 cells/mmc | 0.44 | 0.15–1.30 | 0.138 |
| HCV coinfection | 1.01 | 0.39–2.58 | 0.986 |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; yrs, years; cART, combined antiretroviral therapy; cps, copies; ATV, boosted atazanavir; ATVub, unboosted Atazanavir; DRV, boosted darunavir; LPV, boosted lopinavir; fAPV, boosted fosamprenavir; DTG, dolutegravir; RAL, raltegravir; EVG, elvitegravir; TDF/FTC, tenofovir disoproxil fumarate/emtricitabine; ABC/3TC, abacavir/lamivudine.
Metabolic impact
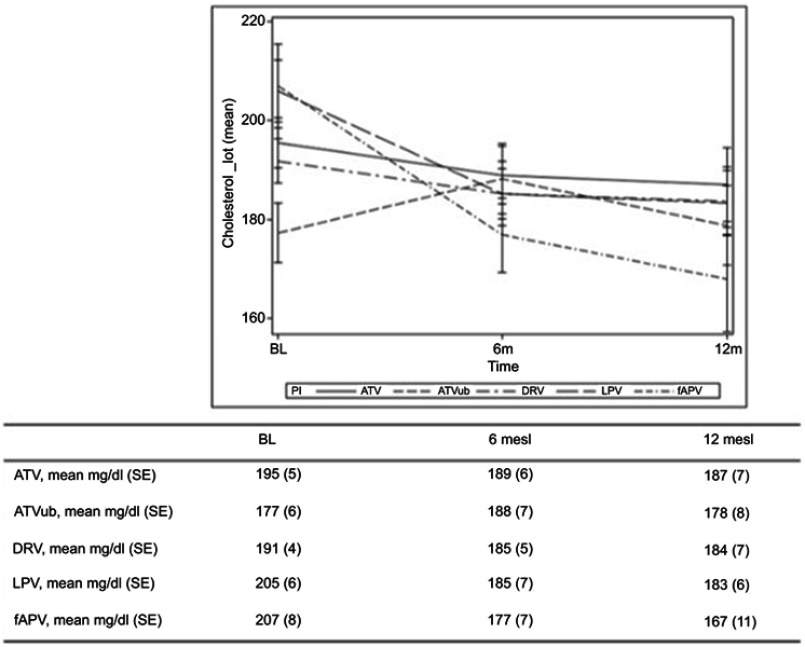
Figure 2 shows the changes from baseline TC levels by previous PI. Sixty-eight patients were being treated with statins before the switch to an INI and five started on statins during the study period; the 22 patients who discontinued statins during the study period (32.3%) were excluded from the metabolic analysis.
Figure 2.
Median TC levels from the time of the switch and after 6 and 12 months according to the PI of provenience.
Note: TC levels are expressed in mg/dL.
Abbreviations: tot, total; BL, baseline; m, months; ATV, boosted atazanavir; ATVub, unboosted atazanavir; DRV, boosted darunavir; fAPV, boosted fosamprenavir; LPV, boosted lopinavir.
Multivariate analysis revealed a correlation between the changes in TC levels and the discontinuation of tenofovir disoproxil fumarate (TDF) at the time of the switch from a PI- to an INI-containing regimen (estimate 9.52 mg/dL, standard error [SE] 2.75; p=0.0006), and between TC levels and a previous antiretroviral regimen containing boosted LPV (estimate −9.16 mg/dL, SE 3.43; p=0.008) and boosted fAPV (estimate −21.59 mg/dL, SE 4.34; p<0.0001) (Table 4).
Table 4.
multivariable linear mixed effects regression model for the variation of total cholesterol after the switch from a PI- to an INI-containing regimen
| Estimate mg/dL |
SE | p value | |
|---|---|---|---|
| Female vs male | 4.26 | 2.26 | 0.061 |
| Age (x one yrs more) | 0.07 | 0.10 | 0.502 |
| Baseline cholesterol (x 1 unit more) | 0.80 | 0.02 | <0.0001 |
| Statins utilization yes vs no | –4.13 | 2.42 | 0.089 |
| Concomitant TDF interruption | 9.52 | 2.75 | 0.0006 |
| Time* | –1.66 | 2.25 | 0.461 |
| *ATVub vs ATV | 6.52 | 3.42 | 0.057 |
| *DRV vs ATV | –0.35 | 3.55 | 0.920 |
| *LPV vs ATV | –9.16 | 3.43 | 0.008 |
| *fAPV vs ATV | –21.59 | 4.34 | <0.0001 |
Note: *The estimates refer to parameters associated to the interaction between PIs and the time unit inserted in the model which is 6 months.
Abbreviations: SE, standard error; yrs, years; ATV, boosted atazanavir; ATVub, unboosted Atazanavir; DRV, boosted darunavir; LPV, boosted lopinavir; fAPV, boosted fosamprenavir; TDF, tenofovir disoproxil fumarato; PIs, protease inhibitors; INI, integrase inhibitors.
Multivariate analysis also revealed a correlation between the change from baseline TG levels and previous exposure to boosted LPV (estimate −32.94 mg/dL, SE 13.23; p=0.013) and boosted fAPV (estimate −36.81 mg/dL, SE 17.02; p=0.031) (Table 5).
Table 5.
multivariable linear mixed effects regression model for the variation of triglycerides levels after the switch from a PI- to an INI-containing regimen
| Estimate mg/dL | SE | p value | |
|---|---|---|---|
| Female vs male | –6.0 | 8.50 | 0.475 |
| Age (x one yrs more) | –0.25 | 0.37 | 0.499 |
| Baseline triglycerides (x 1 unit more) | 0.61 | 0.02 | <0.0001 |
| Statins utilization yes vs no | 15.75 | 9.26 | 0.093 |
| Concomitant TDF interruption | 4.95 | 10.05 | 0.622 |
| Time* | –10.62 | 8.76 | 0.226 |
| *ATVub vs ATV | 2.90 | 13.26 | 0.826 |
| *DRV vs ATV | –2.77 | 13.68 | 0.839 |
| *LPV vs ATV | –32.94 | 13.23 | 0.013 |
| *fAPV vs ATV | –36.81 | 17.02 | 0.031 |
Note: *The estimates refer to parameters associated to the interaction between PIs and the time unit inserted in the model which is 6 months.
Abbreviations: SE, standard error; yrs, years; ATV, boosted atazanavir; ATVub, unboosted Atazanavir; DRV, boosted darunavir; LPV, boosted lopinavir; fAPV, boosted fosamprenavir; TDF, tenofovir disoproxil fumarato; PIs, protease inhibitors; INI, integrase inhibitors.
Only female gender was associated with the changes in HDL cholesterol levels in the multivariate model (estimate 1.82 mg/dL, SE 0.69; p=0.009).
Ten-year cardiovascular risk was assessed in 172 patients using the Framingham algorithm. There was no difference between the risk calculated at the time of the switch from a PI- to an INI-containing regimen and the risk calculated 12 months later (8.5%, IQR 4.5–19.1 vs 8.8%, IQR 4.3–18; p=0.836). After correcting for the previous PI, statin use, TDF discontinuation at the time of the switch, and a high baseline 10-year cardiovascular risk (>10%), the factors associated with risk modification In the multivariable model were age (estimate 0.04%, SE 0.02; p=0.038) and baseline cardiovascular risk (estimate 0.94%, SE 0.01; p<0.0001).
Discussion
The findings of this study of a single-centre cohort of HIV-positive, antiretroviral- experienced patients suggest that switching from a PI- to an INI-containing regimen is safe, long-lasting, and leads to a significant improvement in metabolic profiles.
The overall rate of INI discontinuation due to all causes was comparable with that reported in other cohort studies.13 Initial concerns were raised during the post-marketing use of DTG because the incidence of adverse neuropsychiatric events was higher than that observed in clinical trials.20,21 Our finding that discontinuations due to neurological toxicity accounted for 4.3% of all discontinuations would seen to allay such concerns, which could lead to unnecessary discontinuation.22,23
Approximately one-third of the patients in our cohort changed their PI-containing regimen because of the occurrence of cART-related toxicity, and 18.6% of the patients were in a condition of virological failure at the time of the switch to an INI-containing regimen. It is well known that the use of INI in patients with a long antiretroviral history and previous virological failures may predispose to an increased risk of INI discontinuation due to virological inefficacy when a regimen with a low genetic barrier is used (ie RAL or EVG).11,24 At the time of the switch, the failing patients received two or three active drugs in respectively 38.6% and 61.4% of cases, and none of the patients received only one active drug on the basis of the interpretation of historical genotypes. The patients treated with RAL had a longer history of previous therapy, and a history of intravenous drug use and HCV co-infection, all of which frequently characterise the patients who received the first INI available for clinical use, and may be associated with a reduced probability of cART adherence and shorter duration of the current regimen.25,26 They also presented a higher rate of uncontrolled HIV viremia at the time of the switch than the patients treated with DTG or EVG, and were thus in suboptimal condition for switching to a low genetic barrier regimen.11,24 Finally, more than one-third of the patients who discontinued a RAL-containing regimen did so for reasons of simplification, and it is likely that the advent of drugs to be administered once a day and the reduced pill burden affected the durability of the RAL-containing regimens.27,28 All of these factors may partially explain the difference in the durability of the DTG- and RAL-containing regimens.
Another factor potentially influencing the greater durability of DTG-containing regimens is the possibility of using DTG in an STR as the patients on an STR were less likely to discontinue an INI-containing regimen than those receiving multiple tablets.29
Our univariate analysis showed that there was an increased risk of virological failure in the patients who switched to RAL than those who switched to DTG. Moreover, approximately one third of the RAL- and EVG-containing regimens were discontinued due to virological failure. However, the significant difference between regimens was lost in the multivariate model, which showed that an increased risk of virlogical failure was associated with uncontrolled HIV-RNA at the time of the switch and a greater number of previous antiretroviral regimens. All of these factors are known to be associated with an increased risk of virological failure in patients on low genetic barrier cART.11,30,31 A trend towards an increased risk of virological failure (albeit of borderline statistical significance) was observed in the case of males, which conflicts with some published data indicating a greater risk among females.32
In terms of metabolic profiles, there was a favourable association with a reduction in TC levels in patients coming from boosted LPV- and boosted fAPV-containing regimens. This confirms the findings of previous studies showing an improvement in metabolic profiles after switch from a boosted PI to an INI.12,30 Accordingly, there was also a favourable association between post-switch TG levels and previous exposure to boosted LPV and boosted fAPV.16,33 HDL cholesterol levels were not associated with previous PI use, but higher levels were associated with female gender.34 It is known that females have higher HDL cholesterol levels than males, which has a cardioprotective function especially in the pre-menopausal period, but the gender-related reasons for the difference in HDL levels are not fully understood.35
There was a significant increase in TC levels in the patients who discontinued TDF at the same time as they switched from a PI- to an INI-containing regimen, thus confirming previous findings showing that TDF has a statin-like effect that is unmasked when it is discontinued.36,37
An association between weight gain and a switch to INI-containing regimens has been postulated,6–8 but a recent report based on the SCOLTA observational cohort did not provide any evidence of a relationship between INIs and weight gain, and it has been shown cabotegravir does not have this effect.38–40 Nevertheless, this potential side effect of INI-containing regimens should be considered and balanced against their potential advantage.
Approximately one-third of the patients in our cohort had HCV co-infection and 24% were intravenous drug users, and a higher percentage of both switched to RAL. The advent of direct antiviral agents (DAAs) has prompted switching from PI- to INI-containing regimens as INIs have fewer drug-to-drug interactions, and RAL is the INI that potentially has fewer drug-to-drug interactions with DAAs.41
The use of ubATV in order to avoid the untoward metabolic effects of the ritonavir booster was once widespread, particularly in Italy, but this strategy has now been replaced by new INI-containing simplification strategies that have been demonstrated to be safe in elderly patients at high cardiovascular risk.16,42,43 The switch from ubATV to an INI-containing regimen led to greater durability (albeit of borderline statistical significance) than a switch to boosted ATV. This may have been partially due to the large number of patients switched for reasons of simplification or toxicity in this sub-group of patients.
Approximately one-quarter of the patients in our cohort were taking lipid-lowering statins at the time of the switch. This percentage is higher than that found in previous studies, which have shown the under-prescription of statins to people living with HIV, but in line with the results of a recent study by Gatell et al involving patients at high cardiovascular risk or who were older than fifty years.16,44 However, approximately one-third of our patients discontinued lipid-lowering treatment during the study period.
We did not observe any improvement in 10-year cardiovascular risk as assessed using the Framingham algorithm 12 months after the switch from a PI- to an INI-containing regimen. This is in line with the results of a recent study by Taramasso et al, who found that switching from a boosted PI- or efavirenz-containing regimen to an INI- or rilpivirine-containing regimen did not reduce cardiovascular risk as assessed by the Framingham algorithm despite a significant improvement in lipid profiles.45 However, it must be remembered that this way of assessing cardiovascular risk does not take into account the potential impact of the components of the antiretroviral regimen as some antiretroviral agents (eg abacavir and PIs) have been associated with increased cardiovascular risk, although it is likely that the effect of antiretroviral treatment is more marginal than the classic cardiovascular risk factors. Moreover, it has been shown that antiretroviral agents only affect the risk of patients at higher risk.46,47 Taken together with those of previously published studies, our findings suggest that modifying cART has a limited impact on the cardiovascular risk calculated using the Framingham score, and highlight the importance of concentrating on classic modifiable cardiovascular risk factors.
This study has a number of limitations. First of all, its retrospective design exposes it to potential errors due to omissions or the lack of data in clinical case files. Secondly, its single-centre nature limits the applicability of the results to different settings. Thirdly, the choice of switching from a PI- to an INI-containing regimen was not controlled but based on the clinical judgement of individual physicians. Finally, and as discussed above, the patients treated with RAL had a longer previous antiretroviral history than those treated with DTG or EVG, were exposed to a heterogeneous series of antiretroviral regimens, and were more likely to be co-infected with HCV.
Conclusion
On the basis of our findings, switching from a PI- to an INI-containing regimen can be considered a valid option for patients under virological control. However, the virological efficacy of the regimens may be affected by the number of previous antiretroviral regimens in a patient’s history. DTG-containing regimens seem to be more durable than RAL-containing regimens probably because DTG can be used in a once-daily STR. Patients previously treated with boosted LPV and boosted fAPV showed a significant improvement in lipid profiles. However, as there was no reduction in cardiovascular risk after the switch, it seems that modifying cART is not enough by itself and so attempts to modify classic cardiovascular risk factors should be continued.
Acknowledgment
We would like to thank Mrs Tiziana Formenti for her excellent technical help.
Abbreviation list
PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; INI, integrase inhibitor; RAL, raltegravir; EVG, elvitegravir; DTG, dolutegravir; ub, unboosted; LPV, lopinavir; fAPV, fosamprenavir; ATV, atazanavir; DRV, darunavir; IQR, interquartile range; CI, confidence interval; cART, combined antiretroviral therapy; STR, single tablet regimen; TC, total cholesterol; TG, triglycerides; DAA, direct antiviral agents.
Ethics approval and informed consent
The protocol was reviewed and approved by the Comitato Etico Interaziendale Milano Area 1, and an informed consent form was signed by all of the subjects who participated in this study.
Data availability
Data will be made available upon reasonable request.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
SR has received research grants, consultancy payments and speaker’s fees from Bristol-Myers Squibb, Gilead, ViiV Healthcare, Merck Sharp Dohme, ABBvie and Janssen. MG has received research grants, consultancy payments and speaker’s fees from Bristol-Myers Squibb, Gilead, ViiV Healthcare, Merck Sharp Dohme, ABBvie, Janssen and Roche. The authors report no other conflicts of interest in this work.
References
- 1.Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. Aids. 2013;27:973–979. doi: 10.1097/QAD.0b013e32835cae9c [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–1081. doi: 10.1086/521935 [DOI] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMc063190 [DOI] [PubMed] [Google Scholar]
- 4.Nolan D, Hammond E, Martin A, et al. DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS. 2003;17:1329–1338. doi: 10.1097/01.aids.0000060385.18106.35 [DOI] [PubMed] [Google Scholar]
- 5.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. [DOI] [PubMed] [Google Scholar]
- 6.Norwood J, Turner M, Bofill C, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527–531. doi: 10.1097/QAI.0000000000001525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS. 2017;31(10):1499–1500. doi: 10.1097/QAD.0000000000001495 [DOI] [PubMed] [Google Scholar]
- 8.Debroy P, Sim M, Erlandson KM, et al. Modena HIV metabolic cohort team. Progressive increases in fat mass occur in adults living with HIV on antiretroviral therapy, but patterns differ by sex and anatomic depot. J Antimicrob Chemother. 2019. [Epub ahead of print]. doi: 10.1093/jac/dky551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavie J, Porcher R, Torti C; on behalf of the NEAT Unboosted Atazanavir Cohort Study Group, et al. Efficacy and safety of a switch to unboosted atazanavir in combination with nucleoside analogues in HIV-1-infected patients with virological suppression under antiretroviral therapy. J Antimicrob Chemother. 2011;66(10):2372–2378. doi: 10.1093/jac/dkr316 [DOI] [PubMed] [Google Scholar]
- 10.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of health and human services. Available from http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed March, 9 2019.
- 11.Eron JJ, Young B, Cooper DA, et al. SWITCHMRK 1 and 2 investigators. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet. 2010;375(9712):396–407. doi: 10.1016/S0140-6736(09)62041-9 [DOI] [PubMed] [Google Scholar]
- 12.Martínez E, Larrousse M, Llibre JM; SPIRAL Study Group, et al. Substitution of raltegravir for ritonavir-boosted protease inhibitors in HIV-infected patients: the SPIRAL study. AIDS. 2010;24(11):1697–1707. doi: 10.1097/QAD.0b013e32833a608a [DOI] [PubMed] [Google Scholar]
- 13.Potard V, Simon A, Lacombe JM, Parienti JJ, Costagliola D; French Hospital Database on HIV (FHDH-ANRS CO4). Switching to raltegravir from a virologically effective boosted protease inhibitor regimen: a comparative effectiveness analysis from the french hospital database on HIV (FHDH-ANRS CO4). Clin Infect Dis. 2016;63(9):1254–1261. doi: 10.1093/cid/ciw498 [DOI] [PubMed] [Google Scholar]
- 14.Arribas JR, Pialoux G, Gathe J, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis. 2014;14(7):581–589. doi: 10.1016/S1473-3099(14)70782-0 [DOI] [PubMed] [Google Scholar]
- 15.Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIb study. Antivir Ther. 2017;22(4):295–305. doi: 10.3851/IMP3166 [DOI] [PubMed] [Google Scholar]
- 16.Gatell JM, Assoumou L, Moyle G; NEAT022 Study Group, et al. Immediate vs. deferred switching from a Boosted Protease Inhibitor (PI/r) Based Regimen to a Dolutegravir (DTG) based regimen in virologically suppressed patients with high cardiovascular risk or age ≥50 years: final 96 weeks results of NEAT 022 study. Clin Infect Dis. 2018. doi: 10.1093/cid/ciy505 [DOI] [PubMed] [Google Scholar]
- 17.Raffi F, Esser S, Nunnari G, Pérez-Valero I, Waters L. Switching regimens in virologically suppressed HIV-1-infected patients: evidence base and rationale for integrase strand transfer inhibitor (INSTI)-containing regimens. HIV Med. 2016;17(Suppl 5):3–16. doi: 10.1111/hiv.12440 [DOI] [PubMed] [Google Scholar]
- 18.Andreoni M, Marcotullio S, Puro V, et al. An update on integrase inhibitors: new opportunities for a personalized therapy? The NEXTaimProject. New Microbiol. 2015;38(4):443–490. [PubMed] [Google Scholar]
- 19.D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 20.de Boer MG, van Den Berk GE, van Holten N, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS. 2016;30(18):2831–2834. doi: 10.1097/QAD.0000000000001279 [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18(1):56–63. doi: 10.1111/hiv.12468 [DOI] [PubMed] [Google Scholar]
- 22.Lepik KJ, Yip B, Ulloa AC, et al. Adverse drug reactions to integrase strand transfer inhibitors. AIDS. 2018;32(7):903–912. doi: 10.1097/QAD.0000000000001781 [DOI] [PubMed] [Google Scholar]
- 23.Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS. 2018;13(2):102–111. doi: 10.1097/COH.0000000000000445 [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Frantzell A, Fransen S, Petropoulos CJ. Multiple genetic pathways involving amino acid position 143 of HIV-1 integrase are preferentially associated with specific secondary amino acid substitutions and confer resistance to raltegravir and cross-resistance to elvitegravir. Antimicrob Agents Chemother. 2013;57(9):4105–4113. doi: 10.1128/AAC.00204-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas GM, Cheever LW, Chaisson RE, Moore. RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection J. Acq Immune Def Synd. 2001;27:251–259. doi: 10.1097/00042560-200107010-00006 [DOI] [PubMed] [Google Scholar]
- 26.Taylor LE, Swan T, Matthews GV. Management of hepatitis C virus/HIV coinfection among people who use drugs in the era of direct-acting antiviral–based therapy. Clin Infect Dis. 2013;57(Suppl 2):S118–S124. doi: 10.1093/cid/cit326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills A, Crofoot G, Ortiz R, et al. Switching from twice-daily raltegravir plus tenofovir disoproxil fumarate/emtricitabine to once-daily elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate in virologically suppressed, HIV-1-infected subjects: 48 weeks data. HIV Clin Trials. 2014;15(2):51–56. doi: 10.1310/hct1502-51 [DOI] [PubMed] [Google Scholar]
- 28.Baldin G, Ciccullo A, Capetti A, et al. Efficacy and safety of switching to dolutegravir plus emtricitabine/tenofovir disoproxil fumarate (TDF) or elvitegravir/cobicistat/emtricitabine/TDF in virologically suppressed HIV-infected patients in clinical practice: results from a multicentre, observational study. HIV Med. 2019;20(2):164–168. doi: 10.1111/hiv.12688 [DOI] [PubMed] [Google Scholar]
- 29.Di Biagio A, Lorenzini P, Gustinetti G, et al. Durability of second antiretroviral regimens in the italian cohort naïve antiretrovirals foundation study and factors associated with discontinuation. AIDS Patient Care STDS. 2017;31(12):487–494. doi: 10.1089/apc.2017.0140 [DOI] [PubMed] [Google Scholar]
- 30.Boyd MA, Moore CL, Molina JM; SECOND-LINE study group, et al. Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: an exploratory analysis. Lancet HIV. 2015;Feb(2):e42–51. doi: 10.1016/S2352-3018(14)00061-7 [DOI] [PubMed] [Google Scholar]
- 31.Blanco JL, Gonzalez-Cordón A, Llibre JM, et al. Impact of prior virological failure and nucleos(t)ide genotypic resistance mutations on the efficacy of switching from ritonavir-boosted protease inhibitors to raltegravir. Antivir Ther. 2015;20(5):487–492. doi: 10.3851/IMP2812 [DOI] [PubMed] [Google Scholar]
- 32.Geretti AM, Smith C, Haberl A, et al. Determinants of virological failure after successful viral load suppression in first-line highly active antiretroviral therapy. Antivir Ther. 2008;13(7):927–936. [PubMed] [Google Scholar]
- 33.Martínez E, D’Albuquerque PM, Llibre JM; SPIRAL Trial Group, et al. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS. 2012;26(18):2315–2326. doi: 10.1097/QAD.0b013e328359f29c [DOI] [PubMed] [Google Scholar]
- 34.Bernal E, Masiá M, Padilla S, Gutiérrez F. High-density lipoprotein cholesterol in HIV-infected patients: evidence for an association with HIV-1 viral load, antiretroviral therapy status, and regimen composition. AIDS Patient Care STDS. 2008;22(7):569–575. doi: 10.1089/apc.2007.0186 [DOI] [PubMed] [Google Scholar]
- 35.Pascot A, Lemieux I, Bergeron J, et al. HDL particle size: a marker of the gender difference in the metabolic risk profile. Atherosclerosis. 2002;160(2):399–406. [DOI] [PubMed] [Google Scholar]
- 36.Tungsiripat M, Kitch D, Glesby MJ, et al. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS. 2010;24:1781–1784. doi: 10.1097/QAD.0b013e32833ad8b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbiani M, Bracciale L, Doino M, et al. Lipid-lowering effect of tenofovir in HIV-infected patients. J Antimicrob Chemother. 2011;66(3):682–683. doi: 10.1093/jac/dkq464 [DOI] [PubMed] [Google Scholar]
- 38.Taramasso L, Ricci E, Menzaghi B; CISAI Study Group, et al. Weight gain: a possible side effect of all antiretrovirals. Open Forum Infect Dis. 2017;4(4):ofx239. doi: 10.1093/ofid/ofx239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson A, Spivak A, Presson A, Jamjian C. 549. weight and BMI changes in HIV-infected virologically suppressed adults after switching to an elvitegravir- or dolutegravir-containing regimen. Open Forum Infect Dis. 2018;5(Suppl1):S204. doi: 10.1093/ofid/ofy210.557 [DOI] [Google Scholar]
- 40.Landovitz RJ, Zageneh SZ, Chau G et al. Cabotegravir is not associated with weight gain in HIV-negative individuals: HPTN077. OC 34 LB. CROI Seattle March 4- 72019.
- 41.Smolders EJ, Smit C, de Kanter C, et al. ATHENA national HIV observational cohort. Management of drug interactions with direct-acting antivirals in Dutch HIV/hepatitis C virus-coinfected patients: adequate but not perfect. HIV Med. 2018;19(3):216–226. doi: 10.1111/hiv.12570 [DOI] [PubMed] [Google Scholar]
- 42.Baril J, Conway B, Giguère P, Ferko N, Hollmann S, Angel JB. A meta-analysis of the efficacy and safety of unboosted atazanavir compared with ritonavir-boosted protease inhibitor maintenance therapy in HIV-infected adults with established virological suppression after induction. HIV Med. 2014;15(5):301–310. doi: 10.1111/hiv.12118 [DOI] [PubMed] [Google Scholar]
- 43.Giacomelli A, Oreni L, Franzetti M, et al. Factors involved in continuance of atazanavir-based regimens: results from a cohort of HIV1-positive patients. Antiviral Res. 2016;129:52–57. doi: 10.1016/j.antiviral.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 44.Chastain DB, Stover KR, Riche DM. Evidence-based review of statin use in patients with HIV on antiretroviral therapy. J Clin Transl Endocrinol. 2017;22(8):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taramasso L, Tatarelli P, Ricci E, et al. Improvement of lipid profile after switching from efavirenz or ritonavir-boosted protease inhibitors to rilpivirine or once-daily integrase inhibitors: results from a large observational cohort study (SCOLTA). BMC Infect Dis. 2018;18(1):357. doi: 10.1186/s12879-018-3109-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bavinger C, Bendavid E, Niehaus K, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One. 2013;8(3):e59551. doi: 10.1371/journal.pone.0059551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cerrato E, Calcagno A, D’Ascenzo F, et al Cardiovascular disease in HIV patients: from bench to bedside and backwards. Open Heart. 2015;2:e000174. doi: 10.1136/openhrt-2014-000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.