Abstract
Introduction
The efficacy of cyclosporine A (CsA) in the treatment of idiopathic membranous nephropathy (IMN) is unclear. This meta-analysis was conducted to assess the efficacy and the safety of CsA in the treatment of IMN in Asians.
Methods
We searched the Pubmed, China Biomedical Database, CNKI, Wanfang Data, VIP, and EMBASE (November 30, 2018) systematically to identify the appropriate randomized controlled trials (RCTs) reporting the efficacy and the safety of CsA and glucocorticoid (GC) treatment vs other immunosuppressants and GC on patients with IMN in Asian populations.
Results
The CsA treated group entered complete remission (CR) faster (3 months) than a cyclophosphamide (CTX) group. While the CsA group lower inefficacy rates and higher total remission (TR, CR, or partial remission) than the CTX group in the total treatment (3 months, 6 months, and 12 months), it had a higher relapse rate. As for the CsA group vs the tacrolimus (TAC) group, the TAC had a significant effect in increasing the CR and the TR, with decreased no remission. With the therapeutic regimens of CsA+GC vs CTX+GC, the CsA exhibited better efficacy in lowering the proteinuria levels only at 12 months, not at 3 months or 6 months. Severe events like leucopenia, hemorrhagic cystitis, and alopecia were observed in the CTX group. Gingival hyperplasia, hirsutism, and elevated blood pressure were reported only in the CsA group. Gastrointestinal syndrome, liver function lesion, happened more frequently in the CTX group, and elevated uric acid was more common in the CsA group.
Conclusions
In brief, the CsA has better efficacy than the CTX group in the Asian population, with mild adverse effects but higher relapse rates in short-term treatment.
Keywords: idiopathic membranous nephropathy, cyclosporine A, cyclophosphamide, mycophenolate mofetil, tacrolimus
Introduction
Membranous nephropathy (MN) is the most common pathology in adults with nephrotic syndrome. Idiopathic membranous nephropathy (IMN), which consists of more than 20% of MN, is diagnosed when patients have primary MN without a secondary cause such as hepatitis B related nephritis, lupus nephritis, or malignancy, etc.1,2
Whether to treat IMN has been in debate for decades due to the observation that more than 20% of the patients can achieve spontaneous remission without immunosuppressive treatments.3–5 These phenomena are more frequent in cases with lower baseline proteinuria. Some untreated IMN patients, especially young patients (<50 years old) with proteinuria <4 g/L, stable renal function at 6 months and without the nephrotic syndrome, tend to have a good prognosis. However, some studies report that over 40% of the untreated IMN will develop into end-stage renal disease, especially in old patients with persistent proteinuria >8 g/d and the deteriorating renal function.2–4,6–8
Recently, when treating patients with significant kidney damage or a conservative treatment history, the Italian Ponticelli recommends treatment of IMN, suggesting the combination of the methylprednisolone with the oral cyclophosphamide (CTX).9 However, CTX leads to severe side effects in many patients, such as gonadoinhibition, marrow toxicity, hemorrhagic cystitis, hepatotoxicity, and cancer.10,11
Cyclosporin A (CsA) is composed of a cyclic polypeptide consisting of 11 amino acids, and it is one of the most effective immunosuppressive agents. Clinically, it is mainly used for anti-rejection reaction in kidney, liver, and heart transplantation. It can also be used together with the adrenocortical hormone to treat various immune diseases. As a calcineurin inhibitor, CsA becomes active by integrating with cyclophilin. The CsA-cyclophilin will combine with calcineurin as a competitive inhibition and affect the activity of calcineurin. In this way, the CsA suppresses the production of IL-2, IL-3, and IFN-γ, as well as translocation of nuclear factor-activating T cells. This subsequently dampens T-cell activity, impairs the function of T-lymphocyte helpers, and decreases lymphocyte proliferation. CsA treatment increases the production of vasoconstrictor factors such as the endothelin and thromboxane, which leads to the constriction of blood vessels, decreased filtration rate, and changes in glomerular permeability.12–14 This mechanism pertains to its antiproteinuric effect and toxicity.15,16 Thus, the current meta-analysis was conducted to assess whether CsA is an effective and safe option for IMN compared with other immunosuppressive agents.
Materials and methods
Search strategy
Pubmed, China Biomedical Database, CNKI, Wanfang Data, VIP, and EMBASE were searched using the following keywords: cyclosporine, cyclosporine A, CsA, membranous nephropathy, primary membranous nephropathy, idiopathic membranous nephropathy, MN, and IMN to select appropriate articles. The search extended to November 30, 2018.
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria for the study were as follows: (1) study type: various kinds of randomized controlled trials (RCTs) including prospective, single-blind, or multicenter clinical trials. (2) Patients: all patients were diagnosed with IMN by renal biopsy. Only patients ≥18 years old were included. (3) Drug interventions: different medication comparison, all studies including the application of CsA. (4) Studies in the Asian population.
Exclusion criteria
Exclusion criteria: (1) patients with secondary MN such as lupus nephritis, hepatitis B virus-associated nephritis, and malignancies, etc. (2) Studies that only included CsA without a control group. (3) The diagnostic criteria and detailed scheme were not clear. (4) Either the experimental group or the control group had less than 10 cases. (5) Studies with unclear data.
Outcome measures
Complete remission (CR), partial remission, invalid remission, total remission (TR; CR or partial remission), serum albumin (g/L), 24 hr-proteinuria levels (g), serum creatinine (μmol/L), serum triacylglycerol (nmol/L), serum cholesterol (mmol/L), white blood cell counts and adverse events were used as outcome measures.
Data collection
Two researchers screened the literature (either to read the titles and abstracts or contents) to find appropriate passages according to the designed inclusion criteria and exclusion criteria. At the same time, we organized the group discussion to deal with debatable articles. In this way, we filtered irrelevant studies.
Data analysis and statistical method
To compare and analyze the efficacy and the safety of CsA, we used Review Manager Version 5.3 software to calculate clinical indicators and adverse events of patients with diverse medication treatments. A random effects model was applied to assess data when the P-value of the heterogeneity test was <0.1. Other data were assessed by a fixed model. The weighted mean differences were used to pool the continuous data, and binary data were expressed using the odds ratio (OR). 95% confidence intervals (CIs) were also calculated. χ2-test of heterogeneity <0.05 indicated a significant difference.
Results
Search results
This meta-analysis included a total of 22 randomized and comparative studies17–38 in Asian populations (involving 1,366 patients, with CsA and IMN (Table 1). Sixteen studies17–23,25–30,32–34 reported effects of CsA plus glucocorticoid (GC) vs CTX plus GC. Three studies24,31,35 reported comparisons among CsA plus GC, CTX plus GC, and mycophenolate mofetil (MMF) plus GC. Three studies36–38 compared tacrolimus (TAC) with the CsA.
Table 1.
Characteristics of the included studies
| Author, year | Study design | Treatment strategies | Detailed scheme | Patient characteristics | Main outcome measures | Adverse events |
|---|---|---|---|---|---|---|
| Liu LH, 2013 | Randomized clinical trial | CsA+GC vs CTX+GC | Patients in both groups received oral prednisone at a dose of 0.5−1.0 mg/kg.d. The daily dose of prednisone was tapered after 3 months. The CsA+GC group received CsA (2.5–3.5 mg/kg.d) divided into two times until their urine protein<1 g/d. The daily dose of CsA was tapered by 1/4–1/3 of its dose every 2–3 months to a maintenance dose of 0.5–1.0 mg/d. If the patients’ creatinine clearance elevates more than 50% of their initial levels, then the CsA will be reduced to half of the dose. With this method, patients whose creatinine clearance cannot reverse will stop taking the CsA. For patients in the CTX+GC group, CTX initiated at a dose of 0.6–0.8 g/M (m2 body surface area) and the accumulating dose was 6–8 g. | Thirty patients (23 males, 7 females) with IMN proven by renal biopsy were included. They are divided into two groups, 14 in CsA group, 16 in CTX group. The average age of 30 female patients was 35.4 years. The following qualifications had to be fulfilled: (1) proteinuria ≥3.5 g/day, (2) a creatinine clearance≥142 mL/min/1.73 m2. Exclusion criteria included patients with severe systematic diseases including tertiary hypertension, heart failure, malignancy, hepatitis, and tuberculosis. | CR, PR, NR, TR, serum albumin, 24 hr-proteinuria levels, serum creatinine, serum triacylglycerol, serum cholesterol. | Gastrointestinal syndrome, hirsutism, gingival hyperplasia, elevated transaminase, elevated serum creatinine, elevated uric acid, elevated blood pressure, tremor, leucopenia. |
| Huang ZM, 2016 | Randomized clinical trial | CsA+GC vs CTX+GC | The CsA+GC group received oral CsA (5 mg/kg.d) divided into two times. The blood concentration was monitored after 1 week and would be maintained within 150–200 ng/mL. The daily dose of CsA was tapered after 3 months. The treatment will cease if the drug did not take effect within 6 months. Besides, the CsA+GC group also received oral methylprednisolone at a dose of 0.4 mg/kg.d. For patients in the CTX+GC group, CTX initiated at a dose of 1 g once per month by intravenous drip for 6 months and then once per 3 months. The accumulating dose was 8–10 g. Patients in CTX+GC group received oral methylprednisolone at a dose of 0.8 mg/kg.d. Every patients’ medication will be adjusted according to their condition. | One hundred patients with IMN proven by renal biopsy were included. They were presented with nephrotic syndrome. They are grouped according to random number method into two groups. Group A (median age at study entry, 51.24±12.13 (range 24–67) years) includes 29 males and 21 females. Group B (median age at study entry, 51.34±12.52 (range 24–69) years) includes 28 males and 22 females. | Significant remission, remission, NR, TR rate, 5-year relapse rate, serum albumin, 24 hr-proteinuria levels. | Hypertension, hyperuricemia, hirsutism, gingival hyperplasia, leucopenia, hemorrhagic cystitis, alopecia. |
| Chen ZF, 2014 | Randomized clinical trial | CsA+GC vs CTX+GC | The CsA+GC group received oral CsA (3–5 mg/kg.d) divided into two times. The blood concentration was monitored after 1 week and maintained within 100–175 ng/mL. The daily dose of CsA was tapered after 3 months according to patients’ condition. The treatment will cease if the drug did not take effect within 6 months. Besides, the CsA+GC group also received oral prednisone at a dose of 0.5 mg/kg per day. The daily dose of prednisone was tapered after 8 weeks to a maintenance dose of 5–10 mg/kg.d. For patients in the CTX+GC group, CTX initiated at a dose of 0.6–1.0 g once per month for 6 months and then once 3 months for nine times by intravenous drip and the accumulating dose was 6–8 g. Patients in CTX+GC group received oral prednisone at a dose of 0.8 mg/kg.d. Patients in CTX+GC groups received oral prednisone at a dose of 0.5 mg/kg.d. The daily dose of prednisone was tapered after 8 weeks to a maintenance dose of 5–10 mg/d. | Forty patients (27 males, 13 females) with IMN proven by renal biopsy were included. They (median age at study entry, 51.5±11.6 (range 24–74 years) are divided into two groups randomly. | CR, PR, NR, relapse, CR rate, 24 hr-proteinuria levels, serum albumin, serum cholesterol, serum creatinine, white blood cells count, ALT. | Hypertension, hyperuricemia, hirsutism, gingival hyperplasia, leucopenia, hepatic dysfunction, alopecia. |
| Wu QX,2011 | Randomized clinical trial | CsA+GC vs CTX+GC | Both groups received the same basic treatments such as anticoagulation, ARB, ACEI, etc. Patients in both groups received oral prednisone at a dose of 0.8 mg/kg.d. The daily dose of prednisone was tapered by 5 mg every 8 months to a maintenance dose of 0.5 mg/kg/d for 1–3 months according to their condition. Then, the daily dose of prednisone was tapered by 10% every 2 weeks to 0.4 mg/kg/qod and was stopped according to their condition. The CsA+GC group received CsA capsules at a dose of 3 mg/kg twice per day. The blood concentration was monitored to maintain within 100 –200 g/L. The daily dose of CsA was tapered after 3 months to 2 mg/kg.d for 3 months, then tapered to 1 mg/kg.d for half of year. The treatment would be stopped if the creatinine clearance rises by 30% or liver function is abnormally disabled. For patients in the CTX+GC group, CTX initiated at a dose of 10 mg/kg per day in combination with 0.9% saline for 2 days by intravenous drip. Then the method repeated once every 1 month. The treatment will be stopped if the white blood cells are lower than 3.5×109/L or liver function is abnormally disabled. If the cumulative dose reached 15 (mg.kg)/d, then the medication will change to repeated every 3–6 months. The treatment would stop according to their condition. |
40 patients (29 males,11 females; median age at study entry, 36.2±15.46 (range 20–65) years) with IMN for 6 days to 3 months were included. They are divided into the treatment group and the control group randomly. All of them had to satisfy the diagnosis standard of nephrotic syndrome. Besides, they were proven by renal biopsy as membranous nephritis without tubular damage, had to fulfill qualifications that their liver function, kidney function, blood cell counts should be normal as well as their exclusion criteria included patients with secondary nephrotic syndrome such as hepatitis B related nephritis, lupus nephritis, purpura nephropathy, etc. | CR, PR, significant remission, NR, relapse, CR rate, PR rate, significant remission rate, NR rate, relapse rate, TR rate, 24 hr-proteinuria levels, serum albumin, serum creatinine, blood urea nitrogen, serum cholesterol. | Hirsutism, tremor, gingival hyperplasia, elevated ALT, leucopenia, pneumonia, hemorrhagic cystitis, gastrointestinal syndrome. |
| Liu HT, 2015 | Randomized clinical trial | CsA+GC vs CTX+GC | Patients in the CTX+GC group received oral CTX (100 mg per day) divided into two times. After the accumulating dose is about 6–8 g, the treatment will be adjusted to 50 mg per day. CTX+GC group received oral prednisone at a dose of 0.8–1.0 mg/kg per day. The daily dose of prednisone was tapered by 10% every 2 weeks after 8 weeks to a maintenance dose of 10–20 mg/d. The CsA+GC group received oral CsA (2–3 mg/kg per day) divided into two times. And then we adjusted the medication to maintain blood concentration within 100–200 ng/mL. After 8 weeks, the medication would be tapered by 10% every 2 weeks to the dose of 1–2 mg/kg per day and the blood concentration would be maintained within 80–100 μg/L. Both groups were treated for 12 months. |
84 patients divided into two groups, 42 in CsA group, 42 in CTX group. CsA group includes 29 males and 13 females. CTX group includes 30 males and 12 females. The following qualifications had to be fulfilled: (1) meet the diagnostic criteria and proven as IMN by renal biopsy; (2) have not received systematic treatment; (3) exclude the possibility of toxic hepatitis, tumor, systemic lupus erythematosus or mercury poisoning; (4) exclude patients with secondary nephropathy. | CR, PR, NR, TR rate, recurrence rate, adverse event rate, 24 hr- proteinuria levels, serum albumin, serum creatinine, alanine aminotransferase. | NA |
| Zhang X, 2013 | Randomized clinical trial | CsA+GC vs CTX+GC | For patients in the CTX+GC group, they received oral CTX (1 g per month) for 6 months. And then the treatment will be adjusted to once every 3 months. The accumulating dose is about 6–8 g. CTX+GC group received oral prednisone at a dose of 0.6–1.0 mg/kg per day. After 2–3 months, the dose would reduce gradually. The CsA+GC group received oral prednisone at a dose of 0.15–0.5 mg/kg per day. After 3 months, the dose would reduce gradually. The CsA+GC group received oral CsA (1.0–1.5 mg/kg per day) divided into two times. And then we adjusted the medication to maintain blood concentration within 120–150 ng/mL. The drug dosage will be adjusted according to the patients’ clinical effect and their blood concentration. After the treatment of 2–3 months, we would increase the dose of medication appropriately to patients who did not react to the drug. The dose is up to 2.5 mg/(kg.d). For patients with significant drug efficacy (a reduction in urine protein test <1 g/d), The drug would be decreased by 1/4–1/3 to 0.5–1.0 mg/(kg.d) every 2 to 3 months. Both groups of patients were clinically treated with conventional treatment, including anticoagulation, ACEI, and ARB. |
30 patients with IMN (20 males, 10 females) were divided into experimental group and control group randomly, with 15 cases in each group. Their age ranged from 20 to 70 years old and the average age is 45 years old. The course of disease range from 2 to 49 months and the average disease duration was 23 months. Diagnosed as IMN. All patients had normal liver and kidney functions, normal blood cells, and no secondary kidney disease. The patient was diagnosed as membranous nephropathy from stage I to stage III by renal biopsy. | Significant remission, remission, NR, TR rate, 24 hr- proteinuria levels, serum creatinine, serum triacylglycerol, serum cholesterol, white blood cell counts. | Hirsutism gingival hyperplasia, pneumonia, elevated ALT. |
| Zhang D, 2016 | Randomized clinical trial | CsA with GC vs CTX with GC | Patients in both groups received oral prednisone at a dose of 0.5 mg/kg per day. The daily dose of prednisone was tapered after 8 weeks to a maintenance dose between 5 and 10 mg per day according to patients’ conditions. For patients in the CTX+GC group, CTX initiated at a dose of 8–12 mg/kg, dissolved in intravenous infusion in 250 mL saline, the infusion time should be ≥1 hr, once per day for 3–4 times. The CsA+GC group received CsA (3–5 mg/kg per day) divided into two times to keep a maintenance dose of 150–200 μg/d. If their symptoms were relieved and urine test protein turn to negative, the dose of CsA would be tapered gradually to a maintenance dose of 2 mg/(kg· d) for 9 months. Two groups were treated for 1 year. | 152 patients with IMN proven by renal biopsy, who had no response to treatment with GC monotherapy were included. They are grouped into two groups according to random number method, 76 cases in each group. The CTX group (median age at study entry, 44.68±13.41) includes 47 males, 29 females, whose average disease duration was (11.43±5.65) months. The CsA group (median age at study entry, 44.72±13.73) includes 48 males, 28 females, whose average disease duration was (11.29±5.46) months. | PR, PR rate, CR, CR rate, NR, NR rate TR, TR rate, serum creatinine, serum triacylglycerol, serum cholesterol, white blood cell counts, serum albumin and 24 hr proteinuria. | NA |
| Huang LL, 2017 | Randomized clinical trial | CTX+GC vs CsA+GC | For patients in the CTX+GC group, CTX initiated at a dose of 0.8 g and methylprednisolone at a dose of 500 mg by alternating intravenous drip for 6 months. Every patients’ dose of GC will be adjusted according to their condition. Then the dose of GC will be tapered to a maintenance dose of 30–35 mg/d. The treatment period was 12 months. The CsA+GC group received oral CsA (5 mg/kg per day) divided into two times. The blood concentration was monitored after 1 week and would be maintained within 150–200 ng/mL. The daily dose of CsA was tapered after 3 months to a maintenance dose of 3 mg/kg per day. The dose of GC was the same with the CTX+GC group. |
72 patients with IMN proven by renal biopsy were randomly divided into two groups, 36 cases in each group. The CsA group (median age at study entry, 41.6±6.0) includes 22 males, 14 females, whose average disease duration was (3.5±0.7) months. The CTX group (median age at study entry, 42.8±6.7) includes 24 males, 12 females, whose average disease duration was (3.9±1.0) months. | Significant remission, remission, NR, significant remission rate, remission rate, NR rate, TR rate, relapse, serum creatinine, serum triacylglycerol, serum cholesterol, white blood cell counts, serum albumin, total albumin, urea, fasting plasma glucose and 24 hr proteinuria. | Secondary diabetes, pneumonia, transient renal dysfunction, involuntary tremor, alopecia, hemorrhagic cystitis. |
| Deng SS, 2018 | Randomized clinical trial | CTX+GC vs CsA+GC | For patients in the CTX+GC group, CTX initiated at a dose of 1 g once per month by intravenous drip for 6 months. Then we repeated the treatment once per 3 months. The accumulating dose was 8–10 g. The CsA+GC group received oral CsA (5 mg/kg per day) divided into two times. The blood concentration was monitored after 1 week and would be maintained within 150–200 ng/mL. After 3 months, the daily dose of CsA was tapered gradually. Patients in both groups received oral prednisone at a dose of 0.8 mg/kg per day. | 90 patients with IMN were included. They are grouped into two groups randomly, 45 cases in each group. The CTX group (median age at study entry, 41.6±10.9) includes 29 males,16 females, whose average disease duration was (3.9±1.4) months. The CsA group (median age at study entry, 42.0±11.4) includes 31 males,14 females., whose average disease duration was (4.1±1.7) months. The following qualifications had to be fulfilled:(1) manifested as nephrotic syndrome, proven as IMN by renal biopsy and this time were the first time onset. (2) Patients with malignancy or hepatitis, pregnant women were excluded. | PR, CR, NR, TR, TR rate, relapse rate, side effect. | Hemorrhagic cystitis, alopecia, leucopenia, hypertension, gingival hyperplasia, hyperuricemia. |
| Dong XH, 2017 | Randomized clinical trial | CTX+GC vs CsA+GC | Patients in CTX+GC groups received oral prednisone at a dose of 0.8 mg/kg per day. After 3 months, the dose will be tapered gradually. Oral CTX initiated at a dose of 1 g once per month for 6 months. After 6 months, we repeated the dose once per 3 months. The accumulating dose was 8–10 g. Patients in CsA+GC groups received oral prednisone at a dose of 0.4 mg/kg per day. The CsA+GC group received oral CsA (2.5–3.5 mg/kg per day) divided into two times. The daily dose of CsA was tapered gradually to a maintenance dose of 0.5–1.0 mg/kg per day after the proteinuria<1.0 g/24 hrs. Patients in both groups were treated for 12 months. | 46 IMN patients were included. They are grouped into two groups according to random number method, 23 cases in each group. The CTX group (median age at study entry, 35.9±9.1) includes 15 males, 8 females. The CsA group (median age at study entry, 35.8±9.5) includes 14 males, 9 females. | CR, PR, NR, relapse, 24 hr-proteinuria levels, serum albumin, serum creatinine, serum cholesterol, white blood cell counts, serum creatinine, ALT. | Liver dysfunction, pneumonia, hypertension, gingival hyperplasia, hyperuricemia. |
| Liu JC, 2013 | Randomized clinical trial | CTX+GC vs CsA+GC | The CsA+GC group received oral CsA (2–3 mg/kg per day) divided into two times. The blood concentration was monitored after 1 week, 2 weeks, and every month. And then we adjusted the medication to maintain blood concentration within 100–150 ng/mL. If the patients’ creatinine clearance elevates more than 50% of their initial levels, then the CsA will be reduced to half of the dose. With this method, patients whose creatinine clearance still elevated would stop taking the CsA. After 3 months, if the patients 24 hrs proteinuria<1.0 g, the daily dose of CsA was tapered to a maintenance dose of 1.5–2.0 mg/kg per day. The CsA+GC group received oral prednisone at a dose of 0.5 mg/kg per day. After 8 weeks, the dose would reduce gradually. For patients in the CTX+GC group, CTX initiated at a dose of 1 g once per month, dissolved in intravenous infusion in 250 mL saline for 6 months. The accumulating dose is 6 g. The CTX+GC group received oral prednisone at a dose of 1 mg/kg per day (The maximum dose is 500 mg). After 8 weeks, the dose would reduce gradually. Both groups were treated for 6 months. | 47 patients with IMN proven by renal biopsy, were included. They are grouped into two groups randomly, 24 cases in CsA group, 23 cases in CTX group. The CTX group (median age at study entry, 46.8±9.2) includes 15 males, 9 females. The CsA group (median age at study entry, 46.5±9.8) includes 15 males, 8 females. The following qualifications had to be fulfilled: (1) age ranged from 18 to 70 years old; (2) manifested as nephrotic syndrome (proteinuria>3.5 g/24 hrs, serum albumin<30 g/L); (3) Patients with hepatitis B virus-associated nephritis, hepatitis C virus-associated nephritis, lupus nephritis, malignancy, or other secondary nephropathy; (4) without infection, heart failure, pulmonary failure or other complications. Besides, they all had normal liver function, renal function; (5) they were either initially treated or with a history of short-term prednisone treatment (<2 weeks). |
CR, PR, NR, 24 hr-proteinuria levels, serum albumin, serum creatinine. | Elevated blood pressure and hyperuricemia, increased serum creatinine, gingival hyperplasia, gastrointestinal syndrome, elevated alanine aminotransferase, leucopenia, pneumonia, steroid diabetes. |
| Xie J, 2014 | Randomized clinical trial | CTX+GC vs CsA+GC | Patients in both groups received oral prednisone at a dose of 60 mg once per day. After 2 months, the daily dose of prednisone was tapered by 5 mg every 2 weeks to 40 mg once per month. Then it was tapered by 5 mg every 4 weeks to 20 mg once per month. Finally, it was tapered by 5 mg every 6–8 weeks until we stopped the medication. Patients in CTX+GC groups received oral CTX at a dose of 100 mg once per day for 3 months. After the accumulating dose reached 9 g, the dose will be adjusted to 50 mg once per day for 5–6 month. Finally, we stopped the medication until the accumulating dose reached 16–18 g. During the treatment, the patient’s blood lymphocyte count was monitored every month. If the blood lymphocyte count was less than 0. 6×109/L, then we stopped CTX treatment for 1–2 months. If the blood lymphocyte count returned to 0. 8×109/L, we will continue the CTX treatment. The CsA+GC group received oral CsA (2–3 mg/kg per day) divided into two times. The blood concentration was monitored regularly in the treatment period. And then we adjusted the medication to maintain blood concentration within 80–120 ng/mL. During the treatment, the patient’s blood lymphocyte count was monitored every month. If the blood lymphocyte count was less than 0. 6×109/L, then we adjusted the dose of the CsA to half of the dose. If the blood lymphocyte count returned to 0. 8×109/L, we will continue the CsA treatment to the initial dose. Throughout the follow-up period, patients continued to take the CsA. | Patients are grouped into two groups randomly, 49 cases in CsA group, 56 cases in CTX group. The CTX group (median age at study entry, 47.41±12.09) includes 29 males, 27 females. The CsA group (median age at study entry, 46.61±16.31) includes 24 males, 25 females. The following qualifications had to be fulfilled: (1) manifested as nephrotic syndrome, proven as IMN by renal biopsy; (2) age ranged from 18 to 65 years old; (3) serum creatinine<115 μmol/L or eGFR≥90 mL/(min.1.73 m2); (4) had not receive systematic treatment. (5) Women were negative for the urine pregnancy test before enrollment, and no pregnancy occurred during the treatment. (6) Patients with malignancy or hepatitis, lupus nephritis, mercury poisoning or other secondary nephropathy were excluded. | CR, PR, TR, TR rate, relapse, 24 hr-proteinuria levels, serum albumin, serum creatinine, urea. | No serious infection, severe liver and kidney injury or malignancy. |
| Ding BB, 2014 | Randomized clinical trial | CTX+GC vs CsA+GC | Patients in CTX+GC groups received oral prednisone at a dose of 0.8 mg/kg per day. After 3 months, the dose will be tapered gradually. Oral CTX initiated at a dose of 1 g once per month for 6 months. After 6 months, we repeated the dose once per 3 months. The accumulating dose was 8–10 g. The CsA+GC group received oral CsA (2.5–3.5 mg/kg per day) divided into two times. After the patients 24 hrs proteinuria<1.0 g, the daily dose of CsA was tapered by 1/4–1/3 per 2–3 month to a maintenance dose of 0.5–1.0 mg/kg per day. We adjusted the medication to maintain blood concentration within 100–200 ng/mL. The CsA+GC group received oral prednisone at a dose of 0.4 mg/kg per day. If the patients’ creatinine clearance elevates more than 50% of their initial levels, then the CsA will be reduced to half of the dose. With this method, patients whose creatinine clearance still elevated would stop taking the CsA. | 42 IMN patients are grouped into two groups randomly, 21 cases in each group. The CTX group (median age at study entry, 37.8±17.3) includes 15 males, 6 females, whose average disease duration was (38.2±13.7) months. The CsA group (median age at study entry, 36.6±18.1) includes 13 males, 8 females, whose average disease duration was (39.1±14.1) months. The following qualifications had to be fulfilled: (1) meet the diagnostic criteria of the nephrotic syndrome; (2) exclude hepatitis B related nephritis, diabetes nephritis, lupus nephritis, purpura nephropathy, or other secondary nephropathy; (3) with normal liver or kidney functions; (4) proven by renal biopsy as membranous nephropathy. | PR, CR, NR, TR, TR rate, PR rate, CR rate, NR rate, relapse rate, serum creatinine, serum albumin, blood glucose and 24 hr proteinuria, ALT. | Gastrointestinal syndrome, elevated ALT, leucopenia, hirsutism, elevated serum creatinine, gingival hyperplasia, tremor. |
| Zhang M, 2016 | Randomized clinical trial | CTX+GC vs CsA+GC | Patients in both groups received oral prednisone at a dose of 0.5 mg/kg per day. After 6–8 weeks, the daily dose of prednisone was tapered by 10% every 2–3 weeks to a maintenance dose of 10 mg once per month. We treated the patents with oral prednisone till end of treatment. The CsA+GC group received oral CsA (3–5 mg/kg per day) divided into two times. The blood concentration was monitored after 1 week and would be maintained within 100–175 ng/mL. The daily dose of CsA was tapered after 3 months to a maintenance dose of 2–3 mg/kg per day according to the patients’ condition. After 12 months, if the CsA is ineffective, then we stopped the medication. If the CsA take effect, then the dose of the CsA was tapered gradually and ended. For patients in the CTX+GC group, CTX initiated at a dose of 0.8–1.0 g once per month, dissolved in intravenous infusion in 250 mL saline for 6 months. Then we maintained the medication with a dose of 0.8 once per 3 month. The accumulating dose is 7.2–8.4 g. If the white blood cells count was less than 2.0×109/L or patients get infectious, then we stopped CTX treatment or adjusted the dose of the CsA to half of the dose for 1–2 months. If white blood cells count returned to 3.5×109/L, we will continue the CsA or the CTX with the initial dose. Patients in both groups were treated for 2 years. | 70 patients with membranous nephropathy were included. They are grouped into two groups randomly, 35 cases in each group. The CsA group (median age at study entry, 48.7±5.5) includes 21 males, 14 females. The CTX group (median age at study entry, 49.3±5.2) includes 22 males, 13 females. The following qualifications had to be fulfilled: (1) age ranged from 18 to 70 years old; (2) manifested as nephrotic syndrome and proven by renal biopsy as membranous nephropathy; (3) without a history of therapy with immunosuppressant drugs combined with cytotoxic drugs. Exclusion criteria included: (1) patients with secondary nephropathy; (2) patients who cannot tolerate combination of immunosuppressant drugs with cytotoxic drugs; (3) patients with severe heart cerebrovascular disease, malignancy, organ failure, or immune diseases. |
PR, CR, NR, TR rate, relapse rate, serum creatinine, serum albumin and 24 hr proteinuria, serum cholesterol. | No serious infection, severe liver and kidney injury or malignancy. |
| Liu AQ, 2016 | Randomized clinical trial | CTX+GC vs CsA+GC | Patients in both groups received oral prednisone at a dose of 0.5–1.0 mg/kg per day. The daily dose of prednisone was tapered after 3 months. The CsA+GC group received CsA (2.5–3.5 mg/kg per day) divided into two times until their urine protein<1 g/d. The daily dose of CsA was tapered by 1/4–1/3 of its dose every 2–3 months to a maintenance dose of 0.5–1.0 mg/d. If the patients’ creatinine clearance elevates more than 50% of their initial levels, then the CsA will be reduced to half of the dose. With this method, patients whose creatinine clearance cannot reverse will stop taking the CsA. For patients in the CTX+GC group, CTX initiated at a dose of 0.6–0.8 g/M (m2 body surface area) and the accumulating dose was 6–8 g. | 48 patients with IMN proven by renal biopsy were included. They are grouped into two groups randomly, 24 cases in CsA group, 24 cases in CTX group. The CsA group (median age at study entry, 51.6±8.9) includes 14 males, 10 females. The CTX group (median age at study entry, 51.8±8.5) includes 15 males, 9 females. The following qualifications had to be fulfilled: (1) serum creatinine<142 μmol/L, proteinuria >3.5 g/L; (2) without other severe systematic diseases. | PR, CR, NR, TR rate, relapse rate, serum creatinine, serum albumin, 24 hr proteinuria and side effects. | Gingival hyperplasia, hirsutism, elevated blood pressure, increased serum creatinine, elevated ALT, gastrointestinal syndrome, transient elevation of leukocyte. |
| Sa RL, 2018 | Randomized clinical trial | CTX+GC vs CsA+GC | Patients in CTX+GC groups received oral prednisone at a dose of 1 mg/kg per day. After 3 months, the dose will be tapered gradually according to the patients’ condition. They received oral CTX 50 mg/kg per day) divided into two times. The accumulating dose was 6–8 g. The CsA+GC group received oral CsA (2.0 mg/kg per day) divided into two times. If the patients reach PR or the treatment take effect, then the dose would be decreased gradually after 6 months. Otherwise, we should look for the reasons for treatment failures and avoid them, such as irregular habit of taking medication, use other medications or foods that influence the results, and so on. Without these reasons, we would increase the CsA dose to achieve the best blood concentration and alleviate nephrotic syndrome. The daily dose of CsA was tapered by 1/4–1/3 of its dose every 2–3 months to a maintenance dose of 0.5–1.0 mg/d. If the patients’ creatinine clearance elevates more than 30% of their initial levels, then the CsA will be reduced or stopped. Patients in CsA group without hormone contraindications will receive GC at a dose of 0.5 mg/kg/d. Patients in CsA group with hormone contraindications accepted the CsA monotherapy. | 52 patients with IMN proven by renal biopsy were included. They are grouped into two groups randomly. The CsA group (median age at study entry, 51.2±8.7) includes 15 males, 11 females. The CTX group (median age at study entry, 50.6±8.3) includes 16 males,10 females. All patients without other severe systematic diseases. | Serum creatinine, serum albumin, 24 hr proteinuria. | Hepatotoxicity, hirsutism, elevated blood pressure, elevated serum creatinine. |
| Yang J, 2013 | Randomized clinical trial | CsA+GC vs CTX+GC vs MMF+GC | Both groups received oral prednisone at a dose of 0.8–1.0 mg/kg per day. The dose would be reduced gradually after 8–12 weeks. The total course of treatment of three groups is 12–18 months. For patients in the CTX+GC group, CTX initiated at 0.75 g/M (m2 body surface area) by intermittent intravenous drip per month for 6 months. Then their treatment would be extended according to their condition. The accumulating dose is ≤9 g. The CsA+GC group received CsA (3–5 mg/kg per day) divided into two times. And then we adjusted the medication to maintain blood concentration within 125–175 ng/mL for 2–3 months. Then the daily dose of CsA was tapered gradually to the minimal but effective dose for more than 3 months. For patients in GC+MMF group, patients received MMF at an initial dose of 1.5–2.0 g/d for 6–12 months. Both groups were given ACEI, ARB, if their serum creatinine was lower than 265 µmol/L. All three groups were given regular treatments such as lipid-lowering drugs, anti-platelet adhesion drugs, and hypotensive drugs (controlling blood pressure below 130/80 mmHg). |
Renal tissue specimens of 46 cases were shown as IMN by light microscopy, immunofluorescence, or electron microscopy. Among them, there were 30 males and 16 females. Their ages ranged from 34 to 72 years old. Their average age was (56.3±18.6) years old, and 29 patients (63.1% of them) were over 50 years old. The course of disease was 14 days to 1.5 years, with an average of (8.2±7.5) months. There were 10 cases with hypertension, accounting for 21.7%, all of which had high-risk factors. The high-risk factors were defined as renal insufficiency (Scr >125.0 mol/L and/or 24 hr- proteinuria>8.0 g). The pathological changes of patients were divided into stages I, II, III, and IV. Patients of stage I and II accounting for 88.9%. The follow-up period was no less than 12 months. The patients enrolled in the study were randomly divided into three groups. Exclusion criteria: (1) patients with autoimmune diseases, tumors, infections and drug-induced diseases or other secondary factors; (2) patients with no history of ACEI and ARB medication. | CR, PR, NR, CR rate, PR rate, NR rate, TR rate, recurrence rate, 24 hr-proteinuria levels, serum albumin, serum creatinine. | NA |
| Li LL, 2016 | Randomized clinical trial | CsA+GC vs CTX+GC vs MMF+GC | Patients were randomized into three groups, GC+CTX group, GC+CsA group, GC+MMF group. CTX was used by intravenous drip once per month. The GC+CsA group received CsA at a dose of 3–5 mg/kg per day. After 2–3 months, the dose would reduce gradually. For patients in GC+MMF group, the initial dose of MMF is 1.5–2.0 g/d. Patients in both groups received oral prednisone at a dose of 0.8–1.2 mg/kg per day. The daily dose of prednisone was tapered after 2–3 months. All groups were given drugs to lower blood pressure, anti-platelet adhesion, and lower lipid. | 42 patients with IMN, aged from 33 to 71 years, included 20 males and 22 females. Their average age was (58.3±6.8) years old, the duration of their disease ranged from 20 days to 2 years. They were randomly divided into three groups, 14 cases in the CTX+GC group (7 males, 7 females; median age at study entry, (57.3±4.6) years); 14 cases in the CsA+GC group (6 males, 8 females; median age at study entry, (56.3±6.6)years); 14 cases in the MMF+GC group (7 males, 7 females; median age at study entry, (58.2±4.3) years). Their immunofluorescence and renal tissue specimens were examined as IMN. | Significant remission, remission, NR, significant remission rate, remission rate, NR rate, TR rate, blood pressure, serum creatinine, 24 hr-proteinuria levels. | NA |
| Choi JY, 2017 | Multi-center, randomized controlled trial | MMF+GC vs CsA+GC | Treatment consisted of oral prednisolone 0.15 mg/kg up to a maximum dose of 15 mg/day for patients in both groups. Prednisolone was maintained at a minimum of 5 mg/day during the study period. Concurrent treatment was not standardized; however, blood pressure and proteinuria were primarily treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Statins were used to decrease serum cholesterol levels. In the MMF group, oral MMF was added to corticosteroids. The MMF treatment regimen consisted of 500 mg twice daily in patients weighing less than 50 kg, or 750–1,000 mg twice daily in patients weighing more than 50 kg. The dose of MMF was adjusted in a range of 500–1,000 mg twice daily based on laboratory findings, at the discretion of the attending physician. The dose of MMF was reduced by 25–33% of the previous dose when a patient had moderate to severe diarrhea. MMF was withheld when the WBC count was less than 4,000/mm3 or the patient had intolerable gastrointestinal symptoms, and was restarted at a 50% dose at least 2 weeks after recovery. MMF was discontinued when the patient showed severe adverse events, more than doubled values in liver function tests, newly developed malignancy, or increased serum creatinine level by over 50%. In the CsA group, oral CsA was added to corticosteroids. CsA was started at a dose of 4 mg/kg and the dose was adjusted to maintain a 100±50 ng/mL trough blood level. The trough level of CsA was not an absolute target and the dose could be adjusted at the physician’s discretion. The dose was decreased in cases in which serum CsA level ≥250 ng/mL, there was an increase in serum creatinine level of more than 0.3 mg/dL compared to baseline, elevated serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level, or increased serum bilirubin level ≥2 mg/dL. CsA was discontinued when serum creatinine level did not improve 4 weeks after the dose was reduced in cases of increased serum creatinine ≥30% over baseline. CsA was also discontinued when the patient had severe adverse events or uncontrolled high blood pressure despite the use of three or more anti-hypertensive drugs at maximally tolerated doses. | Patients with biopsy-proven IMN were assessed for eligibility for this study at multiple centers in the Republic of Korea. We also included patients who underwent a biopsy within the preceding 12 months, experienced worsening proteinuria, and exhibited deteriorating renal function, but satisfied the following inclusion criteria. Patients who were ≥18 years old were enrolled in this study if they had proteinuria >8 g/day. Patients with proteinuria <8 g/day were also enrolled if they met three or more of the following criteria: (1) eGFR <60 mL/min/1.73 m2; (2) hypertension (blood pressure ≥140/90 mmHg or ≥120/80 with anti-hypertensive drugs); (3) 24 hr urinary protein >5.0 g/day or spot urine protein to creatinine ratio >5.0 g/g; (4) serum albumin <3.0 g/dL; (5) selectivity index >0.2. eGFR was calculated by the modification of diet in renal disease (MDRD) equation. The selectivity index was calculated using the following equation: urine IgG × serum albumin/serum IgG × urine albumin. Exclusion criteria included the presence of moderate to severe gastrointestinal disorder at screening; a history of allergy to mycophenolate mofetil or cyclosporine; acute or chronic allergy within 4 weeks; presence of serious life-limiting comorbid disorders such as malignancy or uncontrollable active infection; drug or alcohol addiction within 6 months; uncontrolled high blood pressure (≥160/100 mmHg); eGFR ≤30 mL/min/1.73 m2 at screening; absolute neutrophil count <1,500/mm3 or white blood cell <2,500/mm3; platelets <100,000/mm3; three-times greater than normal liver function test values; pregnancy; or lactation. Patients who had received immunosuppressive agents within 6 months for secondary MN with systemic disorder, or had a life expectancy of less than 1 year were also excluded. |
CR, PR, CR rate, PR rate, 24 hr proteinuria, serum albumin, serum creatinine total cholesterol, eGFR, relapse rate, anti-PLA2R Ab levels. | Anemia, diarrhea, epigastric discomfort, infections, new onset diabetes mellitus, malignancy (gastric cancer, bladder tumor). |
| Guo Y, 2016 | Randomized clinical trial | TAC +GC vs CsA + GC | TAC+GC group: The initial dose of oral TAC is 0.1 mg/(kg.d) in the morning and evening. After 3 weeks, the physician would adjust its dosage according to patients’ blood concentrations of FK506 to maintain a stable dose of 5–10 ng/mL for 6 months. The dose would be reduced gradually after 6 months of treatment to maintain their blood concentration within 2–5 ng/mL. The initial dose of oral prednisolone is 1 mg/(kg.d). After 8–12 weeks of treatment, the original dose is reduced by 10% to 20 mg/d, and reached the minimal dose of 10 mg/d after 6 months. If the patient’s serum creatinine levels elevate more than 30% of the baseline value or the blood drug concentration exceeds 10 ng/mL, the TAC dose would be reduced. For patients with hyperglycemia, we adjust their dose or provide them with hypoglycemic agents. Control group: CsA combined with GC treatment: the initial dose of oral CsA dose is 5 mg/(kg.d) in the morning and evening. After 3 months of treatment, the dose will be reduced to 1–3 mg/(kg.d). The blood concentration should be measured during the treatment and maintained at 250–350 ng/mL. Prednisolone usage is consistent with the study group. 42 patients were treated with ACEI, ARB, anticoagulation, Statins, and diuretic drug, either to control high blood pressure, lower their proteinuria levels or lower their lipid levels. |
42 patients with IMN were randomly divided into study group and control group, with 21 cases in each group. The study group (median age at study entry, 67.23±13.92 (range 54–78) years) consisted of 13 males and 8 females. The control group (median age at study entry, 68.02±10.57 (range 52–80) years) includes 12 males and 9 females. | Significant remission, remission, NR, significant remission rate, remission rate, NR rate, TR rate, 24 hr-proteinuria levels, serum albumin, endogenous creatinine clearance, serum creatinine, serum triacylglycerol, serum cholesterol. | Hyperglycemia, nephrotoxicity. |
| Li QH, 2017 | Randomized clinical trial | TAC +GC vs CsA + GC | Patients in the TAC +GC group received oral TAC (0.05–0.1 mg/kg per day) divided into two times. The blood concentration would be monitored every week in the first month and then every month. The dose of the TAC would be adjusted to maintain blood concentration within 5–10 ng/mL. If patients achieve remission, low-dose of medication is acceptable. Patients in the CsA+GC group received oral CsA (3–5 mg/kg per day) divided into two times. The blood concentration would be monitored every week in the first month and then every month. The dose of the CsA would be adjusted to maintain blood concentration within 100–200 ng/mL. Patients in both groups received oral prednisone at a dose of 0.5 mg/kg per day (the maximum dose is 60 mg/d). The observation time were 6 months. The daily dose of prednisone was tapered by 5 mg/d to 10 mg/d every month. This dose would be maintained till the treatment ended. |
31 patients with IMN proven by renal biopsy are divided into two groups randomly, 15 in CsA group, 16 in TAC group. The following qualifications had to be fulfilled: (1) age ranged from 18 to 60 years old. (2) Proven by renal biopsy and laboratory examination indexes as IMN. (3) Persistent 24 hr-proteinuria>8 g/24 hrs. manifested as nephrotic syndrome (proteinuria>3.5 g/24 hrs, serum albumin<30 g/L). (4) Serum creatinine clearance<133 μmol/L. (5) Were willing to enter this research and signed up informed consent voluntarily. Exclusion criteria: (1) with severe complication such as thromboembolism, infections, kidney failure, etc. (2) patients with HIV, heart failure, chronic hepatitis B virus-associated nephritis, chronic hepatitis C virus-associated nephritis, elevated liver enzyme detection value (elevated more than 2 times than normal value), diabetes mellitus diseases and other kidney diseases. (3) with a history of therapy with immunosuppressant drugs or cytotoxic drugs. (4) patients with hormone or immunosuppressant drugs contraindications, such as gastric ulcer, active gastrointestinal bleeding, severe infection, etc. (5) pregnant, be ready to pregnant or lactating women. (6) patients allergic to medication or patients with poor drug compliance. | PR, CR, NR, TR rate, relapse rate, serum creatinine, serum albumin and 24 hr proteinuria, serum cholesterol, blood glucose, serum triacylglycerol. | Infection, hepatotoxicity, gastrointestinal syndrome, hypertension, hyperglycemia, tremor, hirsutism, gingival hyperplasia, reversible nephrotoxicity, hyperuricemia. |
| Hu QF, 2016 | Randomized clinical trial | TAC+GC vs CsA + GC | Patients in the TAC+GC group received oral TAC (0.3 mg/kg per day), which was administered half an hour after meals. The blood concentration would be monitored every 2 weeks in the first month and then every 2 months. The blood concentration of the TAC would be maintained within 4.88–11.34 ng/mL. Patients in TAC groups received prednisone at a dose of 0.5 mg/kg per day. The daily dose of prednisone was tapered till the treatment ended. Patients in the CsA+GC group received oral CsA 20 mg per day before breakfast. The blood concentration would be monitored every 2 weeks in the first month and then every month. The blood concentration of the CsA would be maintained within 100–200 ng/mL till the treatment ended. Patients in CsA groups received prednisone at a dose of 0.5 mg/kg per day. The daily dose of prednisone was tapered till the treatment ended. Adjustment and withdrawal criteria of TAC (1) patients with renal function deterioration during treatment, the dose should be reduced to one-third of the initial dose. After 2 weeks, if patients did not get clinical improvement, then the medication would be stopped. (2) If the blood concentration was >10 ng/mL, the dose should be reduced to half of the dose. After 2 weeks, if the blood concentration was <5 ng/mL, we could increase the dose to maintained the blood drug concentration within 5–10 ng/mL. (3) For patients with leucopenia, if the 2-week intervention did not effect, then the patients would withdrawal from the test. (4) For patients with abnormal blood glucose, diarrhea and other symptoms that needed for other treatments, if the symptoms were serious, then the patients would withdrawal. (5) patients with serious infection or pregnancy would withdrawal. |
77 patients (age ranged from 23 to 50 years old) with IMN proven by renal biopsy were included. They are divided into two groups randomly, 32 in CsA group, 40 in TAC group (five cases quit the treatment because of their personal reasons.) The following qualifications had to be fulfilled: (1) age ranged from 23 to 50 years old, pathological stage ranged from stage I to III. (2) After six months of anti-hypertensive therapy and anti-proteinuria therapy, the proteinuria still>4 g/d. (3) All patients underwent biopsy under aseptic operation and ran corresponding light and electron microscopy. (4) Serum creatinine clearance<221 μmol/L. (5) Without pregnant women and strict birth control measures were taken during the treatment. (6) Did not use immunosuppressant for nearly 3 months. (7) Patient was familiar with the whole experiment and agreed to enter it. Exclusion criteria: (1) with secondary membranous nephropathy. (2) Use immunosuppressant for nearly 1 month. (3) With malignancy. (4) With severe kidney diseases. (5) Patients allergic to macrolides. (6) Patients with chronic hepatitis or elevated liver enzyme detection value (elevated more than two times than normal value). (7) Serum creatinine clearance>309 μmol/L persistently. (8) Pregnant or lactating women. |
PR, CR, NR, TR rate, serum creatinine, serum albumin and 24 hr proteinuria, serum cholesterol, blood glucose, serum triacylglycerol. | Gastrointestinal syndrome, fever, infection, rash, leukopenia. |
Abbreviations: CsA, cyclosporine A; CTX, cyclophosphamide; MMF, Mycophenolate Mofetil; GC, glucocorticoid; TAC, tacrolimus; CR, complete remission; PR, partial remission; NR, no remission; TR, total remission; OR, odds ratio; IMN, Idiopathic membranous nephropathy; Scr, serum creatinine clearance; eGFR, estimated glomerular filtration rate; ACEI, Angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor antagonist; ALT, alanine aminotransferase.
CR (complete remission)
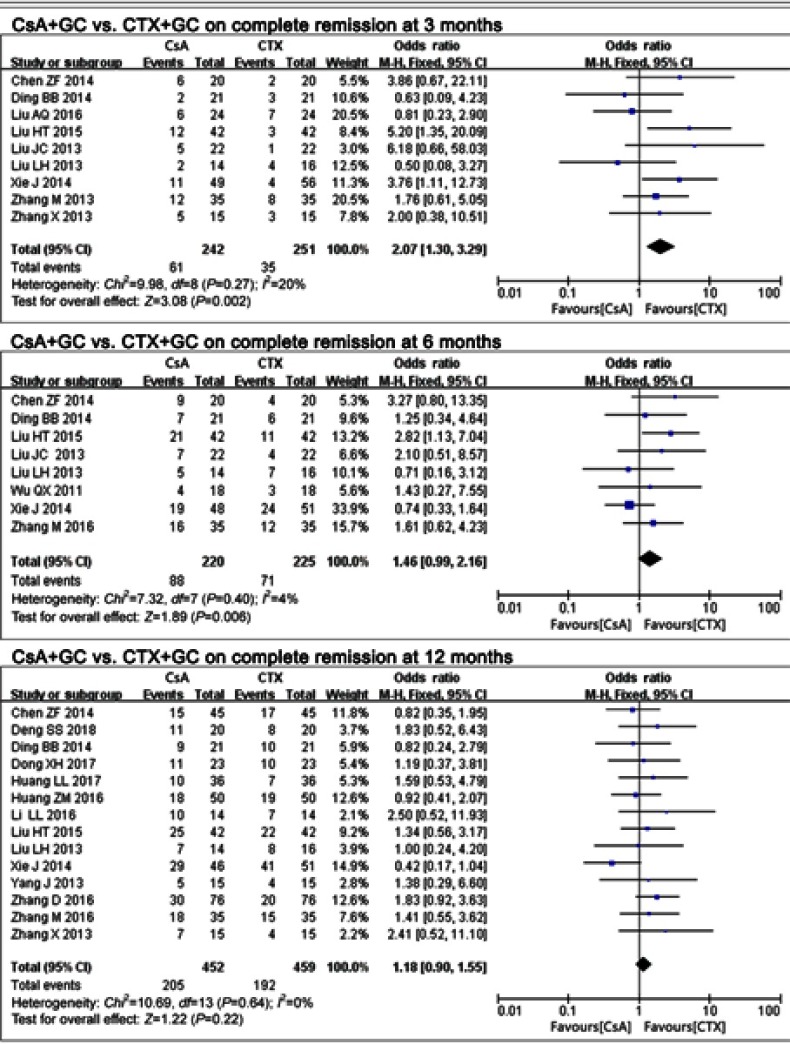
Seventeen studies17–28,30–34 assessed the effects of CsA+GC vs CTX+GC on IMN, and the CR rate was calculated. The CR rate in the CsA group was significantly higher when compared with that in the CTX group at 3 months (OR=2.07, 95% CI: 1.30–3.29; P=0.002; Figure 1 and Table 2).17,19,23,25–28,33,34 While at 6 months,17,19,23,26–28,30,33 (OR =1.46, 95% CI:0.99–2.16, P=0.06; Figure 1 and Table 2) and 12 months17-24,27,28,32–34 (OR=1.18,95% CI: 0.90–1.55, P=0.22; Figure 1 and Table 2), this advantage was not present.
Figure 1.
The effect of CsA+GC vs CTX+GC on CR in patients with IMN in Asian populations.
Abbreviations: CsA, cyclosporine A; CTX, cyclophosphamide; GC, glucocorticoid; CR, complete remission; IMN, idiopathic membranous nephropathy.
Table 2.
Meta-analysis of the efficacy of CsA in the therapy of IMN in the Asian population
| Therapeutic | Indicators | Studies | Q test | Model | OR/WMD | P |
|---|---|---|---|---|---|---|
| Regimen | Number | P-value | Selected | (95%CI) | ||
| CsA+GC vs CTX+GC | CR 3 months | 9 | 0.27 | Fixed | 2.07 (1.30,3.29) | 0.002 |
| CR 6 months | 8 | 0.40 | Fixed | 1.46 (0.99,2.16) | 0.06 | |
| CR 12 months | 14 | 0.64 | Fixed | 1.18 (0.90,1.55) | 0.22 | |
| NR 3 months | 9 | 0.23 | Fixed | 0.52 (0.36,0.74) | 0.0004 | |
| NR 6 months | 8 | 0.15 | Fixed | 0.62 (0.41,0.93) | 0.02 | |
| NR 12 months | 14 | 0.50 | Fixed | 0.51 (0.36,0.73) | 0.0002 | |
| TR 3 months | 9 | 0.23 | Fixed | 1.94 (1.35,2.80) | 0.0004 | |
| TR 6 months | 8 | 0.15 | Fixed | 1.62 (1.07,2.46) | 0.02 | |
| TR 12 months | 14 | 0.39 | Fixed | 1.81 (1.29,2.53) | 0.0006 | |
| Relapse | 10 | 0.10 | Fixed | 3.06 (1.84,5.07) | P<0.0001 | |
| Serum protein 3 months | 6 | 0.008 | Random | 0.16 (−0.27,0.59) | 0.48 | |
| Serum protein 6 months | 6 | 0.09 | Random | 0.56 (0.21,0.90) | 0.002 | |
| Serum protein 12 months | 8 | <0.00001 | Random | 1.40 (0.15,2.64) | 0.03 | |
| Urinary protein 3 months | 6 | 0.007 | Random | −0.35 (−0.78,0.08) | 0.11 | |
| Urinary protein 6 months | 6 | 0.02 | Random | −0.01 (−0.41,0.39) | 0.96 | |
| Urinary protein 12 months | 8 | P<0.00001 | Random | −0.66 (−1.24,-0.08) | 0.02 | |
| Serum creatinine 3 months | 6 | 0.29 | Fixed | 0.07 (−0.16,0.30) | 0.56 | |
| Serum creatinine 6 months | 6 | 0.21 | Fixed | 0.12 (−0.12,0.35) | 0.34 | |
| Serum creatinine12 months | 8 | 0.69 | Fixed | 0.13 (−0.04,0.31) | 0.14 | |
| Cholesterol 3 months | 2 | 0.78 | Fixed | −0.49 (−0.96,-0.01) | 0.04 | |
| Cholesterol 6 months | 3 | 0.26 | Fixed | −0.55 (−0.94,−0.16) | 0.006 | |
| Cholesterol 12 months | 6 | <0.00001 | Random | −0.55 (−1.14,0.03) | 0.06 | |
| Triacylglycerol 12 months | 4 | 0.01 | Random | −0.96 (−1.50,−0.41) | 0.0006 | |
| WBC 12 months | 4 | 0.51 | Fixed | 1.31 (1.04,1.58) | <0.00001 | |
| ALT 3 months | 3 | 0.84 | Fixed | 0.02 (−0.28,0.33) | 0.88 | |
| ALT 6 months | 3 | 0.99 | Fixed | 0.05 (−0.25,0.36) | 0.73 | |
| ALT 12 months | 4 | 0.99 | Fixed | −0.00 (−0.27,0.27) | 1.00 | |
| BUN | 2 | 0.82 | Fixed | −0.00 (−0.38,0.38) | 0.99 | |
| CsA vs MMF | CR | 3 | 0.21 | Fixed | 0.92 (0.41,2.05) | 0.83 |
| NR | 3 | 0.13 | Fixed | 0.55 (0.22,1.36) | 0.20 | |
| TR | 3 | 0.13 | Fixed | 1.82 (0.73,4.49) | 0.20 | |
| CsA vs TAC | CR | 3 | 0.36 | Fixed | 0.42 (0.21,0.85) | 0.02 |
| NR | 3 | 0.96 | Fixed | 3.27 (1.40,7.64) | 0.006 | |
| TR | 3 | 0.96 | Fixed | 0.31 (0.13,0.71) | 0.006 | |
| Serum protein | 2 | 0.01 | Random | −1.17 (−2.23,-0.12) | 0.03 | |
| Urinary protein | 2 | 0.32 | Fixed | 0.36 (−0.01,0.73) | 0.06 | |
| Triacylglycerol | 2 | 0.02 | Random | 1.01 (0.11,1.92) | 0.03 | |
| Cholesterol | 2 | 0.14 | Fixed | 0.34 (−0.03,0.72) | 0.07 |
Abbreviations: CsA, cyclosporine A; CTX, cyclophosphamide; MMF, Mycophenolate Mofetil; GC, glucocorticoid; TAC, tacrolimus; CR, complete remission; PR, partial remission; NR, no remission; TR, total remission, CR or PR; OR, odds ratio.
Three studies24,31,35 assessed the CR rate of CsA+GC vs MMF+GC. The CsA group had similar efficacy to the MMF group (OR=0.92,95%CI: 0.41–2.05, P=0.83; Table 2).
Another three studies24,36–38 compared CsA+GC with the TAC+GC. The CsA group had lower CR rate when compared with the TAC group (OR=0.42, 95%CI: 0.21–0.85, P=0.02; Table 2).
NR (no remission)
Seventeen studies17–28,30–34 were included in the comparison of CsA+GC vs CTX+GC. The NR rate of the CsA+GC group was less than the CTX+GC group at 3 months17,19,23,25–28,33,34 (OR=0.52, 95%CI: 0.36–0.74, P=0.0004; Table 2), 6 months17,19,23,26–28,30,33 (OR=0.62, 95% CI:0.41–0.93, P=0.02; Table 2) and 12 months17–24,27,28,32–34 (OR=0.51, 95% CI:0.36–0.73, P=0.0002; Table 2).
Three studies24,31,35 compared CsA+GC vs MMF+GC. The NR rate of the MMF group did not differ from the CsA group (OR=0.55, 95%CI:0.22–1.36, P=0.20; Table 2).
Three studies24,36–38 calculated the NR rate of CsA+GC vs TAC+GC. Compared with the CsA group, TAC presented a decreased NR rate (OR=3.27, 95% CI:1.40–7.64, P=0.006; Table 2).
TR (total remission)
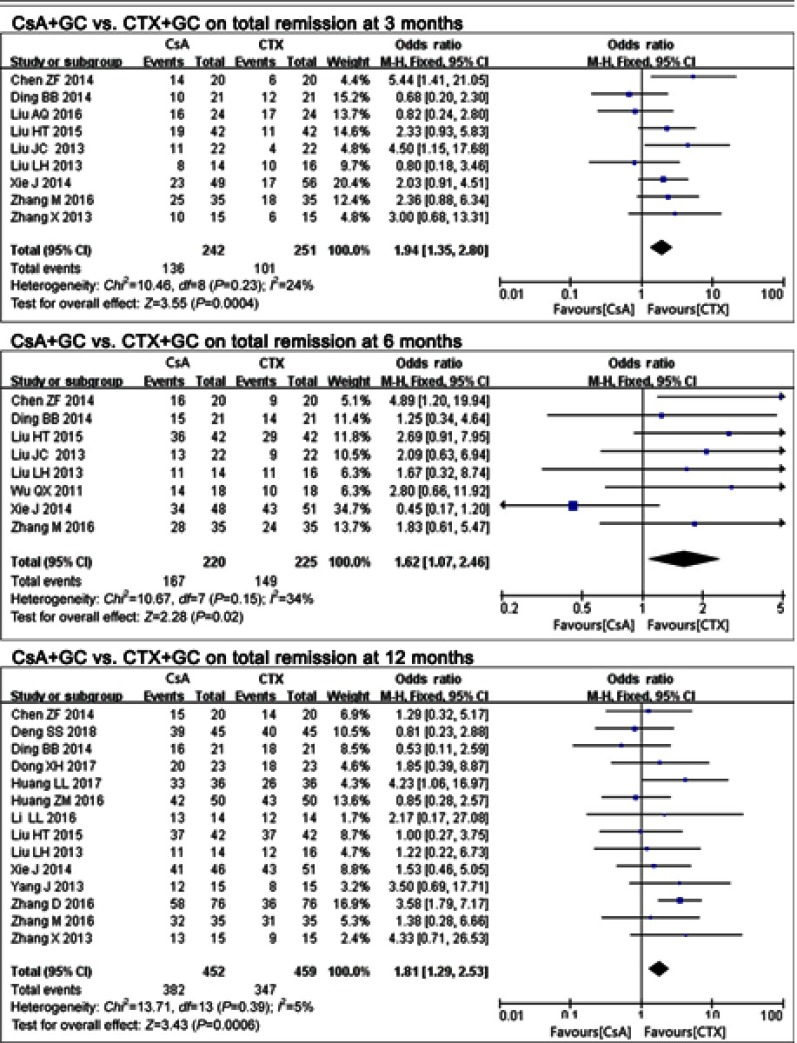
Seventeen studies17–28,30–34 compared CsA+GC vs CTX+GC. TR rate at the 3 time-points suggested diverse outcomes. The CsA group had a higher rate of TR at all 3 time-points, including 3 months17,19,23,25–28,33,34 (P=0.0004, OR=1.94, 95% CI:1.35–2.80; Figure 2 and Table 2), 6 months17,19,23,26–28,30,33 (p=0.02, OR=1.62,95% CI:1.07–2.46; Figure 2 and Table 2) and 12 months17–24,27,28,32–34 (P=0.0006, OR=1.81, 95% CI:1.29–2.53; Figure 2 and Table 2).
Figure 2.
The effect of CsA+GC vs CTX+GC on TR in patients with IMN in Asian populations.
Abbreviations: CsA, cyclosporine A; CTX, cyclophosphamide; GC, glucocorticoid; TR, total remission; IMN, idiopathic membranous nephropathy.
Three studies24,31,35 compared CsA+GC vs MMF+GC. Compared with the MMF group, we found no significant difference in the TR rate of the CsA group (P=0.20, OR=1.82, 95% CI: 0.73–4.49; Table 2).
Three included studies24,36–38 compared the TR rate of TAC+GC with CsA+GC. The CsA group was less effective than the TAC group (P=0.006, OR=0.31, 95%CI:0.13–0.71; Table 2).
Relapse rate
Ten studies17–23,28,30,33 compared relapse rates in CsA+GC vs CTX+GC. A higher relapse rate was observed in the CsA+GC group compared to the CTX+GC group (P<0.0001, OR=3.06, 95% CI: 1.84–5.07; Table 2).
Proteinuria
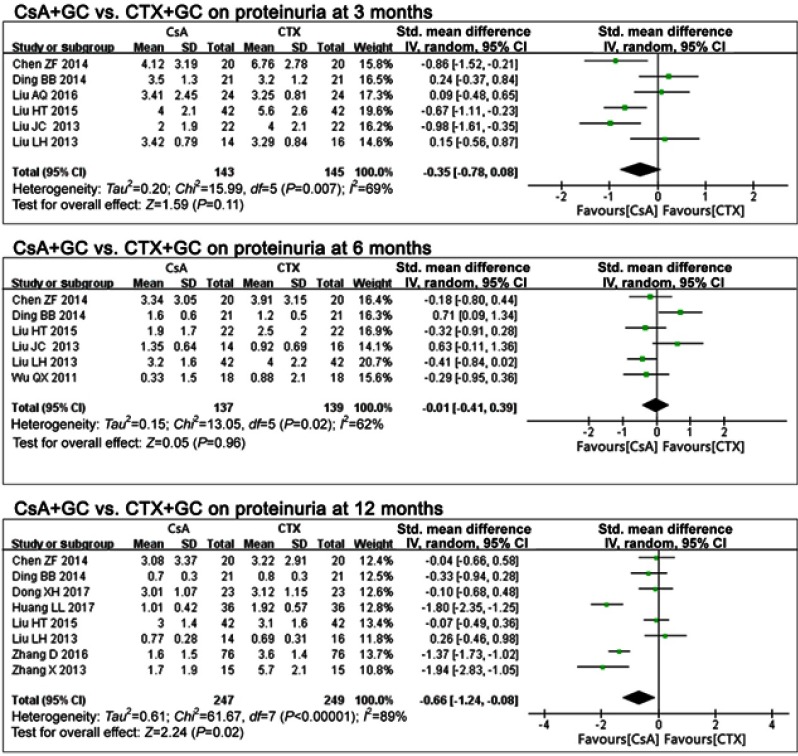
In comparing CsA+GC vs CTX+GC, 11 studies concluded that IMN patients with CsA treatment presented with decreased proteinuria at 12 months17,19–21,27,28,32,34 (P=0.02), with a pooled mean difference of −0.66 (95%CI: −1.24, −0.08; Figure 3 and Table 2), but no significant difference at 3 months17,19,25–28 (P=0.11, OR=−0.35, 95%CI: −0.78,0.08; Figure 3 and Table 2), or 6 months17,19,26–28,30 (P=0.96, OR=−0.01, 95%CI: −0.41 to 0.39; Figure 3 and Table 2).
Figure 3.
The effect of CsA+GC vs CTX+GC on proteinuria in patients with IMN in Asian populations.
Abbreviations: CsA, cyclosporine A; CTX, cyclophosphamide; GC, glucocorticoid; IMN, idiopathic membranous nephropathy.
Two studies36,37 comparing CsA+GC with TAC+GC reported no differences in proteinuria (P=0.06, OR=0.36, 95%CI: −0.01 to 0.73; Table 2).
Serum albumin
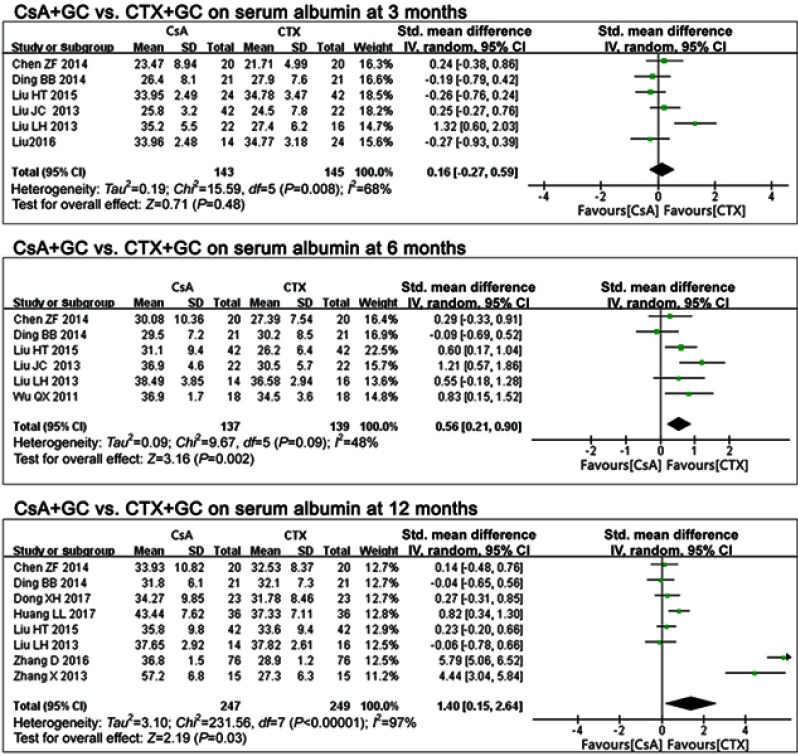
In comparing CsA+GC vs CTX+GC,17,19,25–28 there were no differences in serum albumin at 3 months (P=0.48, OR=0.16, 95%CI:-0.27,0.59; Figure 4 and Table 2).
Figure 4.
The effect of CsA+GC vs CTX+GC on serum albumin in patients with IMN in Asian populations.
Abbreviations: CsA, cyclosporine A; CTX, cyclophosphamide; GC, glucocorticoid; IMN, idiopathic membranous nephropathy.
At 6 months17,19,26–28,30 and 12 months,17,19–21,27,28,32,34 the CsA group exhibited higher serum albumin levels (P=0.002 at 6 months, P=0.03 at 12 months; Figure 4 and Table 2). The pooled mean differences were 0.56 (95%CI: 0.21, 0.90) and 1.40 (95% CI: 0.15, 2.64), respectively.
Two studies36,37 compared CsA+GC vs TAC+GC. The CsA had lower levels of serum albumin (P=0.03, MD=−1.17, 95% CI: −2.23, −0.12; Table 2).
Serum creatinine
In comparing CsA+GC vs CTX+GC, serum creatinine levels at 3 months17,19,25–28 (P=0.56, MD=0.07, 95%CI:-0.16,0.30; Table 2), 6 months17,19,26–28,30 (P=0.34, MD=0.12, 95%CI: −0.12,0.35; Table 2) and 12 months17,19–21,27,28,32,34 (P=0.14, MD=0.13, 95%CI: −0.04 to 0.31; Table 2) indicated that the CsA group had similar efficacy to the CTX group in preserving renal function.
Serum cholesterol
In comparing CsA+GC vs CTX+GC, serum cholesterol of the CsA group at 3 months17,27 (P=0.04, MD=−0.49, 95%CI:-0.96, -0.01; Table 2) and at 6 months17,27,30 (P=0.006, MD=−0.55, 95%CI: −0.94, −0.16; Table 2) was significantly different than the CTX group. This difference was not present at 12 months17,20,21,27,32,34 (P=0.06, MD=−0.55, 95%CI: −1.14 to 0.03; Table 2).
Two studies36,37 assessed the cholesterol levels of CsA+GC vs TAC+GC, which were not different (P=0.07, MD=0.34, 95%CI: −0.03 to 0.72; Table 2).
Serum triacylglycerol
In comparing CsA+GC vs CTX+GC, four studies21,27,32,34 assessed serum triacylglycerol at 12 months. CsA was consistent in its effects (P=0.0006 at 12 months; Table 2). Its pooled mean difference was as follows: −0.96 (95% CI:-1.50, -0.41; Table 2) at 12 months.
The comparison of CsA+GC vs TAC+GC in two studies36,37 indicated that the TAC+GC group had lower serum triacylglycerol levels (P=0.03, MD=1.01, 95%CI:0.11 to 1.92; Table 2).
Other clinical indicators of CsA+GC vs CTX+GC
Comparison of CsA+GC vs CTX+GC, revealed difference in alanine aminotransferase (ALT) at 3 months (P=0.88, MD=0.02, 95%CI: −0.28 to 0.33; Table 2),17,19,28 6 months17,19,28 (P=0.73, MD=−0.05, 95%CI: −0.25 to 0.36; Table 2) or 12 months (P=1.00, MD=−0.00, 95%CI: −0.27 to 0.27; Table 2).17,19,20,28
However, four studies17,20,32,34 indicated that white blood cell levels were lower with CTX treatment (P<0.00001, MD=1.31,95%CI: 1.04 –1.58; Table 2) at 12 months.
Two studies21,30 reported no difference in urea nitrogen (P=0.99, MD=−0.00, 95% CI: −0.38 to 0.38; Table 2).
Adverse events
In comparing CsA+GC vs CTX+GC, the CsA group had more hirsutism17,19,22,27,29,30,34 (P=0.0001, OR=9.28, 95%CI=3.00, 28.69; Table 3), gingival hyperplasia17–19,22,25–27,30,34 (P=0.01, OR=3.96, 95%CI=1.37, 11.43; Table 3), elevated uric acid17,18,20,22,26,27 (P=0.003,OR=5.59,95%CI:1.79,17.41; Table 3), and higher blood pressure17,18,20,22,25–27,29 (P=0.0002, OR=7.10, 95%CI:2.50, 20.14; Table 3).
Table 3.
Meta-analysis of the safety of CsA in induction therapy of patients with IMN (CsA vs CTX)
| Indicators | Studies | Q test | Q test | OR | P |
|---|---|---|---|---|---|
| Number | P-value | P-value | (95%CI) | ||
| Hirsutism | 7 | 0.97 | Fixed | 9.28 (3.00,28.69) | 0.0001 |
| Alopecia | 3 | 0.81 | Fixed | 0.12 (0.02,0.67) | 0.02 |
| Gingival hyperplasia; | 9 | 1.00 | Fixed | 3.96 (1.37,11.43) | 0.01 |
| Hemorrhagic cystitis | 3 | 0.83 | Fixed | 0.13 (0.02,0.74) | 0.02 |
| Liver function lesion | 9 | 0.73 | Fixed | 0.27 (0.12,0.61) | 0.002 |
| Elevated serum creatinine | 4 | 0.97 | Fixed | 4.43 (0.91,21.43) | 0.06 |
| Elevated blood pressure | 8 | 0.88 | Fixed | 7.10 (2.50,20.14) | 0.0002 |
| Elevated uric acid | 6 | 0.62 | Fixed | 5.59 (1.79,17.41) | 0.003 |
| Tremor | 4 | 0.52 | Fixed | 1.97 (0.52,7.41) | 0.32 |
| Gastrointestinal syndrome | 6 | 0.93 | Fixed | 0.05 (0.02,0.17) | <0.00001 |
| Leucopenia | 9 | 0.96 | Fixed | 0.10 (0.04,0.28) | <0.00001 |
Abbreviations: CsA, cyclosporine A; CTX, cyclophosphamide; IMN, idiopathic membranous nephropathy.
Gastrointestinal syndrome19,22,25–27,30 (P<0.00001, OR=0.05, 95%CI=0.02,0.17; Table 3), hemorrhagic cystitis18,22,30 (P=0.02, OR=0.13, 95%CI=0.02,0.74; Table 3), hepatic lesion17,19,20,25–27,29,30,34 (P=0.002, OR=0.27, 95%CI=0.12,0.61; Table 3), leucopenia17-20,22,26,27,30,34 (P<0.00001, OR=0.10, 95%CI=0.04,0.28; Table 3) as well as alopecia17,18,22 (P=0.02, OR=0.12, 95%CI=0.02,0.67; Table 3) were more frequent in the CTX group.
There were no differences in tremor19,22,27,30 (P=0.32, OR=1.97, 95% CI=0.52,7.41; Table 3) or serum creatinine25–27,29 (P=0.06, OR=4.43, 95%CI=0.91,21.43; Table 3).
Discussion
In the current meta-analysis, comparison of the CsA vs the CTX, even though CsA had more rapid effects on CR compared to CTX at 3 months, there were no differences in CR at 6 months or 12 months. While CsA had lower inefficacy rates and a higher TR rate than the CTX group across the total treatment period (3 months, 6 months, and 12 months), it also had a higher relapse rate. There were no differences in CR, NR, or TR rates between CsA and MMF. As for the CsA compared to TAC, TAC had significantly higher CR and TR rates, and decreased NR.
The differences in the clinical index of the CsA group vs the CTX group are as follows: CsA has greater efficacy in lowering proteinuria levels only at 12 months, not at 3 or 6 months, Similar patterns were observed with increased serum protein levels and decreased serum cholesterol levels, which only differed in the later periods (6 and 12 months) but not at 3 months. White blood cells count at 12 months with CTX was significantly lower. CsA tended to be more effective in decreasing serum triacylglycerol. There were no differences in other clinical indicators, such as serum creatinine levels and the ALT, and urea nitrogen.
In comparing CsA+GC vs TAC+GC, TAC improved serum protein levels as well as reduced serum triacylglycerol compared to CsA. There were no differences in proteinuria, serum creatinine, or the serum cholesterol levels.
Adverse event rates also differed between CsA and CTX treated groups. Leucopenia, hemorrhagic cystitis, and alopecia were only observed in the CTX group. Gingival hyperplasia, hirsutism, elevated blood pressure, and elevated blood creatinine were only reported with CsA treatment. All of these adverse events manifested apparent diversity, except the elevated blood creatinine. Rates of other adverse events, including blood creatinine and tremor, were not significantly different. However, gastrointestinal syndrome, liver function lesion, was observed more often in the CTX group. Elevated uric acid levels were more common with CsA. To conclude, patients treated with CTX were more likely to have severe side effects compared to the CsA group.
Overall, CsA had better efficacy than CTX, with milder adverse effects but a higher relapse rate with short-term treatment. There were no significant differences between CsA and MMF in NR, CR, or TR rates. Although the TAC group had higher TR and CR, the NR rate was less than the CsA group.
Previous, meta-analyses have assessed the effects of CsA in treating IMN. Qiu et al,39 calculated the TR and observed that the CsA took effect more quickly than CTX, but this was confined to the first 6 months of treatment in IMN patients (eight studies). However, there were no increased effects of CsA on TR rate (six studies), serum albumin or proteinuria at 12 months. Ren et al,40 also performed a meta-analysis and concluded that CsA (six RCTs) was not superior to CTX in therapeutic effects (including CR and PR rates), when compared with non-immunosuppressive treatment. Xie et al,41 reported that CsA had no effect improvement in CR rate, but better responsiveness by pooling two included studies (116 patients). In the current meta-analysis, we assessed 16 RCTs (1,094 patients) and found that CsA had lower inefficacy rates and higher TR rates when compared with CTX. CsA also had enhanced long-term efficacy in elevating serum protein levels and lowering proteinuria levels (at 12 months), similar to studies of Ren et al.40 Qiu et al,39 observed that CsA patients were more likely to relapse in comparison with CTX or TAC, and our study reported a higher relapse rate with CsA+GC compared to CTX+GC. Qiu et al,39 also indicated a tendency for differences in adverse events (more severe adverse events like leukopenia, alopecia and liver damage with CTX, other events like hirsutism, gingival hyperplasia, worsening hypertension, and hyperuricemia occurred more often with CsA).
Compared with the above meta-analyses, our meta-analysis included a greater number of RCTs to assess the safety and efficacy of CsA, which enhanced the strength of our conclusions. This meta-analysis was the first to compare TAC with CsA in patients with IMN. We carefully assessed longitudinal effects, and provided greater details on adverse events.
However, there was some limitations in this meta-analysis. The quality of some of the included studies was low, and some sample sizes were small. The sample sizes of some specific comparisons for assessing adverse events were small, making it difficult to detect differences.
CsA has recently attracted the attention of a wide range of researchers’, and results of clinical trials of CsA suggest that it could replace CTX as the primary medication for treating IMN. Our study summarized the existing randomized clinical trial data and calculated whether CsA performed better than CTX, TAC, and MMF in IMN in Asian populations. In conclusion, CsA group has the potential to replace CTX, but nephrotoxicity should be carefully considered. Additional studies of the efficacy and safety of other medications such as MMF, TAC, and AZA are needed.
Acknowledgments
This study was supported by Guangzhou Medical Key Discipline Construction Project (2017-2019), the Natural Science Foundation of the Guangdong Province (no. 2015A030310386), and Guangdong Medical Science and Technology Research Fund Project (no. A2018336). Shujun Lin and Hong-Yan Li are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zeng C, Chen H, Wang R, et al. Etiology and clinical characteristics of membranous nephropathy in Chinese patients. Am J Kidney Dis. 2008;52(4):691–698. doi: 10.1053/j.ajkd.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 2.Alfaadhel T, Cattran D. Management of membranous nephropathy in Western countries. Kidney Dis (Basel). 2015;1(2):126–137. doi: 10.1159/000437287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polanco N, Gutierrez E, Covarsi A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21(4):697–704. doi: 10.1681/ASN.2009080861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quaglia M, Stratta P. Idiopathic membranous nephropathy: management strategies. Drugs. 2009;69(10):1303–1317. doi: 10.2165/00003495-200969100-00002 [DOI] [PubMed] [Google Scholar]
- 5.Daniel C. Management of membranous nephropathy: when and what for treatment. J Am Soc Nephrol. 2005;16(5):1188–1194. doi: 10.1681/ASN.2005010028 [DOI] [PubMed] [Google Scholar]
- 6.Glassock RJ. The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am J Kidney Dis. 2004;44(3):562–566. [PubMed] [Google Scholar]
- 7.Jha V, Ganguli A, Saha TK, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18(6):1899–1904. doi: 10.1681/ASN.2007020166 [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Remuzzi G. Idiopathic membranous nephropathy: back to the future? Lancet. 2013;381(9868):706–708. doi: 10.1016/S0140-6736(12)62033-9 [DOI] [PubMed] [Google Scholar]
- 9.Waldman M, Austin HR. Treatment of idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(10):1617–1630. doi: 10.1681/ASN.2012010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu L, Liu L, Yao L, et al. Efficacy and safety of tacrolimus versus cyclophosphamide for primary membranous nephropathy: a meta-analysis. Drugs. 2017;77(2):187–199. doi: 10.1007/s40265-016-0683-z [DOI] [PubMed] [Google Scholar]
- 11.van Den Brand JAJG, Ruggenenti P, Chianca A, et al. Safety of rituximab compared with steroids and cyclophosphamide for idiopathic membranous nephropathy. J Am Soc Nephrol. 2017;28(9):2729. doi: 10.1681/ASN.2016080886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goumenos DS. What have we learned from the use of ciclosporin A in the treatment of nephrotic patients with idiopathic membranous nephropathy? Expert Opin Pharmacother. 2008;9(10):1695–1704. doi: 10.1517/14656566.9.10.1695 [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan J, Halevy D. Cyclosporin treatment of glomerular diseases. Expert Opin Investig Drugs. 2000;9(5):1053–1063. doi: 10.1517/13543784.9.5.1053 [DOI] [PubMed] [Google Scholar]
- 14.Kshirsagar AV, Nachman PH, Falk RJ. Alternative therapies and future intervention for treatment of membranous nephropathy. Semin Nephrol. 2003;23(4):362–372. [DOI] [PubMed] [Google Scholar]
- 15.Ponticelli C. Cyclosporine: from renal transplantation to autoimmune diseases. Ann N Y Acad Sci. 2005;1051:551–558. [DOI] [PubMed] [Google Scholar]
- 16.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481–508. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZF, Zhang JW, Xia NN, et al. Comparison on the therapeutic effect of cyclosporine A and cyclophosphamide in the treatment of idiopathic membranous nephropathy. China Med Pharm. 2014;4(16):16–18. [Google Scholar]
- 18.Deng SS, Zhu MZ, Bo M. Clinical observation of cyclosporine A versus cyclophosphamide in the treatment of idiopathic membranous nephropathy. Chin J Mod Drug Appl. 2018;12(14):125–127. [Google Scholar]
- 19.Ding BB. Comparison of the effects of two therapeutic regimen in the treatment of idiopathic membranous nephropathy. Chin J Prim Med Pharm. 2014;21(21):3238–3241. [Google Scholar]
- 20.Dong XH, Miu JL, Chen HL. The comparison of the clinical effect of cyclosporine A versus cyclophosphamide in the treatment of idiopathic membranous nephropathy. PJCCPVD. 2017;25(S1):80–81. [Google Scholar]
- 21.Huang LL. The observation of long-term efficacy and safety of cyclosporine A combined with glucocorticoid in the treatment of idiopathic membranous nephropathy. Hebei Med. 2017;23(07):1202–1206. [Google Scholar]
- 22.Huang ZM, Chen KJ. The observation of the efficacy, relapse rate and the side effect of cyclosporine A versus cyclophosphamide in the treatment of idiopathic membranous nephropathy. J North Pharm. 2016;13(9):66. [Google Scholar]
- 23.Xie J, Guo W, Zhang QD, et al. Clinical effect of cyclophosphamide or tacrolimus combined with glucocorticoids in the treatment of idiopathic membranous nephropathy. Chin J Integr Tradit West Nephrol. 2014;15(08):716–718. [Google Scholar]
- 24.Li LL. Clinical comparison of the hormone combined with different immunosuppressive agents in the treatment of idiopathic membranous nephropathy. Guide China Med. 2016;14(16):180–181. [Google Scholar]
- 25.Liu AQ, Liu GP, Yu L, et al. Clinical observation of cyclosporine A in the treatment of idiopathic membranous nephropathy. Inner Mongolia Med J. 2016;48(12):1505–1506. [Google Scholar]
- 26.Liu JC, An ZM, Zhang LC, et al. Efficacy of cyclosporine A combined with glucocorticoid in treatment of membranous nephropathy. J Qiqihar Univ Med. 2013;34(13):1910–1912. [Google Scholar]
- 27.Liu LH, Ma SY, Liu ZY, et al. The observation of efficacy of cyclosporine A in the treatment of idiopathic membranous nephropathy. Anhui Med J. 2013;34(8):1217–1219. [Google Scholar]
- 28.Liu HT. The observation of efficacy of cyclosporine A combined with glucocorticoid in the treatment of idiopathic membranous nephropathy. Chin J Prim Med Pharm. 2015;22(16):2467–2470. [Google Scholar]
- 29.Sa RL. The analysis of safety and efficacy of cyclosporine A in the treatment of idiopathic membranous nephropathy. China Health Care Nutr. 2018;28(1):325–326. [Google Scholar]
- 30.Wu QX, Gong ZF. Clinical observation of 20 cases of membranous nephropathy treated with low and medium dose cyclosporine. China Pharm. 2011;14(1):115–117. [Google Scholar]
- 31.Yang J, Liu Y, Zhang Y, et al. Glucocorticoid and immunosuppressive therapy with high risk factors for idiopathic membranous nephropathy in 46 cases. Prog Mod Biomed. 2013;13(14):2710–2712. [Google Scholar]
- 32.Zhang D. The observation of clinical efficacy of cyclosporine A versus cyclophosphamide in treatment of membranous nephropathy. Health Med Res Prac. 2016;13(04):50–51. [Google Scholar]
- 33.Zhang M. Clinical observation of cyclophosphamide combined with glucocorticoid on membranous nephropathy. Chin J Biochem Pharm. 2016;36(1):121–123. [Google Scholar]
- 34.Zhang X. Low-dose of cyclosporine and low-dose glucocorticoid in the treatment of idiopathic membranous nephropathy. Guide China Med. 2013;11(30):121–122. [Google Scholar]
- 35.Choi J, Kim DK, Kim Y, et al. The effect of mycophenolate mofetil versus cyclosporine as combination therapy with low dose corticosteroids in high-risk patients with idiopathic membranous nephropathy: a multicenter randomized trial. J Korean Med Sci. 2018;33(9):e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y. The observation of efficacy of the tacrolimus in the treatment of idiopathic membranous nephropathy. J North Pharm. 2016;13(9):61. [Google Scholar]
- 37.Hu QF The Clinical Observation of the cyclosporine A Versus Tacrolimus Combined with Half-Dose of Glucocorticoid in the Treatment of Idiopathic Membranous Nephropathy [doctoral dissertation]. Xinxiang: Xinxiang Medical University; 2016. [Google Scholar]
- 38.Li QH The Clinical Observation of the cyclosporine A Versus Tacrolimus Combined with Half-Dose of Glucocorticoid in the Treatment of Idiopathic Membranous Nephropathy [doctoral dissertation]. Zhengzhou: Zhengzhou University; 2017. [Google Scholar]
- 39.Qiu TT, Zhang C, Zhao HW, et al. Calcineurin inhibitors versus cyclophosphamide for idiopathic membranous nephropathy: a systematic review and meta-analysis of 21 clinical trials. Autoimmun Rev. 2017;16(2):136–145. [DOI] [PubMed] [Google Scholar]
- 40.Ren S, Wang Y, Xian L, et al. Comparative effectiveness and tolerance of immunosuppressive treatments for idiopathic membranous nephropathy: a network meta-analysis. PLoS One. 2017;12(9):e184398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie G, Xu J, Ye C, et al. Immunosuppressive treatment for nephrotic idiopathic membranous nephropathy: a meta-analysis based on Chinese adults. PLoS One. 2012;7(9):e44330. [DOI] [PMC free article] [PubMed] [Google Scholar]