Abstract
Background:
Improved lung function and fewer pulmonary exacerbations (PEx) were observed with lumacaftor/ivacaftor (LUM/IVA) in patients with cystic fibrosis homozygous for F508del. It is unknown whether PEx reduction extends to patients without early lung function improvement.
Methods:
Post hoc analyses of pooled phase 3 data (NCT01807923, NCT01807949) categorized LUM/IVA-treated patients by percent predicted forced expiratory volume in 1 second (ppFEV1) change from baseline to day 15 into threshold categories (absolute change ≤0 vs >0; relative change <5% vs ≥5%) and compared PEx rates vs placebo.
Results:
LUM (400 mg q12h)/IVA (250 mg q12h)–treated patients (n=369) experienced significantly fewer PEx vs placebo, regardless of threshold category. With LUM/IVA, PEx rate per patient per year was 0.60 for those with absolute change in ppFEV1 >0 and 0.85 for those with absolute change ≤0 (respective rate ratios vs placebo [95% CI]: 0.53 [0.40–0.69; P<0.0001], 0.74 [0.55–0.99; P=0.0441]).
Conclusions:
LUM/IVA significantly reduced PEx, even in patients without early lung function improvement.
Keywords: cystic fibrosis, pulmonary exacerbations, percent predicted forced expiratory volume in 1 second, lumacaftor, ivacaftor
1. INTRODUCTION
In patients with cystic fibrosis (CF), pulmonary exacerbations (PEx) are characterized by acute worsening of pulmonary symptoms, decreased pulmonary function, fatigue, and weight loss [1,2]. Pulmonary exacerbations are associated with a progressive loss of lung function [3–7], a negative impact on quality of life [8, 9], and an increased risk of future PEx [10,11]. Importantly, PEx are a significant, independent predictor of mortality in patients with CF [12–14].
Management of PEx often involves intravenous (IV) antibiotic therapy and hospitalization [15]. According to the US CF Patient Registry 2015 Annual Data Report, the median (range) total duration of IV antibiotic therapy for PEx was 14.0 (5.0–27.0) days for those aged 18 years and older, with a median (range) of 8.3 (4.0–14.3) days spent in the hospital [15]. Reducing PEx is an important therapeutic goal in patients with CF and may help to decrease morbidity and mortality [2,7,10,11]. However, even with extensive IV antibiotic therapy and lengthy hospitalizations, PEx in patients with CF are associated with a progressive decrease in lung function, which is often irreversible [4,5,7].
Recent therapeutic approaches have targeted the underlying mechanism of CF [16,17]. Cystic fibrosis is caused by mutations in the CF transmembrane conductance regulator (CFTR) ion channel that disrupt the balance of ions, resulting in altered viscosity of luminal secretions in a variety of organs [18]. The most common CF-causing mutation in CFTR, F508del, results in both a primary folding defect and a secondary chloride-conductance defect [17]. In two 24-week, phase 3 studies (TRAFFIC and TRANSPORT) with identical study designs, treatment with a combination of lumacaftor (LUM), a folding corrector, and ivacaftor (IVA), a potentiator, significantly improved percent predicted forced expiratory volume in 1 second (ppFEV1; primary endpoint) from 2.6 to 4.0 percentage points compared with placebo in patients aged ≥12 years with CF who were homozygous for the F508del mutation (P<0.001) [17].
The rate of PEx after 24 weeks of LUM/IVA therapy in all patients with CF who were homozygous for the F508del mutation was 30% to 39% lower compared with placebo (P≤0.001) [17]. However, it is unknown if the beneficial effects on PEx outcomes extend to the subset of patients with little or no improvement in ppFEV1 during the first 2 weeks of treatment or is different by subgroups related to age, sex, and other baseline characteristics. The goal of this analysis was to determine whether there was an improvement in PEx (rate, severity, and number of days) in LUM/IVA-treated patients with little or no early improvement in ppFEV1 when compared with placebo-treated patients, using a post hoc analysis of pooled data from the TRAFFIC and TRANSPORT studies, or whether the impact of LUM/IVA on PEx differed by baseline subgroups.
2. METHODS AND MATERIALS
The full methodology and results of TRAFFIC and TRANSPORT have been previously reported [17]. Briefly, TRAFFIC and TRANSPORT were phase 3, multinational, randomized, double-blind, placebo-controlled, parallel-group studies with identical study designs conducted between April 2013 and April 2014 (Clinicaltrials.gov identifiers: NCT01807923 and NCT01807949). The studies were conducted in accordance with International Conference on Harmonization Good Clinical Practice guidelines and with local applicable laws and regulations. Each patient and/or their caregiver provided written informed consent before study participation. Eligible patients had a confirmed diagnosis of CF, were homozygous for the F508del mutation, were ≥12 years of age, and had an FEV1 of predicted normal values of 40% to 90% (inclusive) at screening. Patients were randomized 1:1:1 to receive treatment with LUM 600 mg once daily with IVA 250 mg once every 12 hours (LUM 600 mg qd/IVA 250 mg q12h), LUM 400 mg q12h/IVA 250 mg q12h, or matching placebo for 24 weeks.
The primary endpoint in TRAFFIC and TRANSPORT was the absolute change from baseline in ppFEV1 through 24 weeks of LUM/IVA treatment, which was calculated by averaging the mean absolute change at weeks 16 and 24 [17]. Key secondary endpoints in TRAFFIC and TRANSPORT included the percentage of patients with a relative increase from baseline in ppFEV1 ≥5% and the number of PEx through week 24 (expressed as a rate over 48 weeks; inclusive of events that happened on treatment and after treatment discontinuation). The total number of days receiving IV antibiotics and the total number of days hospitalized for PEx events were recorded through week 24. Although there is no universally accepted standard for the diagnosis or treatment of PEx [19–21], a standardized definition was used in the clinical studies. A PEx event was defined as new or changed antibiotic therapy for any 4 or more of the following sinopulmonary signs or symptoms: change in sputum; new or increased hemoptysis; increased cough; increased dyspnea; malaise, fatigue, or lethargy; temperature >38°C; anorexia or weight loss; sinus pain or tenderness; change in sinus discharge; change in physical examination of the chest; decrease in pulmonary function by 10%; or radiographic changes indicative of a pulmonary infection [16,22].
2.1. Outcomes analyses
Prespecified pooled subgroup analyses, defined according to TRAFFIC and TRANSPORT [17] baseline characteristics including age, ppFEV1, select CF-related medications, and select infections, were performed for the key secondary endpoint of number of PEx through week 24 for LUM/IVA-treated patients vs those receiving placebo.
Post hoc analyses assessed the rates of PEx stratified by early changes in ppFEV1 for patients treated with LUM/IVA vs placebo; patients in the active treatment groups were categorized by their change in ppFEV1 from baseline to day 15 (ie, into threshold categories). Threshold categories were specified for change in ppFEV1 from baseline to day 15: absolute change in ppFEV1 ≤0 vs >0 percentage points and relative change in ppFEV1 <5% vs ≥5%. Pulmonary exacerbation event rates and total number of days with a PEx event per patient per 48 weeks were calculated for each threshold category and for placebo; 48 weeks was considered equivalent to 1 year for the analysis. Analyses were conducted for all PEx events as well as those requiring treatment with IV antibiotics and hospitalization. Rate ratios for event rates were calculated for each threshold category vs placebo, and number of days with PEx was compared between the threshold groups treated with LUM/IVA and those treated with placebo.
Because of the interdependence of ppFEV1 and PEx in CF and methodologic limitations whereby the outcome of interest (in this case, PEx) may occur before the stratification variable (in this case, change from baseline in ppFEV1), the earliest postbaseline ppFEV1 measure (day 15) was used in these analyses of PEx outcomes to minimize confounding. Exploratory assessments categorizing patients based on the change from baseline in ppFEV1 at different time points (week 4 and the average of weeks 16 and 24) were also performed.
2.2. Statistical analyses
All patients who were randomized and received at least 1 dose of study drug were included in the analysis. Prespecified pooled subgroup analyses of PEx rates by baseline characteristics were performed using a negative binomial regression model that included study, treatment, sex, age, and ppFEV1 severity at screening (<70 vs ≥70; omitting the corresponding variables of sex, age, and ppFEV1 severity from the model when performing those particular subgroup analyses), with the log of time spent in the study as an offset.
For the analyses by threshold group, event rates and rate ratios were calculated using negative binomial regression models that included study, treatment category (placebo, LUM 400 mg q12h/IVA 250 mg q12h, or LUM 600 mg qd/IVA 250 mg q12h + threshold category [absolute change in ppFEV1 ≤0 vs >0 or relative change in ppFEV1 <5% vs ≥5%]), sex, age (<18 vs ≥18 years), and ppFEV1 severity at screening (<70 vs ≥70), with the log of time spent in the study treated as an offset. A negative binomial regression model was also used to predict the number of PEx events based on absolute change from baseline in ppFEV1 at day 15 as a continuous variable; the model included study, sex, age (<18 vs ≥18 years), ppFEV1 severity at screening (<70 vs ≥70), and absolute change from baseline in ppFEV1 at day 15, with the log of time spent in the study treated as an offset.
A comparison of the annualized proportion of days with PEx events for each threshold category vs placebo was assessed using a Wilcoxon rank sum test stratified by study, sex, age, and ppFEV1 at screening. The total number of days was normalized for time spent in the study by multiplying the observed proportion of days with event by the total number of expected study days (eg, 168 days for 24 weeks). Data reported here are for the commercially available dose of LUM/IVA (LUM 400 mg q12h/IVA 250 mg q12h). Data for the LUM 600 mg qd/IVA 250 mg q12h dose are reported in the online data supplement.
3. RESULTS
In TRAFFIC and TRANSPORT [17], 1446 patients were screened, 1122 of whom were randomized. In total, 1108 patients received at least 1 dose of study drug and were included in this analysis. When measured at day 15, 146 of the 369 patients who received LUM 400 mg q12h/IVA 250 mg q12h had an absolute change in ppFEV1 ≤0 and 223 had an absolute change >0; 228 patients had a relative change in ppFEV1 <5% and 141 had a relative change ≥5%. Baseline characteristics remained well balanced among treatment groups across threshold ppFEV1 categories (Table 1).
Table 1.
Key Baseline Characteristics for Patients Receiving LUM 400 mg q12h/IVA 250 mg q12h or Placebo by Change in ppFEV1 Threshold Category
| LUM 400 mg q12h/IVA 250 mg q12h |
|||||
|---|---|---|---|---|---|
| Placebo (n=371) |
Absolute change ≤0 (n=146) |
Absolute change >0 (n=223) |
Relative change <5% (n=228) |
Relative change ≥5% (n=141) |
|
| Female sex, n (%) | 181 (48.8) | 73 (50.0) | 109 (48.9) | 110 (48.2) | 72 (51.1) |
| Age group, n (%), y | |||||
| 12 to <18 | 96 (25.9) | 34 (23.3) | 64 (28.7) | 59 (25.9) | 39 (27.7) |
| ≥18 | 275 (74.1) | 112 (76.7) | 159 (71.3) | 169 (74.1) | 102 (72.3) |
| Baseline ppFEV1 subgroup, n (%)* | |||||
| <40 | 28 (7.5) | 8 (5.5) | 21 (9.4) | 13 (5.7) | 16 (11.3) |
| ≥40 | 338 (91.1) | 134 (91.8) | 202 (90.6) | 211 (92.5) | 125 (88.7) |
| Inhaled antibiotic use before first dose, n (%)† | 258 (69.5) | 90 (61.6) | 135 (60.5) | 137 (60.1) | 88 (62.4) |
| Inhaled hypertonic saline before first dose, n (%)† | 220 (59.3) | 89 (61.0) | 138 (61.9) | 140 (61.4) | 87 (61.7) |
| Use of dornase alfa before first dose, n (%)† | 281 (75.7) | 107 (73.3) | 166 (74.4) | 165 (72.4) | 108 (76.6) |
| P aeruginosa positive, n (%) | 276 (74.4) | 120 (82.2) | 166 (74.4) | 179 (78.5) | 107 (75.9) |
IVA, ivacaftor; LUM, lumacaftor; P aeruginosa, Pseudomonas aeruginosa; ppFEV1, percent predicted forced expiratory volume in 1 second; q12h, every 12 hours.
For patients in whom a ppFEV1 measure was available before the first dose of study drug.
Includes medication received before the first dose of study drug; patients may or may not have continued to receive the medication at the time the first dose was administered.
As previously reported, treatment with LUM/IVA reduced the rate of PEx vs placebo; the PEx rate ratio was 0.61 for patients who received LUM 400 mg q12h/IVA 250 mg q12h (P<0.001) [17]. Subgroup analyses demonstrated that the reduced PEx rate favored LUM/IVA therapy over placebo irrespective of patient baseline characteristics including ppFEV1, age, sex, medication use, and Pseudomonas aeruginosa status (Figure 1).
Figure 1.
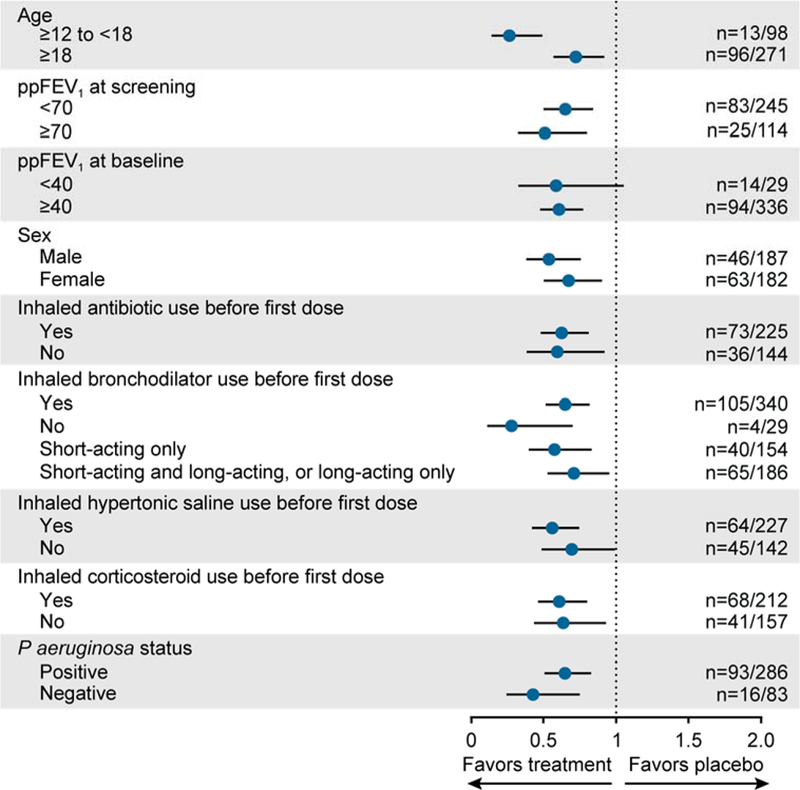
Subgroup analysis of PEx rate ratio for LUM/IVA vs placebo at week 24. Data shown are rate ratio vs placebo from the pooled TRAFFIC and TRANSPORT studies for patients treated with LUM 400 mg q12h/IVA 250 mg q12h. Error bars represent 95% CIs. IVA, ivacaftor; LUM, lumacaftor; P aeruginosa, Pseudomonas aeruginosa; PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 second.
The relationship between early changes in ppFEV1 and PEx event rate was further evaluated by treatment and threshold category. Overall, patients treated with LUM 400 mg q12h/IVA 250 mg q12h experienced fewer PEx events than patients receiving placebo, regardless of the absolute change in ppFEV1 at day 15 (Figure 2). Although PEx event rates were numerically higher in patients with an absolute change ≤0 than in those with an absolute change >0, no statistically significant differences were observed between the threshold categories (P=0.0556), and LUM/IVA-treated patients in both categories had significantly fewer PEx events compared with those receiving placebo. The rate of PEx leading to IV antibiotics was similar between LUM/IVA-treated patients with early absolute change ≤0 and >0 (0.29 and 0.23 events per year, respectively) and significantly lower in both categories than in patients who received placebo (0.58 events per year). The rate of PEx requiring hospitalization was also similar between LUM/IVA-treated patients with early absolute change ≤0 and >0 (0.18 and 0.17 events per year, respectively) and significantly lower than in placebo-treated patients (0.45 events per year; Figure 2A). Similar findings were observed in patients treated with LUM/IVA compared with placebo, regardless of the early relative change in ppFEV1 from baseline to day 15 of <5% or ≥5% (Figure 2B). Results of the negative binomial regression model confirmed that the absolute change from baseline in ppFEV1 to day 15 was not a predictor of PEx risk (coefficient [95% CI]: 0.00 [−0.03, 0.02]).
Figure 2.

PEx rates and rate ratios by treatment with LUM 400 mg q12h/IVA 250 mg q12h or placebo and early change in ppFEV1 threshold category. Event rates are described per year by treatment group and ppFEV1 threshold category of the relative change from baseline to day 15 in ppFEV1 of (A) ≤0 vs >0 and (B) <5% vs ≥5%. Forty-eight weeks was considered equivalent to 1 year for the analysis. Tables show rate ratios (95% CI) for the treatment group vs placebo by ppFEV1 threshold category. Abs Δ, absolute change; IV, intravenous; IVA, ivacaftor; LUM, lumacaftor; PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 second; q12h, every 12 hours; Rel Δ, relative change.
Results of exploratory analyses, in which the change in ppFEV1 measured at week 4 and at the average of weeks 16 and 24 was used to categorize treated patients, were generally similar to results reported using the change in ppFEV1 measured at day 15 (Figure S1).
The relationship between early changes in ppFEV1 by treatment and number of days with PEx (normalized for time spent in the study by multiplying the observed percent days with event by the total study days expected [eg, 168 days for 24 weeks]) was also examined. The mean number of days with PEx, days receiving IV antibiotics due to PEx, and days hospitalized for PEx was lower in patients treated with LUM 400 mg q12h/IVA 250 mg q12h than placebo, regardless of the absolute change (≤0 or >0) or relative change in ppFEV1 (<5% or ≥5%) to day 15 (P≤0.0005 vs placebo; Table 2). The mean number of days with PEx for patients with an absolute change ≤0 was approximately twice that in those with an absolute change >0; however, patients in both threshold categories had substantially fewer PEx days compared with those treated with placebo.
Table 2.
Mean Number of Days of PEx by Treatment With LUM 400 mg q12h/IVA 250 mg q12h or Placebo and Change in ppFEV1 Threshold Category*
| LUM 400 mg q12h/IVA 250 mg q12h |
|||||
|---|---|---|---|---|---|
| Placebo (n=371) |
Absolute change ≤0 (n=146) |
Absolute change >0 (n=223) |
Relative change <5% (n=228) |
Relative change ≥5% (n=141) |
|
| Mean days with PEx (SD) | 15.7 (24.8) | 11.5 (23.1) | 5.9 (11.9) | 8.9 (19.8) | 6.9 (12.6) |
| P value vs placebo | — | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Mean days on IV antibiotics for PEx (SD) | 10.1 (20.5) | 5.4 (15.8) | 2.8 (8.2) | 3.8 (13.1) | 3.7 (9.5) |
| P value vs placebo | — | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Mean days hospitalized for PEx (SD) | 7.6 (18.8) | 3.6 (13.7) | 1.8 (6.3) | 2.6 (11.3) | 2.4 (7.2) |
| P value vs placebo | — | <0.0001 | <0.0001 | <0.0001 | 0.0005 |
IV, intravenous; IVA, ivacaftor; LUM, lumacaftor; PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 second; q12h, every 12 hours; SD, standard deviation.
Change in ppFEV1 from baseline to day 15.
Similar findings to those reported with the commercially available dose of LUM/IVA were also observed in patients treated with LUM 600 mg qd/IVA 250 mg q12h. These results are available in the online data supplement (Tables S1 and S2; Figure S2).
4. DISCUSSION
Given the complexity of pathophysiologic changes associated with pulmonary disease progression in individual patients with CF, it is important to consider the totality of clinical outcome measures (eg, lung function, PEx rate, quality of life) when assessing therapeutic benefit in clinical practice. In this subgroup analysis, LUM/IVA-treated patients who did not experience an early increase in lung function, as measured by ppFEV1, had a higher PEx rate and mean number of days with PEx than those with an increase in lung function; however, the treatment benefit in both categories was significant relative to placebo. It is not possible to know for certain whether patients who did not have an early increase in ppFEV1 were at higher risk of PEx than those with an early increase in ppFEV1 due to unidentified factors not measured in the clinical study. Regardless, treated patients included in all categories had fewer PEx than those in the placebo group, and this trend did not exist when looking at the subset of PEx events requiring IV antibiotics or hospitalization. This suggests that the clinical benefits of LUM/IVA therapy extend to patients who did not experience an early increase in lung function. The treatment benefit was also seen in the subset of PEx requiring IV antibiotics and/or hospitalization, where mean number of days with PEx was similar in patients with and without an increase in ppFEV1. These results may assist clinicians in evaluating whether LUM/IVA therapy is beneficial to individual patients.
Potential methodologic limitations exist whereby the outcome variable we are evaluating (PEx) may occur before the stratification variable (ppFEV1), which may lead to overestimation or underestimation of treatment effects. In patients with CF, the interdependence of PEx and ppFEV1 has been established, such that higher lung function is associated with a lower PEx risk and that PEx impacts the rate of lung function decline [6,23,24]. Therefore, we conducted post hoc analyses of PEx outcomes by threshold category using the earliest postbaseline ppFEV1 measure obtained in the studies (ie, day 15) to limit the number of PEx events that occur before stratification by ppFEV1 and minimize any bias introduced by stratifying in this way. Additional exploratory analyses were performed using threshold categories for change in ppFEV1 measured at week 4 and at the average of weeks 16 and 24. Despite the methodologic shortcomings of these analyses, results were generally similar to the day 15 analyses reported. In addition, a time-varying Cox proportional hazard model of time to first PEx event, with the time-varying covariate being the ppFEV1 measurements over time, was explored, and results were consistent with those reported in the results.
To test whether stratification by >0 and ≤0 was a robust way to assess treated patients, an analysis was conducted in which the quartiles of ppFEV1 changes were used to categorize treated patients into 4 categories. These results were consistent with the results reported above. Pulmonary exacerbation rates for patients receiving placebo varied little across the same quartiles. This justifies the comparison of treated patients’ categories with the pooled placebo patients’ results.
Prespecified subgroup analyses revealed that reductions in the PEx rate were greater in patients treated with LUM/IVA vs placebo irrespective of patient baseline characteristics. These data indicate that treatment benefits are observed in patients regardless of baseline age, ppFEV1 level, medication use, and P aeruginosa status.
CFTR modulators have been shown to impact a multitude of clinical outcomes, including ppFEV1 and PEx [16,17,25]. Results from this post hoc analysis demonstrate the importance of considering the totality of outcomes when evaluating the benefit of CFTR modulators in individual patients. This is especially true considering PEx are clinically meaningful events associated with a progressive loss of lung function, increased risk of future PEx, reduced quality of life, and increased risk of death.
5. CONCLUSIONS
In summary, phase 3 studies of LUM/IVA in patients aged ≥12 years with CF who are homozygous for the F508del mutation demonstrated increases in ppFEV1 and reductions in PEx, including PEx requiring IV antibiotics and/or hospitalization; this post hoc analysis showed that PEx were reduced even among patients who did not experience early increases in ppFEV1 in these phase 3 studies. Furthermore, these reductions were observed irrespective of patient baseline characteristics. While measurements of ppFEV1 are critical to assess lung function, these findings also underscore that CFTR modulators confer additional important benefits to treated patients.
Supplementary Material
ACKNOWLEDGMENTS
Editorial assistance was provided under the direction of the authors by Scott Houck, PhD, Heidi Schreiber, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, with support from Vertex Pharmaceuticals Incorporated.
FUNDING
This work was supported by Vertex Pharmaceuticals Incorporated.
ABBREVIATIONS
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- IV
intravenous
- LUM/IVA
lumacaftor/ivacaftor
- PEx
pulmonary exacerbations
- ppFEV1
percent predicted forced expiratory volume in 1 second
- q12h
every 12 hours
Footnotes
Declaration of interest: SAM received personal fees from Novartis, AbbVie, and Vertex Pharmaceuticals Incorporated outside of the submitted work. MWK received consulting fees from Vertex Pharmaceuticals Incorporated, AbbVie, Albumedix, Anthera Pharmaceuticals, Celtaxsys, Chiesi, Corbus, Genentech, Laurent Pharmaceuticals, Merck, Novartis, Paranta Biosciences, Protalix Biotherapeutics, PTC Therapeutics, and Savara outside of the submitted work. BWR received consulting fees from Aridis Pharmaceuticals, Celtaxsys, KaloBios, Flatley Discovery Lab LLC, Vertex Pharmaceuticals Incorporated, Laurent Pharmaceuticals, Nilvalis Therapeutics, and Synedgen outside of the submitted work. JSE received speaker fees from Vertex Pharmaceuticals Incorporated, grants from Novartis, and grants and consulting fees from ProQR during the conduct of the study. MPB received grants from Vertex Pharmaceuticals Incorporated during the conduct of the study, personal fees and nonfinancial support from Genentech, personal fees and nonfinancial support from Gilead, personal fees and nonfinancial support from Savara, and personal fees and nonfinancial support from Vertex Pharmaceuticals Incorporated outside of the submitted work. CEW received grant income on a per patient basis for conducting studies, personal fees, and travel support from Vertex Pharmaceuticals Incorporated during the conduct of the study. She also received personal fees and travel support from Novartis Pharmaceuticals, a research grant from Novo Nordisk, and grant income on a per patient basis for conducting studies from Vertex Pharmaceuticals Incorporated, Boehringer-Ingelheim, and Ablynx NV outside of the submitted work. DW, GM, MVL, JGJ, and JLR are employees of Vertex Pharmaceuticals Incorporated and may own stock or stock options in that company.
REFERENCES
- [1].Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr 2006;148:259–64. [DOI] [PubMed] [Google Scholar]
- [2].Justicia JL, Solé A, Quintana-Gallego E, Gartner S, de Gracia J, Prados C, et al. Management of pulmonary exacerbations in cystic fibrosis: still an unmet medical need in clinical practice. Expert Rev Respir Med 2015;9:183–94. [DOI] [PubMed] [Google Scholar]
- [3].Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, et al. , for the Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr 2007;151:134–9. [DOI] [PubMed] [Google Scholar]
- [4].Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sanders DB, Hoffman LR, Emerson J, Gibson RL, Rosenfeld M, Redding GJ, et al. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol 2010;45:127–34. [DOI] [PubMed] [Google Scholar]
- [6].Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J 2012;40:61–6. [DOI] [PubMed] [Google Scholar]
- [7].Collaco JM, Green DM, Cutting GR, Naughton KM, Mogayzel PJ Jr. Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. Am J Respir Crit Care Med 2010;182:1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bradley JM, Blume SW, Balp MM, Honeybourne D, Elborn JS. Quality of life and healthcare utilisation in cystic fibrosis: a multicentre study. Eur Respir J 2013;41:571–7. [DOI] [PubMed] [Google Scholar]
- [9].Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002;121:64–72. [DOI] [PubMed] [Google Scholar]
- [10].VanDevanter DR, Morris NJ, Konstan MW. IV-treated pulmonary exacerbations in the prior year: an important independent risk factor for future pulmonary exacerbation in cystic fibrosis. J Cyst Fibros 2016;15:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].VanDevanter DR, Pasta DJ, Konstan MW. Treatment and demographic factors affecting time to next pulmonary exacerbation in cystic fibrosis. J Cyst Fibros 2015;14:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buzzetti R, Alicandro G, Minicucci L, Notarnicola S, Furnari ML, Giordano G, et al. Validation of a predictive survival model in Italian patients with cystic fibrosis. J Cyst Fibros 2012;11:24–9. [DOI] [PubMed] [Google Scholar]
- [13].Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001;153:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, et al. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J 2015;45:670–9. [DOI] [PubMed] [Google Scholar]
- [15].Cystic Fibrosis Foundation. Cystic Fibrosis Foundation patient registry 2015 annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2016. [Google Scholar]
- [16].Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. , for the VX08–770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. New Engl J Med 2011;365:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. , for the TRAFFIC and TRANSPORT Study Groups. Lumacaftor–Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. New Engl J Med 2015;373:220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kreda SM, Davis CW, Rose MC. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb Perspect Med 2012;2:a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kraynack NC, Gothard MD, Falletta LM, McBride JT. Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. Pediatr Pulmonol 2011;46:870–81. [DOI] [PubMed] [Google Scholar]
- [20].Stenbit AE, Flume PA. Pulmonary exacerbations in cystic fibrosis. Curr Opin Pulm Med 2011;17:442–7. [DOI] [PubMed] [Google Scholar]
- [21].VanDevanter DR, Konstan MW. Outcome measures for clinical trials assessing treatment of cystic fibrosis lung disease. Clin Investig (Lond). 2012;2:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. New Engl J Med 1994;331:637–42. [DOI] [PubMed] [Google Scholar]
- [23].de Boer K, Vandemheen KL, Tullis E, Doucette S, Fergusson D, Freitag A, et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011;66:680–5. [DOI] [PubMed] [Google Scholar]
- [24].Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 2007;62:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 2017;5:107–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


