Abstract
We surveyed Society of Cardiovascular Anesthesiologists members regarding anticoagulation practices for cardiopulmonary bypass and attitudes on heparin resistance. Of 550 respondents (18.5% response rate), 74.9% (95% CI, 71.3%–78.5%) used empiric weight-based dosing of heparin, and 70.7% (95% CI, 66.9%–74.5%) targeted an activated clotting time of either 400 or 480 seconds to initiate cardiopulmonary bypass. Of note, 17.1% (95% CI, 13.9%–20.2%) of respondents reported activated clotting time targets lower than those recommended by recent 2018 Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists/American Society of Extracorporeal Technology guidelines or failed to monitor heparin effects at all. When heparin resistance was encountered, 54.2% of respondents (95% CI, 50.0%–58.4%) administered antithrombin concentrates as a first-line therapy.
Despite its longevity of use for anticoagulation during cardiac surgery, significant variability in heparin dosing and monitoring has been reported. A 1993 survey of members of the Society of Cardiovascular Anesthesiologists and American Society of Extracorporeal Technology demonstrated that, although the activated clotting time was used by 99% of respondents, acceptable values for the conduct of cardiopulmonary bypass (CPB) ranged from 240 to 1000 seconds.1 A survey of 54 North American institutions administered in 2008 by the Duke Clinical Research Institute and published by Lobato et al2 reported a similarly wide range of target activated clotting times, with values of 400–480 seconds being used by approximately 70% of respondents. Variability in anticoagulation practices is likely the result of a gap in evidence and any formal recommendations. Only recently have the Society of Cardiovascular Anesthesiologists, American Society of Extracorporeal Technology, and the Society of Thoracic Surgeons released clinical practice guidelines for CPB anticoagulation.3
Decreased heparin responsiveness, frequently termed “heparin resistance,” is an area that lacks consensus in either diagnosis or treatment. The reported incidence during CPB use ranges from 4% to 26%4 and is highly dependent on the initial bolus dose of heparin and the desired activated clotting time target for CPB initiation. Therapies for heparin resistance include antithrombin concentrates, fresh frozen plasma, and the administration of additional heparin.4 The 2011 blood conservation guidelines from the Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists recommend administration of antithrombin concentrates in preference to fresh frozen plasma in instances of antithrombin-mediated heparin resistance.5 However, the acceptance and impact of this recommendation remain unknown. In the report by Lobato et al,2 fresh frozen plasma was the predominant treatment for heparin resistance in 85% of US and Canadian centers.2
Recently, Miles et al6 conducted a global survey of CPB priming practices and reported that 72.7% of respondents used an activated clotting time target between 400 and 500 seconds for initiation of CPB. That particular survey, however, asked only 1 question regarding activated clotting time values and contained no items related to heparin dosing or responsiveness. We conducted the present survey to describe both (1) current practice of anticoagulation for initiation and maintenance of CPB and (2) clinician attitudes on heparin resistance and their current use of antithrombin concentrates. In addition, since 2018 Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists/American Society of Extracorporeal Technology CPB anticoagulation guidelines have just been released,3 we wanted to use the collected survey data to determine what percentage of practitioners are already compliant with these recommendations.
METHODS
Survey Creation and Validation
The authors used SurveyMonkey online software (San Mateo, CA) to create a pilot survey, which was administered to members of the Division of Cardiothoracic Anesthesiology at Emory University. Answers pertaining to heparin dosing and antithrombin concentrate usage were compared to known institutional protocols to ensure that the survey instrument was accurately capturing actual clinical practice. The final iteration of the survey had 20 questions and is provided in Supplemental Digital Content 1, Survey, http://links.lww.com/AA/C680. The survey was reviewed and approved by the Society of Cardiovascular Anesthesio logists’s Research Committee and the Emory School of Medicine’s Institutional Review Board. The requirement for written informed consent was waived by both entities.
Survey Distribution
The target population was members of the Society of Cardiovascular Anesthesiologists at the “Active” and “Associate” levels, representing licensed physicians actively taking care of cardiac surgical patients. Members at the “Fellow/Resident” and “Career Scientist” levels were excluded. The Society of Cardiovascular Anesthesiologists membership office distributed an electronic link to the survey to all eligible members via an initial e-mail communication on August 2, 2017. Members had approximately 2 months to complete the survey, with follow-up reminder e-mails sent on August 24, 2017 and September 29, 2017. The survey closed on October 1, 2017. Members were asked to complete the survey only once.
Determination of Guideline Compliance
The recent Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists/American Society of Extracorporeal Technology guidelines for CPB anticoagulation contain items related to “Heparin dosing for initiation and maintenance of CPB.”3 The recommendations state that a clotting time should be measured and demonstrate “adequate anticoagulation” before initiation and at regular intervals during CPB.3 Guidance on minimal acceptable activated clotting time values is given as either >480 or >400 seconds when whole blood is maximally activated or microcuvette technology is used.3 Thus, respondents who either did not use activated clotting times or targeted activated clotting times <400 seconds either before initiation of CPB or during CPB (survey questions 8, 9, and 11) were considered not compliant with these recommendations.
Completion Incentive
Responses were anonymous, but participants had the option of entering an e-mail address into a random drawing for 1 of 10 Amazon gift cards valued at $125 and 1 of 5 annual memberships to the Society of Cardiovascular Anesthesiologists (value of $270).
Responses Analyzed
Respondents who indicated on the survey that they did not care for adult patients undergoing cardiac surgery using CPB were excluded. Responses with identical e-mail addresses entered for the incentive drawing were considered to be duplicates, and only the first completed survey was used for purposes of the incentive drawing and the analysis. Respondents who entered only demographic information for the purposes of the incentive drawing were not used in the analysis.
Statistical Analysis
Based on an estimated membership population of 3000, CIs of 95%, and a sample proportion of 0.5, we calculated that we would need 501 responses to achieve a global margin of error of 4%. Descriptive statistics, including frequency tables with proportions and bar graphs, were used to summarize the data. Wald CIs for proportions were set at 95%. Responses to individual questions that were left blank were classified as “unknown.” All calculations were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
The electronic link to the survey was distributed to 2972 Society of Cardiovascular Anesthesiologists members. We received 580 responses, of which 550 were used for analysis, giving an effective response rate of 18.5% (Supplemental Digital Content 2, Figure 2, http://links.lww.com/AA/C681). Respondent demographics are provided in the Table and closely mirrored the membership composition of the Society of Cardiovascular Anesthesiologists (Supplemental Digital Content 3, Figure 3A, http://links.lww.com/AA/C682), with 83.8% of responses coming from North America. Responses from all states within the United States were received, with the exceptions of Delaware, Idaho, North Dakota, South Dakota, and Wyoming (Supplemental Digital Content 3, Figure 3B, http://links.lww.com/AA/C682).
Table.
Survey Responses
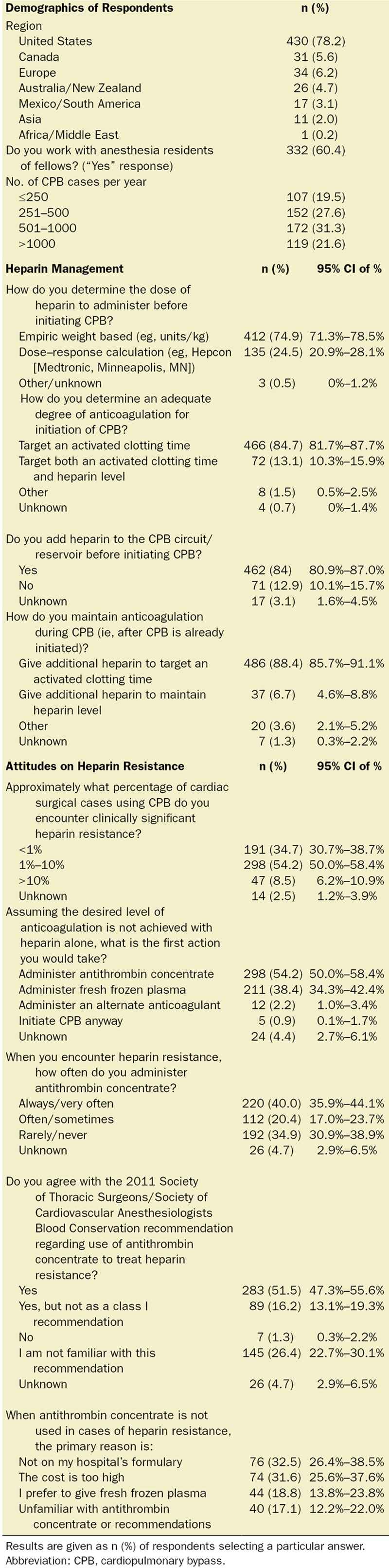
Answers to specific heparin management questions are provided in the Table. An empiric weight-based approach to determining the pre-CPB heparin bolus was used by 74.9% of respondents (95% CI, 71.3%–78.5%). Empiric weight-based doses of heparin used for initiation of CPB are provided in Figure 1A. The maximum dose of heparin that respondents would administer before initiating additional therapy to achieve a target activated clotting time is provided in Figure 1B. Heparin was also added to the CPB circuit by the majority of respondents (84%; 95% CI, 80.9%–87.0%). Adequate anticoagulation was assessed by targeting a specific activated clotting time for “initiation” of CPB and “during” CPB by 84.7% (95% CI, 81.7%–87.7%) and 88.4% (95% CI, 85.7%–91.1%) of respondents, respectively. In addition to an activated clotting time, 13.1% (95% CI, 10.3%–15.9%) also used a heparin level to determine adequate anticoagulation for initiation of CPB, and 6.7% (95% CI, 4.6%–8.8%) maintained a specific heparin level during CPB.
Figure 1.
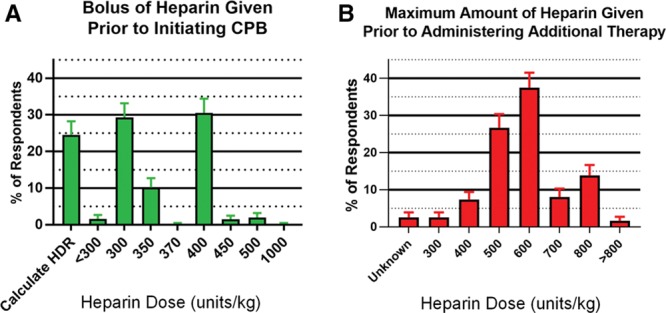
Bar graphs of (A) percentage of respondents indicating either empiric weight-based dose or a calculated dose based on heparin dose–response (HDR) curve, which is typically administered before the initiation of cardiopulmonary bypass (CPB), and (B) percentage of respondents indicating what the maximum dose of heparin they would give before administering additional therapy to achieve the desired level of anticoagulation for CPB. Doses are given in U/kg. Error bars indicate Wald 95% CIs of the proportion.
For those respondents who used an activated clotting time to determine adequate anticoagulation for CPB initiation, either with or without a heparin level (538 of 550), an activated clotting time value of 480 or 400 seconds was used by 70.7% (95% CI, 66.9%–74.5%). The full range of activated clotting time targets for initiation and maintenance of CPB is provided in Figure 2. Of the 550 respondents, 17.1% (95% CI, 13.9%–20.2%) provided answers inconsistent with 2018 Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists/American Society of Extracorporeal Technology guidelines regarding activated clotting time targets for initiation and/or maintenance of CPB.
Figure 2.
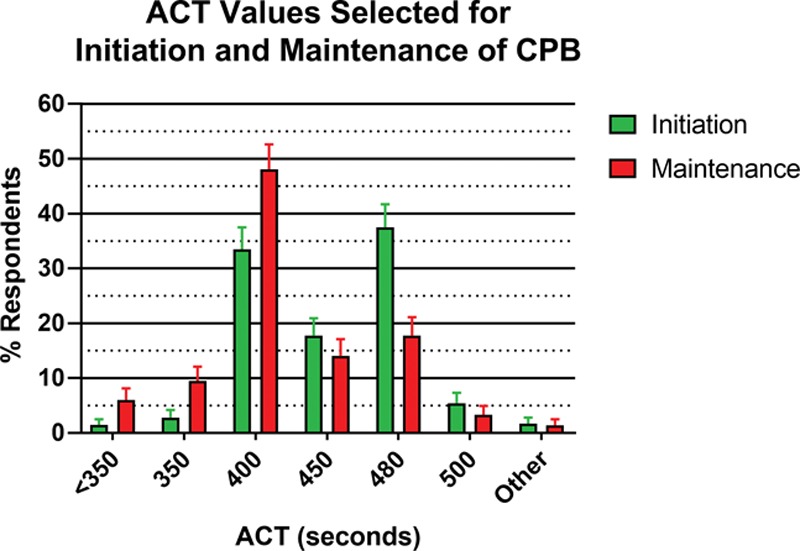
Bar graph of percentage of those respondents who utilized activated clotting times (ACTs) targeting a specific ACT value for initiation (green bars) and maintenance (red bars) of cardiopulmonary bypass (CPB). Error bars indicate Wald 95% CIs of the proportion.
Specific answers to questions regarding heparin resistance are provided in the Table. In terms of frequency, about half of respondents felt that they encountered it in 1%–10% of the CPB cases they performed. More clinicians administered antithrombin concentrate as a first-line treatment (54.2%; 95% CI, 50.0%–58.5%) compared to those who administered fresh frozen plasma as a first-line treatment (38.4%; 95% CI, 34.3%–42.4%). The majority of respondents were in agreement with the 2011 Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists recommendation to use antithrombin concentrate, although about one-third rarely or never utilized it in cases of heparin resistance. The primary reason antithrombin concentrates were not used in this situation was cost being too high and antithrombin concentrates not being on the hospital formulary.
DISCUSSION
Our survey of Society of Cardiovascular Anesthesiologists members regarding anticoagulation practices for CPB consisted mostly of clinicians in North America but included a balanced distribution of small-, medium-, and large-volume centers, as well as a close split between programs with and without trainees. Like the survey conducted in 2008 by Lobato et al,2 we found that empiric dosing of heparin with activated clotting time targets of 400 or 480 seconds is used by the majority of practitioners. Of note, however, is that >15% of respondents reported using anticoagulation therapy falling outside of recent 2018 Society of Thoracic Surgeons/Society of Cardiovascular Anesthesiologists/American Society of Extracorporeal Technology guidelines3; it may be useful to use this number as a starting point to gauge adoption of these recommendations. It is also interesting to note that more clinicians now use antithrombin concentrate in cases of heparin resistance compared to 10 years ago. In conclusion, while anticoagulation targets for CPB, as determined by the activated clotting time, have remained steady over the past decade, antithrombin concentrate utilization has increased when those targets are felt to be inadequately met.
ACKNOWLEDGMENTS
We thank Kathryn Egan, CRN (Department of Anesthesiology, Emory University School of Medicine, Atlanta, Georgia), for her regulatory assistance and Francisco M. Mota Villaplana, PharmD, MSc (Grifols, S.A., Barcelona, Spain), for his advocacy of this project.
DISCLOSURES
Name: Roman M. Sniecinski, MD, MSc.
Contribution: This author helped design the study, interpret the data, and write the manuscript.
Conflicts of Interest: R. M. Sniecinski has received research funding from Grifols, S.A., and Shire ViroPharma.
Name: Elliott Bennett-Guerrero, MD.
Contribution: This author helped design the study and write the manuscript.
Conflicts of Interest: None.
Name: Linda Shore-Lesserson, MD.
Contribution: This author helped design the study and write the manuscript.
Conflicts of Interest: None.
This manuscript was handled by: Nikolaos J. Skubas, MD, DSc, FACC, FASE.
Supplementary Material
Footnotes
Published ahead of print 11 December 2018.
Funding: This work was supported by Grifols, S.A., in the form of a research grant. The sponsor had no involvement in either data analysis or manuscript preparation.
Conflicts of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Despotis GJ, Gravlee G, Filos K, Levy J. Anticoagulation monitoring during cardiac surgery: a review of current and emerging techniques. Anesthesiology. 1999;91:1122–1151. [DOI] [PubMed] [Google Scholar]
- 2.Lobato RL, Despotis GJ, Levy JH, Shore-Lesserson LJ, Carlson MO, Bennett-Guerrero E. Anticoagulation management during cardiopulmonary bypass: a survey of 54 North American institutions. J Thorac Cardiovasc Surg. 2010;139:1665–1666. [DOI] [PubMed] [Google Scholar]
- 3.Shore-Lesserson L, Baker RA, Ferraris VA. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: clinical practice guidelines-anticoagulation during cardiopulmonary bypass. Anesth Analg. 2018;126:413–424. [DOI] [PubMed] [Google Scholar]
- 4.Finley A, Greenberg C. Review article: heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg. 2013;116:1210–1222. [DOI] [PubMed] [Google Scholar]
- 5.Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. [DOI] [PubMed] [Google Scholar]
- 6.Miles LF, Coulson TG, Galhardo C, Falter F. Pump priming practices and anticoagulation in cardiac surgery: results from the global cardiopulmonary bypass survey. Anesth Analg. 2017;125:1871–1877. [DOI] [PubMed] [Google Scholar]


