Supplemental Digital Content is available in the text.
Keywords: biomarker, detection, diagnosis, Sequential Organ Failure Assessment, systemic inflammatory response syndrome, white blood count
Abstract
Objectives:
Most septic patients are initially encountered in the emergency department where sepsis recognition is often delayed, in part due to the lack of effective biomarkers. This study evaluated the diagnostic accuracy of peripheral blood monocyte distribution width alone and in combination with WBC count for early sepsis detection in the emergency department.
Design:
An Institutional Review Board approved, blinded, observational, prospective cohort study conducted between April 2017 and January 2018.
Setting:
Subjects were enrolled from emergency departments at three U.S. academic centers.
Patients:
Adult patients, 18–89 years, with complete blood count performed upon presentation to the emergency department, and who remained hospitalized for at least 12 hours. A total of 2,212 patients were screened, of whom 2,158 subjects were enrolled and categorized per Sepsis-2 criteria, such as controls (n = 1,088), systemic inflammatory response syndrome (n = 441), infection (n = 244), and sepsis (n = 385), and Sepsis-3 criteria, such as control (n = 1,529), infection (n = 386), and sepsis (n = 243).
Interventions:
The primary outcome determined whether an monocyte distribution width of greater than 20.0 U, alone or in combination with WBC, improves early sepsis detection by Sepsis-2 criteria. Secondary endpoints determined monocyte distribution width performance for Sepsis-3 detection.
Measurements and Main Results:
Monocyte distribution width greater than 20.0 U distinguished sepsis from all other conditions based on either Sepsis-2 criteria (area under the curve, 0.79; 95% CI, 0.76–0.82) or Sepsis-3 criteria (area under the curve, 0.73; 95% CI, 0.69–0.76). The negative predictive values for monocyte distribution width less than or equal to 20 U for Sepsis-2 and Sepsis-3 were 93% and 94%, respectively. Monocyte distribution width greater than 20.0 U combined with an abnormal WBC further improved Sepsis-2 detection (area under the curve, 0.85; 95% CI, 0.83–0.88) and as reflected by likelihood ratio and added value analyses. Normal WBC and monocyte distribution width inferred a six-fold lower sepsis probability.
Conclusions:
An monocyte distribution width value of greater than 20.0 U is effective for sepsis detection, based on either Sepsis-2 criteria or Sepsis-3 criteria, during the initial emergency department encounter. In tandem with WBC, monocyte distribution width is further predicted to enhance medical decision making during early sepsis management in the emergency department.
Sepsis is a leading cause of hospital mortality and is a major financial burden on healthcare systems worldwide (1–3). The initial encounter for the vast majority of sepsis patients occurs in the emergency department (ED) (4). Delays in the diagnosis and treatment of sepsis are common in the acute care setting due to the lack of detection.
Delays in the timing of sepsis interventions provided during the earliest phases of the disease strongly correlate with adverse clinical outcomes and with higher costs of care (5–8). Thus, novel protocols and biomarkers are proposed to enhance the early detection of sepsis in the ED (9). Regrettably, existing biomarkers are of limited utility for early sepsis detection because they cannot accurately distinguish sepsis from other common conditions encountered in the ED setting (9–11).
As infections progress in severity, clinical signs of escalation of the host’s immune response become apparent, such as fever, tachycardia, tachypnea, and elevation of the circulating WBC count, collectively referred to as the systemic inflammatory response syndrome (SIRS). During the transition to sepsis, the WBCs also increase in size (12, 13). A feasibility study recently conducted at a single academic center showed that acute changes in monocyte size, referred to as the monocyte distribution width (MDW), best discriminated sepsis (e.g., compared with neutrophil volume changes) from other acute illnesses in the ED and further suggested that the combined performance of MDW and WBC for early sepsis detection was superior to either MDW or WBC alone (14). Based on these encouraging preliminary results further developed in a second pilot study that established the optimal MDW cutoff value for sepsis (see Digital Supplement, Supplemental Digital Content 1, http://links.lww.com/CCM/E574), we prospectively sought to validate the performance of the MDW alone and in combination with the WBC for early sepsis detection in the ED in a large multisite clinical trial.
METHODS
Patient Enrollment
The study, registered with ClinicalTrials.gov (NCT03145428) and approved by the Western Institutional Review Board (protocol number C03747; Puyallup, WA), wherein informed consent was waived, was a blinded, prospective cohort study conducted at three academic centers, such as Hackensack University Medical Center (Hackensack, NJ), The Ohio State University Wexner Medical Center (Columbus, OH), and University of Pittsburgh Medical Center Shadyside Hospital (Pittsburgh, PA). The study enrolled adults, age 18–89 years, whose evaluation included a complete blood count (CBC) with differential upon presentation to the ED. Exclusion criteria were as follows: inadequate blood samples (e.g., analyzed > 2 hr after collection), readmission to the ED within 12 hours, discharged from the ED within 12 hours (i.e., incomplete data for sepsis classification), prisoners, and prior study enrollment. Based on a feasibility trial of similar design (14), we estimated that a minimum of 250 septic patients were needed for a target sensitivity of 75% with the lower limit of the 95% two-sided CI of 65% and 90% power (15). The total number of ED subjects recruited depended on the prevalence of sepsis in the trial.
White Cell Volume Determination
All blood samples were analyzed on a UniCel DxH 800 analyzer (Beckman Coulter, Inc., Brea, CA) with Version 3.0 software within 2 hours of collection. This instrument measures specific cell volume variables and the distribution of cell volumes within a group of cells (sFig. 1, Supplemental Digital Content 2, http://links.lww.com/CCM/E575; legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E574).
Validation of the MDW Cutoff Value for Sepsis Detection
The optimal MDW cutoff value for sepsis detection (i.e., > 20.0 U) was previously established in an independent pilot study conducted at the aforementioned sites enrolling 505 subjects (none of whom were included in the present study), of whom 67 met Sepsis-2. More detailed information relating to pilot study demographics and Sepsis-2 and Sepsis-3 subclassifications is provided in sTable 1 (Supplemental Digital Content 3, http://links.lww.com/CCM/E576), sTable 2 (Supplemental Digital Content 4, http://links.lww.com/CCM/E577), and sTable 3 (Supplemental Digital Content 5, http://links.lww.com/CCM/E578). MDW greater than 20.0 showed optimal sensitivity and specificity for Sepsis-2 and Sepsis-3 (sTable 4, Supplemental Digital Content 6, http://links.lww.com/CCM/E579, and sTable 5, Supplemental Digital Content 7, http://links.lww.com/CCM/E580) and area under the curve (AUC) (sFig. 2, Supplemental Digital Content 8, http://links.lww.com/CCM/E581—legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E574, and sFig. 3, Supplemental Digital Content 9, http://links.lww.com/CCM/E582—legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E574). Note that this pilot study was distinct from the aforementioned feasibility study (14) and from the current study. Namely, none of the pilot study subjects were included in the current study.
Data Abstraction
Medical record access was limited to independent honest brokers, who entered clinical information under an anonymized number in compliance with Institutional Review Board approved data storage and analysis policies, such that members of the research team were blinded to the identity of the study subjects. A schematic representation of the honest broker system used for these investigations is provided (sFig. 4, Supplemental Digital Content 10, http://links.lww.com/CCM/E583; legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E574).
Clinical Classification of ED Patients
Study subjects were categorized based on the “Sepsis-2” consensus criteria (16), such as non-SIRS (i.e., zero or one SIRS criterion [SIRS criteria are as follows: WBC > 12,000 or < 4,000 or > 10% bands; pulse > 90; respiratory rate > 20; and temperature < 96.8°F or > 100.4°F]) and no infection, SIRS (≥ 2 SIRS criteria), sepsis (infection plus SIRS) (including sepsis [no organ failures], severe sepsis [sepsis with one or more organ failures], and septic shock [sepsis with refractory hypotension]), and infection but no sepsis (i.e., zero or one SIRS criterion), and based on the Sepsis-3 criteria (17), such as controls, infection, and sepsis (based on Sequential Organ Failure Assessment SOFA (SOFA or SOFA score [sTable 6, Supplemental Digital Content 11, http://links.lww.com/CCM/E584])] criteria). The presence of infection was determined based on the retrospective chart review of tests performed and clinical data available within the first 12 hours of ED presentation. If no workup for infection was initiated within 12 hours, the patient was categorized as “not infected” by the adjudicator. Test results were extracted from the records 7–10 days later, including cultures, molecular tests (e.g., polymerase chain reaction and antigens), relevant imaging, and tissue pathology (such test results, particularly cultures, were often reported days after admission).
In order to characterize sepsis as being present upon ED admission, sepsis criteria had to be fulfilled within 12 hours of the initial CBC in patients with suspected infection (as reflected by initiation of diagnostic infection workup) and appropriate clinical categorization was verified by expert review of the extracted electronic medical record data by at least two independent investigators at each site. Discordances were arbitrated by a third independent physician reviewer.
Statistical Approach
General descriptive statistics and box plots were calculated for cell population variables. Diagnostic ability was evaluated in terms of the AUC, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) along with their 95% CIs. The score approach was used to calculate CI for sensitivity, specificity, PPV, and NPV.
The following three independent approaches were used to demonstrate the added value of MDW in comparison to WBC alone: 1) Differences in areas under the curve (AUC): calculated using a one-predictor variable logistic model with WBC and a two-predictor variables logistic model with both WBC and MDW, as the predictor, and using sepsis status as the response. AUC comparisons along with their CIs was calculated as described by DeLong et al (18); 2) Decision curve analysis: The number of true positives (TPs) and false positives (FPs) was calculated from the two logistic models (WBC alone and WBC and MDW). The net benefit for a given probability (p, 0 ≤ p ≤ 1) was calculated for each model as
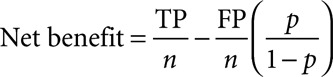 |
where n is the total number of subjects and p is the threshold probability. Decision curves (net benefit vs threshold probability) were plotted for each model and compared with each other (19, 20); and 3) Posttest predicted probability for sepsis: Pretest predicted probability depends on the physical/clinical conditions of each patient who are within inclusion/exclusion criteria of the trial. The average pretest probability (P0) is the prevalence of sepsis in the trial calculated as
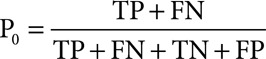 |
Posttest probability (P1) is calculated based on the positive likelihood ratio (LR+) and pretest probability as
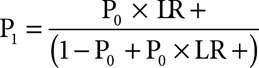 |
Positive likelihood ratios are calculated for WBC (abnormal range: WBC > 12 × 103/μL or WBC < 4 × 103/μL) and WBC and MDW (abnormal range: MDW > 20). SAS 9.4 (SAS Institute, Cary, NC) statistical program was used for data analyses.
RESULTS
Patient Demographics
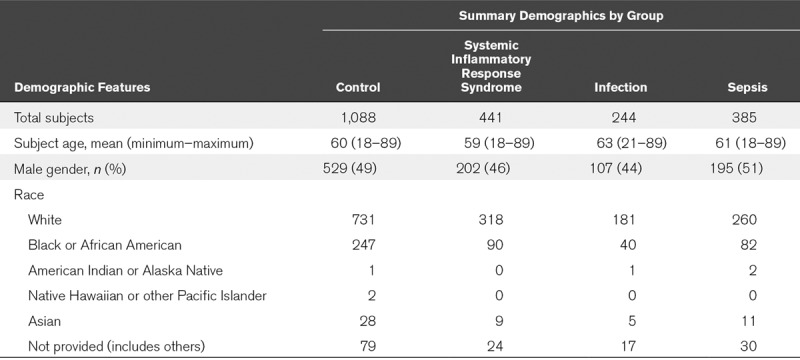
As shown in Figure 1, 2,212 patients were screened, of whom 54 patients were excluded due to inadequate sample collection, prior enrollment, prisoner status, or screening errors. Thus, 2,158 were ultimately enrolled, of whom 385 patients met the diagnosis of Sepsis-2 (17.8% prevalence) and 243 patients met Sepsis-3 criteria (11.3% prevalence). Patient demographics and preexisting medical conditions likely to predispose for sepsis are provided in Table 1, and details of the probable infectious causes of sepsis are provided in sTable 7 (Supplemental Digital Content 12, http://links.lww.com/CCM/E585) and sTable 8 (Supplemental Digital Content 15, http://links.lww.com/CCM/E588). Of the 385 Sepsis-2 patients (74% sepsis, 22% severe sepsis, and 4% septic shock), 86% had bacterial cultures performed, of which 43% were positive.
Figure 1.
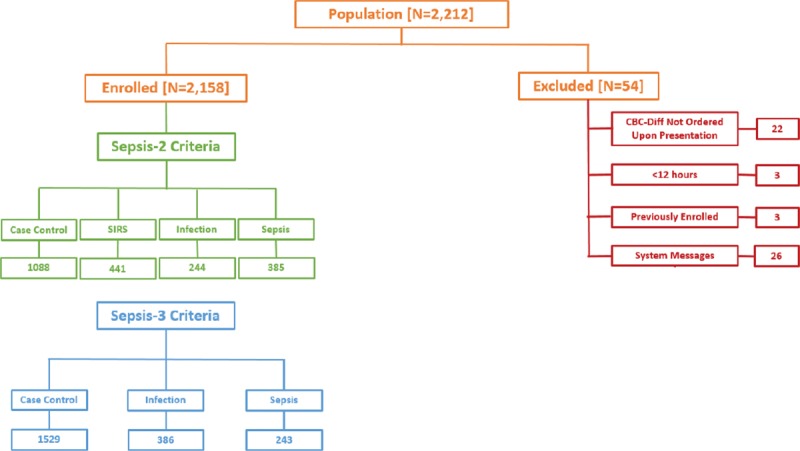
Flow diagram describing patient screening and enrollment. The study was conducted between April 2017 and January 2018. Among all subjects screened, 2.5% were excluded for various reasons, as noted earlier, such that 97.5% of subjects screened were enrolled in the study. CBC = complete blood count, SIRS = systemic inflammatory response syndrome.
TABLE 1.
Study Demographics by Sepsis-2 Criteria
MDW for Sepsis Detection
The MDW values, represented as box plots (Fig. 2), were significantly higher in the sepsis group, regardless of the sepsis criteria used. Analysis of area receiver operating characteristic (ROC) curves for Sepsis-2 and Sepsis-3 is shown in Figure 3, A and B, respectively. The MDW performance for sepsis detection was not influenced by gender (sFig. 5, Supplemental Digital Content 13, http://links.lww.com/CCM/E586; legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E574).
Figure 2.
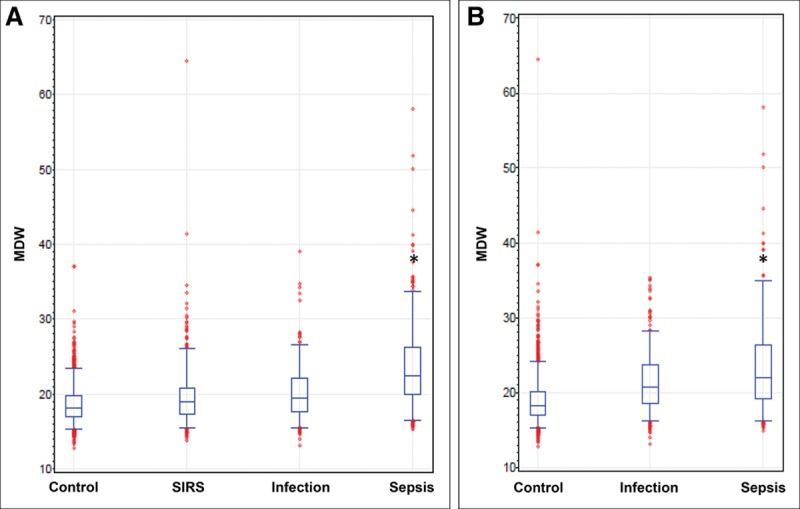
Box plots for monocyte distribution width (MDW) conforming to Sepsis-2 and Sepsis-3 criteria. A, Box plot representation of MDW values showing significantly higher values for patients meeting Sepsis-2 criteria compared with all other emergency department (ED) patient populations. B, MDW was statistically higher than those fulfilling Sepsis-3 criteria compared with other ED patient populations (*p < 0.05 compared with each of the other groups). SIRS = systemic inflammatory response syndrome.
Figure 3.
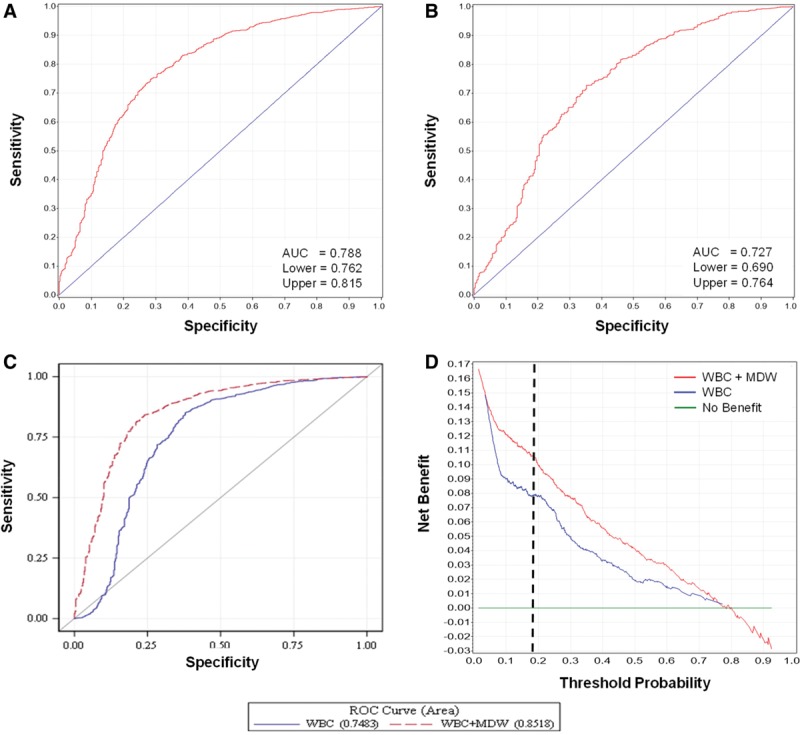
Performance of monocyte distribution width (MDW) for Sepsis-2 and Sepsis-3 detection. Receiver operating characteristic (ROC) curves for MDW conforming to Sepsis-2 (A) and Sepsis-3 (B) criteria and comparing WBC alone and in combination with MDW for Sepsis-2 detection (C). D, A decision curve analysis plots the net benefits of WBC and MDW for sepsis detection compared with WBC alone. Note that the pretest (threshold) probability of sepsis in this cohort was 17.8%, and the added benefit prediction is reflected by the distance between the two plots measured at the black dotted line. AUC = area under the curve.
MDW Performance in Combination With Commonly Used Clinical Variables
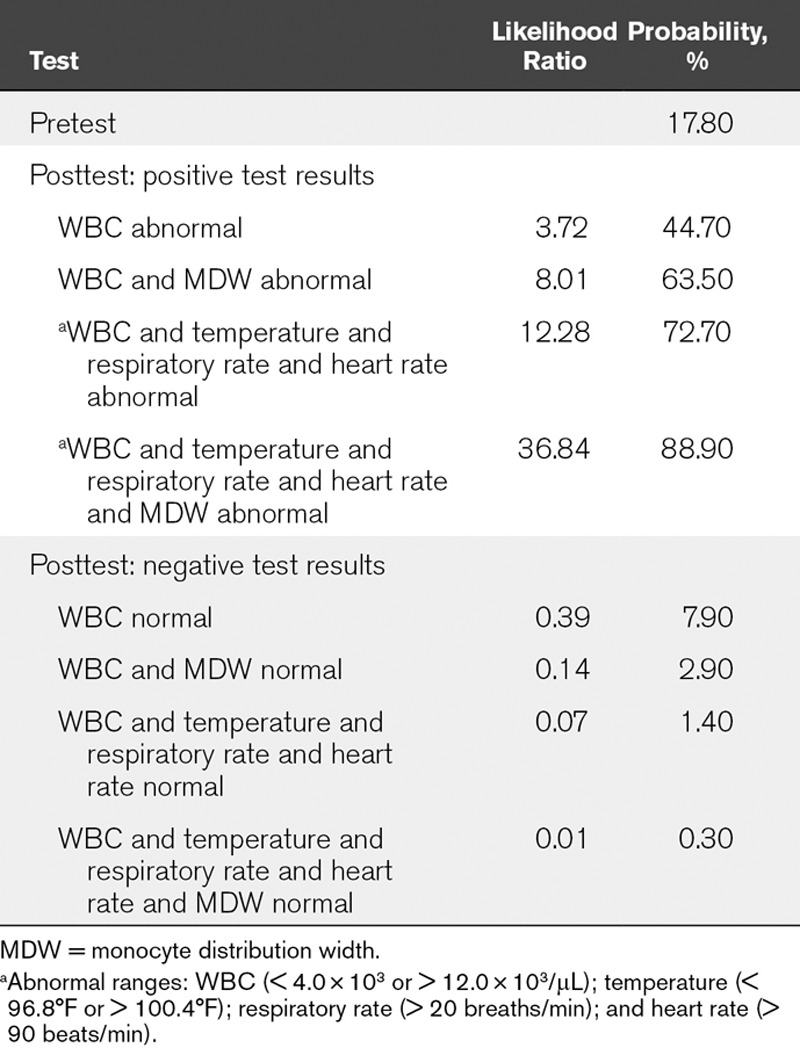
Since MDW and WBC are simultaneously reported as components of the CBC with differential, we evaluated the performance of MDW in tandem with WBC for the detection of sepsis. When both MDW and WBC were outside of their normal ranges, the detection of Sepsis-2 was further enhanced, as reflected by 1) The ROC curve comparison (AUC for abnormal WBC and MDW compared with abnormal WBC alone: 0.85 [95% CI, 0.83–0.88] vs 0.79 [95% CI, 0.76–0.82]; p < 0.05) (Fig. 3C); 2) The WBC and MDW model showed greater benefit in comparison to the WBC alone model particularly when the risk of sepsis was in the range of 5–80%, as was observed in the ED patient population (Fig. 3D). Likewise, the pretest probability of Sepsis-3 in the ED cohort was 11.3%, at which MDW added value to WBC for Sepsis-3 detection based on the separation of curves (sFig. 6, Supplemental Digital Content 14, http://links.lww.com/CCM/E587; legend, Supplemental Digital Content 1, http://links.lww.com/CCM/E574); and 3) An abnormal WBC result increased the probability of a patient having or developing sepsis to 44.7% when the pretest probability was 17.8% (the prevalence of sepsis in the clinical study), with a LR+ of 3.7 (LR+ = sensitivity/1−specificity). The posttest probability increased to 63.5% when both WBC and MDW were abnormal, with an LR+ of 8.0. Conversely, a normal WBC test was associated with a 7.9% sepsis risk and, when both WBC and MDW were normal, further reduced to 2.9% (six-fold lower than the pretest probability). Finally, compared with abnormal SIRS criteria alone, the combined performance of abnormal MDW and SIRS criteria increased the LR+ for sepsis from 12.3 to 36.8 (Table 2).
TABLE 2.
Pretest and Posttest Probabilities and Likelihood Ratios for Sepsis-2 Based Upon aSystemic Inflammatory Response Syndrome Criteria, Alone and in Combination With Monocyte Distribution Width
Predictive Capacity of MDW for Infection to Sepsis Transition
Progression at 72 hours was determined based on results from a single adjudicator (72 hr diagnosis was not arbitrated). Among 379 patients presenting with infection (nonseptic) to the ED based on Sepsis-3 criteria, as judged by adjudicator A, 63 patients progressed to sepsis-3 within 72 hours. A total of 221 patients had MDW greater than 20.0, and 45 of these patients (20%) progressed to Sepsis-3 within 72 hours. In contrast, only 18 of 158 patients (11%) presenting with infection and MDW less than or equal to 20.0 U progressed to Sepsis-3 within 72 hours. Notably, progression from infection (i.e., no evidence of sepsis during the first 12 hr of ED admission) to sepsis within 12–72 hours of ED admission was predicted by an elevated MDW in 71% of patients (45/63).
Added Predictive Value of MDW for Infection Relative to WBC Alone
The first step in the detection of sepsis in the ED patient population is to establish the probability of infection. We evaluated the added benefit of MDW relative to WBC alone for the detection of all infections during the first 12 hours of ED admission (i.e., infection + Sepsis-2). Based on abnormal WBC alone (WBC ≥ 12 or < 4), the probability of infection was 36.7%. When both WBC and MDW were abnormal, the probability of infection increased to 57.6%. Conversely, when WBC was within the normal range, the probability of infection was 12.0% and further decreased to 7.9% when both MDW and WBC were within the normal range. Thus, abnormal MDW is associated with a higher probability of infection at the time of ED admission.
DISCUSSION
This multicenter clinical trial validated the results of a recent single-site feasibility trial (14) that were further developed in a multisite pilot study (see Digital Supplement, Supplemental Digital Content 1, http://links.lww.com/CCM/E574) demonstrating that the MDW alone was effective for the early detection of sepsis in the ED regardless of the diagnostic sepsis criteria used. Early detection has important implications for the initiation of standardized sepsis care bundles, wherein strong correlations exist between delayed treatment and higher mortality (8). This study further showed that incorporation of MDW into clinical decision making would likely enhance the clinical utility of the WBC and other SIRS criteria for early sepsis detection in the ED based on statistical decision curve analyses.
Relative to the prior feasibility study, which was inclusive of all ED patients, this study was enriched for ED patients with greater acute illness severity, as reflected by admission to the ED and/or hospital for at least 12 hours after initial CBC testing. This aspect of the study design was necessary for the proper classification of sepsis in all study subjects and explains the relatively high prevalence of sepsis (17.8%) in this trial compared with prior ED sepsis studies (14, 21, 22). Given that most sepsis patients benefit from hospital admission for the delivery of standardized care (8) and many patients who were initially characterized as infected, nonseptic subsequently progressed to sepsis, we submit that this ED patient subpopulation was most appropriate for sepsis biomarker evaluation.
The guiding principle during the development of sepsis diagnostic criteria is to identify reliable and convenient variables by which potentially life-threatening infections are readily detected in the clinical setting. The foundation of all sepsis definitions, from Sepsis-1 to Sepsis-3, rests on the activation of the host’s immune response during the transition from a localized inflammatory response to a systemic immune response, as indicated by the mobilization of circulating immune cells (elevated WBC) and clinical manifestations, such as hyperthermia, tachycardia, and tachypnea, comprising the SIRS criteria. SIRS reflects the systemic release of proinflammatory cytokines regulating the host’s immune response to pathogens (23, 24). Unfortunately, SIRS is a manifestation of many other acute noninfectious illnesses confronted in the ED setting. Indeed, in this trial, the prevalence of sepsis-related SIRS was equivalent to other noninfectious causes of SIRS in the ED population. Thus, SIRS criteria are of limited value for sepsis detection due to poor specificity.
The concept of monitoring changes in the morphology of circulating immune cells as an early sign of infection has been previously recognized. In response to microbial “danger signals” (e.g., bacterial endotoxins), circulating immune cells, particularly monocytes, and neutrophils are rapidly activated, as reflected by changes in their size and shape (12, 13) and the release of chemokines and cytokines in order to, respectively, recruit and activate other immune cells (25, 26). Another possible explanation is that sepsis provokes the release of larger, immature monocytes (e.g., monoblasts) into the circulation (27). Regardless of the mechanism, increased immune cell size during severe infections likely precedes the onset of SIRS, as indicated by the observation that elevated MDW predicted progression from infection at ED admission to Sepsis-3 within 72 hours 71% of the time.
The MDW biomarker is predicted to provide added value to current sepsis detection protocols. A CBC with differential is routinely obtained in most patients presenting to the ED to screen for acute illness and guide the clinician while generating a differential diagnosis, including decisions to pursue a diagnosis of sepsis. The WBC serves as a current laboratory standard for the initial detection of severe infections, wherein an abnormal WBC is ~88% sensitive for sepsis detection, but its specificity for sepsis detection is low. In this study, it was shown that MDW enhanced the performance of WBC for early sepsis detection based on the following three different statistical approaches: 1) differences in the AUC, 2) probability and likelihood ratios, and 3) a statistical “added value analysis.” In contrast to currently available biomarkers of sepsis, such as procalcitonin, C-reactive protein, and lactic acid, which are typically used to confirm the presence of sepsis after the initial patient encounter in the ED (28), the MDW could be automatically reported with the CBC and differential to serve as means of sepsis detection during the initial ED encounter.
Another important finding of this study was the demonstration of enhanced detection of sepsis based on the new Sepsis-3 definition (17). Relative to Sepsis-2, Sepsis-3 is a more advanced phase of systemic infection associated with organ failures and higher mortality risk (29). As shown in Figure 3, the detection of Sepsis-3 based on an MDW of greater than 20.0 U was comparable to the performance for Sepsis-2 (AUC = 0.73 vs 0.79 for Sepsis-3 vs Sepsis-2, respectively) and early detection of Sepsis-3 has important implications for prioritizing the care of community-acquired sepsis (30).
Finally, we acknowledge limitations of this study. For instance, the nature of the infectious agent could have important implications for the MDW response. However, in vitro studies show that a wide variety of microbial antigens, representing diverse pathogens, are capable of rapidly inducing monocyte activation (31) and this study included an array of sepsis etiologies, including bacterial, viral, and fungal (sTable 3, Supplemental Digital Content 5, http://links.lww.com/CCM/E578). Thus, it is likely that MDW detects a broad spectrum of microbial pathogens causing sepsis. Another potential limitation is the added value analysis of MDW performance compared with WBC alone for sepsis detection, which does not consider all clinical input (e.g., other SIRS criteria, patient history, and examination findings) that could potentially influence the early detection of sepsis by ED healthcare providers. Prospective clinical utility studies are required to confirm the added clinical value predictions provided herein.
CONCLUSIONS
Changes in the volume of peripheral blood monocytes, specifically an increase in MDW, effectively identifies septic patients and infected patients who are at increased risk for progression to sepsis in the ED. In conjunction with the WBC, as components of the CBC with differential, the performance of MDW further improves and is predicted to enhance healthcare decisions relating to the early detection and treatment of sepsis in the ED.
ACKNOWLEDGMENTS
We acknowledge the substantial contributions of the following persons: Justin Rohrbach, MS, for his efforts to facilitate encoded data transfer and data management; Iris Castro, PhD, for her efforts with monitoring and work with honest brokers; Darla Lower, BS, MT-ASCP, Justin Murphy, BS, MT-ASCP, and Lydia Contis, MD, for providing oversight and procurement of residual clinical blood samples and performance of quality procedures for this study; Raghavan Murugan, MD, Ali Al-Khafaji, MD, and David Huang, MD, for assisting with adjudication and arbitration of sepsis patients; JoAnna Williams, MD, for oversight of the process, whereby unused clinical blood samples were made available in a timely fashion for this research; John Christman, MD, and Michael Samoszuk, MD, for assisting with final editing of the article; and Michael Hill and Jeffrey Caterino, MD, for oversight of the honest brokers and related data quality.
Supplementary Material
Footnotes
*See also p. 1152.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Supported, in part, by a grant from Beckman Coulter.
Drs. Crouser’s, Parrillo’s, Bicking’s, Peck-Palmer’s, Julian’s, Kleven’s, Raj’s, and Procopio’s institutions received funding from Beckman Coulter. Dr. Crouser’s institution received funding from Foundation for Sarcoidosis Research and the National Institutes of Health (NIH), received funding from ATyr Pharmaceutical (consulting), and disclosed that he designed the trial in coordination with Beckman Coulter. Dr. Parrillo received funding from National Heart, Lung, and Blood Institute-NIH Heart Failure Network, consulting fees for some of the work performed, and Asahi-Kasei America (consulting). Dr. Seymour’s institution received funding from the NIH and received support for article research from the NIH. Drs. Seymour, Angus, and Esguerra received funding from Beckman Coulter. Drs. Bicking, Esguerra, Kleven, Raj, Procopio, and Tejidor disclosed off-label product use of Beckman Coulter equipment used to measure monocyte distribution width, the entity under study and described in the article. Dr. Esguerra received speaker honoraria for presenting data related to the pilot study to audiences internationally in Brussels and in Hong Kong. Dr. Peck-Palmer’s institution received funding from Roche Diagnostics. Dr. Magari disclosed that he and his spouse are Beckman Coulter employees. Drs. Magari and Tejidor disclosed work for hire. Drs. Careaga and Tejidor disclosed that they are Beckman Coulter employees.
REFERENCES
- 1.Torio CM, Moore BJ; Healthcare Cost and Utilization Project (HCUP): National Inpatient Hospital Costs: The Most Expensive Conditions by Payer. Statistical Brief 204. 2013. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf. Accessed March 19, 2019
- 2.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 312:90–92 [DOI] [PubMed] [Google Scholar]
- 3.Paoli CJ, Reynolds MA, Sinha M, et al. Epidemiology and costs of sepsis in the United States – an analysis based on timing of diagnosis and severity level. Crit Care Med 2018; 46:1889–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang HE, Shapiro NI, Angus DC, et al. National estimates of severe sepsis in United States emergency departments. Crit Care Med 2007; 35:1928–1936 [DOI] [PubMed] [Google Scholar]
- 5.Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med 2017; 196:856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour CW, Kahn JM, Martin-Gill C, et al. Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med 2017; 45:759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones SL, Ashton CM, Kiehne L, et al. Reductions in sepsis mortality and costs after design and implementation of a nurse-based early recognition and response program. Jt Comm J Qual Patient Saf 2015; 41:483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruinelli L, Westra BL, Yadav P, et al. Delay within the 3-hour surviving sepsis campaign guideline on mortality for patients with severe sepsis and septic shock. Crit Care Med 2018; 46:500–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churpek MM, Snyder A, Sokol S, et al. Investigating the impact of different suspicion of infection criteria on the accuracy of quick sepsis-related organ failure assessment, systemic inflammatory response syndrome, and early warning scores. Crit Care Med 2017; 45:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haydar S, Spanier M, Weems P, et al. Comparison of QSOFA score and SIRS criteria as screening mechanisms for emergency department sepsis. Am J Emerg Med 2017; 35:1730–1733 [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharjee P, Edelson DP, Churpek MM. Identifying patients with sepsis on the hospital wards. Chest 2017; 151:898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MJ, Cheng G, Agrawal DK. Cl- channels are expressed in human normal monocytes: A functional role in migration, adhesion and volume change. Clin Exp Immunol 2004; 138:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leckie MJ, Bryan SA, Khan J, et al. Automated quantitation of circulating neutrophil and eosinophil activation in asthmatic patients. Thorax 2000; 55:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crouser ED, Parrillo JE, Seymour C, et al. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest 2017; 152:518–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson NL, Kotz S, Kemp AW. Univariate Discrete Distributions. 1992Second Edition Hooken, NJ, John Wiley & Sons. [Google Scholar]
- 16.Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee: American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20:864–874 [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44:837–845 [PubMed] [Google Scholar]
- 19.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 2006; 26:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moons KG, de Groot JA, Linnet K, et al. Quantifying the added value of a diagnostic test or marker. Clin Chem 2012; 58:1408–1417 [DOI] [PubMed] [Google Scholar]
- 21.Wang HE, Jones AR, Donnelly JP. Revised national estimates of emergency department visits for sepsis in the United States. Crit Care Med 2017; 45:1443–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezende E, Silva JM, Jr, Isola AM, et al. Epidemiology of severe sepsis in the emergency department and difficulties in the initial assistance. Clinics (Sao Paulo) 2008; 63:457–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skerrett SJ, Bagby GJ, Schmidt RA, et al. Antibody-mediated depletion of tumor necrosis factor-alpha impairs pulmonary host defenses to Legionella pneumophila. J Infect Dis 1997; 176:1019–1028 [DOI] [PubMed] [Google Scholar]
- 24.Nakane A, Okamoto M, Asano M, et al. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun 1995; 63:1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee R, Kanti Barman P, Kumar Thatoi P, et al. Non-classical monocytes display inflammatory features: Validation in sepsis and systemic lupus erythematous. Sci Rep 2015; 5:13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mifsud EJ, Tan AC, Jackson DC. TLR agonists as modulators of the innate immune response and their potential as agents against infectious disease. Front Immunol 2014; 5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goasguen JE, Bennett JM, Bain BJ, et al. ; International Working Group on Morphology of Myelodysplastic Syndrome: Morphological evaluation of monocytes and their precursors. Haematologica 2009; 94:994–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljungström L, Pernestig AK, Jacobsson G, et al. Diagnostic accuracy of procalcitonin, neutrophil-lymphocyte count ratio, C-reactive protein, and lactate in patients with suspected bacterial sepsis. PLoS One 2017; 12:e0181704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serafim R, Gomes JA, Salluh J, et al. A comparison of the quick-SOFA and systemic inflammatory response syndrome criteria for the diagnosis of sepsis and prediction of mortality: A systematic review and meta-analysis. Chest 2018; 153:646–655 [DOI] [PubMed] [Google Scholar]
- 30.Ranzani OT, Prina E, Menéndez R, et al. New sepsis definition (Sepsis-3) and community-acquired pneumonia mortality. A validation and clinical decision-making study. Am J Respir Crit Care Med 2017; 196:1287–1297 [DOI] [PubMed] [Google Scholar]
- 31.Vu T, Rahimian A, Stybayeva G, et al. Reconfigurable microfluidic device with integrated antibody arrays for capture, multiplexed stimulation, and cytokine profiling of human monocytes. Biomicrofluidics 2015; 9:044115. [DOI] [PMC free article] [PubMed] [Google Scholar]


