Abstract
Mesenchymal stem cell (MSC)-mediated immunomodulation affects both innate and adaptive immune systems. These responses to environmental cues, such as pathogen-associated molecular patterns, damage-associated molecular patterns, or proinflammatory cytokines, are crucial for resolution of inflammation, as well as successful tissue healing and regeneration. We observed that intermittent, repeated exposure of MSCs to LPS induced stronger NF-κB activation than singular stimulation. A similar phenomenon, named innate immune memory or trained immunity, has been reported with macrophages. However, the potential regulation of “immune memory” in nonclassic immune cells, such as MSCs, has not been reported. In the current study, we chose IFN-γ plus TNF-α restimulation-induced iNOS expression as a model of MSC activation, because IFN-γ and TNF-α play crucial roles in MSC-mediated immunomodulation. The iNOS expression was enhanced in LPS-trained MSCs, 3 d after a washout period following primary stimulation. LPS-trained MSCs enhanced the anti-inflammatory (arginase 1 and CD206) marker expression, but decreased the proinflammatory marker (TNF-α, IL-1β, iNOS, and IL-6) expression using an MSC-macrophage coculture model. In contrast, LPS-trained MSCs demonstrated a defective regulation on CD4 T-cell proliferation. Mechanistic studies suggested that histone methylation and the JNK pathway are involved in LPS-trained immunomodulation in MSCs. Our results demonstrate differential immunomodulatory effects of trained MSCs on macrophages and T cells. These immunomodulatory consequences are critical, because they will have a major impact on current MSC-based cell therapies.—Lin, T., Pajarinen, J., Kohno, Y., Huang, J.-F., Maruyama, M., Romero-Lopez, M., Nathan, K., Yao, Z., Goodman, S. B. Trained murine mesenchymal stem cells have anti-inflammatory effect on macrophages, but defective regulation on T-cell proliferation.
Keywords: trained immunity, immunomodulation, T lymphocyte
Mesenchymal stem cells (MSCs) were first identified from bone marrow and are characterized by their multilineage differentiation abilities into mesodermal tissues, including bone and cartilage (1, 2). MSCs are distributed in the perivascular niche of most tissues (3). MSCs are capable of sensing environmental cues, and their cellular responses are crucial for the resolution of inflammation and successful tissue healing and regeneration (4). MSC-mediated immunomodulation includes both the innate and adaptive immune systems. For example, crosstalk between MSCs and macrophages is critical for initiation of protective mechanisms during septic shock (5). MSCs can also inhibit the adaptive immune response via suppression of T-cell proliferation, induction of T-cell apoptosis, and recruitment of regulatory T cells (6). The potential of MSC-based therapy in immune-related diseases has been demonstrated in more than 400 clinical trials (7).
The cellular responses of MSCs to pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns, or proinflammatory cytokines underscores the resolution of inflammation (8). Németh et al. (5) demonstrated that LPS, the gram-negative bacteria-derived endotoxin, enhanced NF-κB activation, iNOS expression, and prostaglandin E2 production in MSCs using in vitro and in vivo models. Activated MSCs induced the secretion of the anti-inflammatory cytokine IL-10 by macrophages through paracrine and cell-to-cell contact regulations. Ren et al. (9) showed that IFN-γ plus one of the proinflammatory cytokines (TNF-α, IL-1β, or IL-1α) synergistically induced iNOS expression by MSCs, which is essential for the suppression of T-cell proliferation.
Recent studies indicate that proinflammatory stimulation may have prolonged effects on the function of MSCs. MSCs preconditioned by transient exposure to inflammatory cytokines alone or combined with PAMP enhanced immunomodulation and tissue regeneration (10–12). We recently observed that when MSCs were exposed to LPS repeatedly, NF-κB activation was significantly increased compared with single LPS exposure (13). A similar phenomenon of intermitted stimulus leading to dissimilar and nonstereotypical responses in innate immunity has been reported in studies conducted with macrophages exposed to PAMPs such as β-glucans and LPS and has been named “innate immune memory” or “trained immunity” (14–16). Such cellular responses raise the possibility that immune memory may not only exist in the adaptive immune system but also in innate immune cells (16). However, the potential regulation of immune memory in nonclassic immune cells, such as MSCs, has not been reported.
In the current study, we found that LPS-trained MSCs have enhanced immunomodulatory capabilities on the proinflammatory response of macrophages. We also observed that LPS-trained MSCs have a defective regulation on T-cell proliferation. The potential involvement of signal transduction and epigenetic regulation on gene activation in LPS-trained MSCs was also characterized.
MATERIALS AND METHODS
Cells
The methods of isolating murine bone marrow derived MSCs have been previously described (17). Stanford’s Administrative Panel on Laboratory Animal Care approved this isolation protocol (APLAC 17566), and institutional guidelines for the care and use of laboratory animals were observed in all aspects of this project. In brief, bone marrow was collected from the femurs and tibias of 8–10 wk-old C57BL/6 or Balb/c male mice. For MSC isolation, the cells were carefully suspended and passed through a 70 µm strainer, spun down, and resuspended in α-minimal essential medium supplied with 10% MSC certified fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) and antibiotic-antimycotic solution (100 units of penicillin, 100 µg of streptomycin, and 0.25 µg of amphotericin B per milliliter, HyClone; Thermo Fisher Scientific). Medium was replaced the next day to remove the unattached cells (passage 1). The immunophenotype of isolated MSCs [spinocerebellar ataxia type 1 (Sca1+)/CD105+/CD44+/CD45−/CD34−/CD11b−] was characterized by an LSR II Flow Cytometer (BD Biosciences, San Jose, CA, USA) at passage 4.
For macrophage isolation, the bone marrow cells were washed 3 times with culture medium [Roswell Park Memorial Institute (RPMI) 1640 medium, supplemented with 10% heat inactivated FBS, and the antibiotic-antimycotic solution], resuspended in the culture medium containing 30% of L929 cell conditioned medium and 10 ng/ml mouse macrophage colony stimulation factor (R&D Systems, Minneapolis, MN, USA), and replated in T-175 culture flasks at a concentration of 4 × 107 cells per flask. Cells were allowed to expand for 5–7 d, with a medium change at the second day to remove nonadherent cells.
For CD4 T-cell isolation, splenocytes were collected from 8–10 wk-old C57BL/6 male mice using sterile technique. Red blood cells were first depleted by using red blood cell lysis buffer (MilliporeSigma, Burlington, MA, USA). After washing the cells, CD4+ T cells were isolated by CD4 negative selection magnetic microbeads (StemCell Technologies, Vancouver, BC, Canada). The instructions for cell isolation system were followed carefully. After isolation, the cells were resuspended in the RPMI medium (Thermo Fisher Scientific) containing 1 mM sodium pyruvate (Thermo Fisher Scientific), 10% heat inactivated FBS (Thermo Fisher Scientific), 55 µM 2-ME (Thermo Fisher Scientific), and antibiotic-antimycotic solution (9). The isolated cells were activated before being cocultured with MSCs, as described in CD4 T-cell proliferation assay.
Mouse embryonic fibroblasts (from C57BL/6 mice) at passage 1 were purchased from MilliporeSigma and were cultured in DMEM, supplemented with 10% heat inactivated FBS, and the antibiotic-antimycotic solution. Cells at passages 2–4 were used in the experiments.
Reagents
LPS (L3137), anacardic acid, and pargyline were purchased from MilliporeSigma. L-α- and D-α-hydroxyglutaric acid, 5′-deoxy-5′-methylthioadenosine, protein kinase B (Akt) inhibitor VIII, PD98059 (MEK inhibitor), SB202190 [p38 mitogen-activated protein kinase (p38) inhibitor], SP600125 (JNK inhibitor), and IKK2 inhibitor VI were purchased from Cayman Chemical (Ann Arbor, MI, USA). Recombinant mouse IFN-γ and TNF-α were purchased from R&D Systems.
Preparation of trained MSCs and macrophages
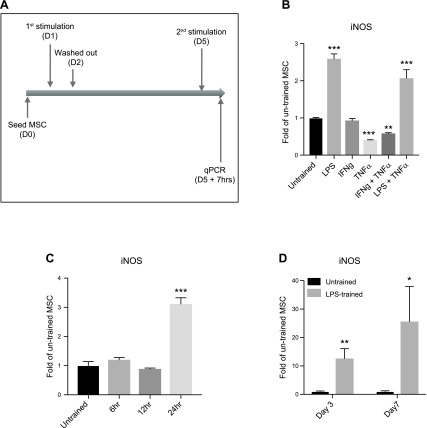
Mouse MSCs were treated with 20 μg/ml LPS for 24 h or otherwise as indicated below. The dose of LPS was determined based on a previous study (10). The trained MSCs were washed by PBS and incubated with fresh medium without LPS for 3 d before the secondary stimulations (Fig. 1A). The LPS-trained or tolerant macrophages were prepared as illustrated in Supplemental Fig. S2A.
Figure 1.
Increased iNOS expression in LPS-trained MSCs. A) Illustration of experimental outline. qPCR, quantitative PCR; D, day. B) The expression of iNOS in MSCs trained with different first stimulations and restimulated with IFN-γ plus TNF-α. C) The expression of iNOS in LPS-trained MSCs with different training time (6, 12, or 24 h). D) The expression of iNOS in LPS-trained MSCs at 3 or 7 d later. *P < 0.05, **P < 0.01, ***P < 0.005 compared with the untrained MSCs.
RNA extraction and quantitative PCR
The trained or control MSCs were treated with 20 ng/ml IFN-γ plus 20 ng/ml TNF-α (9) or 1 μg/ml LPS (5) for 7 h at 3 or 7 d later. Cellular RNAs were extracted by using RNeasy RNA Purification Kit (Qiagen, Venlo, The Netherlands). RNAs were reverse transcribed into cDNA using a high-capacity cDNA archive kit (Thermo Fisher Scientific). Probes for 18S rRNA, TNF-α, IL-1β, IL-1Ra, IL-6, iNOS, arginase 1 (Arg1), CD206, C-C motif chemokine ligand 2 (CCL2), C-X-C motif chemokine ligand 9 (CXCL9) (9), cyclooxygenase-2 (COX2) (5), SMAD-specific E3 ubiquitin protein ligase 2 (Smurf2) (18), tafazzin (TAZ) (19), programmed death-ligand 1 (20), indoleamine 2,3-deoxygenase (IDO) (21), and TGF-β (22) were purchased from Thermo Fisher Scientific. RT-PCR was performed in an ABI 7900HT Sequencing Detection System (Thermo Fisher Scientific), using the 18S rRNA as the internal control. The −ΔΔCt relative quantitation method was used to evaluate gene expression level. The samples in each group were prepared in triplicate. The experiments were repeated independently in different batches of MSCs from C57BL/6 male mice or Balb/c male mice.
Macrophage-MSC coculture system
The macrophage-MSC coculture system was set up based on previous studies (Fig. 2A) (5). Primary macrophages were seeded and allowed to attach for 1 h of incubation. The trained or control MSCs were then seeded and incubated overnight. The cocultured cells were treated with 1 μg/ml LPS. The supernatant was collected 5 h later, and the secretion of IL-10 and TNF-α was examined by ELISA (R&D Systems). Macrophage polarization marker expression was examined by quantitative PCR. The samples in each group were prepared in triplicate, and the experiments were repeated twice independently.
Figure 2.
LPS-trained MSCs have enhanced immunomodulation on proinflammatory macrophages. A) Illustration of the murine macrophages (Mac) and MSC (LPS-trained or control) coculture system; D, day. B, C) Secretions of proinflammatory (TNF-α) (B) and anti-inflammatory (IL-10) (C) cytokine in the macrophage-MSC coculture system stimulated with 1 μg/ml LPS were quantified by ELISA. D, E) The transcriptions of proinflammatory (M1) and anti-inflammatory (M2) markers in macrophage alone (D) or macrophage-MSC cocultured cells (E) were examined by quantitative PCR (qPCR). *P < 0.05, **P < 0.01, ***P < 0.005 compared with the untrained MSCs. #P < 0.05, ##P < 0.01, ###P < 0.005 compared with the LPS-treated macrophages.
T-cell proliferation assay
The proliferation assay of T cells cocultured with MSCs was modified from the previous study (9). The isolated CD4 T cells were labeled with CellTrace carboxyfluorescein succinimidyl ester dye (Thermo Fisher Scientific) and activated by using Dynabeads Mouse T-Activator CD3/CD28 (Thermo Fisher Scientific) supplemented with 200 U/ml IL-2 (R&D Systems) for 48 h. The activated T cells were then cocultured with the trained or control MSCs at ratios of 1:20 and 1:60 (MSC:T cell; Fig. 3 and Supplemental Fig. S2) for 5 d. The medium was supplemented with or without 20 ng/ml TNF-α and 20 ng/ml IFN-γ during the coculture. T-cell proliferation was analyzed by LSRII Flow Cytometer (BD Biosciences). The experiments were repeated twice independently.
Figure 3.
LPS-trained MSC failed to suppress CD4 T-cell proliferation in the T-cell-MSC cocultured system with IFN-γ plus TNF-α stimulation. A) Illustration of the murine CD4 T cells and MSCs (LPS-trained or untrained) coculture system with IFN-γ plus TNF-α (I + T) restimulation. The activated CD4 T cells were labeled with carboxyfluorescein succinimidyl ester (CSFE) dye, and the cell proliferation was examined 5 d after the coculture by fluorescence-activated cell sorting (FACS); D, day. B) Each peak represents a new passage of proliferated T cells. The youngest populations were gated and compared. FITC-A, fluorescein isothiocyanate A; MSC:T, MSC:T cell ratio.
Lactate assay
Lactate levels in the culture supernatants were quantified by a Lactate Assay Kit (MilliporeSigma). The manufacturer’s instructions were followed carefully. The optical densities were determined using a Bio-Rad 3550-UV microplate reader (Bio-Rad, Hercules, CA, USA) set at 570 nm.
Promoter analysis
The murine iNOS promoter sequence was obtained from Ensembl (ensembl.org). The sequence including 6000 bases upstream from the iNOS gene (mouse C57BL/6, ENSMUSG00000020826) was included for the analysis using the default setting. The regulator motif binding and histone methylation pattern were documented.
Statistical analysis
Nonpaired Student’s t tests were performed for data with 2 groups, and a 1-way ANOVA with a Tukey’s post hoc test was performed for data with 3 or more groups. The statistical analysis was conducted using Prism 7 (GraphPad Software, La Jolla, CA, USA). Data are reported as means ± sd; P < 0.05 was chosen as the threshold of statistical significance.
RESULTS
LPS-trained MSCs had enhanced iNOS expression induced by IFN-γ plus TNF-α
We choose IFN-γ plus TNF-α induced iNOS expression as a model of activation because of their crucial role in MSC-mediated immunomodulation (9). MSCs were treated with 20 μg/ml LPS alone, LPS plus 20 ng/ml TNF-α, 20 ng/ml IFN-γ, 20 ng/ml TNF-α, or IFN-γ plus TNF-α for 24 h, and restimulated with IFN-γ plus TNF-α after a washout for 3 d (Fig. 1A). The expression of iNOS was increased in LPS treated MSCs regardless of their combination with TNF-α and decreased in TNF-α or IFN-γ plus TNF-α treated MSCs (Fig. 1B). Shorter exposure time to LPS (6 or 12 h) was not sufficient to enhance iNOS expression in MSCs upon restimulation, suggesting the effect may require downstream gene expression activation by LPS (Fig. 1C). Restimulation at 7 d after washout still showed enhanced iNOS expression in LPS-trained MSCs, indicating the effect could remain for at least 1 wk (Fig. 1D).
LPS-trained MSCs reduced the proinflammatory response in macrophages
Attenuation of sepsis by MSCs is mediated through suppression of proinflammatory response in macrophages via direct cell contact and paracrine regulations (5). The established MSC-macrophage direct coculture model was used to characterize the effect of LPS-trained MSCs on the proinflammatory response with LPS restimulation (Fig. 2A) (5). Decreased TNF-α (Fig. 2B) and increased IL-10 (Fig. 2C) secretions were found in the MSC-macrophage coculture model compared with macrophages alone; TNF-α secretion was reduced further in the LPS-trained MSC-macrophage cocultures compared with the untrained MSC-macrophage systems. Successful M1 macrophage polarization was characterized by increased M1 marker (TNF-α/IL-1β/iNOS/IL-6) and reduced M2 marker (Arg1/CD206) transcription in macrophages (Fig. 2D). Decreased M1 marker (TNF-α/IL-1β/iNOS/IL-6) and increased M2 marker (Arg1) transcriptions were found in the LPS-trained MSC-macrophage cocultures compared with the untrained controls (Fig. 2E).
LPS-trained MSCs failed to suppress CD4 T-cell proliferation in vitro
Induction of iNOS expression by IFN-γ plus TNF-α (or other proinflammatory cytokines) in MSCs is essential for the subsequent reduction of T-cell proliferation (9), which is an important feature in clinical applications of MSC-based therapy. The MSC-CD4 T-cell coculture system was used to examine whether increased iNOS expression in the LPS-trained MSCs could enhance the suppressive effect on T-cell proliferation (Fig. 3A and Supplemental Fig. S1A). Consistent with previous findings (9), untrained MSCs only reduced the T-cell proliferation at lower ratios (MSC:T cell = 1:20) with IFN-γ plus TNF-α stimulation (8.45%, Fig. 3B). No significant reduction was observed at higher ratios (42.2%, 1:60) or unstimulated controls (Supplemental Fig. S1B). Despite the increased iNOS expression, LPS-trained MSCs showed minimal suppressive effect on T-cell proliferation at 1:20 (34.0%) and 1:60 (39.7%) ratios with IFN-γ plus TNF-α stimulation (Fig. 3B).
Increased iNOS expression in trained MSCs is associated with the JNK pathway and histone methylation
To clarify the potential mechanisms of immunomodulation in the LPS-trained MSCs, selected NF-κB target gene expression was examined, because we previously observed increased NF-κB activity in the LPS-trained MSCs (Fig. 4A) (13). Among 9 selected genes, LPS-trained MSCs showed increased expression of iNOS, IL-1β, and IL-6 (Fig. 4B) under IFN-γ plus TNF-α restimulation, and no significant difference was found in other NF-κB target genes, including TNF-α, CCL2, CXCL9, COX2, Smurf2, and TAZ. LPS-trained MSCs showed decreased expression of proinflammatory genes under LPS restimulation, and no significant difference was found in COX2, Smurf2, and TAZ genes (Supplemental Fig. S3). The gene expression changes (iNOS, IL-1β, and IL-6) were not observed in LPS-trained primary mouse embryonic fibroblast under IFN-γ plus TNF-α restimulation (Fig. 4C), suggesting the regulation may be limited to MSCs but not in other mesenchymal cells. We further examined other immunosuppressive factor expressions in LPS-trained MSCs because the induced iNOS expression was not associated with the phenotypes on T-cell modulation (Fig. 3). Interestingly, LPS-trained MSCs showed decreased IDO expression, and no significant difference was found in programmed death-ligand 1 and TGF-β expression (Fig. 4D).
Figure 4.
Selective gene induction in the LPS-trained MSC or mouse embryonic fibroblast (MEF) with second IFN-γ plus TNF-α (I + T) stimulation. A) Illustration of LPS-trained MSC or MEF and restimulation; D, day. B–D) The expression of iNOS, IL-1β, IL-6, TNF-α, CCL2, CXCL9, COX2, Smurf2, TAZ, programmed death-ligand 1 (PDL1), IDO, and TGF-β in MSC (B, D) or MEF (C) was examined by quantitative PCR (qPCR). *P < 0.05, **P < 0.01, ***P < 0.005 compared with the untrained MSCs.
Trained immunity in innate immune cells is mediated by epigenetic modifications (16). A mechanistic study using the epigenetic or signal transduction inhibitors is illustrated in Fig. 5A. The enhanced expression of iNOS in LPS-trained MSCs was blocked by treatment with MTA, a histone methyltransferase inhibitor (Fig. 5B). Treatment of D-α-hydroxyglutaric acid, an inhibitor of α-ketoglutarate dependent dioxygenase and consequent epigenetic modification including DNA and histone demethylation, further increased iNOS expression in LPS-trained MSCs. Treatments of L-α-hydroxyglutaric acid, pargyline [histone 3 lysine 4 (H3K4) demethylase inhibitor], and anacardic acid (histone deacetylase inhibitor) had no effect on iNOS expression.
Figure 5.
Involvement of histone methylation and JNK signaling pathway in the induction of iNOS expression in LPS-trained MSCs. A) Illustration of mechanistic study with epigenetic or signaling pathway inhibitors. I + T, IFN-γ plus TNF-α; qPCR, quantitative PCR; D, day; W/WO, with/without. B) The expression of iNOS in LPS-trained MSCs with epigenetic inhibitors. AA, anacardic acid; D-α, D-α-hydroxyglutaric acid; L-α, L-α-hydroxyglutaric acid; MTA, 5′-deoxy-5′-methylthioadenosine; Par, pargyline. C) Downstream signaling pathway of TLR-4 activation. D) The expression of iNOS in LPS-trained MSCs with pathway inhibitors. Akt I, Akt inhibitor VIII; IKK2I, IKK2 inhibitor VI; MEK I, PD98059, JNK I, SP600125; p38 I, SB202190. *P < 0.05, **P < 0.01, ***P < 0.005 compared with the untrained MSCs; ##P < 0.01 compared with the LPS-trained MSCs.
Binding of LPS to TLR-4 activates multiple signaling pathways (Fig. 5C). Inhibition of JNK activation suppressed iNOS expression in LPS-trained MSCs (Fig. 5D). Inhibition of other TLR-activated pathways, including NF-κB (IKK2 inhibitor), Akt, MEK, and p38 signaling, did not affect iNOS expression. These results suggested that histone methylation and JNK activation are involved in the regulation of enhanced iNOS expression in LPS-trained MSCs.
DISCUSSION
Our findings provide the first evidence of trained immunomodulation, or innate immune memory, in bone marrow–derived MSCs. Despite its crucial roles in the modulation of innate and adaptive responses, MSCs are not classic immune cells such as macrophages or lymphocytes. The trained response of MSCs to PAMPs or other environmental cues suggests that MSCS play important roles in regulating the inflammatory status and tissue repair processes, considering their unique tissue distribution around perivascular niches (3).
One important question with respect to trained immunity is how long the memory can persist in the immune cells. Circulating monocytes and macrophages are cells with short half-lives. The trained immune response was observed up to 3 mo poststimulation in an in vivo model (23), indicating that the innate immune memory may also occur in innate immune precursor cells. Although the turnover rate of endogenous MSCs remains unclear, it is likely that MSCs live longer than macrophages because MSCs have self-renewal abilities and can differentiate into long-lived tissues, including bone. We showed that increased iNOS expression in the LPS-trained MSCs persisted for at least 7 d. The LPS-trained MSCs may potentially have long-term immunomodulatory effects, though this has not yet been characterized in vivo.
Previous studies showed that macrophage exposed to PAMPs could either induce trained immunity with enhanced responses or lead to tolerance to restimulation (14, 15). β-glucan–trained macrophages have increased proinflammatory responses when restimulated with the same or different PAMPs after a washout of the β-glucan for few days (14). On the other hand, LPS induced a tolerance response in macrophages with continuous LPS restimulation at lower doses (15). We confirmed that continuous LPS stimulation (Supplemental Fig. S2A, upper) induced a tolerance response in TNF-α, iNOS, and IL-1β expression in macrophages (Supplemental Fig. S2B, left). On the other hand, restimulation of LPS after 3 d the washout period (LPS-trained, Supplemental Fig. S2A, bottom) only induced tolerance in IL-1β expression but had no effect on TNF-α or iNOS expression (Supplemental Fig. S2B, right). Our results and previous studies suggest that the phenotypes of tolerance and trained immunity are determined by the type of PAMPs, the exposed cell type, and the exposure time course.
LPS-trained MSCs showed different roles in the regulation of innate and adaptive immune responses. The enhanced modulation of proinflammatory response with LPS restimulation suggests a protective role of MSCs in, for example, sepsis. However, the impaired regulation of T-cell proliferation of LPS-trained MSCs may potentially increase the risk of T-cell–mediated diseases (24). On the other hand, MSC infiltration is associated with cancer progression via suppression of adaptive immunity (25). The behavior of LPS-trained MSCs may also provide 1 potential mechanism by which endotoxin exposure might affect cancer formation and progression (26).
Previous studies showed that MSCs preconditioned with inflammatory cytokines such as TNF-α or IL-1β have enhanced potential for tissue regeneration using in vitro and in vivo models (14–16). Naik et al. (27) recently reported that acute inflammation hastens the epithelial stem cell–mediated skin repair after tissue damage. These studies suggest that inflammatory cytokines can sensitize the tissue regeneration abilities of MSCs and other lineage-specific stem cells. Compared with exogenous ligands (PAMPs) and inflammatory cytokines, the potential effects of endogenous ligands (damage-associated molecular patterns) on the memory of nonclassic immune cells remain unclear.
In contrast to the conventional adaptive immune response, innate immune memory can lead to differential responses to the same or different stimulations (16). The memory in the innate immune cells is often mediated through epigenetic modifications or transcriptional factor–based regulations without genetic change. These trained or tolerance responses in macrophages are mediated by histone methylation (H3K4 methylation) or acetylation (16). Our data showed that inhibition of histone methylation blocked the enhanced iNOS expression in LPS-trained MSCs, suggesting that the epigenetic mark of gene activation, such as H3K4 methylation, is involved in the trained immunity in MSCs. Increased glycolysis and accumulated metabolites lead to epigenetic changes in trained macrophages. However, we did not observe increased glycolysis in the LPS-trained MSCs (Supplemental Fig. S4). Analysis of murine iNOS promoter showed multiple c-Jun (transcription factor of JNK pathway) binding sites and the epigenetic marks of H3K4 methylation (Supplemental Fig. S5). However, additional studies are required to confirm the potential correlation between JNK activation and H3K4 methylation on the regulation of iNOS expression in LPS-trained MSC.
There are limitations to this preliminary study. First, the biologic response of MSCs trained by different PAMPs, such as β-glucan (14) and others, was not characterized. Second, the increased iNOS expression in the LPS-trained MSCs was not correlated to the phenotype observed in the MSC-T-cell coculture model. The potential correlation of decreased IDO expression with the phenotype of impaired T-cell modulation remains to be confirmed. Third, the detailed mechanisms, including the potential correlation between JNK pathway activation and histone modifications, and the histone methylation pattern changes in LPS-trained MSC, remain to be clarified. Last, the experimental model is limited to in vitro murine cells. Investigation of the effects of LPS-trained MSC in translational models is required in future studies.
In summary, the current studies demonstrate differential immunomodulatory effects of trained MSCs on macrophages and T cells. These immunomodulatory consequences are critical, because they will have a major impact on current MSC-based cell therapies.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Katie Hsu, Sai-Wen Tang, and Magdiel Perez Cruz (all from Stanford University) for consulting on the T-cell proliferation assay. The authors also thank Piera Smeriglio (Stanford University) for consulting on the mechanistic study of epigenetic regulation. This work was supported by U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 1R01AR063717 (to S.B.G.) and the Ellenburg Chair in Surgery at Stanford University. J.P. was supported by a grant from the Jane and Aatos Erkko Foundation. The authors declare no conflicts of interest.
Glossary
- Akt
protein kinase B
- Arg1
arginase 1
- CCL2
C-C motif chemokine ligand 2
- COX2
cyclooxygenase-2
- CXCL9
C-X-C motif chemokine ligand 9
- FBS
fetal bovine serum
- H3K4
histone 3 lysine 4
- IDO
indoleamine 2,3-deoxygenase
- MSC
mesenchymal stem cell
- p38
p38 mitogen-activated protein kinase
- PAMP
pathogen-associated molecular pattern
- Smurf2
SMAD-specific E3 ubiquitin protein ligase 2
- TAZ
tafazzin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. Lin drafted the manuscript; T. Lin and J. Pajarinen designed and conducted the experiments; J. Pajarinen, Y. Kohno, J.-F. Huang, M. Maruyama, M. Romero-Lopez, K. Nathan, and Z. Yao contributed significantly to data interpretation and manuscript editing; and S. B. Goodman supervised the experiments and helped in interpretation of the data and manuscript writing.
REFERENCES
- 1.Jiang Y., Jahagirdar B. N., Reinhardt R. L., Schwartz R. E., Keene C. D., Ortiz-Gonzalez X. R., Reyes M., Lenvik T., Lund T., Blackstad M., Du J., Aldrich S., Lisberg A., Low W. C., Largaespada D. A., Verfaillie C. M. (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49; erratum: 447, 879–880 [DOI] [PubMed] [Google Scholar]
- 2.Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 3.Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P. N., Traas J., Schugar R., Deasy B. M., Badylak S., Buhring H. J., Giacobino J. P., Lazzari L., Huard J., Péault B. (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 [DOI] [PubMed] [Google Scholar]
- 4.Bernardo M. E., Fibbe W. E. (2013) Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13, 392–402 [DOI] [PubMed] [Google Scholar]
- 5.Németh K., Leelahavanichkul A., Yuen P. S., Mayer B., Parmelee A., Doi K., Robey P. G., Leelahavanichkul K., Koller B. H., Brown J. M., Hu X., Jelinek I., Star R. A., Mezey E. (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 15, 42–49; erratum: 462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal S., Pittenger M. F. (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 [DOI] [PubMed] [Google Scholar]
- 7.Squillaro T., Peluso G., Galderisi U. (2016) Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 25, 829–848 [DOI] [PubMed] [Google Scholar]
- 8.Caplan A. I., Correa D. (2011) The MSC: an injury drugstore. Cell Stem Cell 9, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A. I., Zhao R. C., Shi Y. (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 [DOI] [PubMed] [Google Scholar]
- 10.Lin T., Pajarinen J., Nabeshima A., Lu L., Nathan K., Jämsen E., Yao Z., Goodman S. B. (2017) Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res. Ther. 8, 277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redondo-Castro E., Cunningham C., Miller J., Martuscelli L., Aoulad-Ali S., Rothwell N. J., Kielty C. M., Allan S. M., Pinteaux E. (2017) Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 8, 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saparov A., Ogay V., Nurgozhin T., Jumabay M., Chen W. C. (2016) Preconditioning of human mesenchymal stem cells to enhance their regulation of the immune response. Stem Cells Int. 2016, 3924858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin T., Pajarinen J., Nabeshima A., Lu L., Nathan K., Yao Z., Goodman S. B. (2017) Establishment of NF-κB sensing and interleukin-4 secreting mesenchymal stromal cells as an “on-demand” drug delivery system to modulate inflammation. Cytotherapy 19, 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintin J., Saeed S., Martens J. H. A., Giamarellos-Bourboulis E. J., Ifrim D. C., Logie C., Jacobs L., Jansen T., Kullberg B. J., Wijmenga C., Joosten L. A. B., Xavier R. J., van der Meer J. W. M., Stunnenberg H. G., Netea M. G. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster S. L., Hargreaves D. C., Medzhitov R. (2007) Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978; erratum: 451, 102 [DOI] [PubMed] [Google Scholar]
- 16.Netea M. G., Joosten L. A., Latz E., Mills K. H., Natoli G., Stunnenberg H. G., O’Neill L. A., Xavier R. J. (2016) Trained immunity: a program of innate immune memory in health and disease. Science 352, aaf1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin T. H., Sato T., Barcay K. R., Waters H., Loi F., Zhang R., Pajarinen J., Egashira K., Yao Z., Goodman S. B. (2015) NF-κB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Eng. Part A 21, 875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang J., Liu F., Lee M., Wu B., Ting K., Zara J. N., Soo C., Al Hezaimi K., Zou W., Chen X., Mooney D. J., Wang C. Y. (2013) NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc. Natl. Acad. Sci. USA 110, 9469–9474; erratum: 110, 13690-13691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H. H., Shin K. K., Kim Y. J., Song J. S., Kim J. M., Bae Y. C., Kim C. D., Jung J. S. (2010) NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J. Cell. Physiol. 223, 168–177 [DOI] [PubMed] [Google Scholar]
- 20.Davies L. C., Heldring N., Kadri N., Le Blanc K. (2017) Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells 35, 766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling W., Zhang J., Yuan Z., Ren G., Zhang L., Chen X., Rabson A. B., Roberts A. I., Wang Y., Shi Y. (2014) Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 74, 1576–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engela A. U., Baan C. C., Dor F. J., Weimar W., Hoogduijn M. J. (2012) On the interactions between mesenchymal stem cells and regulatory T cells for immunomodulation in transplantation. Front. Immunol. 3, 126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L. A., Ifrim D. C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H. G., Xavier R. J., van der Meer J. W., van Crevel R., Netea M. G. (2012) Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 109, 17537–17542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zappia E., Casazza S., Pedemonte E., Benvenuto F., Bonanni I., Gerdoni E., Giunti D., Ceravolo A., Cazzanti F., Frassoni F., Mancardi G., Uccelli A. (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106, 1755–1761 [DOI] [PubMed] [Google Scholar]
- 25.Djouad F., Plence P., Bony C., Tropel P., Apparailly F., Sany J., Noël D., Jorgensen C. (2003) Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102, 3837–3844 [DOI] [PubMed] [Google Scholar]
- 26.Lundin J. I., Checkoway H. (2009) Endotoxin and cancer. Environ. Health Perspect. 117, 1344–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naik S., Larsen S. B., Gomez N. C., Alaverdyan K., Sendoel A., Yuan S., Polak L., Kulukian A., Chai S., Fuchs E. (2017) Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.