Abstract
Biallelic inactivating mutations in DOCK8 cause a combined immunodeficiency characterized by severe pathogen infections, eczema, allergies, malignancy, and impaired humoral responses. These clinical features result from functional defects in most lymphocyte lineages. Thus, DOCK8 plays a key role in immune cell function. Hematopoietic stem cell transplant (HSCT) is curative for DOCK8 deficiency. While previous reports have described clinical outcomes for DOCK8 deficiency following HSCT, the effect on lymphocyte reconstitution and function has not been investigated. Our study determined whether defects in lymphocyte differentiation and function in DOCK8-deficient patients were restored following HSCT. DOCK8-deficient T and B lymphocytes exhibited aberrant activation and effector function in vivo and in vitro. Frequencies of αβ T and MAIT cells were reduced, while γδT cells were increased in DOCK8-deficient patients. HSCT improved abnormal lymphocyte function in DOCK8-deficient patients. Elevated total and allergen-specific IgE in DOCK8-deficient patients decreased over time following HSCT. Our results document the extensive catalog of cellular defects in DOCK8-deficient patients and the efficacy of HSCT in correcting these defects, concurrent with improvements in clinical phenotypes. Overall, our findings reveal mechanisms at a functional cellular level for improvements in clinical features of DOCK8 deficiency after HSCT, identify biomarkers that correlate with improved clinical outcomes, and inform the general dynamics of immune reconstitution in patients with monogenic immune disorders following HSCT.
Keywords: Immunology, Infectious disease
Keywords: Adaptive immunity, Cellular immune response, Stem cell transplantation
Stem cell transplantation effectively restores cellular functional defects in patients with DOCK8 deficiency, predicting disease improvement and resolution of clinical symptoms.
Introduction
Primary immunodeficiencies (PIDs) are rare conditions caused by mutations in a single gene that cripples the development and/or function of immune cells (1, 2). Currently, more than 350 genes have been identified that, when mutated, can result in immune dysregulation (1, 3). Although these inborn errors of immunity have been typically associated with heightened susceptibility to disease due to recurrent pathogen infections, the phenotype of PIDs is much broader and can include autoimmunity, autoinflammation, allergy, and malignancy (1, 4–6). While PIDs due to a specific gene defect are rare, the rapid discovery of the molecular causes of novel PIDs, the ongoing appreciation of the diversity of clinical presentations of these conditions, and the application of newborn screening across several countries are revealing that collectively the incidence of PIDs is much greater than typically reported (2, 3, 7, 8). For these reasons, it is important to understand the biology and pathogenesis of individual PIDs, and have a thorough knowledge of the optimal treatments and subsequent outcomes for PIDs resulting from mutations in specific pathways.
Severe combined immunodeficiencies (SCID), due to mutations in IL2RG, JAK3, ADA, RAG, or IL7R, or combined immunodeficiencies (CIDs), due to mutations in, e.g., DOCK2, STK4, MALT1, CARD11, or IKBKB, are usually fatal unless early therapeutic intervention such as hematopoietic stem cell transplant (HSCT), gene therapy, or enzyme replacement is applied (2). The first HSCTs for PID were performed in 1968 (9, 10). While results from these initial transplants for PID were disappointing (9, 10), remarkable advances have been made over the past 50 years, such that the overall survival of SCID/CID patients following HSCT can exceed 95% (11–16). However, depending on the age at time of transplant, incidence of infection, and nature and source of the donor, mortality after HSCT can remain substantial, with 5- to 10-year survival ranging from <40% to ~80% (11–13, 15, 16). Thus, in order to improve therapeutic outcomes, it is critical to identify correlates or biomarkers of successful immune cell reconstitution in PID patients following HSCT.
DOCK8 is a guanine nucleotide exchange factor with key roles in regulating cytoskeletal rearrangement, cell activation, migration, and survival (17, 18). Despite its broad expression, DOCK8 has a critical and nonredundant role in immunity, as revealed by the discovery that biallelic DOCK8 mutations cause a CID characterized by recurrent mucocutaneous viral, bacterial, and fungal infections (80%–90% of cases), severe eczema (>95%), allergies (~70%), hyper-IgE (98%), and increased susceptibility to malignancy (HPV-induced carcinoma, EBV-associated lymphoma) and autoimmunity (17–22).
Numerous studies have investigated cellular defects in DOCK8 deficiency to understand both the nonredundant roles of DOCK8 in lymphocyte biology and mechanisms of disease in DOCK8-deficient patients. These investigations revealed dysregulated survival, proliferation, differentiation, migration, and senescence/exhaustion of CD4+ and CD8+ T cells (19, 23–27), decreased Treg function (28), NK cell cytotoxicity (29, 30) and NKT cell development (31), and reduced B cell activation in vitro and memory B cell generation in vivo (32, 33).
Similar to other CIDs, outcomes for DOCK8 deficiency are poor, with >95% mortality by 40 years (median survival ~10–20 years), and the incidence of life-threatening infections and malignancy increases every decade (21, 22). Consequently, HSCT is the standard of care for the life-threatening infections and related immune complications associated with DOCK8 deficiency (22). Several studies have examined outcomes of HSCT in DOCK8 deficiency, with generally positive results (~80% survival), but varying degrees of clinical improvement. Eczema, cutaneous viral and bacterial infections, responses to vaccines, and levels of serum IgM, IgG, and IgA all markedly improved after HSCT (34–45). In contrast, allergic disease following HSCT is highly variable, resolving (32, 40, 46), improving (32, 34, 35, 37), or persisting (32, 41, 47). Clinical improvements in transplanted DOCK8-deficient patients have been associated with both mixed (40, 44, 47) and complete (34, 36, 41, 42) donor chimerism.
In this study, we used DOCK8 deficiency as a model to delineate mechanisms underlying disease pathogenesis before HSCT and improvement of clinical features of PID after HSCT, and identify correlates of immune reconstitution and function following HSCT. This allowed us to extensively catalog cellular defects due to DOCK8 deficiency and investigate quantitative and qualitative improvement of these defects after HSCT. Cellular improvements correlated with reconstitution of DOCK8 protein expression and clinical outcomes in these patients. To date, this is, to our knowledge, the largest study of its kind and provides insights into the functional changes that may predict successful immune reconstitution and guide ongoing treatments and management of DOCK8-deficient patients following HSCT. Furthermore, our study provides proof of principle for performing high-dimensional multifunctional cellular analyses before and after therapy in other PIDs to understand treatment-induced alterations in cellular behavior and clinical outcomes and guide implementation of optimal treatments for these conditions.
Results
DOCK8 is constitutively expressed by lymphocytes in healthy donors and DOCK8-deficient patients after HSCT.
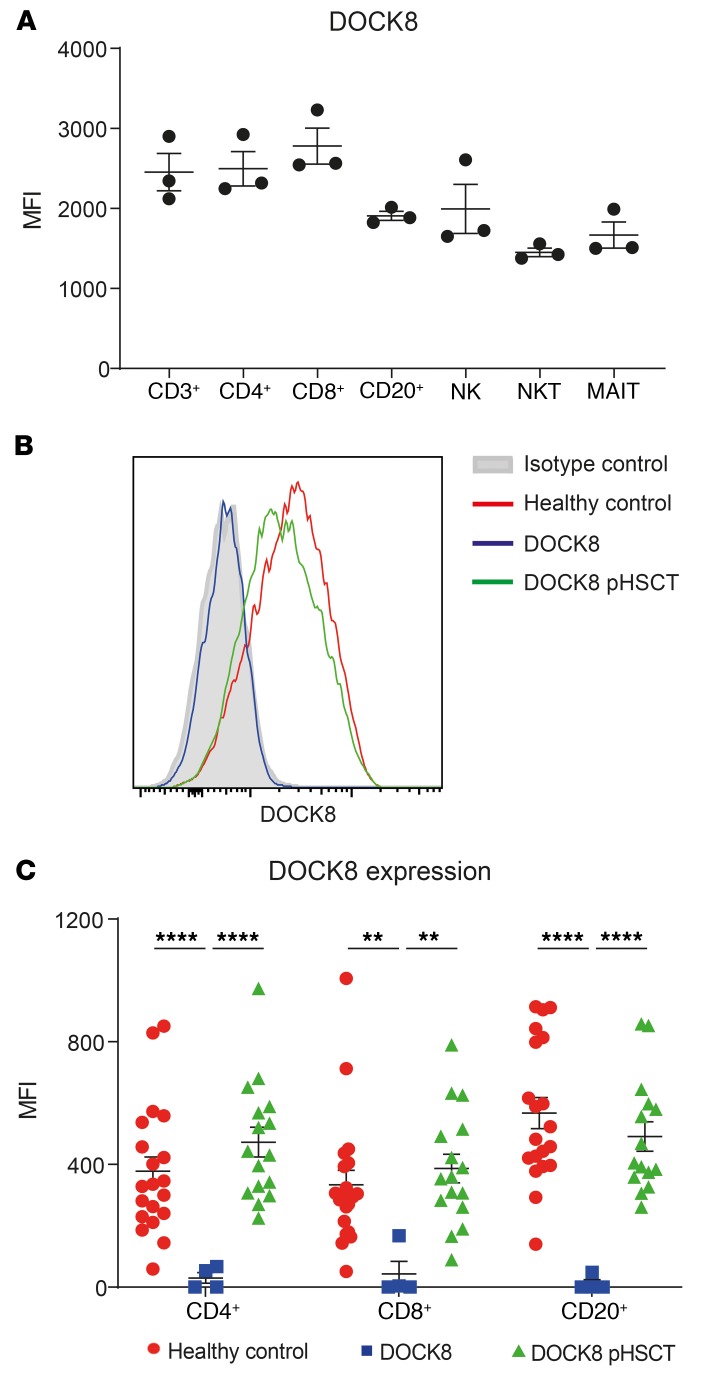
To gain insight into the role of DOCK8 in immune function, we first determined DOCK8 expression in the major lymphocyte subsets in PBMCs of healthy volunteers. DOCK8 was highly and comparably expressed in total T cells, CD4+ and CD8+ T cells, B cells, and NK cells (Figure 1A) (48, 49). We also established that DOCK8 is constitutively expressed in NKT and mucosal associated invariant T (MAIT) cells (Figure 1A). Next, we confirmed lack of expression in patients with DOCK8 mutations and assessed restoration of DOCK8 expression following HSCT. Patients studied here exhibited near-undetectable levels of DOCK8 protein, with expression in lymphocytes (Figure 1B), CD4+ T cells, CD8+ T cells, and CD20+ B cells (Figure 1C) being drastically reduced compared with those from healthy volunteers. Importantly, DOCK8 expression in these lymphocyte populations from transplanted patients was restored to levels similar to those of lymphocytes from healthy volunteers (Figure 1, B and C).
Figure 1. DOCK8 is highly expressed in lymphocyte subsets, absent in DOCK8-deficient patients and restored following HSCT.
(A) PBMCs from healthy donors (n = 3) were stained with Abs against CD3, CD4, CD8, CD20, CD56, CD161, and TCR Vα24, Vβ11, and Vα7.2. The cells were then fixed, permeabilized, and stained with anti-DOCK8 mAb. Expression of intracellular DOCK8 in total T cells (CD3+), CD4+ T cells (CD3+CD4+CD8–), CD8+ T cells (CD3+CD4–CD8+), B cells (CD20+CD3–), NK cells (CD3–CD56+), NKT cells (CD3+TCRVα24+Vβ11+), and MAIT cells (CD3+CD161+TCRVα7.2+) was then determined. Data represent the average geometric MFI ± SEM of different lymphocyte subsets from 3 unrelated donors labeled with anti-DOCK8 mAb less the MFI of cells labeled with isotype control mAb. (B and C) PBMCs from healthy donors (n = 20) or DOCK8-deficient patients before (n = 4) or following HSCT (pHSCT; n = 15–16) were stained with Abs against CD4, CD8, and CD20 before fixing, permeabilization, and staining for DOCK8. DOCK8 expression was determined in total lymphocytes (B), as well as in CD4+ T cells, CD8+ T cells, and CD20+ B cells (C). The histogram in B depicts DOCK8 expression in total lymphocytes from 1 representative healthy donor, and lymphocytes from the same DOCK8-deficient patient before and after transplant as well as an isotype control. The graph in C represents the mean MFI ± SEM of DOCK8 expression (minus MFI of isotype control mAb). Statistical analysis was performed using unpaired t test with Welch’s correction; **P < 0.01, ****P < 0.001.
Clinical characteristics of DOCK8-deficient patients — impact of HSCT.
We studied an international cohort of DOCK8-deficient patients (Table 1) who had either confirmed biallelic mutations in DOCK8 (n = 18) or lacked DOCK8 protein in leukocytes (n = 2). In total, immune cells were examined in 18 DOCK8-deficient patients after HSCT; matched PBMC samples were available from 7 patients before and after HSCT, and 2 patients at 2 or more time points after HSCT. The source of transplant was haploidentical (n = 6), matched unrelated (n = 7), or matched related (n = 6) donors. Consistent with a recent study of HSCT for a large cohort of DOCK8-deficient patients (32), no correlations were observed between the source of the transplant and the overall clinical outcome of the patients after HSCT (Table 2). All DOCK8-deficient patients studied here experienced recurrent viral and bacterial infections (Table 1). Candidiasis was reported in 30%, allergies in 80%, and impaired vaccine responses in >90% of patients (Table 1). Following HSCT, infections were reduced, and vaccine responses improved in all DOCK8-deficient patients (Table 2). Allergies improved in only 1 of 11 patients tested (Table 2); however, this was an underestimate, as many of the patients did not undergo formal clinical allergy testing after HSCT. Consistent with flow cytometric analysis of DOCK8 expression, which revealed comparable expression in patients after HSCT and healthy donors (Figure 1, B and C), donor engraftment following transplant was >90% in all patients (Table 2).
Table 1. Clinical details of DOCK8-deficient patients before HSCT.
Table 2. Clinical details of DOCK8-deficient patients after HSCT.
DOCK8-deficient lymphocytes exhibit a unique phenotype typical of aberrant in vivo differentiation.
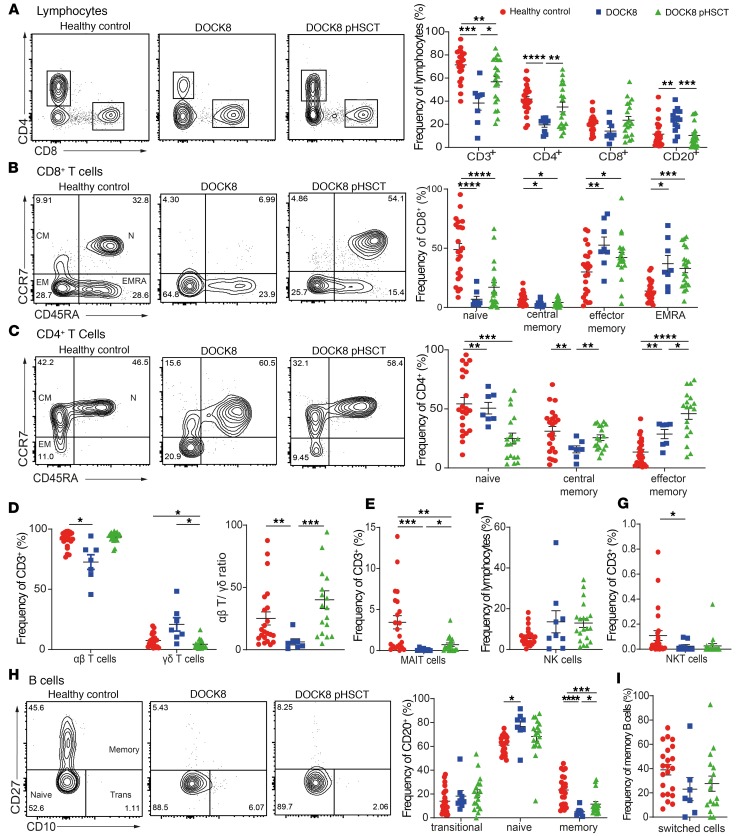
To elucidate the effects of DOCK8 deficiency on lymphocytes and the impact of HSCT on these defects, we undertook extensive phenotypic analysis. Consistent with previous observations (20, 26), we found reductions in proportions of CD3+ T cells in DOCK8-deficient patients (Figure 2A), largely due to reduced frequencies of CD4+ T cells (Figure 2A). Analysis of T cell subsets confirmed skewing of DOCK8-deficient CD8+ T cells to effector memory (TEM) and effector memory CD45RA+ (TEMRA) cells at the expense of naive and central memory (TCM) cells (Figure 2B). In contrast, proportions of naive CD4+ T cells were comparable to healthy controls, while CD4+ TCM cells were decreased and CD4+ TEM cells were increased (Figure 2C). Tregs were proportionally increased in DOCK8-deficient patients compared with controls (data not shown). DOCK8 deficiency also affected αβ and γδ T cells, with reductions and increases, respectively, in these subsets in patients compared with controls, resulting in a skewed αβ/γδ T cell ratio (Figure 2D). Furthermore, DOCK8-deficient patients had ~10-fold fewer MAIT cells than healthy controls (Figure 2E); frequencies of NK cells and NK cell subsets were normal (Figure 2F; data not shown; and refs. 20, 29); and NKT cells were reduced (Figure 2G) (31). In contrast, proportions of total (Figure 2A) and naive (Figure 2H) B cells were significantly increased in patients compared with healthy donors; however, DOCK8-deficient patients had significantly decreased proportions of total memory B cells (Figure 2H). Interestingly, frequencies of Ig class-switched memory B cells were unaffected by DOCK8 deficiency (Figure 2I).
Figure 2. Effect of HSCT on lymphocyte phenotype and differentiation in DOCK8-deficient patients.
PBMCs from healthy donors (n = 22–24), untransplanted DOCK8-deficient patients (n = 7–9), or DOCK8-deficient patients following HSCT (DOCK8 pHSCT) (n = 18–20) were labeled with mAbs against CD3, CD4, CD8, CD20, CD56, CD45RA, CCR7, CD10, CD27, IgM, IgD, TCRαβ, TCRγδ, TCRVα24, TCRVβ11, CD161, and TCR Vα7.2. Proportions of (A) CD3+ cells, CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B cells (CD20+); (B) CD8+ naive (CD45RA+CCR7+), central memory (TCM; CD45RA–CCR7+), effector memory (TEM; CD45RA–CCR7–), and CD45RA+ revertant memory (TEMRA; CD45RA+CCR7–) cells; (C) CD4+ naive, TCM, and TEM cell subsets; (D) αβ and γδ TCR+ T cells; (E) MAIT cells (CD3+TCRVα7.2+CD161+); (F) NK cells (CD3–CD56+); (G) NKT cells (CD3+TCRVα24+ Vβ11+); (H) transitional (Trans; CD20+CD10+CD27–), naive (CD20+CD10–CD27–), and memory (CD20+CD10–CD27+) B cell subsets; and (I) Ig class-switched memory (CD20+CD27+ IgD–IgM–) B cells were then determined by flow cytometric analysis. Contour plots represent 1 representative normal donor and 1 DOCK8-deficient patient before and after HSCT. Data are mean ± SEM. Statistical analysis was performed with GraphPad Prism using unpaired t test with Welch’s correction; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Impact of HSCT on lymphocyte differentiation in vivo in DOCK8-deficient patients.
Detailed analysis of immune cells in 18 DOCK8-deficient patients 6–43 months after HSCT (mean, 15 months) revealed that most of these defects in lymphocyte differentiation were improved. Specifically, CD3+ T cell proportions were significantly increased due to the recovery of total CD4+ T cells, although they remained reduced overall compared with controls (Figure 2A). However, proportions of CD4+ T cells tended to reached normal levels in patients ≥12 months after HSCT (Supplemental Figure 1A; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.127527DS1). The loss of naive CD8+ T cells and skewing to a CD8+ TEM phenotype in untransplanted patients improved following HSCT (Figure 2B). While at the level of the total cohort, these values were not significantly different compared with healthy controls (Figure 2B), they normalized in patients ≥12 months after HSCT (Supplemental Figure 1C). CD4+ TCM cells and Tregs were normalized after HSCT, but CD4+ TEM cells persisted at an increased frequency at the expense of naive CD4+ T cells (Figure 2C and data not shown) until ≥23 months after HSCT (Supplemental Figure 1D). Proportions of αβ and γδ T cells were promptly reestablished by HSCT (Figure 2D and Supplemental Figure 1E); MAIT cells in transplanted DOCK8-deficient patients were significantly increased compared with pre-HSCT levels, but remained significantly reduced relative to healthy donors in patients irrespective of time of analysis after HSCT (Figure 2E and Supplemental Figure 1F). Strikingly, proportions of NKT cells remained unchanged in transplanted DOCK8-deficient patients, being significantly decreased at all times after HSCT (Figure 2G and Supplemental Figure 1H).
HSCT corrected the increased frequencies of total (Figure 2A) and naive (Figure 2H) B cells in DOCK8-deficient patients. Importantly, memory B cells were significantly increased after transplant compared with untransplanted patients, but remained decreased compared with healthy donors (Figure 2H). The kinetics of the concurrent decline in proportions of transitional and increases in naive and memory B cells in transplanted DOCK8-deficient patients (Supplemental Figure 1B) is reminiscent of the temporal reappearance of these B cell subsets in individuals undergoing HSCT for hematological malignancies (50, 51). Thus, DOCK8 deficiency affects the generation and differentiation of a wide range of lymphocytes and HSCT improves most defects, but some improvements are likely to be more time dependent.
Memory T cells in DOCK8-deficient patients exhibit signs of exhaustion, some of which persist after HSCT.
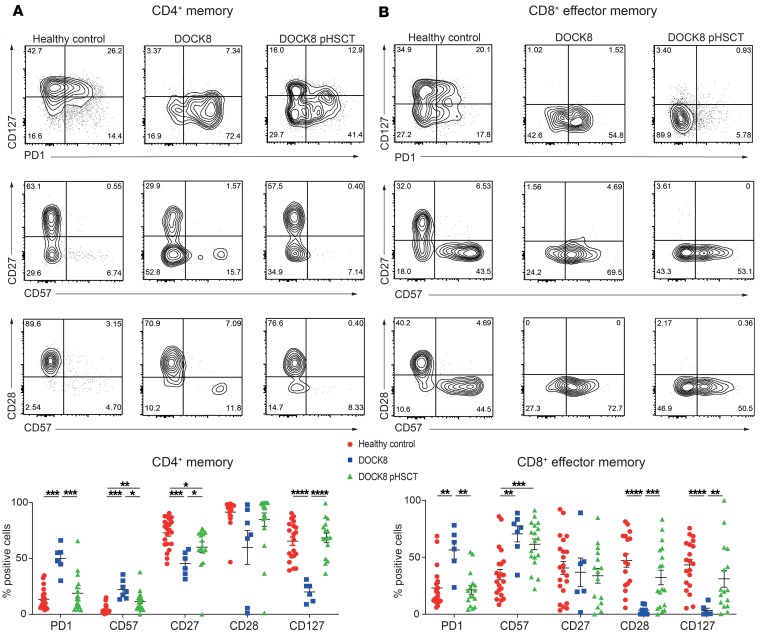
Memory T cells from DOCK8-deficient patients exhibit phenotypic features of chronic activation or exhaustion/senescence (23, 26), which likely impedes their effector function (52, 53). Thus, frequencies of DOCK8-deficient memory CD4+ and CD8+ TEM cells expressing CD57 and PD-1 were significantly increased compared with healthy volunteers (Figure 3, A and B). This was coupled with significantly decreased expression of CD127, CD27, and/or CD28 on DOCK8-deficient CD4+ memory and CD8+ TEM cells (Figure 3, A and B). Proportions of patient memory CD4+ T cells expressing PD-1 and CD127 normalized after HSCT, and CD57 was significantly decreased following HSCT but continued to exceed that of healthy volunteers (Figure 3A). CD27 expression on patient memory CD4+ T cells improved after HSCT but remained lower than that on memory CD4+ T cells from healthy volunteers (Figure 3A). Similar phenotypic changes were observed for CD8+ TEM cells, with HSCT normalizing PD-1, CD28, and CD127 and partially correcting CD57 expression (Figure 3B). Taken together, the results indicated that HSCT partially restored the exhausted/senescent phenotype of CD4+ and CD8+ T cells in DOCK8-deficient patients.
Figure 3. DOCK8-deficient memory CD4+ and CD8+ T cells exhibit signs of exhaustion, which decline after HSCT.
PBMCs from healthy donors (n = 17–22) and DOCK8-deficient patients either before (n = 6–7) or after (n = 16–19) HSCT were labeled with mAbs against CD4, CD8, CD45RA, CCR7, CD127, CD27, CD28, PD-1, and CD57. Coexpression of CD127 and PD-1, CD27 and CD57, and CD28 and CD57 by (A) memory CD4+ T cells (CD4+CD8–CD45RA–CCR7+/–) or (B) TEM CD8+ T cells (CD8+CD4–CD45RA–CCR7–) was determined. Contour plots are representative of 1 healthy donor and the same DOCK8-deficient patient assessed before and after HSCT. The graphs show mean ± SEM. Statistical analysis was performed with Prism using unpaired t test with Welch’s correction; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
Defects in proliferation, acquisition of cytotoxic effector function, and cytokine secretion by CD8+ T cells in DOCK8-deficient patients are improved by HSCT.
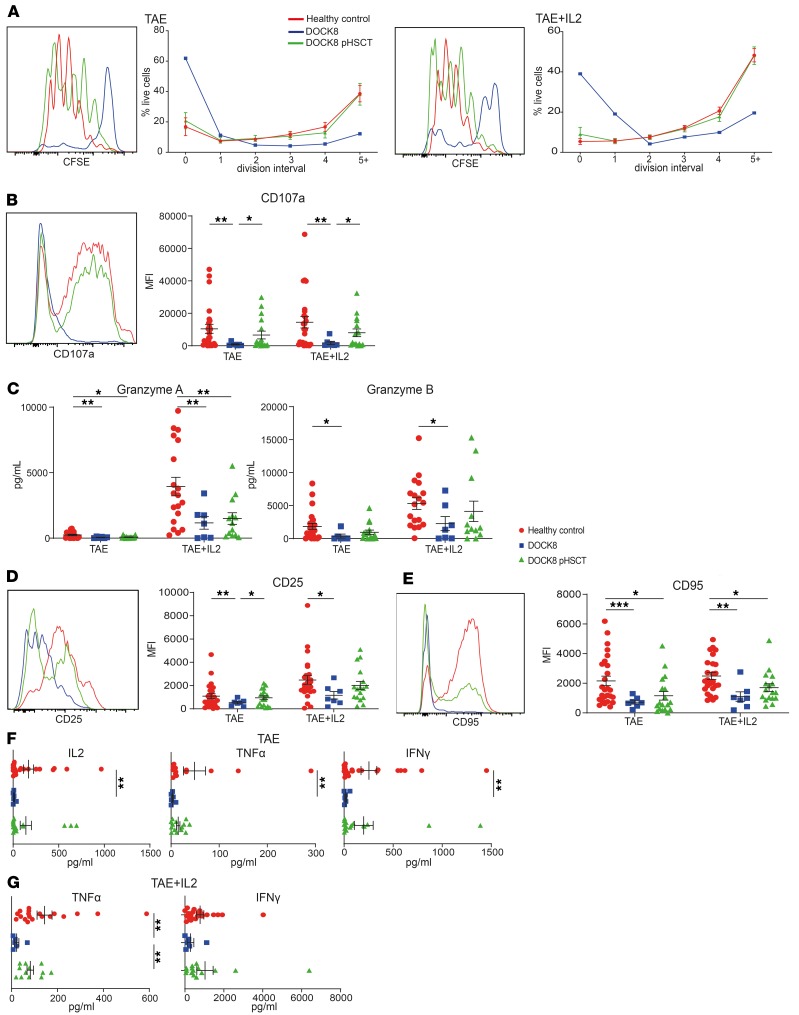
While proliferative, cytoskeletal, and survival defects of CD8+ T cells have been proposed to underlie impaired antiviral immunity in DOCK8 deficiency (23, 24), the role of DOCK8 in CD8+ T cell responses has only been assessed in a few patients (19). To investigate CD8+ T cell dysfunction due to DOCK8 deficiency, and the effect of HSCT on these functions, CD8+ T cells were labeled with CFSE and cultured for 5 days with anti-CD2/CD3/CD28 mAbs (TAE beads). Analysis of CFSE dilution revealed reduced proliferation of DOCK8-deficient CD8+ T cells (Figure 4A, left panels) (23). While exogenous IL-2 improved proliferation, the extent of division of DOCK8-deficient CD8+ T cells remained diminished compared with healthy control CD8+ T cells (Figure 4A, right panels).
Figure 4. HSCT overcomes CD8+ T cell functional defects due to DOCK8 deficiency.
CD8+ T cells were sorted from the peripheral blood of healthy donors (n = 18–26), untransplanted DOCK8-deficient patients (n = 2–7), and DOCK8-deficient patients following HSCT (DOCK8 pHSCT) (n = 13–18); labeled with CFSE; and then cultured with TAE (anti-CD2/CD3/CD28) beads in the absence or presence of IL-2. After 5 days, culture supernatants were collected, and cells were harvested and then restimulated with PMA/ionomycin for 6 hours, with brefeldin A, monensin, and anti-CD107a mAb being added after 1 hour. (A) The frequency of cells in each division was determined by dilution of CFSE. (B) Expression of CD107a and (C) secretion of granzyme A and granzyme B were determined by flow cytometry and cytometric bead arrays, respectively. (D) Surface expression of CD25 and (E) CD95 was determined by flow cytometry. (F and G) Secretion of IFN-γ, TNF-α, and IL-2 was determined by cytometric bead arrays. Histograms in A, B, D, and E are representative of one healthy donor and one paired DOCK8-defcient patient before and after HSCT. Data represent the mean ± SEM. Statistics performed using Prism unpaired t test with Welch’s correction *P < 0.05, **P < 0.01, ***P < 0.005.
We next measured acquisition of a cytotoxic phenotype following in vitro activation. CD107a expression, an indicator of degranulation in CD8+ T cells (54), and granzyme A/B secretion (Figure 4, B and C) were significantly decreased for DOCK8-deficient CD8+ T cells compared with healthy volunteers. DOCK8-deficient CD8+ T cells also exhibited generalized activation defects, with significantly reduced expression of CD25 and CD95 following in vitro stimulation (Figure 4, D and E). IL-2, IFN-γ, and TNF-α secretion by DOCK8-deficient CD8+ T cells was also significantly lower than in healthy controls (Figure 4F). While exogenous IL-2 increased secretion of granzymes (Figure 4C), TNF-α and IFN-γ (Figure 4G), and CD25 expression (Figure 4D) by CD8+ T cells, the overall response of IL-2–treated DOCK8-deficient CD8+ T cells remained significantly lower than in healthy control CD8+ T cells.
Post-HSCT, CD8+ T cells from DOCK8-deficient patients proliferated as well as those from healthy controls even without addition of IL-2 (Figure 4A). Furthermore, degranulation (Figure 4B), granzyme B expression (Figure 4C), CD25 induction (Figure 4D), and IFN-γ, TNF-α, and IL-2 secretion (Figure 4F) were all restored to normal levels. However, granzyme A secretion (Figure 4C) and CD95 expression (Figure 4E) remained significantly decreased. Thus, multiple effector functions of CD8+ T cells were severely compromised in DOCK8-deficient patients, but HSCT largely restored functionality to levels similar to those in healthy controls.
Dysregulated cytokine production by CD4+ T cells in DOCK8-deficient patients is normalized by HSCT.
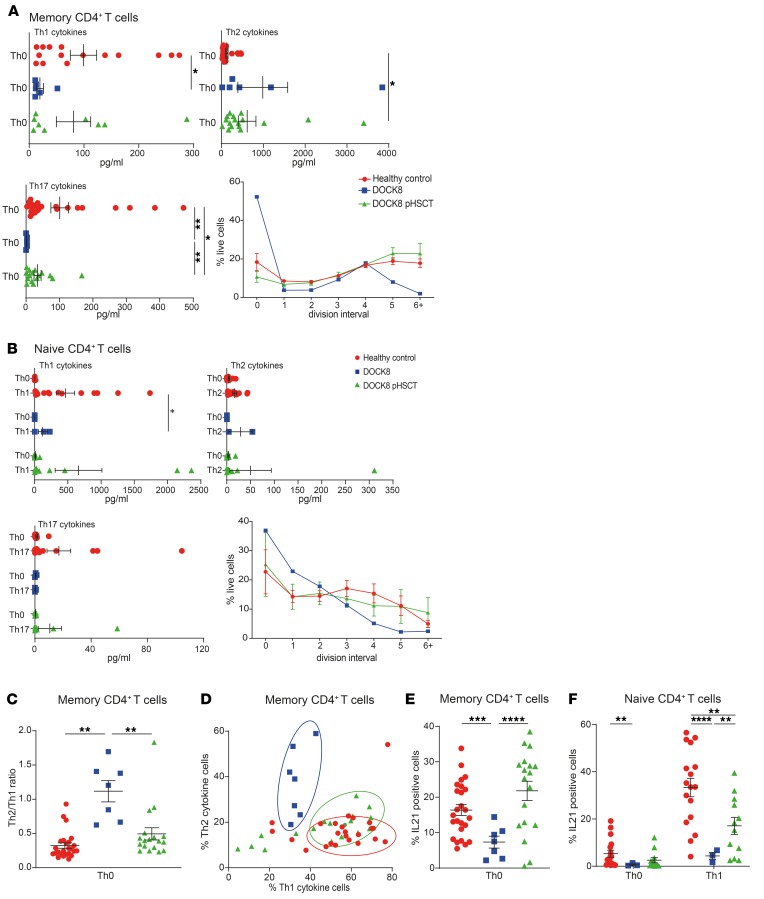
Cytokine production by in vitro activated memory CD4+ T cells provides information about their differentiation in vivo (55). Memory CD4+ T cells from DOCK8-deficient patients showed significantly reduced production of Th1 (IFN-γ/TNF-α) and Th17 (IL-17A/IL-17F) but increased Th2 (IL-4, IL-5, IL-13) cytokines compared with healthy controls (Figure 5A and Supplemental Figure 2A) (25, 26).
Figure 5. Dysregulated cytokine production by DOCK8-deficient CD4+ T cells is greatly improved following HSCT.
Naive and memory CD4+ T cells were sort-purified from the peripheral blood of healthy donors (n = 7–25), untransplanted DOCK8-deficient patients (n = 2–7), and DOCK8-deficient patients following HSCT (DOCK8 pHSCT) (n = 6–18). The cells were labeled with CFSE and then cultured under Th0 conditions (TAE beads; naive and memory), or Th1- (+IL-12), Th2- (+ IL-4), or Th17-polarizing (IL-1β, IL-6, IL-21, IL-23, TGF-β, prostaglandin E2) conditions (naive only) for 5 days. (A and B) Cells and culture supernatants were harvested to assess proliferation of (CFSE dilution) and cytokine secretion of (A) Th1 cytokines (IFN-γ/TNF-α), Th2 cytokines (IL-4/IL-5/IL-13), or Th17 cytokines (IL-17A/IL-17F) by memory CD4+ T cells, and of (B) of Th1 cytokines (IFN-γ/TNF-α), Th2 cytokines (IL-5/IL-13), or Th17 cytokines (IL-17A/IL-17F) naive CD4+ T cells. (C and D) Cells were restimulated with PMA/ionomycin before permeabilization and intracellular staining to determine proportions of cells expressing Th1 (IFN-γ, TNF-α) and Th2 (IL-4, IL-13) cytokines. Data are presented as (C) the ratio of cells expressing Th2 versus Th1 cytokines and (D) the combined percentage of cells from individual donors and patients expressing Th1 (i.e., %IFN-γ+/TNF-α+/IFN-γ+TNF-α+ cells) versus Th2 (i.e., %IL-4+/IL-13+/IL-4+IL-13+ cells) cytokines. (E and F) Intracellular expression of IL-21 by memory CD4+ T cells (E) and naive CD4+ T cells cultured under Th0- or Th1-polarizing conditions (F) cells was measured. Graphs show mean ± SEM. Statistical performed with Prism using unpaired t test with Welch’s correction; *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001.
The defect in generating Th17-type cells in vivo in DOCK8-deficient patients was intrinsic, as revealed by impaired induction of IL-17A/F by DOCK8-deficient naive CD4+ T cells cultured under Th17-polarizing conditions (Figure 5B). Th2 cytokines (IL-5, IL-13) were normal (Figure 5B). IFN-γ expression by DOCK8-deficient naive CD4+ T cells under Th1 conditions was intact (Supplemental Figure 2B), despite reduced secretion (Figure 5B), thus suggesting that an extrinsic defect underlies poor generation of Th1 cells in DOCK8-deficient patients in vivo (26).
Consistent with our previous work, both DOCK8-deficient naive (Figure 5B) and memory (Figure 5A) CD4+ T cells showed defects in proliferation compared with normal healthy controls cells (23, 26). Since this could impact cytokine production (56), we investigated IFN-γ expression by memory and Th1-stimulated naive CD4+ T cells in the context of cell division. IFN-γ production was decreased across all divisions and hence did not result from proliferative defects (Supplemental Figure 2, C and D).
Given the implication of the Th1/Th2 axis in allergy (57), we further explored Th1 and Th2 cytokine production by memory CD4+ T cells at the single-cell level. We used intracellular staining to calculate the ratio of cells producing Th2 (IL-4/IL-13) versus Th1 (IFN-γ/TNF-α) cytokines. DOCK8-deficient memory CD4+ T cells showed a significantly increased Th2/Th1 ratio compared with controls (Figure 5C). In a plot representing each donor and patient showing proportions of Th2 versus Th1 cells, DOCK8-deficient memory CD4+ T cells formed a distinct cluster away from control memory cells (Figure 5D). This also revealed that the perturbed Th2/Th1 ratio in each patient resulted from increased Th2 and corresponding decreased Th1 cytokine production, further supporting skewed differentiation in vivo.
HSCT greatly improved CD4+ T cell function in vivo in DOCK8-deficient patients. First, proliferation of naive and memory CD4+ T cells from transplanted patients was comparable to controls (Figure 5, A and B). Second, Th1 cytokine production (Figure 5A) by memory CD4+ T cells was recovered, while these cells produced significantly increased Th17 and decreased Th2 cytokines (Figure 5A and Supplemental Figure 2A). CD4+CD45RA–CXCR3–CCR6+ Th17 cells (55) also increased after HSCT (before: 4.3% ± 2.1% [n = 8]; after: 10.4% ± 6.1% [n = 17]). Third, naive CD4+ T cells from DOCK8-deficient patients after HSCT produced normal levels of Th1, Th2, and Th17 cytokines following appropriate polarization (Figure 5B and Supplemental Figure 2A). Fourth, the Th2/Th1 cytokine ratio was normalized after HSCT (Figure 5C). This was due to coincident decreases in Th2 and increases in Th1 cells, as evidenced by a population clustered between DOCK8-deficient patients and controls (Figure 5D). Thus, CD4+ T cell differentiation defects in DOCK8-deficient patients were restored to normal or near-normal levels by HSCT.
Defective production of IL-21 by DOCK8-deficient CD4+ T cells.
IL-21 potently induces B cell activation, differentiation, and Ab production (58). IL-21 production by DOCK8-deficient memory CD4+ T cells was significantly decreased compared with healthy donors (Figure 5E). This defect was also cell intrinsic, since IL-21 induction in naive DOCK8-deficient CD4+ T cells in vitro was also significantly impaired (Figure 5F). Reduced IL-21 production by DOCK8-deficient naive CD4+ T cells was not due to diminished proliferation, as fewer IL-21+ cells were detected across all divisions measured compared with those from healthy donors (Supplemental Figure 1D). Strikingly, following HSCT, production of IL-21 (Figure 5E) by memory CD4+ T cells in DOCK8-deficient patients was restored, while IL-21 induction in naive CD4+ T cells was significantly increased (Figure 5F).
HSCT overcomes the B cell–intrinsic impairment in survival, proliferation, and differentiation due to DOCK8 deficiency.
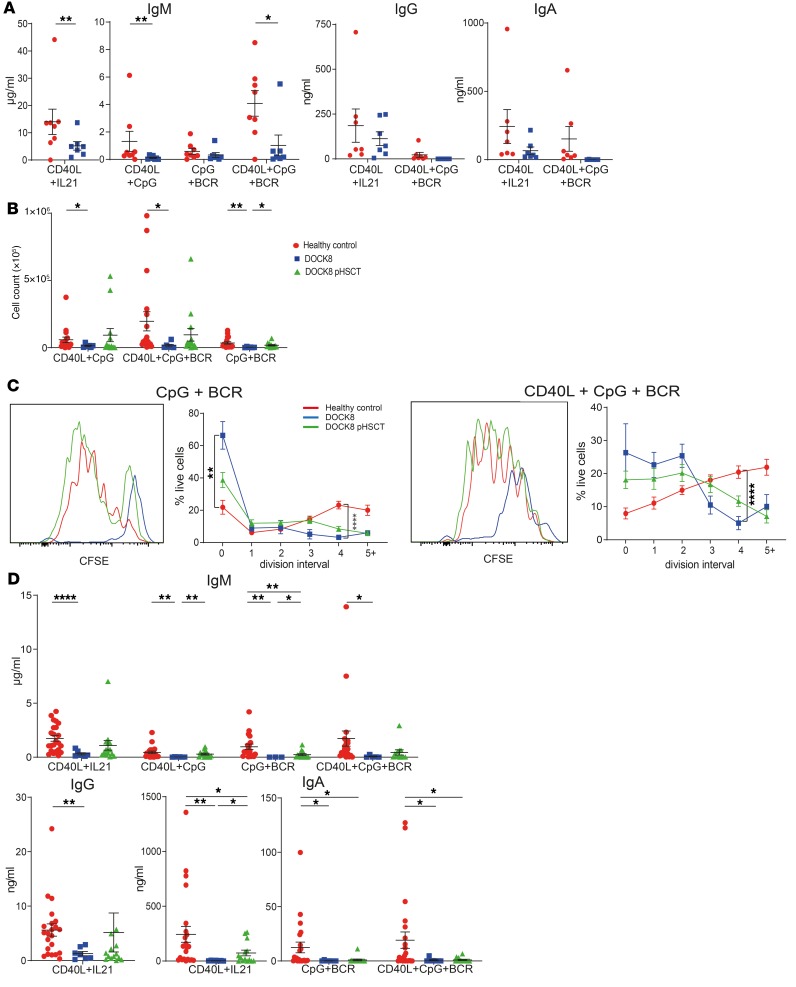
Many DOCK8-deficient patients have impaired antibody responses to vaccines (19, 21, 33) (Table 1). Dock8 is required in murine B cells to generate germinal centers and long-lived humoral immunity (59), and studies of DOCK8-deficient humans reported reductions in memory B cells (33) and compromised in vitro activation of naive B cells and identified DOCK8 as an adaptor for TLR signaling (33, 60, 61). To extend these findings, we examined the impact of DOCK8 deficiency on naive B cell function by assessing Ig secretion in vitro. When stimulated with mimics of T cell help (CD40L/IL-21), TLR ligands (CpG), and BCR agonists, naive B cells from healthy volunteers secreted IgM (Figure 6A). CD40L/IL-21 or CD40L/CpG/BCR stimulation also induced switching to IgG and IgA (Figure 6A), with CD40L/CpG/BCR being less effective than CD40L/IL-21 (Figure 6A). DOCK8-deficient naive B cells secreted significantly lower levels of IgM, with a trend toward decreased IgG and IgA under most in vitro conditions (Figure 6A). DOCK8-deficient naive B cells also exhibited significantly compromised survival and proliferation in vitro compared with control naive B cells (Figure 6, B and C). These data demonstrate that DOCK8-deficient B cells are defective in responding not only to TLR- (33, 61) and BCR-mediated (60) signals, but also those delivered via CD40 and cytokines. Survival, proliferation, and secretion of IgM and IgG by naive B cells isolated from transplanted DOCK8-deficient patients were largely restored (Figure 6, B and C). In contrast, IgA secretion by naive B cells from transplanted patients remained significantly lower than controls (Figure 6D). These findings reveal that DOCK8 deficiency intrinsically impairs naive B cell survival, proliferation, and differentiation, establishing that DOCK8-dependent signals are elicited in B cells downstream of numerous stimulatory receptors. However, these key functions are almost completely regained following HSCT, thus explaining improved humoral immune responses in DOCK8 deficiency after HSCT (Table 2).
Figure 6. B cell functional defects due to DOCK8 deficiency are improved following HSCT.
(A) Naive B cells were sort-purified from healthy donors and DOCK8-deficient patients (n = 7) and then cultured with CD40L/IL-21, CD40L/CpG, CpG/BCR agonist (Staphylococcus aureus Cowan I), or CD40L/CpG/BCR. After 11 days, culture supernatants were harvested and levels of secreted IgM, IgG, and IgA then determined. Data represent mean ± SEM. (B–D) Naive B cells were sort-purified from healthy donors (n = 18–23), untransplanted DOCK8-deficient patients (n = 5–7), and DOCK8-deficient patients following HSCT (DOCK8 pHSCT) (n = 12–15); labeled with CFSE; and then cultured with combinations of CD40L, IL-21, CpG, and BCR stimulus for 5 days. After this time, cells and culture supernatants were harvested. (B) Cell number was determined using Calibrite beads. (C) Frequency of cells in each division was determined by CFSE dilution. Histogram plots show CFSE dilution from 1 representative healthy donor and 1 paired DOCK8-deficient patient before and after HSCT. (D) Secretion of IgM, IgG, and IgA was determined by ELISAs. Data in each graph represent mean ± SEM. Statistical analysis was performed with Prism using unpaired t test with Welch’s correction; *P < 0.05, **P < 0.01, ****P < 0.001.
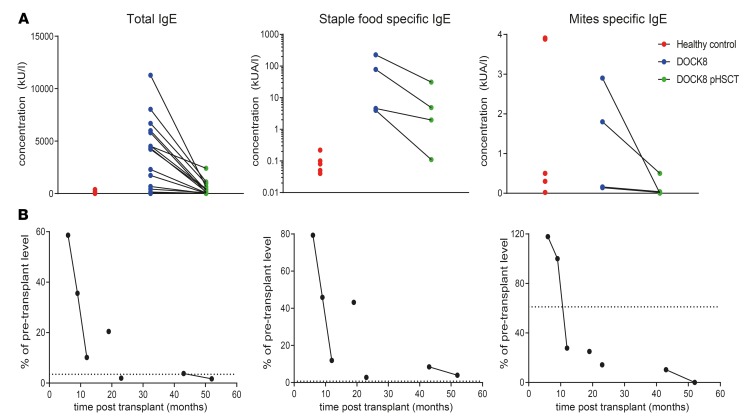
Elevated serum IgE and allergen-specific IgE levels decrease in a time-dependent manner following HSCT of DOCK8-deficient patients.
Most DOCK8-deficient patients have extremely high levels of total and allergen-specific IgE, consistent with severe allergies (19, 20, 26). Indeed, total and food allergen–specific IgE levels were increased 50- to 1000-fold in the DOCK8-deficient patients studied here compared with healthy controls (Table 1 and Figure 7A). However, dust mite–specific IgE was normal or moderately increased in DOCK8-deficiency (Figure 7A). After HSCT, all patients showed decreased total and food allergen–specific IgE compared with pre-HSCT levels (Tables 1 and 2 and Figure 7A). One patient had moderately positive dust mite allergen–specific IgE levels before HSCT, which was also reduced following HSCT (Figure 7A).
Figure 7. Elevated total and allergen-specific serum IgE levels decrease in a time-dependent manner in DOCK8-deficient patients following HSCT.
(A) Serum from healthy donors (n = 5) and DOCK8-deficient patients (n = 4–18) collected before and after HSCT was analyzed for concentrations of total IgE and IgE specific for staple foods and dust mites. (B) Data in A expressed as a percentage of pre-transplant levels of total and allergen-specific IgE for each patient and plotted against the time after HSCT. Points joined by a line are from the same patient (patients 16 and 18 in Table 1) assayed at different times after HSCT. Dotted line indicates average of healthy control values as percentage of average of patient pre-transplant values.
HSCT has been reported to have mixed outcomes on allergy in DOCK8 deficiency (32, 46, 47). We also noted variability in reductions in total and allergen-specific IgE, ranging from 2- to >1000-fold (Figure 7A). To investigate this further, we expressed IgE levels following HSCT as a percentage of pre-transplant levels for each patient and as a function of time after HSCT. For this, we could also study 2 patients longitudinally. This revealed that the magnitude of the reduction in total and specific IgE levels in DOCK8-deficient patients was time dependent after transplant (Figure 7B). In general, food-specific IgE levels declined at a slower rate than total IgE levels (Figure 7B). Hence, elevated IgE levels in DOCK8-deficient patients were decreased with HSCT and tended to normalize over time.
Impact of HSCT on lymphocyte reconstitution in other PID patients.
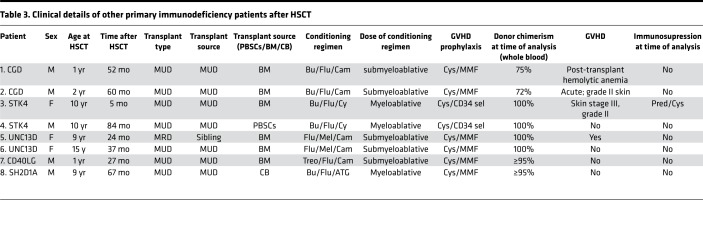
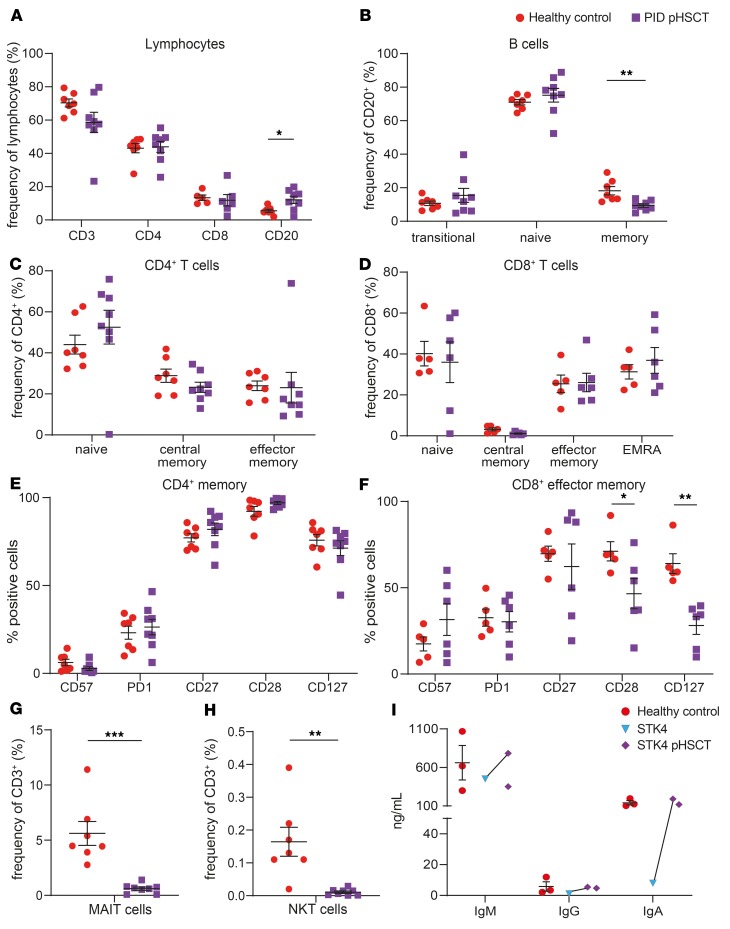
It was possible that some of the impairments in lymphocyte differentiation in transplanted DOCK8-deficient patients were unique to DOCK8 deficiency or a general consequence of HSCT in PID. To differentiate between these possibilities, we examined lymphocytes in 8 additional PID patients who underwent HSCT due to loss-of-function mutations in UNC13D (n = 2), STK4 (n = 2), CYBB (n = 2), CD40LG (n = 1), or SH2D1A (n = 1) (Table 3) (62–64). Time after HSCT ranged from 5 to 84 (mean 44.5) months (Table 3). Proportions of CD3+, CD4+, and CD8+ T cells in these transplanted PID patients were comparable to healthy controls, while B cells were increased (Figure 8A). Memory B cells were reduced following HSCT (Figure 8B), but differentiation of CD4+ and CD8+ T cells was normal (Figure 8, C and D). Expression of exhaustion/senescence markers on CD4+ memory T cells in these transplanted PID patients was comparable to that on CD4+ memory T cells from healthy donors (Figure 8E); however, patient CD8+ TEM cells showed decreased expression of CD28 and CD127 relative to controls (Figure 8F). Interestingly, these PID patients exhibited decreased frequencies of MAIT (Figure 8G) and NKT cells (Figure 8H) after HSCT.
Table 3. Clinical details of other primary immunodeficiency patients after HSCT.
Figure 8. Lymphocyte phenotype and differentiation in PID patients pHSCT.
PBMCs from healthy donors (n = 5–7) or patients with mutations in UNC13D (n = 2), STK4 (n = 2), CYBB (n = 2), CD40LG (n = 1), or SH2D1A (n = 1) who had previously undergone HSCT (PID pHSCT) (n = 6–8) were labeled with mAbs against CD3, CD4, CD8, CD20, CD45RA, CCR7, CD10, CD27, CD28, CD57, CD127, PD-1, TCRVα24, TCRVβ11, CD161 and TCR Vα7.2 (see Figure 1 legend). Proportions of (A) CD3+ cells, CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), B cells (CD20+); (B) transitional, naive and memory B cell subsets; (C) CD4+ naive, TCM, and TEM cell subsets; (D) CD8+ naive, TCM, TEM, and TEMRA cell subsets; (E) CD4+ memory and (F) CD8+ TEM cells expressing CD57, PD-1, CD27, CD28, and CD127; (G) MAIT cells; and (H) NKT cells were then determined by flow cytometric analysis. (I) Naive B cells were sorted from healthy donors (n = 3), STK4-deficient patients (n = 1), or STK4-deficient patients following HSCT (STK4 pHSCT) (n = 2) and cultured with CD40L + IL-21 for 5 days. After this time, culture supernatants were harvested, and secretion of IgM, IgG and IgA was determined by ELISAs. Points joined by lines represent data from the same patient before and after HSCT. Data are mean ± SEM. Statistical analysis was performed with Prism using unpaired t test; *P < 0.05, **P < 0.01, ***P < 0.005.
We investigated STK4-deficient patients in more detail, as STK4 deficiency shares clinical features with DOCK8 deficiency, including susceptibility to viral infections, elevated serum IgE, naive T cell lymphopenia, and reduced memory B cells (18, 62). Naive B cells from STK4-deficient patients exhibit impaired production of IgM, IgG, and IgA following in vitro stimulation with CD40L/IL-21 (62) (Figure 8I). However, Ig production by naive B cells isolated from these patients and activated in vitro was normalized following HSCT (Figure 8I). Thus, analysis of patients with DOCK8 deficiency or other inborn errors of immunity allowed us to identify specific and general outcomes for reconstitution of lymphocyte development, differentiation, and function following HSCT.
Discussion
The study of cellular defects due to DOCK8 deficiency is essential to reveal mechanisms underlying the constellation of clinical features in these patients. Previous studies showed that DOCK8 deficiency compromises memory B cell generation, NK cell cytotoxicity, NKT cell development, CD8+ T cell differentiation, CD4+ T cell cytokine production, and Treg function (23, 25–31, 33). We have confirmed and substantially extended these findings to produce a comprehensive catalog of phenotypic and functional defects in DOCK8-deficient lymphocytes, including impaired CD8+ T cell cytotoxicity, reduced B cell survival and proliferation, poor induction of Tfh-type cells, and defective generation of MAIT cells. Collectively, these defects explain the poor control of pathogen infections, impaired humoral immunity, and severe atopic disease in individuals with DOCK8 mutations.
HSCT is the only treatment capable of curing DOCK8 deficiency. Cellular mechanisms underlying clinical improvements after HSCT have not previously been established. DOCK8-deficient patients assessed in our study reported poor vaccine responses prior to HSCT (Table 1) but normal responses after HSCT (Table 2). This correlated with functional improvements in naive B cell survival, proliferation, and differentiation in vitro and a significant, albeit incomplete, increase in memory B cell formation in vivo. CD19 expression is reduced on DOCK8-deficient human B cells, and it has been proposed that this contributes to poor B cell responses in vivo (60). We confirmed this reduction in CD19 expression and noted that HSCT increased CD19 on patient B cells (data not shown); this may also facilitate improved B cell behavior in DOCK8-deficient patients after HSCT. Due to the kinetics of memory B cell generation after HSCT (50, 51) and the increases in serum IgG and IgA during the first years of life (65), reconstitution of the memory compartment in DOCK8-deficient patients is expected to increase with time following HSCT. Indeed, we observed that a normal frequency of memory B cells was achieved in patients who were ≥23 months after transplant (Supplemental Figure 2B). This was also observed for the non-DOCK8 PID patients examined, inasmuch as the patients who were tested ≥60 months after HSCT had the highest proportions of memory B cells in peripheral blood (Figure 8B). It is likely that the increased ability of naive and memory CD4+ T cells from HSCT DOCK8-deficient patients to produce IL-21 would also contribute to improved B cell function, given the potency of IL-21 in inducing human B cell proliferation and differentiation into plasma cells (PCs) (58).
The prevalence and severity of infections in DOCK8-deficient patients before transplant were dramatically reduced following HSCT. Many cellular changes are likely responsible for improved host defense, including increased frequencies, proliferation, and normalized differentiation of CD4+ T cells. Increased Th1 and Th17 cytokines would contribute to effective control of bacterial, viral, and fungal infections in transplanted DOCK8-deficient patients. Furthermore, restored differentiation and production of cytokines and cytolytic mediators by DOCK8-deficient CD8+ T cells would reduce viral infections after HSCT. The mild recovery of MAIT cells may also be important in host defense in DOCK8 deficiency after HSCT (66). Last, resolution of severe skin inflammation after HSCT could in part be attributed to decreased Th2 skewing and concordant reductions in total and allergen-specific IgE. IL-21R–deficient mice and humans have increased serum IgE (67, 68), and administering IL-21 to Dock8-deficient mice alleviates disease in an allergic asthma model (69). Thus, increased IL-21 production by CD4+ T cells in transplanted DOCK8-deficient patients may not only contribute to improved humoral immunity, but also mediate improvements in allergy and reductions in serum IgE. Similarly, since CpG activation suppresses IgE production by CD40L/IL-4–stimulated human naive B cells (61), restored TLR signaling in B cells after HSCT may also contribute to reductions in serum IgE in DOCK8-deficient patients.
Malignancies occur in 10%–15% of DOCK8-deficient patients. Indeed, ~30% of patients studied here reported malignancies prior to HSCT. Incidence of malignancies in transplanted patients has not been described, likely due to insufficient follow-up times. However, the lack of malignancy among patients in our study and other reports (37, 43, 44) suggests that by restoring CD8+ T cell cytotoxic function, HSCT also protects against tumorigenesis in DOCK8 deficiency.
Despite effective control of infections due to improved cellular function in patients after HSCT, memory T cells still exhibited modest signs of chronic activation/exhaustion. This could reflect reactivation of latent viruses. Indeed, EBV reactivation occurs in ~20% of HSCT patients (70), while CMV reactivation following HSCT is associated with exhausted CD8+ T cells compared with patients who did not experience viral reactivation after HSCT (71). Interestingly, 2 patients we studied had CMV reactivation after HSCT (DOCK8 patients 15 and 18), but no further complication. Additionally, exhaustion of CD4+ and CD8+ memory T cells in DOCK8-deficient patients was reduced ≥12 months after HSCT (Supplemental Figure 1, I and J), suggesting that the effects of viral reactivation are short lived due to functional reconstitution of immune cells.
HSCT has been reported to have variable outcomes for allergic disease in DOCK8-deficient patients. Thus, it has been reported that HSCT led to resolution (34, 37, 72), improvement (35, 44), or persistence (41, 47, 73) of allergies in these patients. However, a recent study of 56 transplanted DOCK8-deficient patients found that food allergies resolved or improved in 61% of cases (32). Furthermore, as a substantial proportion of this cohort avoided allergen exposure after HSCT, the actual level of allergy improvement was nearly 80% (34 of 43 patients) (32), suggesting that HSCT positively impacts allergic disease in DOCK8 deficiency. Our findings of decreased production of Th2 cytokines by memory CD4+ T cells and corresponding reductions in serum levels of allergen-specific IgE in DOCK8-deficient patients with time are consistent with decreased allergic disease in cohorts of transplanted DOCK8-deficient patients. Furthermore, the kinetics of reduction in total and allergen-specific IgE could explain conflicting results regarding the impact of HSCT on allergic disease in DOCK8 deficiency. Long-lived host PCs in bone marrow may produce IgE in DOCK8-deficient patients after HSCT (40, 44, 47). They may survive conditioning but be displaced from survival niches by newly generated PCs, resulting in apoptosis (74). Hence, the time-dependent decrease in allergen-specific IgE in transplanted patients could result from the gradual turnover of IgE+ PCs by the reconstituted donor immune system. Continued longitudinal assessment of transplanted patients will establish the long-term efficacy of HSCT on allergic disease in DOCK8-deficient patients.
Successful immune reconstitution following HSCT often depends on high donor chimerism (75). Previous reports detected >95% chimerism in CD4+ and CD8+ T cells (34, 36, 40, 41), but varying (0%–100%) levels for B cells (38, 41), in HSCT DOCK8-deficient patients. Despite this, DOCK8-deficient patients with mixed B cell chimerism showed improvements comparable to patients with complete donor chimerism (40), indicating that high donor chimerism is not the sole determinant of restored immune function following HSCT of these patients. Interestingly, some DOCK8-deficient patients undergo somatic reversion and reexpress functional DOCK8 protein in leukocytes (48). DOCK8 was expressed in high proportions (>50%) of memory T cells from somatically reverted patients, but in fewer (<10%) naive CD4+ T cells, B cells, and NK cells (48). Although they had DOCK8+ lymphocytes, clinical improvements in DOCK8-revertant patients were mild, with all continuing to have significant disease and some undergoing HSCT (48). This may be because the revertant population continues to express an exhausted/senescent phenotype (48), which may compromise their response to antigenic stimuli. These data also suggest that DOCK8 is required in most lymphocyte populations, including naive T and B cells, to achieve significant clinical improvements. Indeed, the finding that the TCR repertoire of DOCK8-revertant cells is dominated by a few Vβ clonotypes (48) implies that a restricted repertoire is not conducive to eliciting robust immune responses in DOCK8-revertant patients. We detected >90% donor chimerism and normal levels of DOCK8 expression in all transplanted patients. This likely explains the dramatic differences in disease outcome in DOCK8-deficient patients who have undergone HSCT and those with somatic reversion in only some lymphoid cells.
A caveat of our study was that we could not analyze pre- and post-HSCT samples from all DOCK8-deficient patients. However, there were several matched patients. Examination of these individual patients before and after transplant revealed results identical to those from all untransplanted and transplanted DOCK8-deficient patients (Supplemental Figure 3). This indicates that our collective results are representative of analysis derived from matched patients.
Comparison of our results with data from other PID patients who underwent HSCT highlights outcomes that are a general consequence of the transplant process and those that are unique to DOCK8 deficiency. The shared characteristic of reduced frequencies of memory B cells across both cohorts of patients was consistent with the kinetics of B cell reconstitution after HSCT in other clinical settings (50). The lack of complete reconstitution of MAIT and NKT cells after HSCT in both the DOCK8-deficient and the other PID patients after HSCT has similarly been reported in patients undergoing HSCT for hematological malignancies as late as 12–24 months following transplant (76, 77). This may result from increased sensitivity of MAIT cells to GVHD prophylactic immunosuppressive drugs (77). These observations for MAIT and NKT cells in DOCK8 deficiency and the other PIDs examined are reminiscent of the persistent deficiency of other populations of innate-type lymphocytes, including NK cells and ILCs, in X-SCID and JAK/SCID patients decades after HSCT (78). Thus, an alterative explanation is an inability of precursors of different innate lymphoid cell populations to adequately seed developmental niches following HSCT to enable the reconstitution of these immune cell lineages. In contrast to these findings, DOCK8-deficient patients continued to exhibit greater signs of T cell exhaustion/senescence after HSCT, with CD8+ T cells in transplanted DOCK8-deficient patients remaining skewed toward TEM and TEMRA and being enriched for CD57+CD127dim cells.
In conclusion, we detail the numerous adverse effects of DOCK8 deficiency on the differentiation and function of CD8+ and CD4+ T, MAIT, NK, and B cells that underlie clinical features of patients with DOCK8 mutations. By demonstrating that effective restoration of key functional defects in adaptive immune cells following HSCT corresponds to improved clinical features, we have identified cellular mechanisms for the clinical efficacy of HSCT as a treatment for DOCK8 deficiency. Comparison to other PID patients who have also undergone HSCT identified unique and general consequences of HSCT in PIDs. Combining our defined readouts of cellular function with clinical features after HSCT may facilitate predicting long-term outcomes for DOCK8-deficient patients undergoing potentially curative HSCT. Such an approach has been applied to SCID patients, with several clinical, cellular, and functional improvements being established as predictors of successful outcomes of HSCT (12, 79, 80). Collectively, our results underscore the value and importance of determining the impact of monogenic mutations on immune cell function and applying these findings following therapeutic interventions in order to ensure optimal patient management and outcomes.
Methods
For antibodies and reagents used in this study, see Supplemental Table 1.
Human samples.
PBMCs and plasma were from healthy volunteers (Australian Red Cross) and DOCK8-deficient patients before and/or after HSCT (Tables 1 and 2). HSCT conditioning is detailed in Table 2. PBMCs were available from 7 patients before and after HSCT, and one patient at 2 time points and another patient at 3 time points after HSCT. Plasma was analyzed for total and allergen-specific IgE (food or mite mix) (26). PBMCs were also collected from patients with recessive mutations in CYBB, STK4, or UNC13D or hemizygous CD40LG or SH2D1A mutations who had previously undergone HSCT (Table 3) (62–64).
Phenotyping.
Proportions of CD3+, CD4+ T (CD3+CD4+), CD8+ T (CD3+CD8+), B (CD20+) cells; naive (N; CD45RA+CCR7+), central memory (TCM; CD45RA–CCR7+), effector memory (TEM; CD45RA–CCR7–), CD45RA+ revertant memory (TEMRA; CD45RA+CCR7–) cells; αβ (CD3+TCRαβ+) and γδ (CD3+TCRγδ+) T cells; mucosal associated invariant T (MAIT; CD3+TCRVα7.2+CD161+), NK (CD3–CD56+), and NKT (CD3+TCRVα24+ Vβ11+) cells; transitional (CD20+CD10+CD27–), naive (CD20+CD10–CD27–), memory (CD20+CD10–CD27+), and class-switched (CD20+CD27+ IgD–IgM–) B cell subsets were determined by flow cytometry (23, 51, 53, 55, 66). PD-1, CD57, CD27, CD28, and CD127 expression was examined on T cells to examine exhaustion (23, 53).
Analysis of DOCK8 expression.
PBMCs were labeled with CD3, CD4, CD8, CD20, CD56, TCRVα24, TCRVβ11, CD161, and Vα7.2 mAbs, fixed, permeabilized, and stained with anti-DOCK8. Alternatively, PBMCs were labeled with mouse IgG2 mAbs to CD4, CD8, and CD20; fixed; permeabilized; and stained with anti-DOCK8 or isotype control IgG1, then with PE–anti-mouse IgG1.
Lymphocyte isolation and functional analysis.
Naive and memory CD4+ T cells were isolated after excluding Tregs (CD25hiCD127lo) and sorting CD4+CD45RA+CCR7+ and CD4+CD45RA– cells, respectively (55). CD8+ T and naive B cells were isolated as CD8+CD4– and CD20+CD10–CD27–IgG– cells (23, 51), respectively. B cells were cultured with CD40L (200 ng/ml), 50 ng/ml IL-21, 1 μg/ml CpG 2006, and/or 0.05% Staphylococcus aureus Cowan (SAC) to crosslink the BCR (51, 81). Cells were harvested, stained with Zombie Aqua dye to determine viability, and enumerated with Calibrite beads. Ig secretion was determined as described previously (51). CD8+ T cells were cultured as described previously (23, 53, 54). CD4+ T cells were cultured under Th0-, Th1-, Th2-, or Th17-polarizing conditions, and cytokine production wa determined (26, 55). Cells were labeled with CFSE (23, 26); proliferation was determined by assessing CFSE dilution (26).
Statistics.
All statistical analysis was performed with GraphPad Prism using unpaired t test with Welch’s correction. P values less than 0.05 were considered significant.
Study approval.
This study was approved by the Sydney Local Health District RPAH Zone Human Research Ethics Committee and Research Governance Office, Royal Prince Alfred Hospital, Camperdown, New South Wales, Australia (protocol X16-0210/LNR/16/RPAH/257); the South East Sydney Local Health District Human Research Ethics Committee, Prince of Wales/Sydney Children’s Hospital (protocol HREC/11/POWH/152); the Royal Children’s Hospital Melbourne Human Research Ethics Committee, Parkville, Victoria, Australia (protocol 33146A) and the National Institute of Allergy and Infectious Diseases Institutional Review Board, Bethesda, Maryland, USA (protocol 95-I-0066). Written informed consent was obtained from participants or their guardians.
Author contributions
BAP designed the research, performed experiments, analyzed and interpreted results, and wrote the manuscript; DTA performed experiments and analyzed results; JMS, TC, SC, DC, PEG, KF, RM, TGP, MW, DEC, PH, JBZ, JP, FA, AJC, CP, JB, BN, GU, PDA, JLC, HCS, AFF, NS, and DDH provided patient samples and clinical details and managed patient care; SGT and CSM designed the research, analyzed and interpreted results, wrote the manuscript, and provided funding for the project; all authors commented on the manuscript.
Supplementary Material
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia (NHMRC) (1060303; 1088215; 1127157; 1139865), the Office of Health and Medical Research of the New South Wales Government, the Jeffrey Modell Foundation, the John Cook Brown Foundation, and the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases and National Cancer Institute, NIH. BAP is supported by a Research Training Program Scholarship awarded by the Australian Government. TGP is a Senior Research Fellow (no. 1155678) and SGT was a Principal Research Fellow (no. 1042925) of the NHMRC. CSM is supported by an Early-Mid Career Research Fellowship from the New South Wales Government.
Version 1. 04/25/2019
In-Press Preview
Version 2. 06/06/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019 American Society for Clinical Investigation
Reference information: JCI Insight. 2019;4(11):e127527. https://doi.org/10.1172/jci.insight.127527.
Contributor Information
Bethany A. Pillay, Email: b.pillay@garvan.org.au.
Danielle T. Avery, Email: d.priestley@garvan.org.au.
Joanne M. Smart, Email: j.smart@rch.gov.au.
Theresa Cole, Email: Theresa.Cole@rch.org.au.
Sharon Choo, Email: Sharon.Choo@rch.org.au.
Damien Chan, Email: Damien.Chan@sa.gov.au.
Paul E. Gray, Email: Paul.Gray@SESIAHS.HEALTH.NSW.GOV.AU.
Katie Frith, Email: catherine.frith@health.nsw.gov.au.
Richard Mitchell, Email: richard.mitchell@unsw.edu.au.
Tri Giang Phan, Email: t.phan@garvan.org.au.
Melanie Wong, Email: melanie.wong@health.nsw.gov.au.
Dianne E. Campbell, Email: dianne.campbell1@health.nsw.gov.au.
Peter Hsu, Email: peter.hsu@health.nsw.gov.au.
John B. Ziegler, Email: j.ziegler@unsw.edu.au.
Jane Peake, Email: j.peake@uq.edu.au.
Frank Alvaro, Email: Frank.Alvaro@hnehealth.nsw.gov.au.
Capucine Picard, Email: capucine.picard@inserm.fr.
Jacinta Bustamante, Email: jacinta.bustamante@inserm.fr.
Benedicte Neven, Email: benedicte.neven@nck.aphp.fr.
Andrew J. Cant, Email: Andrew.Cant@nuth.nhs.uk.
Gulbu Uzel, Email: guzel@niaid.nih.gov.
Peter D. Arkwright, Email: peter.arkwright@nhs.net.
Jean-Laurent Casanova, Email: Jean-Laurent.Casanova@rockefeller.edu.
Helen C. Su, Email: Hsu@niaid.nih.gov.
Nirali Shah, Email: nirali.shah@nih.gov.
Dennis D. Hickstein, Email: hicksted@mail.nih.gov.
Stuart G. Tangye, Email: s.tangye@garvan.org.au.
Cindy S. Ma, Email: c.ma@garvan.org.au.
References
- 1.Picard C, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee report on inborn errors of immunity. J Clin Immunol. 2018;38(1):96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer A, Notarangelo LD, Neven B, Cavazzana M, Puck JM. Severe combined immunodeficiencies and related disorders. Nat Rev Dis Primers. 2015;1:15061. doi: 10.1038/nrdp.2015.61. [DOI] [PubMed] [Google Scholar]
- 3.Meyts I, et al. Exome and genome sequencing for inborn errors of immunity. J Allergy Clin Immunol. 2016;138(4):957–969. doi: 10.1016/j.jaci.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer A, Provot J, Jais JP, Alcais A, Mahlaoui N, members of the CEREDIH French PID study group Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J Allergy Clin Immunol. 2017;140(5):1388–1393.e8. doi: 10.1016/j.jaci.2016.12.978. [DOI] [PubMed] [Google Scholar]
- 5.Lyons JJ, Milner JD. Primary atopic disorders. J Exp Med. 2018;215(4):1009–1022. doi: 10.1084/jem.20172306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayor PC, et al. Cancer in primary immunodeficiency diseases: cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol. 2018;141(3):1028–1035. doi: 10.1016/j.jaci.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puck JM. Newborn screening for severe combined immunodeficiency and T-cell lymphopenia. Immunol Rev. 2019;287(1):241–252. doi: 10.1111/imr.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seleman M, Hoyos-Bachiloglu R, Geha RS, Chou J. Uses of next-generation sequencing technologies for the diagnosis of primary immunodeficiencies. Front Immunol. 2017;8:847. doi: 10.3389/fimmu.2017.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2(7583):1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 10.Hong R, Cooper MD, Allan MJ, Kay HE, Meuwissen H, Good RA. Immunological restitution in lymphopenic immunological deficiency syndrome. Lancet. 1968;1(7541):503–506. doi: 10.1016/s0140-6736(68)91468-2. [DOI] [PubMed] [Google Scholar]
- 11.Brown L, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117(11):3243–3246. doi: 10.1182/blood-2010-08-300384. [DOI] [PubMed] [Google Scholar]
- 12.Haddad E, et al. SCID genotype and 6-month posttransplant CD4 count predict survival and immune recovery. Blood. 2018;132(17):1737–1749. doi: 10.1182/blood-2018-03-840702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimall J, et al. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood. 2017;130(25):2718–2727. doi: 10.1182/blood-2017-05-781849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002;99(3):872–878. doi: 10.1182/blood.V99.3.872. [DOI] [PubMed] [Google Scholar]
- 15.Pai SY, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014;371(5):434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dvorak CC, et al. Comparison of outcomes of hematopoietic stem cell transplantation without chemotherapy conditioning by using matched sibling and unrelated donors for treatment of severe combined immunodeficiency. J Allergy Clin Immunol. 2014;134(4):935–943.e15. doi: 10.1016/j.jaci.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su HC, Jing H, Angelus P, Freeman AF. Insights into immunity from clinical and basic science studies of DOCK8 immunodeficiency syndrome. Immunol Rev. 2019;287(1):9–19. doi: 10.1111/imr.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.et al. Human inborn errors of the actin cytoskeleton affecting immunity: way beyond WAS WIP. Immunol Cell Biol. 2019;97(4):389–402. doi: 10.1111/imcb.12243. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361(21):2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhardt KR, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124(6):1289–302.e4. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelhardt KR, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2015;136(2):402–412. doi: 10.1016/j.jaci.2014.12.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aydin SE, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options — a review of 136 patients. J Clin Immunol. 2015;35(2):189–198. doi: 10.1007/s10875-014-0126-0. [DOI] [PubMed] [Google Scholar]
- 23.Randall KL, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208(11):2305–2320. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, et al. DOCK8 regulates lymphocyte shape integrity for skin antiviral immunity. J Exp Med. 2014;211(13):2549–2566. doi: 10.1084/jem.20141307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keles S, et al. Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes T. J Allergy Clin Immunol. 2016;138(5):1384–1394.e2. doi: 10.1016/j.jaci.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tangye SG, et al. Dedicator of cytokinesis 8-deficient CD4. J Allergy Clin Immunol. 2017;139(3):933–949. doi: 10.1016/j.jaci.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambe T, et al. DOCK8 is essential for T-cell survival and the maintenance of CD8+ T-cell memory. Eur J Immunol. 2011;41(12):3423–3435. doi: 10.1002/eji.201141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen E, et al. DOCK8 deficient patients have a breakdown in peripheral B cell tolerance and defective regulatory T cells. J Allergy Clin Immunol. 2014;134(6):1365–1374. doi: 10.1016/j.jaci.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizesko MC, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2013;131(3):840–848. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearney CJ, et al. DOCK8 drives Src-dependent NK cell effector function. J Immunol. 2017;199(6):2118–2127. doi: 10.4049/jimmunol.1700751. [DOI] [PubMed] [Google Scholar]
- 31.Crawford G, et al. DOCK8 is critical for the survival and function of NKT cells. Blood. 2013;122(12):2052–2061. doi: 10.1182/blood-2013-02-482331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aydin SE, et al. Hematopoietic stem cell transplantation as treatment for patients with DOCK8 deficiency. J Allergy Clin Immunol Pract. 2019;7(3):848–855. doi: 10.1016/j.jaip.2018.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jabara HH, et al. DOCK8 functions as an adaptor that links Toll-like receptor–MyD88 signaling to B cell activation. Nat Immunol. 2012;13(6):612–620. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boztug H, et al. Clinical and immunological correction of DOCK8 deficiency by allogeneic hematopoietic stem cell transplantation following a reduced toxicity conditioning regimen. Pediatr Hematol Oncol. 2012;29(7):585–594. doi: 10.3109/08880018.2012.714844. [DOI] [PubMed] [Google Scholar]
- 35.Metin A, et al. Successful bone marrow transplantation for DOCK8 deficient hyper IgE syndrome. Pediatr Transplant. 2012;16(4):398–399. doi: 10.1111/j.1399-3046.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 36.Cuellar-Rodriguez J, et al. Matched related and unrelated donor hematopoietic stem cell transplantation for DOCK8 deficiency. Biol Blood Marrow Transplant. 2015;21(6):1037–1045. doi: 10.1016/j.bbmt.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuşkonmaz B, Ayvaz D, Tezcan İ, Yüce A, Sanal Ö, Çetinkaya DU. Successful hematopoietic stem cell transplantation after myeloablative conditioning in three patients with dedicator of cytokinesis 8 deficiency (DOCK8) related Hyper IgE syndrome. Bone Marrow Transplant. 2018;53(3):339–343. doi: 10.1038/s41409-017-0040-1. [DOI] [PubMed] [Google Scholar]
- 38.Shah NN, et al. Haploidentical related donor hematopoietic stem cell transplantation for dedicator-of-cytokinesis 8 deficiency using post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2017;23(6):980–990. doi: 10.1016/j.bbmt.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S, et al. Haploidentical stem cell transplantation in DOCK8 deficiency — successful control of pre-existing severe viremia with a TCRaß/CD19-depleted graft and antiviral treatment. Clin Immunol. 2014;152(1-2):111–114. doi: 10.1016/j.clim.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Al-Herz W, et al. Hematopoietic stem cell transplantation outcomes for 11 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2016;138(3):852–859.e3. doi: 10.1016/j.jaci.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman AF, et al. Haploidentical related donor hematopoietic stem cell transplantation with post-transplantation cyclophosphamide for DOCK8 deficiency. J Allergy Clin Immunol Pract. 2016;4(6):1239–1242. doi: 10.1016/j.jaip.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uygun DFK, et al. Hematopoietic stem cell transplantation from unrelated donors in children with DOCK8 deficiency. Pediatr Transplant. 2017;21(7):e13015. doi: 10.1111/petr.13015. [DOI] [PubMed] [Google Scholar]
- 43.Gatz SA, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46(4):552–556. doi: 10.1038/bmt.2010.169. [DOI] [PubMed] [Google Scholar]
- 44.Bittner TC, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr. 2010;222(6):351–355. doi: 10.1055/s-0030-1265135. [DOI] [PubMed] [Google Scholar]
- 45.Al Shekaili L, et al. Novel mutation in DOCK8-HIES with severe phenotype and successful transplantation. Clin Immunol. 2017;178:39–44. doi: 10.1016/j.clim.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Azık F, et al. Resolution of food-induced anaphylaxis in DOCK8-deficient patients following bone marrow transplantation. Turk J Pediatr. 2015;57(1):112–115. [PubMed] [Google Scholar]
- 47.Happel CS, et al. Food allergies can persist after myeloablative hematopoietic stem cell transplantation in dedicator of cytokinesis 8-deficient patients. J Allergy Clin Immunol. 2016;137(6):1895–1898.e5. doi: 10.1016/j.jaci.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jing H, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014;133(6):1667–1675. doi: 10.1016/j.jaci.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pai SY, et al. Flow cytometry diagnosis of dedicator of cytokinesis 8 (DOCK8) deficiency. J Allergy Clin Immunol. 2014;134(1):221–223. doi: 10.1016/j.jaci.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuss AK, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol. 2006;176(3):1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 51.Suryani S, et al. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood. 2010;115(3):519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- 52.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards ESJ, et al. Activating PIK3CD mutations impair human cytotoxic lymphocyte differentiation and function and EBV immunity. J Allergy Clin Immunol. 2019;143(1):276–291.e6. doi: 10.1016/j.jaci.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 54.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1-2):65–78. doi: 10.1016/S0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 55.Ma CS, et al. Monogenic mutations differentially affect the quantity and quality of T follicular helper cells in patients with human primary immunodeficiencies. J Allergy Clin Immunol. 2015;136(4):993–1006.e1. doi: 10.1016/j.jaci.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gett AV, Hodgkin PD. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci USA. 1998;95(16):9488–9493. doi: 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol. 2004;113(3):395–400. doi: 10.1016/j.jaci.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 58.Moens L, Tangye SG. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front Immunol. 2014;5:65. doi: 10.3389/fimmu.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Randall KL, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol. 2009;10(12):1283–1291. doi: 10.1038/ni.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun X, et al. Dock8 regulates BCR signaling and activation of memory B cells via WASP and CD19. Blood Adv. 2018;2(4):401–413. doi: 10.1182/bloodadvances.2017007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Massaad MJ, et al. DOCK8 and STAT3 dependent inhibition of IgE isotype switching by TLR9 ligation in human B cells. Clin Immunol. 2017;183:263–265. doi: 10.1016/j.clim.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Moran I, et al. B cell-intrinsic requirement for STK4 in humoral immunity in mice and human subjects. J Allergy Clin Immunol. 2019;null:null. doi: 10.1016/j.jaci.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Gray PE, et al. Cerebral vasculitis in X-linked lymphoproliferative disease cured by matched unrelated cord blood transplant. J Clin Immunol. 2015;35(7):604–609. doi: 10.1007/s10875-015-0194-9. [DOI] [PubMed] [Google Scholar]
- 64.Gray PE, et al. Late-onset non-HLH presentations of growth arrest, inflammatory arachnoiditis, and severe infectious mononucleosis, in siblings with hypomorphic defects in UNC13D. Front Immunol. 2017;8:944. doi: 10.3389/fimmu.2017.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buckley RH, Dees SC, O’Fallon WM. Serum immunoglobulins. I. Levels in normal children and in uncomplicated childhood allergy. Pediatrics. 1968;41(3):600–611. [PubMed] [Google Scholar]
- 66.Wilson RP, et al. STAT3 is a critical cell-intrinsic regulator of human unconventional T cell numbers and function. J Exp Med. 2015;212(6):855–864. doi: 10.1084/jem.20141992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozaki K, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298(5598):1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 68.Kotlarz D, Ziętara N, Milner JD, Klein C. Human IL-21 and IL-21R deficiencies: two novel entities of primary immunodeficiency. Curr Opin Pediatr. 2014;26(6):704–712. doi: 10.1097/MOP.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 69.Wu J, et al. IL-21 alleviates allergic asthma in DOCK8-knockout mice. Biochem Biophys Res Commun. 2018;501(1):92–99. doi: 10.1016/j.bbrc.2018.04.179. [DOI] [PubMed] [Google Scholar]
- 70.Lankester AC. Management of Epstein-Barr virus reactivation following allogeneic stem cell transplantation. Reports of Practical Oncology & Radiotherapy. 2007;12(3):163–165. doi: 10.1016/S1507-1367(10)60052-1. [DOI] [Google Scholar]
- 71.Scherrenburg J, Pietersma F, Jacobi R, Schuurman R, Meijer E, van Baarle D. Cytomegalovirus (CMV)-reactivation influences T-cell differentiation and CMV-specific T-cell reconstitution after stem cell transplantation. J Clin Cell Immunol. 2012;S9:001 [Google Scholar]
- 72.Barlogis V, et al. Successful allogeneic hematopoietic stem cell transplantation for DOCK8 deficiency. J Allergy Clin Immunol. 2011;128(2):420–22.e2. doi: 10.1016/j.jaci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 73.Al-Herz W, et al. Clinical, immunologic and genetic profile of DOCK8-deficient patients in Kuwait. Clin Immunol. 2012;143(3):266–272. doi: 10.1016/j.clim.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radbruch A, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6(10):741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 75.Khan F, Agarwal A, Agrawal S. Significance of chimerism in hematopoietic stem cell transplantation: new variations on an old theme. Bone Marrow Transplant. 2004;34(1):1–12. doi: 10.1038/sj.bmt.1704525. [DOI] [PubMed] [Google Scholar]
- 76.Haraguchi K, et al. Recovery of Valpha24+ NKT cells after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;34(7):595–602. doi: 10.1038/sj.bmt.1704582. [DOI] [PubMed] [Google Scholar]
- 77.Solders M, et al. Mucosal-associated invariant T cells display a poor reconstitution and altered phenotype after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2017;8:1861. doi: 10.3389/fimmu.2017.01861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vely F, et al. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2016;17(11):1291–1299. doi: 10.1038/ni.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Recher M, et al. IL-21 is the primary common γ chain-binding cytokine required for human B-cell differentiation in vivo. Blood. 2011;118(26):6824–6835. doi: 10.1182/blood-2011-06-362533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miggelbrink AM, et al. B-cell differentiation and IL-21 response in. Blood. 2018;131(26):2967–2977. doi: 10.1182/blood-2017-10-809822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romagnani S, et al. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol. 1981;127(4):1307–1313. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.