Abstract
Background
Immunological factors play a unique role in the setting of preeclampsia; there is a rising debate about the performance of interleukin 17 (IL-17) as inflammatory mediator in its pathogenesis. The purpose of this paper was to evaluate the significance of IL-17 in the diagnosis and prognosis of preeclampsia and estimate a cutoff value for better prediction.
Methods
A prospective case control study, 40 patient were enrolled in the study, two groups were designed: a normotensive (control) group (n=20) and preeclampsia group (n=20). Both groups were compared regarding serum IL-17 level to clarify its significance, then ROC curve analysis was done to establish the best cutoff level to predict preeclampsia, with further assessment of its relation to blood pressure to determine its prognostic value.
Results
We noted a statistically significant difference in serum IL-17 (pg/mL) level between the preeclampsia and control group (P<0.05). The best cutoff value of serum IL-17 in preeclampsia was (8.2 pg/mL) with a sensitivity of 100%, specificity 80% and accuracy 89%. There was also significant variation in its concentrations before and after control of blood pressure and a significant positive correlation with systolic blood pressure level (r=0.9).
Conclusion
IL-17 is a significant inflammatory biomarker in preeclampsia with useful prognostic power to predict severity of disease.
Keywords: IL-17, preeclampsia, inflammatory biomarker, hypertension, proteinuria
Introduction
Preeclampsia is hypertension with associated proteinuria before 20 weeks’ gestation. It is considered a critical reason for maternal and fetal morbidity and mortality, and represents a social and medical burden on the community.1 Placental insufficiency owing to impaired implantation has been identified as a cornerstone in the initiation of the disease, but the actual etiologies and the pathologic pathway remain unclear.2 An aggressive maternal inflammatory response has been implicated in the pathologic pathway of such diseases, with activation of both the natural and the adaptive immunity and imbalance between circulating “angiogenic and anti-angiogenic” factors.2,3 The lymphocytic cells that play a key role, with antagonist functions, are T-regulatory cells (Tregs) and T-helper 17 cells (Th17) cells. In normal pregnancy, Tregs prevent the response of maternal immune system against fetal tissue and a decrease in the number of Tregs is associated with improper implantation. Conversely, Th17 cells promote inflammation, autoimmunity and transplant rejection in humans and a significant increase in Th17 cells accompanied by decreased Tregs has been reported in many obstetric complications. The balance and correlation between Th1 cells, Th2 cells, Th17 cells, and Tregs should be maintained to ensure a safe environment for the fetus and a subsequent good obstetric outcome.4
Interleukin-17 is an inflammatory cytokine secreted by Th17 cells and has a proven action in the progression of many inflammatory processes. Human IL-17 was identified first in CD4+ T cells and then in CD8+ T cells, NKT cells and monocytes; it functions as a dynamic player in the recruitment and activation of neutrophils, representing a unique feature of the inflammatory response found in preeclampsia.2,5
But the actual role of IL-17, association with patient characteristics and its correlation with disease control remain to be eludicated.6 Understanding reproductive immunology and the IL-17 mechanism in controlling the pathologic pathway of preeclampsia would assist with early prediction, follow-up and assessment of the emerging new therapies, with the potential to improve outcomes in perinatal care.3,7 We evaluated the significance of IL-17 in the diagnosis and prognosis of preeclampsia and sought to define a cut ff value for better identification of the condition.
Materials and methods
Study design and subjects
A prospective case control study was conducted; the sample size was calculated to be 40 cases using open-Epi at CI 95% and a study power of 80%,2 recruited from patients attending the antenatal outpatient clinics of the Obstetrics and Gynecology department at Zagazig University Hospitals. Subjects were divided into two groups: a normal (control) group, and a preeclampsia group.
The healthy (control) group consisted of 20 normal pregnant women at a gestational age of 28–34 weeks. The preeclampsia group comprised 20 pregnant women with blood pressure ≥140/90 mmHg and proteinuria ≥0.3 gm/24 h, at a gestational age of 28–34 weeks, diagnosed according to the American College of Obstetricians and Gynecologists (ACOG) definition. Cases were considered severe if blood pressure was ≥160/110 mmHg and proteinuria ≥5 mg/24 h. Pregnancies with coexisting autoimmune/inflammatory diseases, diabetes mellitus, angiopathy, renal disorder, maternal infections and fetal congenital anomaly were excluded. All women were submitted to history taking, general examination and obstetric ultrasound to check that they met the inclusion criteria.
Blood pressure was measured for both groups at the time of admission, then reevaluated for preeclamptic patients every 4 hours at starting at 10:00 daily, considering the effect of the circadian rhythm. Patients in the preeclampsia group were offered a management protocol in the form of antihypertensive drugs, antioxidant and close monitoring for 2weeks (magnesium sulphate infusion was not used in our protocol routinely, but reserved for cases of severe preeclampsia, for neuroprotection against fits),8 then IL-17 serum concentration was measured again following further assessment of blood pressure.
Ethical approvals
The study was approved by the institutional review board of the Faculty of Medicine at Zagazig University and all participants provided their written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Methods
Analysis of serum IL-17A levels
Serum IL-17A level measurement was done by collecting a 5-mL blood sample from all participants (both groups) under completely aseptic conditions in the immunology laboratory. Serum samples were separated and stored at −20 °C to avoid loss of bioactive human IL-17A, measured by IL-17 Sandwich ELISA Kit according to the kit manufacturer’s instructions (Thermo Fisher Scientific Inc., USA; BMS2017). Mean absorbance was determined at 450 nm wavelength for each sample. Concentrations of IL-17 were measured as pg/mL in serum samples, with a limit of sensitivity 0.5 pg/mL.
A further blood sample was taken from the preeclampsia group for reassessment of IL-17A after control of blood pressure.
Results
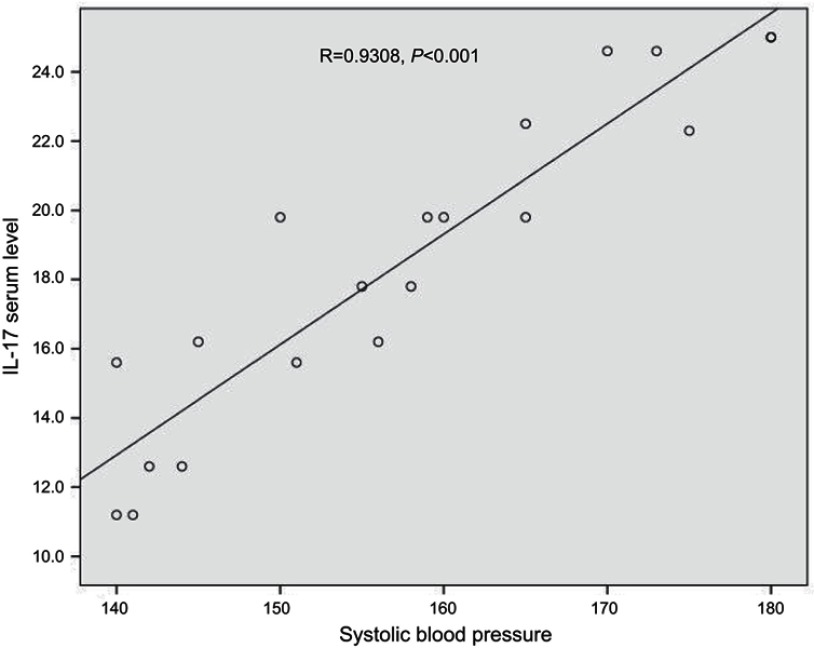
Table 1 summarizes the clinical characteristics of the studied groups. The current study revealed that the mean value of IL-17 in the preeclampsia group was 18.5 pg/mL,; for the control group this was 4.3 pg/mL, representing a strong statistical significant variance between groups (Table 2). There was no statistically significant correlation between concentration of serum IL-17 pg/mL and patient characteristics in either group (Table 3). A receiver operating characteristic (ROC) curve showed the best cutoff value for IL-17 in preeclampsia to be 8.2 pg/mL, with a sensitivity of 100%, specificity 80% and accuracy 89% (AUC=1) (Table 4). A highly significant positive correlation between serum IL-17 level and systolic blood pressure was found using Pearson’s correlation coefficient (Figure 1). Furthermore, a highly significant difference in IL-17 level before and after control of blood pressure was noted in the paired t-test (Table 5).
Table 1.
Clinical characteristics of the studied groups
| Variables | Studied groups | P-value | |
|---|---|---|---|
| Control group (n=20) | Preeclampsia group (n=20) | ||
|
Age (years) Mean ± SD Median (range) |
23.6 ± 5.2 22 (18–38) |
26.2 ± 4.8 25 (20–35) |
0.06 (NS) |
|
Gestational age (weeks) Mean ± SD Median (range) |
33 ± 2.6 34 (28–36) |
33.6 ± 1.7 34 (30–36) |
0.34 (NS) |
|
Parity, n (%) Prim Multigravida |
3 (15) 17 (85) |
5 (25) 15 (75) |
0.5 (NS) |
Abbreviation: NS, nonsignificant.
Table 2.
Comparison between control group and preeclampsia group: serum IL-17 (pg/mL)
| Serum IL-17 (pg/mL) | Control group (n=20) | Preeclampsia group (n=20) | **P-value |
|---|---|---|---|
|
Mean ± SD Median (range) |
4.38 ± 3.6 5 (0.4−0) |
18.5 ± 4.5 18.8 (11.2–25) |
0.001 (S) |
Note: **Mann-Whitney test.
Abbreviation: S, significant.
Table 3.
Significance between serum IL-17 (pg/mL) level and patient characters in the control group versus preeclampsia group
| Patient character | IL-17 level (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Control group | Preeclampsia group | ||||||
| No | Mean ± SD | **P-value | No | Mean ± SD | **P-value | ||
| Age (years) | ≤30 ≥31 |
15 5 |
4.3 ± 3.6 5.1 ± 4.9 |
0.61 (NS) |
16 4 |
19±6.9 20.4±4.8 |
0.73 (NS) |
| Gestational age (weeks) | 28–32 32–34 |
9 11 |
6 ± 2.9 4.6 ± 4.7 |
0.57 (NS) |
4 16 |
13.5±4.7 13.6±8.8 |
0.56 (NS) |
| Parity | Primi Multi |
3 17 |
5.4 ± 3.8 4.3 ± 3.6 |
0.72 (NS) |
5 15 |
11.7±6.2 13.9±6.4 |
0.41 (NS) |
Note: **Mann-Whitney test.
Abbreviation: NS, nonsignificant.
Table 4.
Validity of IL-17 in prediction of preeclampsia
| Validity parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Value | AUC | Cutoff | Sensitivity % | Specificity % | PPV | NPV | Accuracy | P |
| 1 | 8.2 pg/mL | 100% | 80% | 82% | 100% | 89% | 0.0001 | |
Abbreviations: AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value.
Figure 1.
Correlation between IL-17 serum level (pg/ml) and systolic blood pressure.
Table 5.
Comparison between pre and post treatment serum IL-17 (pg/ml) level
| Item | Mean ± SD | Mean (PD) ± SD | Paired t-test | P-value |
|---|---|---|---|---|
| Pre-treatment | 18.5 ± 6.3 | 4.43 ± 3 | 6.591 | 0.0001 (S) |
| Post-treatment | 14.06 ± 4.3 |
Abbreviations: S, significant; PD, paired difference.
Discussion
Recently, the role of immunologic and subsequent inflammatory response at the placental site has emerged from investigations concerning the pathogenesis of many unfavorable sequelae in the third trimester, especially preeclampsia. IL-17 is an inflammatory biomarker that has attracted increasing attention in this context, particularly its relation to changes in blood pressure. Although several studies have reported on IL-17 levels in patients with preeclampsia, ours is the first to describe a decrease in IL-17 levels in response to a successful management protocol (as discussed later).
We enrolled a group of preeclampsia patients in late gestation (28–34 weeks) compared with a control group of healthy pregnant females with a comparable gestational age, in a trial to estimate the diagnostic and prognostic value of IL-17. Our findings demonstrated no significant association between IL-17 levels and patient characteristics – including no correlation with advance in gestational age in late pregnancy, parity, nor even increasing maternal age. This means that IL-17 could be used as a neutral predicting factor in future research. The studies to address this issue are slightly deficient and controversial, but the majority confirm our results.4,6 Martínez-García et al9 reported a significant increase in the serum IL-17 level at the third trimester compared with the first trimester (37.28 versus 14.61 pg/mL), but this does not conflict with our findings, which focus on late pregnancy only. An interesting Chinese study was conducted in 1031 preeclampsia patients and 1298 controls of women in later pregnancy, to investigate the association between genetic variants in IL-17A, IL-17F, and IL-17RA and susceptibility to preeclampsia in Chinese Han women. That study used TaqMan allelic discrimination real-time PCR to genotype the polymorphisms of IL-17A rs2275913, IL-17F rs763780, and IL-17RA rs4819554, but surprisingly no significant differences in genotypic or allelic frequencies were found at the three polymorphic sites between preeclampsia patients and controls.10 Since it was a genetic study restricted to a single ethnic group, the findings cannot be extrapolated more widely: preeclampsia is a complex polygenetic hereditary disease, and many factors may affect and modify the gene expression – resulting in a varying prevalence, especially among different ethnic groups.
The ROC curve recorded a cutoff concentration for IL-17 of 8.2 pg/mL with a sensitivity of 100%, specificity 80%, positive predictive value (PPV) 82%, negative predictive value (NPP) 100% and accuracy 89%. Molvarec et al6 conducted a reasonable trial for finding a similar cutoff value but with much lower sensitivity (54%). Darmochwal-Kolarz et al2 observed an increased IL-17 level in the sera of 34 preeclamptic patient compared with 35 healthy pregnant women; they explained their findings by proposing that the inflammatory immune response in preeclampsia patients is an exacerbation of the normal inflammatory response preceding birth. Conflicting findings were reported by Ozkan et al,11 who claimed that there is a decrease in plasma IL-17 in preeclamptic women; however, the presence of many potentially confounding factors in their assay led them to recommend further confirmatory studies.
Poordast et al12 conducted a similar study to assess T-helper 17-associated cytokines (IL-17, IL-21, IL-23 and TGF-β) in the third trimester of pregnancy. Notably, they worked on three groups (30 preeclampsia patients, 30 normotensive pregnant women and 30 healthy individuals) and found comparable results concerning the serum levels of IL-17 and TGF-β, which were significantly higher in preeclampsia patients compared with the normal pregnant group and healthy individuals (P<0.0001). The opposite was found with IL-23 and IL-21 (P=0.005), with no significant differences between the studied groups. Concerning our results, there was a significant difference in IL-17 levels before and after control of the preeclampsia, with a significant positive association with systolic blood pressure, which may indicate a useful prognostic value for this cytokine in follow-up of the disease. Many studies have addressed this relationship, and most of the reported results align with ours.13 Darmochwal-Kolarz et al2 found that levels of IL-17 positively correlated with systolic blood pressure (r=0.42, P<0.01) in patients with fetal growth restriction (FGR) and preeclampsia. Yet Molvarec et al6 observed no significant variance in serum IL-17 concentrations between patients with mild versus severe preeclampsia, suggesting that the process responsible for elevation of blood pressure is complex and does not depend on IL-17 alone.
In our view, IL-17 is a sensitive cytokine that reflects the magnitude of inflammatory process at the maternal–fetal interface in cases of preeclampsia. Our finding that the decrease in IL-17 level was in line with improved blood pressure could be logically explained by attempted control of the inflammatory process at the placental site resulting in a decrease in inflammatory cytokines such as IL-17, with a subsequent decrease in blood pressure due to reduced release of vasoconstrictor substances, ensuring that the process evades the eclamptic pathway.
Recently, some researchers have reported an important role for magnesium sulphate as a part of preeclampsia management protocols, to help lower inflammatory serum cytokines in those patients. While this is a logical proposal, it has not been thoroughly investigated because most studies have either been conducted in vitro or have focused on other cytokines.14,15 A possible direct relation of serum levels of IL-17 to the magnesium sulphate infusion was beyond the scope of the current study, but will certainly be addressed in future research.
Conclusion
This paper has clearly shown the importance of IL-17 in the pathologic process of preeclampsia, describe a clear cutoff level that may be used in further studies for screening purpose. Our research also clarifies its prognostic role in predicting the severity of disease, which may help in predicting critical complications.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alsamarai AGM, Majeed HM, Alobaidi AH. Interleukin-6 (Il-6), interleukin-17 (Il-17) and angiopoietin in women with bad obstetric history Kirkuk, Iraq. Am Res J Haematol. 2016;1:1–11. [Google Scholar]
- 2.Darmochwal-Kolarz D, Michalak M, Kolarz B, et al. The role of interleukin-17, interleukin-23, and transforming growth factor in pregnancy complicated by placental insufficiency. Biomed Res Int. 2017;1:1–5. Article ID 6904325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molvarec A, Ito M, Shima T, et al. Decreased proportion of peripheral blood vascular endothelial growth factor expressing T and natural killer cells in preeclampsia. Am J Obstet Gynecol. 2010;203(567):e561–e568. doi: 10.1016/j.ajog.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 4.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x [DOI] [PubMed] [Google Scholar]
- 5.Michel ML, Keller AC, Paget C, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molvarec A, Czegle I, Szij´Art´O J, Rigó J. Increased circulating interleukin-17 levels in preeclampsia. J Reprod Immunol. 2015;112:53–57. doi: 10.1016/j.jri.2015.05.007 [DOI] [PubMed] [Google Scholar]
- 7.Molvarec A, Szarka A, Walentin S, Szucs E, Nagy B, Rigo J. Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with preeclampsia. Hypertens Res. 2010;33:892–898. doi: 10.1038/hr.2010.92 [DOI] [PubMed] [Google Scholar]
- 8.National Collaborating Centre for Women’s and Children’s Health (UK) Hypertension in Pregnancy. The Management of Hypertensive Disorders during Pregnancy. London, UK: RCOG Press; 2010. [PubMed] [Google Scholar]
- 9.Martínez-García EA, Chávez-Robles B, Sánchez-Hernández PE, et al. IL-17 increased in the third trimester healthy women with term labor. Am J Reprod Immunol. 2011;65(2):99–103. doi: 10.1111/j.1600-0897.2010.00893.x [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Guo M, Liu F, et al. Role of IL-17 variants in preeclampsia in Chinese Han Women. PLoS One. 2015;10(10):e0140118. doi: 10.1371/journal.pone.0140118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A, Sapmaz E. Plasma IL-17, IL-35, interferon-Y, SOCS3 and TGF- B levels in pregnant women with preeclampsia and their relation with severity of disease. J Maternal Fetal Neonatal Med. 2014;27(15):1513–1517. doi: 10.3109/14767058.2013.861415 [DOI] [PubMed] [Google Scholar]
- 12.Poordast T, Najib FS, Baharlou R, Bijani A, Alamdarloo SM, Poordast A. Assessment of T helper 17-associated cytokines in third trimester of pregnancy. Iran J Immunol. 2017;14(2):172–179. [PubMed] [Google Scholar]
- 13.Foroozanfard F, Soleimani A, Arbab E, Samimi M, Tamadon MR. Relationship between IL-17 and ambulatory blood pressure in polycystic ovary syndrome. Nephropathol. 2017;6(1):15–24. doi: 10.15171/jnp.2017.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q, Zhao M, Guo F, et al. The reduction of circulating levels of IL-6 in pregnant women with preeclampsia by magnesium sulphate and nifedipine: in vitro evidence for potential mechanisms. Placenta. 2015;36(6):661–666. doi: 10.1016/j.placenta.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto J, Romani AM, Valentin-Torres AM, et al. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J Immunol. 2012;188(12). doi: 10.4049/jimmunol.1101765 [DOI] [PMC free article] [PubMed] [Google Scholar]