ABSTRACT
SCN5A is expressed in cardiomyocytes and gastrointestinal (GI) smooth muscle cells (SMCs) as the voltage-gated mechanosensitive sodium channel NaV1.5. The influx of Na+ through NaV1.5 produces a fast depolarization in membrane potential, indispensable for electrical excitability in cardiomyocytes and important for electrical slow waves in GI smooth muscle. As such, abnormal NaV1.5 voltage gating or mechanosensitivity may result in channelopathies. SCN5A mutation G615E – found separately in cases of acquired long-QT syndrome, sudden cardiac death, and irritable bowel syndrome – has a relatively minor effect on NaV1.5 voltage gating. The aim of this study was to test whether G615E impacts mechanosensitivity. Mechanosensitivity of wild-type (WT) or G615E-NaV1.5 in HEK-293 cells was examined by shear stress on voltage- or current-clamped whole cells or pressure on macroscopic patches. Unlike WT, voltage-clamped G615E-NaV1.5 showed a loss in shear- and pressure-sensitivity of peak current yet a normal leftward shift in the voltage-dependence of activation. In current-clamp, shear stress led to a significant increase in firing spike frequency with a decrease in firing threshold for WT but not G615E-NaV1.5. Our results show that the G615E mutation leads to functionally abnormal NaV1.5 channels, which cause disruptions in mechanosensitivity and mechano-electrical feedback and suggest a potential contribution to smooth muscle pathophysiology.
KEYWORDS: Electrophysiology, mechanotransduction, ion channel, voltage-gated sodium channel type 5, functional gastrointestinal disorder, irritable bowel syndrome
Introduction
The voltage-gated mechanosensitive Na+ channel NaV1.5 is expressed by SCN5A in human cardiac myocytes and gastrointestinal (GI) smooth muscle cells (SMCs) [1,2]. SCN5A mutations are well established to cause cardiac conduction disorders, called channelopathies. Interestingly, patients with SCN5A cardiac channelopathies have an increased prevalence of irritable bowel syndrome (IBS) [3], and conversely, functionally abnormal SCN5A mutations are present in 2–3% of IBS patients [4–6].
Mechanosensitivity is important for the function of all cells [7], but it plays an especially important role in organ systems whose primary function is mechanical, such as cardiovascular, gastrointestinal, and urinary. In electrically excitable systems, mechanical forces regulate function by mechano-electrical feedback [8]. For example, mechanical stretch of neurons reversibly depolarizes the resting membrane potential and increases the frequency of action potentials [9], which is balanced by mechanosensitive voltage-gated potassium channels that provide a “mechanical brake” to their excitability [10]. NaV1.5 channels are gated by voltage, but they are also mechanosensitive [11,12], and mechanosensitivity of these channels is particularly relevant because they are expressed in heart and gut, which are mechanically active organs. Indeed, disruptions in NaV1.5 mechanosensitivity may contribute to cardiac conduction disorders [13]. In cardiomyocytes (7), GI SMCs [14,15], and heterologous expression systems [11,12,16], mechanical stimuli alter NaV1.5 function by increasing peak Na+ current (IPEAK), hyperpolarizing the voltage dependence of activation (V1/2A) and availability (inactivation, V1/2I), and accelerating channel kinetics. However, the mechanism of NaV1.5 mechanosensitivity remains unclear, which limits our ability to understand the contributions of NaV1.5 mutations to pathophysiology.
Previous work showed that disease-associated NaV1.5 mutations can disrupt voltage-gating, and a portion also disrupts mechanosensitivity [5,6]. Testing whether any NaV1.5 mutation could affect these two functions separately may illuminate the relationship between these functions. The mutation G615E NaV1.5 was found in several studies to associate with cardiac conduction disorders [17–20] and irritable bowel syndrome [4] but does not appear to lead to significant disruptions in NaV1.5 voltage-dependent function. In this study, we compare the mechanosensitivities of wild-type (WT) and G615E NaV1.5, a missense mutation in the DI-DII linker with normal current density [4] but a potentially disrupted mechanosensitivity [21].
Methods
Molecular biology
Plasmids
A single nucleotide change (c.1844 G→A) was engineered by site-directed mutagenesis in a construct containing the most common splice variant of SCN5A (hH1c1, H558/Q1077del) to substitute G615E in the Na+ channel α-subunit (p.G615E-NaV1.5) using the QuikChange II XL Site-Directed Mutagenesis Kit. The integrity of the construct and the presence of the desired mutation were verified by DNA sequencing.
Heterologous expression and cell culture
Wild-type (WT) NaV1.5 or p.G615E-NaV1.5 (G615E NaV1.5) were co-transfected with pEGFP-C1 into HEK-293 cells using Lipofectamine 2000 (Thermo Fisher Scientific, Massachusetts, USA).
Electrophysiology
Pipette fabrication and data acquisition
Electrodes were pulled to a resistance of 2–5 MΩ from KG12 (Kimble glass, Fisher Scientific, Massachusetts, USA) for whole-cell voltage- or current-clamp or to a resistance of 1–2 MΩ from Garner 8250 glass for cell-attached pressure-clamp on a P97 puller (Sutter Instruments, California, USA) and coated with heat-cured R6101 polymer (Dow Corning, MI). Whole-cell and cell-attached patch data from HEK-293 cells were recorded at 20 kHz with an Axopatch 200B patch clamp, CyberAmp320, Digidata 1550, and pClamp 10.5 software (Molecular Devices, California, USA).
Whole-cell voltage clamp
The intracellular solution contained (in mM): 135 K+, 130 CH3SO3−, 20 Cl−, 5 Na+, 5 Mg2+, 5 HEPES, 2 EGTA; pH 7.0, 290 mmol/kg. The extracellular solution contained (in mM): 15 Na+, 140 Cs+, 160 Cl−, 2.5 Ca2+, 5 K+, 10 HEPES, 5.5 glucose; pH 7.35, 305 mmol/kg. Episodic protocol. To measure peak Na+ current density, cells transfected with WT- or G615E-NaV1.5 were held at −120 mV before pulsed through a 2-stage, 24-step voltage ladder (1) from −80 to +35 mV in 5 mV intervals for 50 ms each and (2) to −30 mV for 50 ms. The times between sweeps and each of 10 runs were 250 ms and 6 s, respectively. Peak currents at each voltage step were normalized to the cell capacitance (pF) dialed in during recording or to the maximum peak inward current without shear. Mechanical stimulation by shear stress. Flow of extracellular (bath) solution was applied by gravity drip, calibrated to a rate of 10 mL/min with intravenous tubing.
Whole-cell current clamp
The intracellular solution contained (in mM): 135 K+, 130 CH3SO3−, 20 Cl−, 5 Na+, 5 Mg2+, 5 HEPES, 2 EGTA; pH 7.0, 290 mmol/kg. The extracellular solution contained (in mM): 150 Na+, 160 Cl−, 5 K+, 2.5 Ca2+, 10 HEPES, 5.5 glucose; pH 7.35, 305 mmol/kg. Gap-free protocol. To measure the change in frequency of spontaneous events, cells transfected with WT- or G615E-NaV1.5 were recorded continuously below the predicted threshold. Briefly, window currents were plotted automatically from whole-cell Na+ currents recorded in voltage-clamp mode. With the range of the window current calculated to determine the threshold to elicit membrane potential spikes, the amplifier was switched to I-clamp mode, and current was continuously injected to hyperpolarize the membrane potential approximately 10–20 mV negative from the window current. Spontaneous activity was recorded with a gap-free protocol. With current injected to keep the resting potential hyperpolarized relative to the half-point of the voltage-dependence of inactivation in order to ensure full availability (WT, −89.9 ± 3.8 mV vs. G615E, −91.4 ± 3.5 mV; n = 8, P = 0.49 by a two-tailed unpaired t-test), spontaneous spike frequencies without shear ranged from 0.1 to 1.0 Hz for 80–90% of all experiments (8 of 9 WT and 18 of 22 G615E); experiments with baseline frequencies outside this range were excluded from the analysis. Episodic protocols. To establish a prediction for the threshold of elicited activity, cells transfected with WT- or G615E-NaV1.5 were held at −15 pA before pulsed through a 9-step current ladder from −15 pA to +25 pA in 5 pA intervals for 50 ms each. The time between sweeps was 1 s. To measure the probability of firing potentials, cells were held at resting current (e.g., −15 pA) and pulsed through 20 repetitions of a five-stage protocol with 5.45 s between sweeps: (1) to above the predicted threshold (e.g., +0 pA) for 50 ms and back to rest for 500 ms, then (2–5) to below the predicted threshold (e.g., −5 pA) for 50 ms and back to rest for 500 ms. Mechanical stimulation. Shear stress was applied by flow of extracellular solution at 10 mL/min, as described above in voltage-clamp mode.
Cell-attached patch pressure clamp
The pipette solution contained (in mM): 150 Na+, 160 Cl−, 5 K+, 2.5 Ca2+, 10 HEPES, 5.5 glucose; pH 7.35, 305 mmol/kg. The bath solution contained (in mM): 15 Na+, 140 Cs+, 160 Cl−, 2.5 Ca2+, 5 K+, 10 HEPES, 5.5 glucose; pH 7.35, 305 mmol/kg. Episodic protocol and mechanical stimulation by pressure. Na+ currents in macroscopic patches were elicited by a sequence of paired voltage ladders (Figure 3(a)) with pressures up to −60 mmHg applied during each step of the second voltage ladder (Figure 3(b)).
Figure 3.
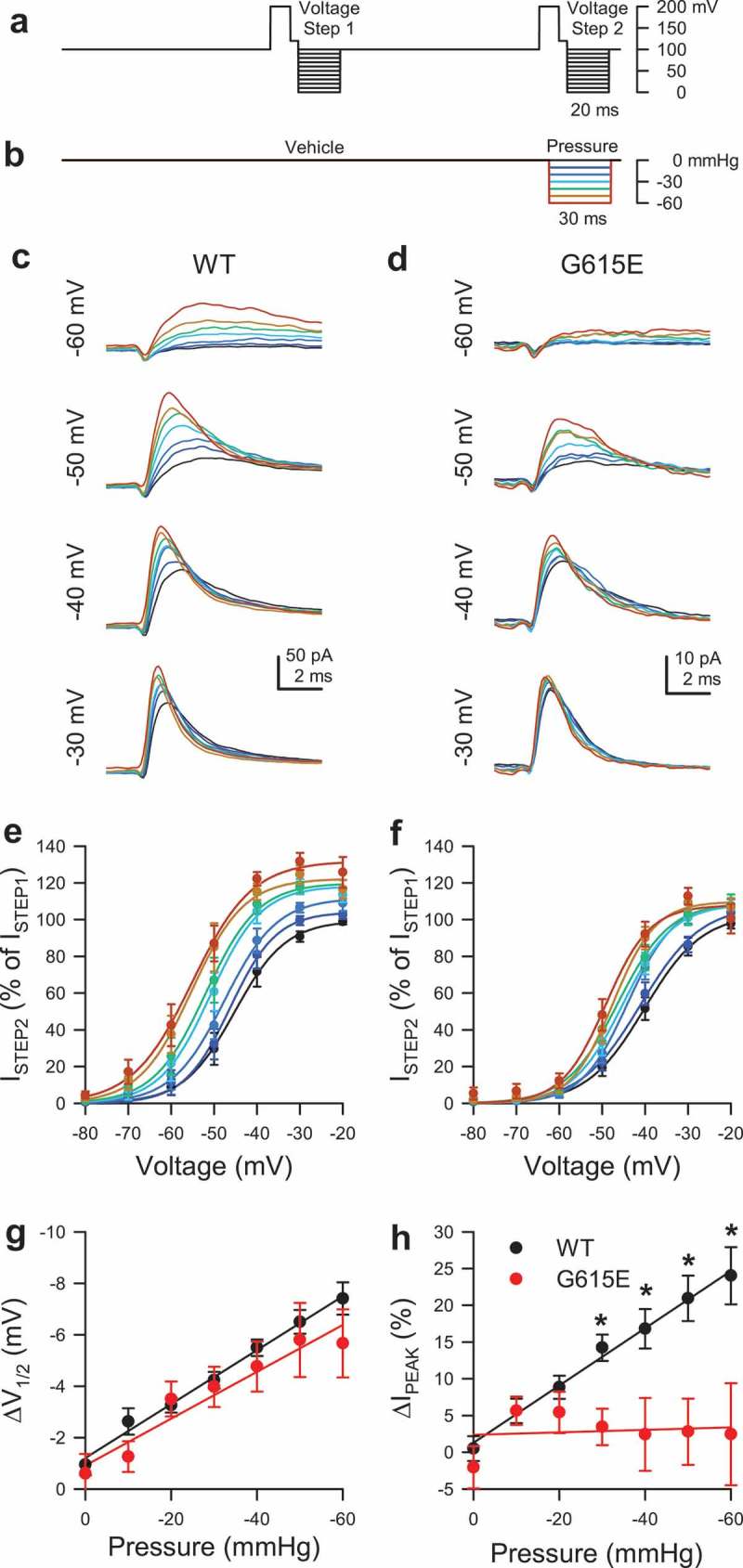
Pressure sensitivity of WT NaV1.5 compared to G615E NaV1.5. (a-b), Voltage-clamp (a) and pressure-clamp (b) protocols were used to elicit macroscopic Na+ currents. Pressure was off during voltage step 1 (vehicle) and on during step 2 (pressure). (c-d), Representative macroscopic patch currents in HEK293 cells transfected with WT- (c) or G615E- NaV1.5 (d), elicited by depolarizations to −60, −50, −40, or −30 mV during voltage step 2 with the pressure indicated by the color gradient defined in (b). (e-f), Steady-state activation curves of macroscopic patch currents for WT (e) or G615E-NaV1.5 (f) during voltage step 2 with the pressure indicated by the color gradient. P < 0.05 effect of pressure or voltage for WT- (d) and G615E-NaV1.5 (e) by a two-way ANOVA with Dunnett post-test. (g), Change in voltage of half-activation (ΔV1/2A) from WT (black) or G615E- NaV1.5 (red), the difference between V1/2A during voltage step 2 and V1/2A during step 1, plotted as a function of the pressure during step 2. P < 0.05 effect of pressure and P > 0.05 effect of genotype by a three-way ANOVA with Tukey post-test. (h), Change in peak current (ΔIPEAK) from WT (black) or G615E- NaV1.5 (red), the % increase in peak currents during voltage step 2 (ISTEP2) normalized to same-sweep peak currents during step 1 (ISTEP1), plotted as a function of the pressure during step 2. *P < 0.05 effect of pressure, genotype by a three-way ANOVA with Tukey post-test.
Data analysis
To calculate whole-cell conductance and voltage dependence of activation, NaV1.5 current-voltage (I-V) plots were fit with the equation: IV = GMAX*(V-EREV)/(1 + e(V-V1/2A)/slope), in which GMAX is the maximum conductance of peak Na+ current, and V1/2A is the voltage of half-activation. To calculate frequency, the number of spontaneous potentials firing past 0 mV in gap-free mode were expressed as a fraction of the 60- to 120-s acquisition time (Hz). To calculate the probability of firing, the number of evoked potentials firing past 0 mV were expressed as a fraction of the number of current stimuli. Response to pressure was measured by the change in peak Na+ current, ISTEP2 – ISTEP1 = ΔIPEAK. I–V curves were examined for any shifts in the V1/2 of activation (ΔV1/2A) versus paired controls without applied pressure. Data are expressed as the mean ± standard error of the mean (SEM). Change as a result of shear stress or pressure was assigned when P < 0.05 for mechano-stimulus to control by a one-sample t-test (Figure 1(f,h); Figure 2(g-i); Figure 4(d); Figure 5(i)), P < 0.05 for G615E to WT by a two-way ANOVA with Dunnett post-test (Figure 3(e-f), Figure 4(c)), or P < 0.05 for G615E to WT by a three-way ANOVA with Tukey post-test (Figure 1(b,d); Figure 2(a-f); Figure 3(g-h); Figure 5(e-h)).
Figure 1.
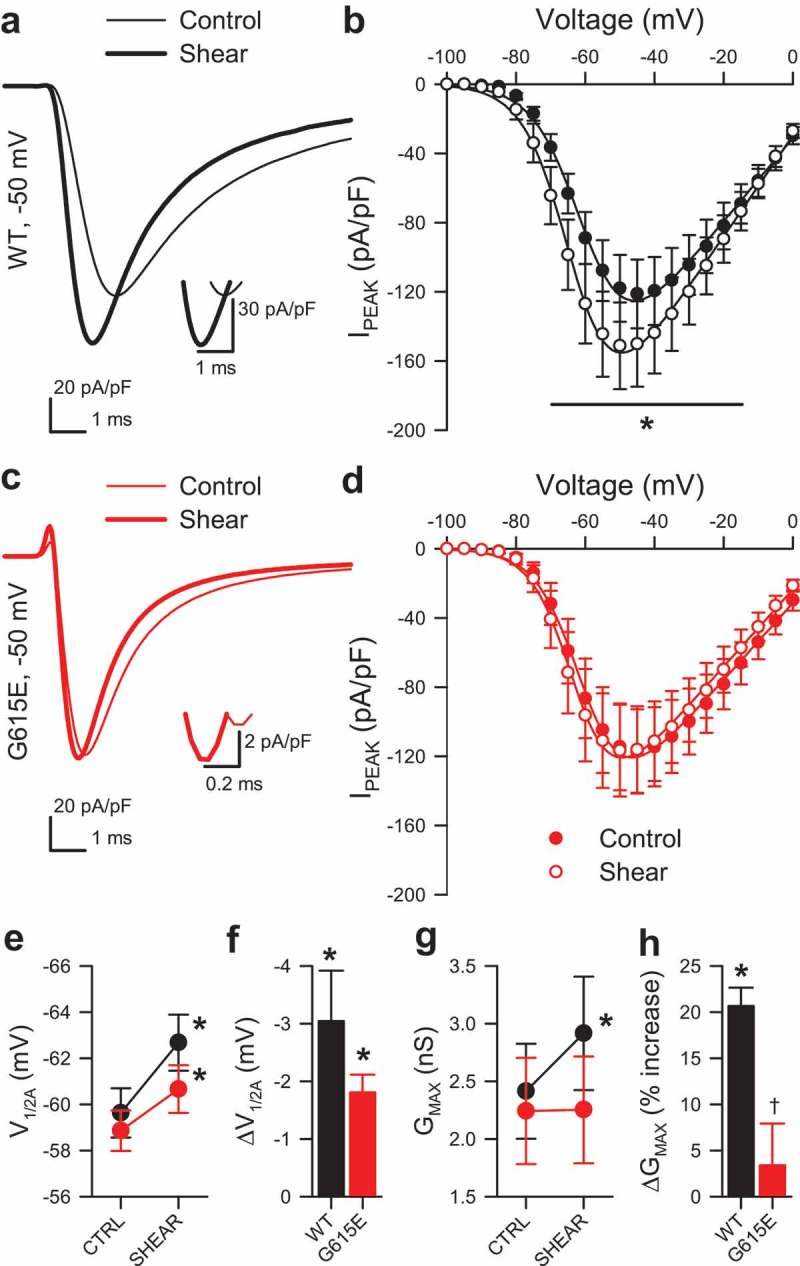
Shear sensitivity of voltage-gating of G615E NaV1.5 compared to WT NaV1.5. (a, c), Na+ current traces from wild-type (WT, a) or G615E (c) NaV1.5 channels elicited by a voltage step from −120 to −50 mV, before (–) or during shear stress (▬) by flow of extracellular solution at 10 mL/min. Insets show the current traces at peak. (b,d), Normalized peak current densities (IPEAK) of WT (b) or G615E (d) Na+ currents before (●) or during (○) shear stress. (e-f), Voltage of half-activation (V1/2A) during control or shear stress (e) and the average shift in V1/2A with shear (f, ΔV1/2A) for WT (black) and G615E (red). (g-h), Peak conductance (GMAX) during control or shear stress (g) and the average change in GMAX with shear (h, ΔGMAX) for WT (black) or G615E (red). (b,d,e,g), n = 12 cells each, *P < 0.05 shear vs. control and P < 0.05 interaction between genotype and shear by a three-way ANOVA with Tukey post-test. (f,h), n = 12 cells each, *P < 0.05% to 0% by a two-tailed one-sample t-test; †P< 0.05 to WT by a two-tailed unpaired t-test.
Figure 2.
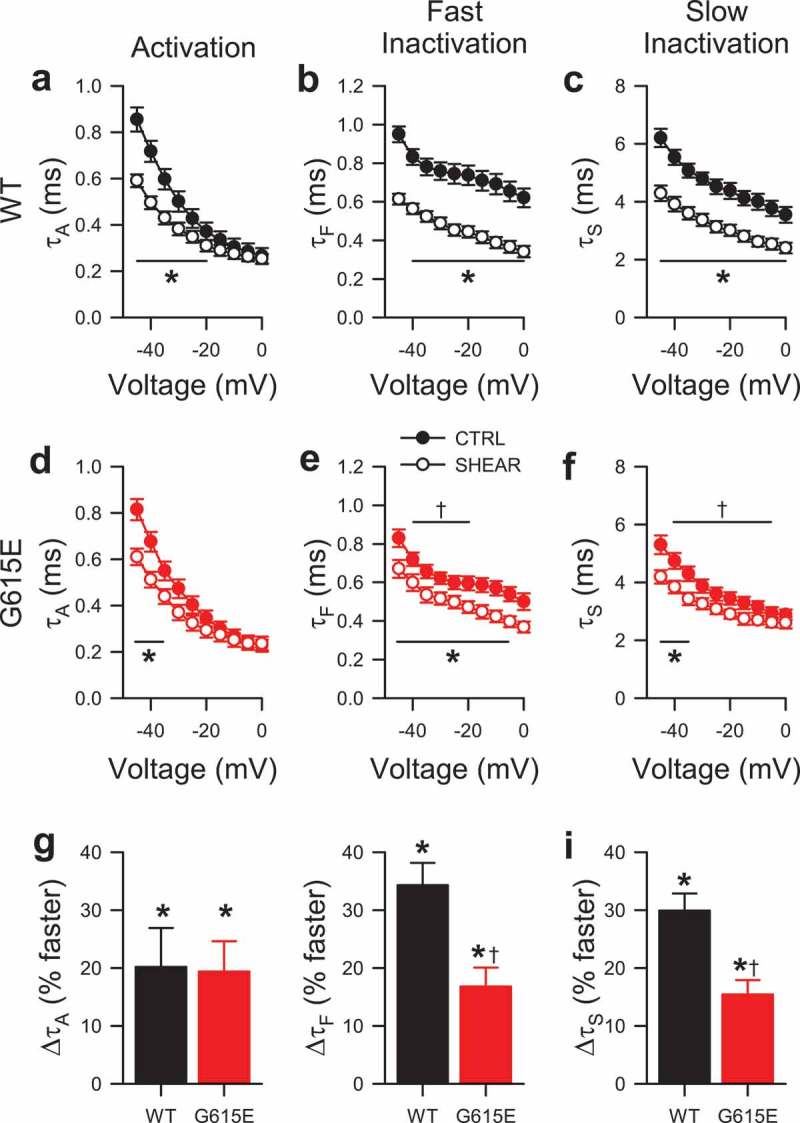
Shear sensitivity of voltage-dependent gating kinetics of G615E NaV1.5 compared to WT NaV1.5. (a-f), Time constants of activation (a, d; τA) and two inactivation components (b-c, e-f) from wild-type (a-c, WT) or G615E (d-f) NaV1.5 currents, before (●) or during shear stress (○) by flow of extracellular solution at 10 mL/min [n = 12 cells each; *P < 0.05 shear stress vs. baseline controls and P < 0.05 interaction between voltage and shear (τA) or between genotype and shear (τF, τS) by a three-way ANOVA with Tukey post-test; †P< 0.05 to WT by a two-tailed unpaired t-test]. (g-i), Average change in time constants of activation (g) and two inactivation components (h-i) of WT (black) or G615E (red) NaV1.5 currents at −30 mV (n = 12 cells each; *P < 0.05% to 0% by a two-tailed one-sample t-test; †P < 0.05 to WT by a two-tailed unpaired t-test).
Figure 4.

Mechanosensitivity of WT and G615E NaV1.5-induced spontaneous electrical excitability. (a-b), Gap-free current clamp recording of spontaneous potentials from an HEK293 cell expressing WT NaV1.5 (a) or G615E (b), before, during (underline), or 35 s after shear stress. (c), Average frequencies of spontaneous potentials from HEK293 cells expressing WT (black) or G615E (red) NaV1.5 channels, before (CTRL), during (SHEAR), or after (POST) shear stress (n = 6 cells each, *P < 0.05 vs. CTRL and P < 0.05 effect of genotype or shear by a two-way ANOVA with Dunnett post-test). (d), Average change in frequency of spontaneous potentials in HEK293 cells expressing WT (black) or G615E (red) NaV1.5 channels (n = 6 cells each, *P < 0.05 WT to 0% by a two-tailed one-sample t-test, NS for G615E).
Figure 5.

Mechanosensitivity of WT and G615E NaV1.5-induced elicited electrical excitability. (a-b), Potentials evoked by a 50-ms current stimulus before (–) or during (▬) shear stress from HEK293 cells expressing either WT (a) or G615E (b) NaV1.5 channels. (c-d), 20 overlapping sweeps of potentials from WT (c) or G615E-NaV1.5 (d) transfected HEK cells, induced by a current stimulus protocol (inset) with one super-threshold (Δ15 pA) 50-ms stimulus followed by four sub-threshold 50-ms stimuli (Δ10 pA), before (control) or during (shear) mechanical stimulation. (e-f), Fraction of potentials from WT (e) or G615E-NaV1.5 (f) transfected HEK cells evoked at the super-threshold 15-pA stimulus or four sub-threshold 10-pA stimuli before (●) or during (○) application of shear stress (n = 6 cells each; *P < 0.05 WT control vs. WT shear and P < 0.05 interaction between stimulus, genotype, and shear by a three-way ANOVA with Tukey post-test). (g-h), Time to peak potential (tPEAK) from cells expressing WT (g) or G615E-NaV1.5 (h) channels evoked at the 15-pA (■) or 10-pA (□) stimulus before (CTRL) or during shear stress (SHEAR) (n = 6 cells each; *P < 0.05, WT control vs. WT shear; P < 0.05, effect of stimulus or effect of shear; and P < 0.05, interaction between genotype and shear by a three-way ANOVA with Tukey post-test). (i), Average change in time to peak potential (ΔtPEAK) induced by shear for WT (black) or G615E-NaV1.5 (red) (n = 6 cells each; *P < 0.05% to 0% by a two-tailed one-sample t-test).
Results
Effect of mechanical stimulation by shear stress on whole-cell WT and G615E NaV1.5 currents
In the absence of shear stress, we found that voltage-dependent peak conductance of G615E NaV1.5 was not different than WT NaV1.5 (GMAX 2.41 ± 0.41 nS, WT vs. 2.24 ± 0.46 nS, G615E; n = 12, P > 0.05) (Figure 1(a,c,g)). As previously described [6,16], whole-cell NaV1.5 conductance increased in response to shear stress (GMAX of WT NaV1.5: 2.41 ± 0.41 nS control to 2.91 ± 0.49 nS shear, 20.7 ± 2.0% increase, n = 12, *P < 0.001 to control) (Figure 1(a-b, g-h)). In contrast, G615E NaV1.5 peak current was unchanged by shear stress (GMAX: 2.24 ± 0.46 nS control to 2.25 ± 0.46 nS shear, 3.4 ± 4.5% change, n = 12, P > 0.05 control vs. shear) (Figure 1(c-d), (g-h)). Both WT and G615E NaV1.5 showed similar small but significant left-shifts of the half-points of voltage-dependence of activation (V1/2A) with shear stress (WT: −59.6 ± 1.1 mV control, −62.7 ± 1.2 mV shear; −3.0 ± 0.9 mV change in V1/2A; n = 12, P < 0.01; G615E: −58.9 ± 0.9 mV control, −60.7 ± 1.0 mV shear, −1.8 ± 0.3 mV change in V1/2A; n = 12, P < 0.01) (Figure 1(b, d-f)).
Having observed an acceleration in the time of peak current (insets, Figure 1(a,c)), we next examined WT and G615E Na+ currents for shear-induced changes to kinetics. Difference currents were constructed by subtracting composites of 12 families of whole-cell Na+ current during shear from the composites of their respective controls (Supplementary Figure 1). The negative deflection in the difference currents from WT was roughly twice the size than that from G615E, while the positive deflections were similar. Examining changes to parameters of Na+ current kinetics in greater detail, we found that the time constants of activation (τA) were similarly faster for WT (+20.2 ± 6.7%, Figure 2(a)) and G615E (+19.4 ± 5.2%, Figure 2(d); n = 12, P < 0.05 by a one-sample t-test, P > 0.05 WT vs. G615E by a two-tailed unpaired t-test, Figure 2(g)); additionally, the time constants of fast (τF) and slow inactivation (τS) each were faster for both WT (τF, +34.3 ± 3.8%; τS, +29.9 ± 3.0%) and G615E channels (τF, +16.8 ± 3.3%; τS, +15.4 ± 2.5%; n = 12, P < 0.05 by one-sample t-tests, Figure 2(h-i)), as shown previously for WT [6,16]. However, shear-induced acceleration of inactivation in G615E was relatively less than in WT (Figure 2(h-i)), which may be because G615E control currents already inactivated faster than WT control currents (τF at −30 mV: WT, 0.76 ± 0.05 vs. G615E, 0.62 ± 0.03 ms; τS at −30 mV: WT, 4.81 ± 0.21 vs. G615E, 3.87 ± 0.23 ms; n = 12, P < 0.05 WT vs. G615E by two-tailed unpaired t-tests) (Figure 2(b-c, e-f, g-h)).
Effect of pressure on WT and G615E NaV1.5 current within a patch
Next, we used another technique to confirm the loss of G615E NaV1.5 mechanosensitivity we found with shear stress. We examined WT and G615E NaV1.5 by simultaneous voltage- and pressure-clamp with on-cell patches [16,22]. With a two-step protocol to determine NaV1.5 pressure dependence [22] (Figure 3(a-b)), the currents elicited by “Voltage Step 1” test the voltage-dependence of NaV1.5, while currents from “Voltage Step 2” test the effect of pressure concurrently with voltage. We found that during Voltage Step 1 (0 mmHg), peak Na+ currents and voltage-dependence of activation were not statistically different between constructs (IMAX: WT, 71.6 ± 15.0 pA vs. G615E, 39.0 ± 12.8 pA, n = 9, P = 0.12; V1/2A: WT, 41.2 ± 4.5 mV vs. G615E, 39.4 ± 1.7 mV, n = 9, P = 0.71 WT to G615E by two-tailed unpaired t-tests). However, as with shear stress, there were significant differences in the responses of WT and G615E NaV1.5 to pressure (Table 1, Figure 3(c-f)). WT NaV1.5 V1/2A was hyperpolarized proportionately with pressure, for a slope of −1.0 ± 0.1 mV per −10 mmHg (P < 0.05 effect of pressure by a three-way ANOVA with Tukey post-test) (Figure 3(e,g)), and WT NaV1.5 macroscopic peak currents increased proportionately with negative patch pressure, for a rate of 3.9 ± 0.7% per −10 mmHg (n = 10 cells, P < 0.05 effect of pressure, voltage, and interaction by a two-way ANOVA with Dunnett post-test) (Figure 3(e,h)). G615E NaV1.5 V1/2A left-shifted with pressure (P < 0.05 effect of pressure and P > 0.05 effect of genotype) (Figure 3(f-g)), and the slope of ΔV1/2A for G615E NaV1.5 did not differ from WT NaV1.5, −0.9 ± 0.3 mV per −10 mmHg (Figure 3(g), Table 1). In contrast to WT NaV1.5, peak Na+ currents of G615E did not respond to pressure, for a rate of only 0.2 ± 1.0% per −10 mmHg (n = 10 cells, P < 0.05 effect of genotype, P < 0.05 effect of interaction between genotype and pressure by a three-way ANOVA with Tukey post-test) (Figure 3(h), Table 1). In all, our data in both whole cell with shear stress and patch with pressure show that G615E NaV1.5, unlike WT NaV1.5, lacks substantial force-dependent changes in mechanically induced peak currents and kinetics, whilst retaining the responses in voltage-dependence.
Table 1.
Effect of negative patch pressure on the change in peak Na+ current (ΔIPEAK) or the change in voltage of half-activation (ΔV1/2A) of WT or G615E NaV1.5.
| Pressure (mmHg) |
ΔIPEAK (%) |
ΔV1/2A (mV) |
||
|---|---|---|---|---|
| WT | G615E | WT | G615E | |
| −00 | 0.5 ± 0.2 | −2.1 ± 2.9 | −0.9 ± 0.4 | −0.6 ± 0.8 |
| −10 | 5.6 ± 1.7 | 5.6 ± 1.9 | −2.6 ± 0.5 | −1.3 ± 0.6 |
| −20 | 8.8 ± 1.6 | 5.4 ± 2.8 | −3.3 ± 0.3 | −3.5 ± 0.7 |
| −30 | 14.2 ± 0.2 | 3.5 ± 2.5* | −4.2 ± 0.3 | −4.0 ± 0.8 |
| −40 | 16.8 ±2.7 | 2.4 ± 5.0* | −5.5 ± 0.3 | −4.8 ± 1.0 |
| −50 | 20.9 ± 3.1 | 2.8 ± 4.5* | −6.5 ± 0.5 | −5.8 ± 1.4 |
| −60 | 24.0 ± 0.4 | 2.4 ± 6.9* | −7.4 ± 0.6 | −5.7 ± 1.3 |
Effect of shear stress on cell electrical excitability
Since NaV1.5 is involved in electrical excitability in the heart and gut, we pursued the effects of mechanical stimulation on NaV1.5 function in current clamp. Recent studies show that electrical excitability can be re-created in mammalian cell lines commonly used for heterologous expression, such as CHO and HEK-293 cells (14, 15). Therefore, we examined the spontaneous spiking of WT or G615E-transfected NaV1.5 HEK-293 cells for changes during shear stress in current-clamp mode (Figure 4(a-b)). For WT NaV1.5, we saw that shear stress reversibly increased the probability of spontaneous spiking events (Figure 4(a-b)); however, for G615E NaV1.5, shear stress failed to produce an increase in spiking frequency (WT NaV1.5: 0.35 ± 0.09 Hz control, to 0.58 ± 0.10 Hz shear, to 0.28 ± 0.05 Hz recovery, n = 8, P < 0.05 control vs. shear; G615E NaV1.5: 0.29 ± 0.03 Hz control, to 0.32 ± 0.06 Hz shear, to 0.24 ± 0.04 Hz recovery, n = 18; P < 0.05 effect of genotype and shear; P < 0.05 interaction between genotype and shear by a two-way ANOVA with Dunnett post-test; Figure 4(c-d)).
We next wanted to test whether elicited excitability is different for WT and G615E NaV1.5 channels. In current clamp mode, a -15-pA injection for 50 ms elicited a singular membrane potential spike in HEK-293 cells transfected with either WT or G615E NaV1.5 (Figure 5(a-b)). However, the shear-induced changes to the membrane potential spiking kinetics of WT NaV1.5 were not observed in cells with G615E NaV1.5 (Figure 5(b)). Since our data suggested an acceleration in NaV1.5 activation and inactivation with mechanical stress, we wanted to test whether submaximal electrical stimulation would result in increased electrical excitability in the presence of mechanical stimulation. Therefore, we designed a protocol that compared a step at maximal (fully activating) stimulation to a sequence of four steps at submaximal stimulation (Figure 5(c-d), top traces). At rest, we saw that at maximal stimulation (15 pA), >90% of steps led to spiking for both WT (98.3 ± 1.7%, n = 6, Figure 5(c,e)) and G615E NaV1.5 (90.8%±7.2%, n = 6, P> 0.05 vs. WT by a two-tailed non-parametric t-test; Figure 5(d,f)). Meanwhile, submaximal stimulation (10 pA) at rest led to spiking in a similar but reduced fraction of stimuli for both WT and G615E (WT, 23.4 ± 9.1% vs. G615E, 17.3 ± 4.6%; n = 6 each, P> 0.05 by a two-tailed non-parametric t-test). In the presence of shear stress, maximal stimulation continued to produce membrane potential spikes for >90% of depolarizations for both WT and G615E NaV1.5 (WT, 92.5 ± 4.8%; G615E, 92.4 ± 5.9%; n = 6 each, P > 0.05 by a two-tailed non-parametric t-test; Figure 5(c-f)), but only in WT and not in G615E did shear stress increase potentials induced by subthreshold stimuli (WT, 71.9 ± 14.4%; G615E 27.2 ± 8.5%; P < 0.05 WT control vs. WT shear and P < 0.05 interaction between stimulus, genotype, and shear by a three-way ANOVA with Tukey post-test).
Shear stress accelerated the spiking upstroke for WT NaV1.5, with the time from 10-pA stimulus to peak potential (tPEAK) decreasing from 51.3 ± 3.7 ms to 35.4 ± 2.6 ms (30.0 ± 5.2% faster) (Figure 5(g,i)). However, shear had no effect on G615E NaV1.5 tPEAK (46.4 ± 3.1 ms to 41.0 ± 1.3 ms; 9.8 ± 5.8% faster) [n = 6 cells each; P < 0.05, WT control vs. WT shear; P > 0.05, G615E control to G615E shear; and P < 0.05, interaction between genotype and shear by a three-way ANOVA with Tukey post-test (g-h); and P < 0.05, WT to 0% change by a one-sample two-tailed t-test (I)] (Figure 5(h-i)). The effect of shear on either depolarization of the baseline (WT, +3.5 ± 3.6 mV vs. G615E, +4.2 ± 0.8 mV) or decrease in peak amplitude (WT, −3.9 ± 1.6 mV vs. G615E, −5.1 ± 2.5 mV) was not different. In all, the current-clamp data suggest that loss of mechanosensitivity in G615E NaV1.5 would lead to a loss of mechanically induced excitability in WT NaV1.5.
Discussion
The goal of the current study was to examine NaV1.5 mechanosensitivity and its role in cellular mechano-electrical feedback. We used a unique SCN5A mutation G615E that is associated with acquired long-QT syndrome [19], sudden cardiac death [17], and irritable bowel syndrome [4]. In previous studies [4,17] and in this one, G615E NaV1.5 had mostly intact voltage-dependent function – normal voltage-dependence of activation, and either no or minor changes in voltage-dependence of inactivation.
On the other hand, we found dramatic differences in mechanosensitivity of voltage-dependent gating between WT and G615E NaV1.5. We tested both constructs in whole cell and patch, using shear stress and patch pressure as mechanical stimuli, respectively. For WT NaV1.5, we saw force produce several changes to voltage-dependent function, similar to previous studies on NaV1.5 [5,11,12,16,22] and NaV1.4 [23], but these changes were nearly absent for G615E NaV1.5. Thus, G615E NaV1.5 joins previously identified disease-associated mutations with abnormal NaV1.5 mechanosensitivity. These associated with long QT syndrome [13] in the heart and with IBS [5,6] in the gut and resulted in NaV1.5 with abnormalities in voltage-dependent function that were further accentuated by mechanical forces. However, to our knowledge, this is the first NaV1.5 mutation that has disrupted mechanosensitivity, while voltage-sensitivity remained mostly intact. Our findings may be relevant for understanding channelopathy mechanisms in cases when NaV1.5 mutations fail to reveal functional changes using voltage-dependence protocols. In such cases, and as we see with G615E NaV1.5, the functional impact may be on mechanical [6] or thermal [24] sensitivity.
Our results provide intriguing mechanistic insights on NaV1.5 mechanosensitivity. First, G615E is located on the intracellular linker connecting DI and DII, which is a novel location for a missense mutation to impact mechanosensitivity. Other NaV1.5 channelopathies like G298S have loss-of-mechanosensitivity and are in linkers as well. However, how these linkers contribute to the mechanism of NaV1.5 mechanosensitivity remains unclear. Second, our findings suggest that mechanisms of voltage- and mechano-sensation by NaV1.5 may be distinct. This is surprising since mechanical stimuli are well established to modulate voltage-sensitivity of voltage-gated channels [11,12,25] but not to introduce a separate mechano-gating paradigm [11,12]. Third, G615E NaV1.5 lost one but not both mechanosensitive responses – it lacked a perfusion-induced current increase but maintained a negative shift in the voltage-dependence of activation. This would suggest that mechanosensitive increases in peak Na+ current may be mechanistically distinct from mechanically induced shifts in voltage-dependence of activation and availability. Fourth, the findings suggest that the mechanosensitivity of the voltage-dependence of activation may be separate from that of availability. Previous and current studies show that mechanical stimuli accelerate the kinetics of activation and inactivation by the same constant. If the acceleration of activation by force is the rate-limiting step [26], it may explain the effect on the acceleration of inactivation. However, G615E NaV1.5 demonstrates an intact mechanosensitivity of activation but a loss of mechanosensitivity of inactivation, suggesting separate mechanisms. In all, our results shed important light on the mechanism of NaV1.5 mechanosensitivity and show that NaV1.5 mechano- and voltage-sensitivity may be targeted separately.
We are ultimately interested in understanding how NaV1.5 mechanosensitivity impacts SMC excitability, also called mechano-electrical feedback [8]. These cells undergo constant repetitive mechanical deformations – they are stretched during diastole and contracted during systole. Stretch is excitatory for WT NaV1.5 channels at the upstroke. But given the acceleration of inactivation, NaV1.5 stretch also results in a more significant current decrease during inactivation [11–13] and slowed recovery from inactivation [11]. Therefore, the overall impact of NaV1.5 mechanosensitivity on SMC function is difficult to judge only from the voltage-dependent operation. We designed current-clamp protocols in a reductionist system, a HEK-293 cell that expressed only WT NaV1.5 or G615E NaV1.5. Similar to previous studies [27,28], we found that these cells had both spontaneous and elicited electrical excitability. Compared to WT, G615E NaV1.5 had decreased mechanosensitivity of both spontaneous and elicited firing, suggesting that NaV1.5 mechanosensitivity may play an important excitatory role in SMC mechano-electrical feedback. However, a note of caution is required. We set the resting potential hyperpolarized to allow for full NaV1.5 availability, but this system limits our ability to determine whether sub-threshold events by either channel can result in firing, as may happen in excitable cells. In a set of preliminary studies, we investigated the potential influence of G615E-NaV1.5 on mechano-electrical feedback in GI SMCs by computational modeling. In silico modeling simulated NaV1.5 voltage- and current-clamp in vitro behavior, and the resulting SMC electrical activity and cytoplasmic Ca2+ concentrations were affected by mechanical forces for WT but not G615E-NaV1.5 (Supplementary Figure 2).
In addition to mechanosensitive voltage-gated sodium channels, cells have other mechanosensitive voltage-gated ion channels, such as potassium (KV) [29] and mechanically gated ion channels [30]. The effects of mechanical forces on these channels are expected to produce various electrical outcomes. For example, KV1.1 mechanosensitivity leads to “mechanical braking” of neuronal excitability [10]. Further, it will be important to determine the precise mechanical energies that the mechanosensitive ion channels encounter in vivo and to replicate these for studies in vitro. It is unclear how the current in vitro mechanostimulation protocols correlate with in vivo physiology. To fully understand mechano-electrical coupling we will need to integrate mechanosensitivity of NaV1.5 and other mechanosensitive ion channels into cell models and to place these models into physiologically relevant mechanical contexts.
In summary, the disease-associated NaV1.5 missense mutation G615E disrupts NaV1.5 mechanosensitivity without a significant impact on voltage-dependent function, which may have important consequences for mechano-electrical coupling in myocytes. This raises the possibility of directly targeting NaV1.5 mechanosensitivity in disease with a drug such as ranolazine [16,22], which does not have a significant effect on NaV1.5 peak current but can inhibit mechanosensitivity [15,16,31].
Funding Statement
This work was supported by the American Gastroenterological Association [RSA]; National Institute of Diabetes and Digestive and Kidney Diseases [DK106456]; National Institute of Diabetes and Digestive and Kidney Diseases [P30DK084567]; National Institute of Diabetes and Digestive and Kidney Diseases [DK052766]; National Institute of Diabetes and Digestive and Kidney Diseases [DK66271].
Acknowledgments
We thank Kristy Zodrow for administrative assistance. We would like to acknowledge Dr. Martin Buist (National University of Singapore) for advice on in silico modeling of SMCs. This work was supported by NIH grants DK052766 (GF), DK106456 (AB), and DK66271 (YS); AGA RSA (AB); the Mayo Clinic Center for Cell Signaling in Gastroenterology (NIDDK P30DK084567).
Author contributions
Peter R. Strege: conceived and designed research, performed experiments, analyzed data, interpreted results of experiments, prepared figures, drafted manuscript, edited and revised manuscript, approved final version of manuscript
Amelia Mazzone: performed experiments, approved final version of manuscript
Arnaldo Mercado-Perez: conceived and designed research, performed experiments, interpreted results of experiments, prepared figures, edited and revised manuscript, approved final version of manuscript
Yuri A. Saito: conceived and designed research, edited and revised manuscript, approved final version of manuscript
Cheryl E. Bernard: approved final version of manuscript
Gianrico Farrugia: conceived and designed research, edited and revised manuscript, approved final version of manuscript
Arthur Beyder: conceived and designed research, analyzed data, interpreted results of experiments, drafted manuscript, edited and revised manuscript, approved final version of manuscript
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Holm AN, Rich A, Miller SM, et al. Sodium current in human jejunal circular smooth muscle cells. Gastroenterology. 2002;122:178–187. [DOI] [PubMed] [Google Scholar]
- [2].Ou Y, Gibbons SJ, Miller SM, et al. SCN5A is expressed in human jejunal circular smooth muscle cells. Neurogastroenterol Motil. 2002;14:477–486. [DOI] [PubMed] [Google Scholar]
- [3].Locke GR 3rd, Ackerman MJ, Zinsmeister AR, et al. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Amer J Gastroenterol. 2006;101:1299–1304. [DOI] [PubMed] [Google Scholar]
- [4].Beyder A, Mazzone A, Strege PR, et al. Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome. Gastroenterology. 2014;146:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saito YA, Strege PR, Tester DJ, et al. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Amer J Physiol. 2009;296:G211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Strege PR, Mazzone A, Bernard CE, et al. Irritable bowel syndrome (IBS) patients have SCN5A channelopathies that lead to decreased NaV1.5 current and mechanosensitivity. Amer J Physiol. 2017;314:G494–G503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sachs F, Morris CE.. Mechanosensitive ion channels in nonspecialized cells. Rev Physiol Biochem Pharmacol. 1998;132:1–77. [DOI] [PubMed] [Google Scholar]
- [8].Kohl P, Sachs F, Franz MR. Cardiac mechano-electric feedback and arrhythmias: from pipette to patient. Philadelphia (PA): Elsevier Saunders; 2005. [Google Scholar]
- [9].Eyzaguirre C, Kuffler SW. Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol. 1955;39:87–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hao J, Padilla F, Dandonneau M, et al. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron. 2013;77:899–914. [DOI] [PubMed] [Google Scholar]
- [11].Beyder A, Rae JL, Bernard C, et al. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J Physiol. 2010;588:4969–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morris CE, Juranka PF. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys J. 2007;93:822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Banderali U, Juranka PF, Clark RB, et al. Impaired stretch modulation in potentially lethal cardiac sodium channel mutants. Channels (Austin). 2010;4:12–21. 10260 [pii]. [DOI] [PubMed] [Google Scholar]
- [14].Strege PR, Holm AN, Rich A, et al. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Amer J Physiol. 2003;284:C60–6. [DOI] [PubMed] [Google Scholar]
- [15].Neshatian L, Strege PR, Rhee P-L, et al. Ranolazine inhibits voltage-gated mechanosensitive sodium channels in human colon circular smooth muscle cells. Amer J Physiol. 2015;309:G506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beyder A, Strege PR, Reyes S, et al. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel NaV1.5: a novel mechanism of drug action. Circulation. 2012;125:2698–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Albert CM, Nam EG, Rimm EB, et al. Cardiac sodium channel gene variants and sudden cardiac death in women. Circulation. 2008;117:16–23. [DOI] [PubMed] [Google Scholar]
- [18].Paulussen AD, Gilissen RA, Armstrong M, et al. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med (Berl). 2004;82:182–188. [DOI] [PubMed] [Google Scholar]
- [19].Yang P, Kanki H, Drolet B, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–1948. [DOI] [PubMed] [Google Scholar]
- [20].Kapplinger JD, Tester DJ, Alders M, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Strege PR, Mazzone A, Loftus YAS, et al. Tu1268 - IBS-associated Scn5A mutation G615E results in Nav1.5 voltage-dependent sodium channels with normal voltage-dependent function and loss of mechanosensitivity. Gastroenterology. 2018;154:S–920. [Google Scholar]
- [22].Beyder A, Strege PR, Bernard C, et al. Membrane permeable local anesthetics modulate NaV 1.5 mechanosensitivity. Channels (Austin). 2012;6:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tabarean IV, Juranka P, Morris CE. Membrane stretch affects gating modes of a skeletal muscle sodium channel. Biophys J. 1999;77:758–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Abdelsayed M, Peters CH, Ruben PC. Differential thermosensitivity in mixed syndrome cardiac sodium channel mutants. J Physiol. 2015. DOI: 10.1113/JP270139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Laitko U, Morris CE. Membrane tension accelerates rate-limiting voltage-dependent activation and slow inactivation steps in a Shaker channel. J Gen Physiol. 2004;123:135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983;306:436–441. [DOI] [PubMed] [Google Scholar]
- [27].Hsu H, Huang E, Yang XC, et al. Slow and incomplete inactivations of voltage-gated channels dominate encoding in synthetic neurons. Biophys J. 1993;65:1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kirkton RD, Bursac N. Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies. Nat Commun. 2011;2:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gu CX, Juranka PF, Morris CE. Stretch-activation and stretch-inactivation of Shaker-IR, a voltage-gated K+ channel. Biophys J. 2001;80:2678–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Strege P, Beyder A, Bernard C, et al. Ranolazine inhibits shear sensitivity of endogenous Na (+) current and spontaneous action potentials in HL-1 cells. Channels (Austin). 2012;6:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


