Abstract
Diet is an important regulator of the gastrointestinal microbiota. Vitamin A and vitamin D deficiencies result in less diverse, dysbiotic microbial communities and increased susceptibility to infection or injury of the gastrointestinal tract. The vitamin A and vitamin D receptors are nuclear receptors expressed by the host, but not the microbiota. Vitamin A and vitamin D mediated regulation of the intestinal epithelium and mucosal immune cells underlies the effects of these nutrients on the microbiota. Vitamin A and vitamin D regulate the expression of tight junction proteins on intestinal epithelial cells that are critical for barrier function in the gut. Other shared functions of vitamin A and vitamin D include the support of innate lymphoid cells that produce IL-22, suppression of IFN-γ and IL-17 by T cells, and induction of regulatory T cells in the mucosal tissues. There are some unique functions of vitamin A and D; for example, vitamin A induces gut homing receptors on T cells, while vitamin D suppresses gut homing receptors on T cells. Together, vitamin A and vitamin D mediated regulation of the intestinal epithelium and mucosal immune system shape the microbial communities in the gut to maintain homeostasis.
Keywords: vitamin A, vitamin D, microbiota, nutrition, gastrointestinal tract, mucosal immune system
A population of nearly 100 trillion dynamic and diverse microbiota—between 500 and 1000 different species—inhabit the human gut (Backhed et al. 2005). The metagenome—the combined genomic content of the intestinal flora—can rapidly vary as a function of diet, tissue location, host genetics and a variety of other factors. Studies using gnotobiotic and germfree (GF) mice have shown that the gut microbiota are essential for normal immune system development, displacement of pathogens, and extraction of additional energy from otherwise non-digestible dietary substrates (Guarner 2006; Tan et al. 2014). Highlighting the importance of the gut microbiota, numerous human diseases have been attributed to significant alterations in the gut microbiota (Guarner 2006). Chronic diseases including inflammatory bowel disease (IBD), diabetes, cardiovascular disease, cancer and neuroinflammatory disorders are associated with dysbiosis and reduced diversity of microbes as compared to healthy individuals (Frank et al. 2007; Shreiner et al. 2015; Jackson et al. 2018).
Gut microbial ecology is regulated by the diet (Turnbaugh et al. 2009; Ooi et al. 2014; Carmody et al. 2015). For example, Bacteroidetes levels increased with weight loss, either by fat- or carbohydrate-restricted low-calorie diets (Ley et al. 2006). Not only do macronutrients in the diet affect the microbiota, but deficiencies in micronutrients (vitamins, selenium, iron) alter the microbial communities in the gut (Ooi et al. 2013; Lv CH et al. 2015; Li et al. 2017; Buret et al. 2019). In addition, dietary fiber is indigestible by the mammalian host, but is readily digested by the gut microbiota (De Filippo et al. 2010; Wu et al. 2011; De Vadder et al. 2016). Diet induced changes in the microbiota result in metabolic changes for the host. Thus, dietary interventions are a potential tool to modulate gut microbiota and have applications in the maintenance of gastrointestinal homeostasis and prevention of chronic diseases.
Diet mediated changes to the microbiota occur rapidly and affect immune mediated disease in mice (Ooi et al. 2014). Extreme changes to the diet (low fat (LF)/plant-polysaccharide diet versus high fat (HF)/high sugar diet) had significant effects on the microbiota within 3 days (Carmody et al. 2015). In addition, there are crucial developmental windows when the microbiota determines future disease susceptibility (obesity and immune mediated disease) (Rook et al. 2017). Changing lifestyles and diets have led to increased incidences of obesity, and immune mediated disease (Rook et al. 2017). Fecal transplants and probiotics are being used as approaches to alter the microbiota and reduce disease burden (Rook et al. 2017). However, any approach that ignores the diet is likely to result in only transient changes to the microbiota. There is an opportunity to identify different nutrients and foods that can be used to shape and maintain the microbiota. Here we review the mechanisms whereby vitamin A and vitamin D maintain gastrointestinal (GI) homeostasis and shape the microbiota.
Mechanisms of GI homeostasis
Intestinal epithelial cells (IEC) make up the lining of the intestine, and the intestinal epithelium functions to absorb nutrients and water and acts as a physical barrier between the host and the intestinal microbiota (Goto and Ivanov 2013). IEC express tight junction molecules that regulate permeability of the epithelium and maintain intestinal integrity (Deplancke and Gaskins 2001; Peterson and Artis 2014; Ahmad et al. 2017). In addition, the IEC of the GI tract produces mucous and anti-bacterial peptides that help protect the host from infection (Wittkopf et al. 2014). If intestinal barrier function becomes compromised, leakage of food antigens and/or bacteria can elicit systemic inflammation. It has been demonstrated that compromised intestinal barrier function contributes to the risk of developing autoimmune diseases (Fasano and Shea-Donohue 2005). Antigens that escape from the gut exacerbate intestinal inflammation and activate immune cells triggering immune mediated disease (Fasano and Shea-Donohue 2005). Leaky GI tracts have been associated with diseases of the intestine including increased susceptibility to GI infection and IBD (Fasano and Shea-Donohue 2005). The IEC create a barrier along the GI tract of the host that protects against infection and maintains intestinal homeostasis.
The gut microbiota is established in the neonate and is critical for the development of secondary lymphoid organs and establishment of tolerogenic responses (Tanaka and Nakayama 2017). Pattern recognition receptors including the toll-like receptors are primarily responsible for mediating early tolerance to the commensals and identifying pathogens (Belkaid and Hand 2014). Alterations to the microbial community can limit the extent to which tolerance develops and instead can result in immune mediated diseases including IBD (Sommer et al. 2017). The innate lymphoid cells (ILC) interact with the microbiota and are early sources of regulatory cytokines including IL-22 from ILC3 cells. IL-22 is a protective cytokine, known for its role in the induction of antimicrobial peptides and maintenance of barrier integrity (Tait Wojno and Artis 2016; Geremia and Arancibia-Cárcamo 2017). T cells and B cells also require the microbiota for development and function. In germfree (GF) mice the CD4+ T cells produce some IL-4 but very little IFN-γ or IL-17 (Niess et al. 2008). The microbiota is required to regulate the IgE and IgA B cell response as well. GF mice have high IgE levels and very little IgA compared to conventional mice (Cahenzli et al. 2013). In addition, there is a population of microbiota specific FoxP3/Rorγt+ expressing T regulatory (reg) cells in the colon lamina propria (LP) that require the microbiota (Ohnmacht et al. 2015). A polysaccharide from Bacteriodes fragilis has been described that induces Th1 cells and inhibits the higher Th2 response that exists in GF mice (Mazmanian et al. 2005). The presence of segmented filamentous bacteria in a mouse colony is sufficient for the development of Th17 cells (Gaboriau-Routhiau et al. 2009; Ivanov et al. 2009). Bacteroides fragilis and Clostridium species in the microbiota have been found to suppress experimental colitis via the induction of T reg cells (Round and Mazmanian 2010; Atarashi et al. 2011). Commensal microbes and microbial products are important for the development of the mucosal immune system.
The mucosal immune system of the GI tract must protect the host from infection while remaining tolerant to commensal microbes and food antigens. The intestine contains many specialized and unique immune cells that maintain homeostasis. The intraepithelial lymphocytes (IEL) in the intestine are primarily CD8+, γδ and αβ T cells (80% T cells) (Cheroutre 2005). There are very few B cells and myeloid cells within the IEL (Cheroutre 2005). Another unique feature of the immune cells in the IEL is the co-expression of CD8αα (Cheroutre and Lambolez 2008). CD8αα expressing T cells have regulatory functions in the GI tract that are critical for the maintenance of tolerance (Cheroutre 2005). The LP of the intestine has T cells, B cells and myeloid cells that play a role in host resistance to infection and tolerance (Eberl 2005; Park and Eberl 2018). Of note in the LP are dendritic cells, αβ CD4+ and CD8+ T cells and innate lymphoid cells (ILC) (Eberl 2005; Park and Eberl 2018). The mucosal immune system and the gut IEC contain specialized cells that are critical for the ability of the host to fight infection while remaining tolerant to commensals in the GI tract.
Vitamin A and vitamin D
Vitamin A and vitamin D are essential fat-soluble micronutrients that play key roles in multiple physiological functions including regulation of GI homeostasis. The vitamin A metabolite retinol can be released from tissue stores into the plasma where it can be absorbed by cells and made into the bioactive metabolite, retinoic acid (RA). Some vitamin D can be manufactured via a photolysis reaction in the skin; however, UV produced vitamin D is extremely variable and vitamin D from sunlight exposure is significantly reduced in northern climates, and especially low during the winter (Clemens et al. 1982; DeLuca 1993). Vitamin D is metabolized to produce 25hydroxyvitamin D in the liver and then metabolized in the kidney to produce the high affinity vitamin D ligand, 1,25dihydroxyvitamin D (1,25D). Vitamin A deficiency continues to be a global health problem in developing countries where foods that contain vitamin A are limited (WHO 2009). Children with vitamin A deficiency have high rates of respiratory and diarrheal infections that can be decreased with vitamin A supplementation (Villamor and Fawzi 2000). Dietary intake of vitamin D can also be problematic, since there are few foods that are naturally rich in vitamin D. Vitamin D deficiency has been linked to higher prevalence of immune mediated diseases including inflammatory bowel disease (IBD) (Cantorna and Mahon 2004). Deficiencies of vitamin A or vitamin D result in increased incidences of disease in the GI tract.
Changes in vitamin A or D status affect the gut microbiota
The major microbial taxa of the human and rodent are Firmicutes and Bacteroidetes and healthy humans have diverse microbial communities in the GI tract (Hillman et al. 2017). Decreased complexity in the microbial communities have been shown in patients with IBD (Frank et al. 2007; Hillman et al. 2017). The reduced frequencies of commensals from the Firmicutes and Bacteroidetes phyla and increased representation of Proteobacteria and Actinobacteria phyla are characteristic of the microbiota in patients with IBD (Frank et al. 2007). Bacteria that are members of the Proteobacteria phyla can cause infection and are commonly found associated with disease in the GI tract (Rizzatti et al. 2017). A complex and diverse community of microbes maintain homeostasis while limiting disease causing bacteria.
Not surprisingly, changes in vitamin A status affect the community of bacteria found in the GI tract of mice (Cha et al. 2010; Tian et al. 2018) and humans (Lv Z et al. 2016). The microbiota in the cecum of vitamin A deficient (A-) mice had significantly lower numbers of Bacteroidetes phyla members than vitamin A sufficient (A+) mice (Tian et al. 2018). Vitamin A sufficient children had microbial communities that were more diverse than vitamin A deficient children (Lv Z et al. 2016). Acetate, propionate and butyrate are the end products of fermentation of dietary fiber by the intestinal microbiota. A+ mice had significantly higher butyrate levels and lower acetate levels in the cecum than A− mice (Tian et al. 2018). The increase in A+ butyrate levels corresponded to higher numbers of the butyrate-producing bacteria Clostridium ramosum (a member of Clostridium_XVIII) in A+ than A− mice (Tian et al. 2018). In addition, bacterial genes associated with butyrate production (but and buk) were higher in the A+ versus A− cecal samples (Tian et al. 2018). Transient vitamin A deprivation of A+ mice for 4 weeks resulted in alterations in the microbial communities in the gut (Hibberd et al. 2017). Bacteriodes vulgaris responded to the transient vitamin A deficiency by increasing its abundance compared to A+ controls (Hibberd et al. 2017). Changes in vitamin A status, even transient changes, result in dysbiosis of the microbial communities in the gut.
Like vitamin A, vitamin D regulates the microbial communities in the GI tract. Mice that were unable to produce 1,25D (Cyp27B1 KO) and VDR KO mice had greater expansion of Proteobacteria and lower abundance of Lachnospiraceae belonging to Firmicutes phylum compared to D+ WT controls (Ooi et al. 2013). Similar to the changes in the microbiota of D- mice; the microbiota of IBD patients had higher Proteobacteria and lower abundance of Lachnospiraceae belonging to Firmicutes phylum as compared to healthy controls (Frank et al. 2007). Colitis severity in D- mice was associated with two-fold higher Helicobacter family members (Proteobacteria phylum) in their feces than D+ mice (Ooi et al. 2013). Helicobacter species have been known to trigger GI intestinal inflammation. Thus, vitamin D affects the communities of microbiota found in the GI tract.
Vitamin A and vitamin D regulation of GI immunity
Within the gastrointestinal tract epithelial cells and immune cells express the vitamin A receptor (retinoic acid receptor, RAR) and the vitamin D receptor (VDR). Both RAR and VDR form heterodimers with the retinoid X receptors (RXR) and all three receptors are part of the steroid/hormone superfamily of nuclear transcription regulators. RA and 1,25D are the high affinity ligands for RAR and VDR respectively. Prokaryotes do not express vitamin A or vitamin D receptors. Therefore, the effects of vitamin A and vitamin D on the microbiota are likely due to indirect effects of the micronutrients on the host that then regulate the microbiota.
There are several studies demonstrating that vitamin A and vitamin D regulate tight junction molecule expression and intestinal barrier function (Kubota et al. 2001; Kong Juan et al. 2008; Lima et al. 2010). Treating IEC cell lines with either RA or 1,25D induced the expression of ZO-1, Occludin, Claudins (RA: Claudin 6 and 7, 1,25D: Claudin 2 and 12) and increased transepithelial resistance in vitro (Kubota et al. 2001; Osanai et al. 2007; Fujita et al. 2008; Kong J. et al. 2008). Supplementing A- children with vitamin A decreased urine lactulose/mannitol levels, suggesting an increase in intestinal integrity (Thurnham et al. 2000; Lima et al. 2010). Similarly vitamin D supplementation improved barrier function and induced the expression of the antibacterial peptide cathelicidin in patients with IBD (Raftery et al. 2015). VDR knockout (KO) mice and mice with the inability to produce 1,25D had impaired intestinal permeability in a mouse model of colitis (Froicu et al. 2006; Ooi et al. 2013). RA and 1,25D have several overlapping functions that regulate expression of ZO-1, Occludin and Claudin tight junction proteins (Table 1). Together the effects of vitamin A and vitamin D on IECs plays a critical role to preserve intestinal barrier functions.
Table 1:
The overlapping and synergistic effects of vitamin A and D on mucosal immunity.
| Parameter | A+/D+ | A− | D− | References |
|---|---|---|---|---|
| Tight junction proteins | ||||
| Occludin | + | − | − | (Kubota et al. 2001; Osanai et al. 2007; Fujita et al. 2008; Kong et al. 2008) |
| Claudin | + | − | − | |
| ZO-1 | + | − | − | |
| Cell type | ||||
| ILC3 | + | − | − | (van de Pavert et al. 2014; Lin et al. 2019) |
| T cells | + | − | + | (McDaniel et al. 2015; Lin et al. 2019) |
| CD8ɑɑ Treg | + | − | − | (Bruce and Cantorna 2011; Larange and Cheroutre 2016) |
| FoxP3+ Treg | + | − | − | (Elias et al. 2008; Kang et al. 2012; Parastouei et al. 2018) |
| Homing receptors | ||||
| α4β7 | + | − | + | (Iwata et al. 2004; Sigmundsdottir et al. 2007) |
| CCR9 | + | − | + | |
| Cytokines | ||||
| IL-17 | + | ++ | ++ | (Lemire 1992; Cantorna et al. 1994; Mundy et al. 2005; Froicu et al. 2006; Elias et al. 2008; Bai et al. 2009; Kang et al. 2012; Mielke et al. 2013; Parastouei et al. 2018; Snyder et al. 2018; Lin et al. 2019) |
| IFN-γ | + | ++ | ++ | |
| IL-22 | + | − | − | |
| IL-10 | + | − | − | |
Vitamin A and vitamin D regulate innate and adaptive immunity (Veldhoen and Ferreira 2015). Vitamin A and vitamin D are required for the normal development and function of ILC3 cells in the GI tract (van de Pavert et al. 2014; Lin et al. 2019). In addition, A- mice have significantly fewer lymphocytes (T and B cells) in the mucosa of the intestine (McDaniel et al. 2015). D- mice do not have changes to the numbers of T and B cells in the uninflamed intestine (Lin et al. 2019). RA increased the expression of the gut homing receptors, α4β7 and CCR9 expression on T cells, which allowed the recruitment of T cells to the gut mucosa (Table 1, (Iwata et al. 2004)). Conversely 1,25D decreased α4β7 and CCR9 expression and blocked T cell homing to the gut (Table 1, (Sigmundsdottir et al. 2007)). RA and 1,25D have opposing function on expression of α4β7 and CCR9 on T cells that likely explain the reduced frequencies of T cells in the gastrointestinal tract of A- but not D- mice (McDaniel et al. 2015; Lin et al. 2019). The source of IL-17 in the GI tract of A+ mice is T cells but in A- mice, with fewer T cells in the intestine, IL-17 is produced by CD11b+ innate cells (Snyder et al. 2018). RA and 1,25D inhibited IFN-γ production from T cells in vitro (Lemire 1992; Cantorna et al. 1994). In addition, A- and D- mice overproduced IFN-γ and IL-17 (Froicu et al. 2006; Snyder et al. 2018; Lin et al. 2019). RA and 1,25D inhibited Th17 cells in vitro and in vivo (Table 1, (Elias et al. 2008; Bai et al. 2009; Ikeda et al. 2010; Qiu et al. 2017; Parastouei et al. 2018)). In vitro, RA and 1,25D induced FoxP3 and IL-10 production (Table 1, (Elias et al. 2008; Kang et al. 2012; Parastouei et al. 2018)). Consistent with the inhibition of IL-17 and IFN-γ, RA and 1,25D treatment of mice reduced colonic inflammation caused by dextran sodium sulfate, C. rodentium infection, and other models of experimental IBD (Bai et al. 2009; Bruce et al. 2011; Cantorna 2012; Ryz et al. 2012; Mielke et al. 2013; Spencer et al. 2014). In addition, RA and 1,25D induced IL-22 production in the gut and the RA treatments resulted in increased production of the anti-bacterial peptides Reg3β and Reg3γ (Table 1, (Bai et al. 2009; Mielke et al. 2013)). VDR KO mice had fewer CD8αα expressing regulatory T cells in the gut and RA expanded T effector memory cells that express CD8αα (Bruce and Cantorna 2011; Larange and Cheroutre 2016). The common mechanisms by which vitamin A and D regulate GI immunity include inhibition of IL-17 and IFN-γ, induction of ILC3 and IL-22, the induction of intestinal CD8αα T cells and the induction of IL-10 and FoxP3+ T reg cells (Table 1, Fig. 1).
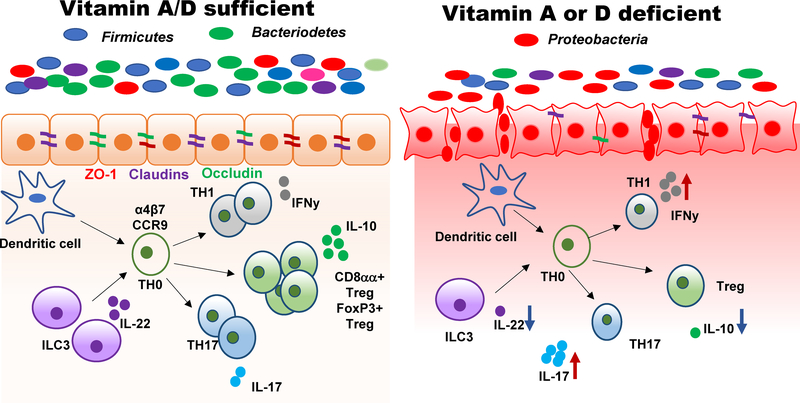
Figure 1. The mechanisms underlying the role of vitamin A and vitamin D in the regulation of GI homeostasis and the microbiota.
Deficiency in either vitamin A or vitamin D results in dysbiosis of the microbiota and increased susceptibility to injury in the GI tract. The effects of vitamin A and vitamin D on the microbiota are as a result of the regulation of gut epithelial and immune cells. Vitamin A or D deficient animals have less diverse microbial communities and increased presence of potentially pathogenic Proteobacteria phylum members. Both vitamin A and vitamin D are important to induce ZO-1, Occludin and Claudin tight junction proteins important for the integrity of the barrier. Deficiency in either vitamin A or vitamin D results in leaky guts. In addition to gut epithelial cells, the mucosal immune system is a target of vitamin A and vitamin D. The development of ILC3 cells that produce IL-22, CD8αα and T reg cells that produce IL-10 also requires vitamin A and vitamin D. Furthermore, vitamin A and vitamin D inhibit the functions of Th1 and Th17 cells in the gut. T cell homing is regulated by vitamin A but not vitamin D. Deficiency in either vitamin A or vitamin D results in impaired barrier function, increased IL-17 and IFN-γ, reduced Treg, ILC3, IL-10 and IL-22 and dysbiosis of the microbiota. The shared effects of vitamin A and vitamin D on the host epithelial and immune cells indirectly affects the community of microbes found in the gut.
Experimental evidence supports beneficial roles for vitamin A and vitamin D in the host response to GI induced injury and infection (Carman et al. 1992; Hall et al. 2011; Restori et al. 2014) (Cantorna and Mahon 2004). RA and 1,25D suppressed IL-17 and IFN-γ which was associated with the resolution of inflammation in the GI tract (Cantorna et al. 2000; Snyder et al. 2018; Lin et al. 2019). Vitamin D deficiency and VDR deficiency have been shown to exacerbate experimental IBD in the IL-10 KO mouse, the T cell transfer model and dextran sodium sulfate induced colitis (Cantorna et al. 2000; Froicu et al. 2003; Froicu and Cantorna 2007). Because of the inhibitory effects of RA and 1,25D on Th1 and Th17 cells we predicted that bacterial infections that require Th1/Th17 cell responses for resistance might be more severe in A- and D- mice. Paradoxically, we found that A- mice and D- mice were unable (A-) or slower (D-) to clear a GI tract infection with enteropathogenic Escherichia coli-like infection (Citrobacter rodentium) (McDaniel et al. 2015; Snyder et al. 2018; Lin et al. 2019). Severe deficiencies of vitamin A or D resulted in the early mortality of mice following C. rodentium infection (Cantorna 2012; McDaniel et al. 2015; Lin et al. 2019). Vitamin A and vitamin D have overlapping roles in the development and function of ILC3 cells that produce IL-22 (Table 1, (van de Pavert et al. 2014; Lin et al. 2019)). IL-22 and ILC3 cells are critical for host resistance to C. rodentium (Mundy et al. 2005). In addition, a failure of infection induced Th17 cells in A- and D- mice results in slower kinetics of clearance in D- mice and a failure to clear in A- mice (McDaniel et al. 2015; Snyder et al. 2018; Lin et al. 2019). Vitamin A and vitamin D have overlapping and essential functions for the protection from GI infection as well as the resolution of inflammation in the GI tract (Table 1 and Fig. 1).
Mechanisms by which vitamin A and D regulate the microbiota
Vitamin A and vitamin D both regulate the microbiota. Deficiency in either vitamin results in less diversity in the microbial community, with reductions in commensal organisms important for the induction of T reg cells and more potentially disease, causing microbiota from the Proteobacteria phylum (Fig. 1). An intact intestinal barrier requires both vitamin A and vitamin D that act in synergy to regulate ZO-1, Occludin and Claudin tight junction proteins (Fig. 1). In addition, ILC3, CD8αα T cells and Treg cells depend on adequate vitamin A and vitamin D (Fig. 1). IL-22 produced by ILC3 or T cells is a protective factor in the GI tract that regulates antibacterial peptides in the small intestine, induces Th17 expansion in the colon and protects the host from C. rodentium infection (Zheng et al. 2008; Sonnenberg et al. 2012). The effect of vitamin A and D on the microbiota is due to the indirect effects of these nutrients to regulate the mucosal barrier and immune cells (Fig. 1). Alterations in either nutrient results in the reduced capacity to respond to chemical or infectious injury of the GI tract effectively (Fig. 1). The result of the inadequate response to injury results in dysbiosis and chronic inflammation (Fig. 1). The immune regulatory functions of vitamin A and vitamin D are the means by which these nutrients shape the microbial communities in the gut.
Conclusions
Vitamin A and vitamin D status are important for GI homeostasis. Deficiencies in either nutrient result in microbial dysbiosis, exacerbated colitis and increased susceptibility to infection of the GI tract. The shared ability of vitamin A and vitamin D to regulate the barrier, ILC3 and T cells underlies the impact of these nutrients on the microbiota. Transient vitamin A deficiency resulted in alterations to the microbial community. Whether there is a similar effect of transient vitamin D deficiency on the microbiota is uncertain. Regardless, the data suggest that improving nutrient status could be a low-cost method to reinstate homeostasis of the GI tract and to maintain the microbial community structure. Future strategies to replace the microbiota using fecal transplants or probiotics should consider incorporating nutrients like vitamin A and vitamin D that would help maintain the diverse community structure associated with health.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (R01AT005378), United States Department of Agriculture (2914–38420-21822) and United States Department of Agriculture National Institute of Food and Agriculture/Hatch Appropriations (Project: #PEN04605, Accession #1018545).
Footnotes
No conflicts of interest to declare.
LITERATURE CITED
- Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. 2017. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 10(2):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y et al. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species [Research Support, Non-U.S. Gov’t]. Science. 331(6015):337–341. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. 2005. Host-bacterial mutualism in the human intestine. Science. 307(5717):1915–1920. eng. [DOI] [PubMed] [Google Scholar]
- Bai A, Lu N, Guo Y, Liu Z, Chen J, Peng Z. 2009. All-trans retinoic acid down-regulates inflammatory responses by shifting the Treg/Th17 profile in human ulcerative and murine colitis [Research Support, Non-U.S. Gov’t]. Journal of leukocyte biology. 86(4):959–969. eng. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand Timothy W. 2014. Role of the Microbiota in Immunity and Inflammation. Cell. 157(1):121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce D, Cantorna MT. 2011. Intrinsic requirement for the vitamin D receptor in the development of CD8alphaalpha-expressing T cells. J Immunol. 186(5):2819–2825. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce D, Yu S, Ooi JH, Cantorna MT. 2011. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling [Research Support, N.I.H., Extramural]. Int Immunol. 23(8):519–528. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buret AG, Motta JP, Allain T, Ferraz J, Wallace JL. 2019. Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: a role for iron? J Biomed Sci. 26(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahenzli J, Koller Y, Wyss M, Geuking MB, McCoy KD. 2013. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels [Research Support, Non-U.S. Gov’t]. Cell Host Microbe. 14(5):559–570. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT. 2012. Vitamin D, multiple sclerosis and inflammatory bowel disease [Review]. Arch Biochem Biophys. 523(1):103–106. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD. 2004. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 229(11):1136–1142. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Munsick C, Bemiss C, Mahon BD. 2000. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease [Research Support, Non-U.S. Gov’t]. J Nutr. 130(11):2648–2652. eng. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Nashold FE, Hayes CE. 1994. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function [Research Support, U.S. Gov’t, P.H.S.]. J Immunol. 152(4):1515–1522. eng. [PubMed] [Google Scholar]
- Carman JA, Pond L, Nashold F, Wassom DL, Hayes CE. 1992. Immunity to Trichinella spiralis infection in vitamin A-deficient mice. J Exp Med. 175(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody RN, Gerber GK, Luevano JM Jr., Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. 2015. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 17(1):72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha HR, Chang SY, Chang JH, Kim JO, Yang JY, Kim CH, Kweon MN. 2010. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 184(12):6799–6806. [DOI] [PubMed] [Google Scholar]
- Cheroutre H 2005. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 206:114–131. [DOI] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F. 2008. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity. 28(2):149–159. [DOI] [PubMed] [Google Scholar]
- Clemens TL, Adams JS, Nolan JM, Holick MF. 1982. Measurement of circulating vitamin D in man. Clin Chim Acta. 121(3):301–308. [DOI] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proceedings of the National Academy of Sciences of the United States of America. 107(33):14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Backhed F, Mithieux G. 2016. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metabolism. 24(1):151–157. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. 1993. Vitamin D. Nutrition Today. 28:6–11. [Google Scholar]
- Deplancke B, Gaskins HR. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 73(6):1131S–1141S. eng. [DOI] [PubMed] [Google Scholar]
- Eberl G 2005. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 5(5):413–420. [DOI] [PubMed] [Google Scholar]
- Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. 2008. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 111(3):1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Shea-Donohue T. 2005. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2(9):416–422. [DOI] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases [Research Support, N.I.H., Extramural]. Proc Natl Acad Sci U S A. 104(34):13780–13785. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Cantorna MT. 2007. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 8:5 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. 2003. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 17(12):2386–2392. [DOI] [PubMed] [Google Scholar]
- Froicu M, Zhu Y, Cantorna MT. 2006. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 117(3):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Sugimoto K, Inatomi S, Maeda T, Osanai M, Uchiyama Y, Yamamoto Y, Wada T, Kojima T, Yokozaki H et al. 2008. Tight junction proteins claudin-2 and −12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol Biol Cell. 19(5):1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G et al. 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 31(4):677–689. [DOI] [PubMed] [Google Scholar]
- Geremia A, Arancibia-Cárcamo CV. 2017. Innate Lymphoid Cells in Intestinal Inflammation. Frontiers in immunology. 8:1296–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Ivanov II. 2013. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol Cell Biol. 91(3):204–214. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F 2006. Enteric flora in health and disease. Digestion. 73 Suppl 1:5–12. eng. [DOI] [PubMed] [Google Scholar]
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M et al. 2011. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 34(3):435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd MC, Wu M, Rodionov DA, Li X, Cheng J, Griffin NW, Barratt MJ, Giannone RJ, Hettich RL, Osterman AL et al. 2017. The effects of micronutrient deficiencies on bacterial species from the human gut microbiota. Science translational medicine. 9(390). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman ET, Lu H, Yao T, Nakatsu CH. 2017. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 32(4):300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, Nishimura T. 2010. 1alpha,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett. 134(1):7–16. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria [Research Support, N.I.H., Extramural [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Support, Non-U.S. Gov’t.
- Research Support, U.S. Gov’t, Non-P.H.S.]. Cell. 139(3):485–498. eng.19836068 [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 21(4):527–538. [DOI] [PubMed] [Google Scholar]
- Jackson MA, Verdi S, Maxan M-E, Shin CM, Zierer J, Bowyer RCE, Martin T, Williams FMK, Menni C, Bell JT et al. 2018. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nature Communications. 9(1):2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SW, Kim SH, Lee N, Lee WW, Hwang KA, Shin MS, Lee SH, Kim WU, Kang I. 2012. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J Immunol. 188(11):5276–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. 2008. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 294(1):G208–216. [DOI] [PubMed] [Google Scholar]
- Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. 2008. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. American Journal of Physiology-Gastrointestinal and Liver Physiology. 294(1):G208–G216. [DOI] [PubMed] [Google Scholar]
- Kubota H, Chiba H, Takakuwa Y, Osanai M, Tobioka H, Kohama G, Mori M, Sawada N. 2001. Retinoid X receptor alpha and retinoic acid receptor gamma mediate expression of genes encoding tight-junction proteins and barrier function in F9 cells during visceral endodermal differentiation. Exp Cell Res. 263(1):163–172. [DOI] [PubMed] [Google Scholar]
- Larange A, Cheroutre H. 2016. Retinoic Acid and Retinoic Acid Receptors as Pleiotropic Modulators of the Immune System. Annu Rev Immunol. 34:369–394. [DOI] [PubMed] [Google Scholar]
- Lemire JM. 1992. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 49(1):26–31. [DOI] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology - Human gut microbes associated with obesity. Nature. 444(7122):1022–1023. [DOI] [PubMed] [Google Scholar]
- Li L, Krause L, Somerset S. 2017. Associations between micronutrient intakes and gut microbiota in a group of adults with cystic fibrosis. Clin Nutr. 36(4):1097–1104. [DOI] [PubMed] [Google Scholar]
- Lima AA, Soares AM, Lima NL, Mota RM, Maciel BL, Kvalsund MP, Barrett LJ, Fitzgerald RP, Blaner WS, Guerrant RL. 2010. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr. 50(3):309–315. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YD, Arora J, Diehl K, Bora SA, Cantorna MT. 2019. Vitamin D Is Required for ILC3 Derived IL-22 and Protection From Citrobacter rodentium Infection. Frontiers in immunology. 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv CH, Wang T, Regmi N, Chen X, Huang K, Liao SF. 2015. Effects of dietary supplementation of selenium-enriched probiotics on production performance and intestinal microbiota of weanling piglets raised under high ambient temperature. J Anim Physiol Anim Nutr (Berl). 99(6):1161–1171. [DOI] [PubMed] [Google Scholar]
- Lv Z, Wang Y, Yang T, Zhan X, Li Z, Hu H, Li T, Chen J. 2016. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr. 59(2):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system [Comparative Study [DOI] [PubMed] [Google Scholar]
- Research Support, N.I.H., Extramural.
- Research Support, Non-U.S. Gov’t.
- Research Support, U.S. Gov’t, P.H.S.]. Cell. 122(1):107–118. eng.16009137 [Google Scholar]
- McDaniel KL, Restori KH, Dodds JW, Kennett MJ, Ross AC, Cantorna MT. 2015. Vitamin A-Deficient Hosts Become Nonsymptomatic Reservoirs of Escherichia coli-Like Enteric Infections [Research Support, N.I.H., Extramural [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Support, Non-U.S. Gov’t]. Infect Immun. 83(7):2984–2991. eng. [Google Scholar]
- Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, Misiak A, Dungan LS, Sutton CE, Streubel G et al. 2013. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation [Research Support, Non-U.S. Gov’t]. J Exp Med. 210(6):1117–1124. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. 2005. Citrobacter rodentium of mice and man. Cell Microbiol. 7(12):1697–1706. eng. [DOI] [PubMed] [Google Scholar]
- Niess JH, Leithauser F, Adler G, Reimann J. 2008. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 180(1):559–568. [DOI] [PubMed] [Google Scholar]
- Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M et al. 2015. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 349(6251):989–993. [DOI] [PubMed] [Google Scholar]
- Ooi JH, Li Y, Rogers CJ, Cantorna MT. 2013. Vitamin D Regulates the Gut Microbiome and Protects Mice from Dextran Sodium Sulfate-Induced Colitis. J Nutr. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi JH, Waddell A, Lin YD, Albert I, Rust LT, Holden V, Cantorna MT. 2014. Dominant effects of the diet on the microbiome and the local and systemic immune response in mice [Research Support, N.I.H., Extramural]. PloS one. 9(1):e86366 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai M, Nishikiori N, Murata M, Chiba H, Kojima T, Sawada N. 2007. Cellular retinoic acid bioavailability determines epithelial integrity: Role of retinoic acid receptor alpha agonists in colitis. Mol Pharmacol. 71(1):250–258. [DOI] [PubMed] [Google Scholar]
- Parastouei K, Mirshafiey A, Eshraghian MR, Shiri-Shahsavar MR, Solaymani-Mohammadi F, Chahardoli R, Alvandi E, Saboor-Yaraghi AA. 2018. The effect of 1, 25(OH)2 D3 (calcitriol) alone and in combination with all-trans retinoic acid on ROR-gammat, IL-17, TGF-beta, and FOXP3 gene expression in experimental autoimmune encephalomyelitis. Nutr Neurosci. 21(3):210–218. [DOI] [PubMed] [Google Scholar]
- Park JH, Eberl G. 2018. Type 3 regulatory T cells at the interface of symbiosis. J Microbiol. 56(3):163–171. [DOI] [PubMed] [Google Scholar]
- Peterson LW, Artis D. 2014. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 14(3):141–153. eng. [DOI] [PubMed] [Google Scholar]
- Qiu YY, Zhou XY, Qian XF, Wu YX, Qin C, Bian T. 2017. 1,25-dihydroxyvitamin D3 reduces mouse airway inflammation of neutrophilic asthma by transcriptional modulation of interleukin-17A. Am J Transl Res. 9(12):5411–5421. [PMC free article] [PubMed] [Google Scholar]
- Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, Meddings J, O’Sullivan M. 2015. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: Results from a randomised double-blind placebo-controlled study. United European Gastroenterology Journal. 3(3):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restori KH, McDaniel KL, Wray AE, Cantorna MT, Ross AC. 2014. Streptococcus pneumoniae-induced pneumonia and Citrobacter rodentium-induced gut infection differentially alter vitamin A concentrations in the lung and liver of mice [Research Support, N.I.H., Extramural]. J Nutr. 144(3):392–398. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. 2017. Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int. 2017:9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G, Backhed F, Levin BR, McFall-Ngai MJ, McLean AR. 2017. Evolution, human-microbe interactions, and life history plasticity. Lancet. 390(10093):521–530. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 107(27):12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryz NR, Patterson SJ, Zhang Y, Ma C, Huang T, Bhinder G, Wu X, Chan J, Glesby A, Sham HP et al. 2012. Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal Th17 responses [Research Support, Non-U.S. Gov’t]. Am J Physiol Gastrointest Liver Physiol. 303(12):G1299–1311. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreiner AB, Kao JY, Young VB. 2015. The gut microbiome in health and in disease. Current opinion in gastroenterology. 31(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. 2007. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 8(3):285–293. [DOI] [PubMed] [Google Scholar]
- Snyder LM, McDaniel KL, Tian Y, Wei CH, Kennett MJ, Patterson AD, Ross AC, Cantorna MT. 2018. Retinoic Acid Mediated Clearance of Citrobacter rodentium in Vitamin A Deficient Mice Requires CD11b+ and T Cells. Frontiers in immunology. 9:3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. 2017. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 15(10):630–638. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, Shibata N, Grunberg S, Sinha R, Zahm AM et al. 2012. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 336(6086):1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF Jr., Wang J, Ramalingam TR et al. 2014. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity [Research Support, N.I.H., Extramural [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Support, N.I.H., Intramural.
- Research Support, Non-U.S. Gov’t.
- Research Support, U.S. Gov’t, Non-P.H.S.]. Science. 343(6169):432–437. eng.24458645 [Google Scholar]
- Tait Wojno ED, Artis D. 2016. Emerging concepts and future challenges in innate lymphoid cell biology. The Journal of Experimental Medicine. 213(11):2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. 2014. The role of short-chain fatty acids in health and disease. Adv Immunol. 121:91–119. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nakayama J. 2017. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 66(4):515–522. [DOI] [PubMed] [Google Scholar]
- Thurnham DI, Northrop-Clewes CA, McCullough FS, Das BS, Lunn PG. 2000. Innate immunity, gut integrity, and vitamin A in Gambian and Indian infants. J Infect Dis. 182 Suppl 1:S23–28. eng. [DOI] [PubMed] [Google Scholar]
- Tian Y, Nichols RG, Cai J, Patterson AD, Cantorna MT. 2018. Vitamin A deficiency in mice alters host and gut microbial metabolism leading to altered energy homeostasis. J Nutr Biochem. 54:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice [Research Support, N.I.H., Extramural [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Support, Non-U.S. Gov’t]. Science translational medicine. 1(6):6ra14 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G et al. 2014. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 508(7494):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Ferreira C. 2015. Influence of nutrient-derived metabolites on lymphocyte immunity. Nat Med. 21(7):709–718. [DOI] [PubMed] [Google Scholar]
- Villamor E, Fawzi WW. 2000. Vitamin A supplementation: implications for morbidity and mortality in children. J Infect Dis. 182 Suppl 1:S122–133. [DOI] [PubMed] [Google Scholar]
- WHO. 2009. Global prevalence of vitamin A deficiency in populations at risk 1995–2005. Geneva: World Health Organization. [Google Scholar]
- Wittkopf N, Neurath MF, Becker C. 2014. Immune-epithelial crosstalk at the intestinal surface. J Gastroenterol. 49(3):375–387. [DOI] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R et al. 2011. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science. 334(6052):105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ et al. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 14(3):282–289. [DOI] [PubMed] [Google Scholar]