Abstract
Background:
Research shows that older adults can have a decline in three key resting state networks: (default mode network, central executive network, and salience network) after total knee arthroplasty and that patients’ pre-surgery brain and cognitive integrity predicts decline.
Objectives:
First, to assess resting state network connectivity decline from the perspective of nodal connectivity changes in a larger older adult surgery sample. Second, to compare pre-post functional connectivity changes in mild cognitive impairment (MCI) versus non-MCI.
Methods:
Surgery (n=69) and non-surgery (n=65) peers completed a comprehensive preoperative neuropsychological evaluation and pre- and acute (within 48 hour) post-surgery/pseudo-surgery functional brain magnetic resonance imaging scan. MCI was classified within both (MCI surgery, n=13; MCI non-surgery, n=10). Using standard coordinates, we defined default mode network, salience network, central executive network, and the visual network (serving as a control network). The functional connectivity of these networks and brain areas (nodes) that make up these networks were examined for pre-post-surgery changes through paired samples t-test and ANOVA.
Results:
There was a decline in RSN connectivity after surgery (p<.05) only in the three cognitive networks (not the visual network). The default mode and salience network showed nodal connectivity changes (p<.01). MCI surgery had greater functional connectivity decline in DMN and SN. Non-surgery participants showed no significant functional connectivity change.
Conclusion:
Surgery with general anesthesia selectively alters functional connectivity in major cognitive resting state networks particularly in DMN and SN. Participants with MCI appear more vulnerable to these functional changes.
Keywords: Mild cognitive impairment, functional magnetic resonance imaging, cognitive dysfunction, anesthesia, orthopedics, dementia, surgery, anesthesia
Introduction
Total knee arthroplasty (TKA) is a common elective surgical procedure for older adults. Although this surgery can improve the quality of life for many individuals, the surgery is associated with negative post-surgical outcomes such as delirium and cognitive decline [1, 2]. The neural mechanisms underlying these negative cognitive outcomes still need investigation.
Recently, Huang, et al. [3] reported that older adults electing total knee arthroplasty (TKA) with general anesthesia show an acute (48 hours post-surgery) decline in three key functional resting state networks (RSNs): the default mode network (DMN), the central executive network (CEN), and the salience network (SN). Compared to non-surgery peers, 23% of surgery participants had particularly large connectivity declines in at least one network with 15% having large declines across all networks. Additionally, the authors used markers of individuals’ brain and cognitive integrity prior to surgery to predict the degree of decline in the DMN; individuals who had lower brain and cognitive integrity prior to surgery had greater decline in functional connectivity.
While the Huang, et al. [3] study was the first to report changes in major RSNs following TKA, it has a number of limitations. First, each RSN was examined through the overall connectivity of multiple network nodes or brain regions. Previous studies have shown that anesthesia disrupts functional connectivity and blood flow in specific brain regions and networks, e.g., the thalamocortical network, resulting in an impairment of integration of information [4–7]. This raised the question that surgery may not affect distributed regions within an RSN uniformly.
Second, although Huang, et al. [3] showed pre-surgery cognitive status predicts post-surgery RSN changes, it is unknown if individuals meeting diagnostic criteria for mild cognitive impairment are particularly vulnerable to resting state network change. It is known that individuals with mild neurocognitive disorder or mild cognitive impairment (MCI) have altered resting state networks [8–11]. Specifically, Lee, et al. [8] found increased DMN connectivity in MCI, and Gardini, et al. [10] and Li, et al. [11] found an increase in DMN connectivity associated with poorer memory performance in an MCI population. Additionally, Rombouts, et al. [9] found less deactivation of the DMN in MCI individuals during a visual encoding and nonspatial working memory task when compared to controls. To what extent the abnormal patterns of brain networks in MCI foreshadow their reduced ability to withstand insults such as major orthopedic surgery remains to be established [12].
The current study had two objectives. First, we sought to expand upon findings from Huang, et al. [3] by focusing on individual regions of resting state networks. A larger sample was included to enhance the statistical rigor of the findings. Second, we sought to test the hypothesis that MCI patients were vulnerable to surgery-related resting state network changes. In particular, the MCI population would show greater decline in DMN connectivity after surgery.
Methods
The University of Florida Institutional Review Board in Gainesville, Florida approved this study. Each participant signed consents and we conducted the study in accordance to principles of the Declaration of Helsinki.
Participants
Participants electing TKA were recruited through University of Florida orthopedic clinics, screened for dementia via the Telephone Interview for Cognitive Status (TICS; [13, 14]), and enrolled between 2013 and May of 2016 as part of an ongoing federally funded investigation. Participants in the non-surgery group were recruited through University of Florida orthopedic clinics, community mailings, and locally posted fliers. Non-surgery participants were recruited through a yoked review process to “match” individual surgery participants on age, education, sex, and ethnicity/race. Non-surgery participants had to abstain from surgery for at least one year. Both groups were recruited over the same period and were tested and scanned at the same time intervals. All participants met the following inclusion/exclusion criteria: 1) aged 60 or older, 2) English as primary language, 3) have osteoarthritis or comparable joint pain, 4) have intact activities of daily living, and 5) have baseline neuropsychological testing unsupportive for dementia criteria per Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition [15]. Additional exclusion criteria included: any other major surgery within the study timeline, history of head trauma/neurodegenerative illness, documented learning or seizure disorder, less than a sixth-grade education, substance abuse in the last year, major cardiac disease, chronic medical illness known to induce encephalopathy, implantable device precluding an MRI, and an unwillingness to complete the MRI. Two doctoral-trained neuropsychologists reviewed the baseline data to confirm that test scores met the expected ranges for non-demented individuals. We report on some of these participants in other publications [3, 16].
Procedures
Figure 1. Participants completed a phone cognitive screening [14] and a comprehensive history/systems interview to confirm inclusion/exclusion criteria, followed by an in-person comorbidity rating [17], activities of daily living [18], neuropsychological assessment, and brain MRI. The surgery group had TKA and the non-surgery group were assigned a pseudo-surgery date. This pseudo surgery date served as an anchor for follow-up assessment. Within 48 hours of surgery or pseudo-surgery date, each participant received a second post-surgery brain MRI. The same examiner completed testing for all participants. Trained raters blind to group condition scored and double entered all data.
Figure 1.
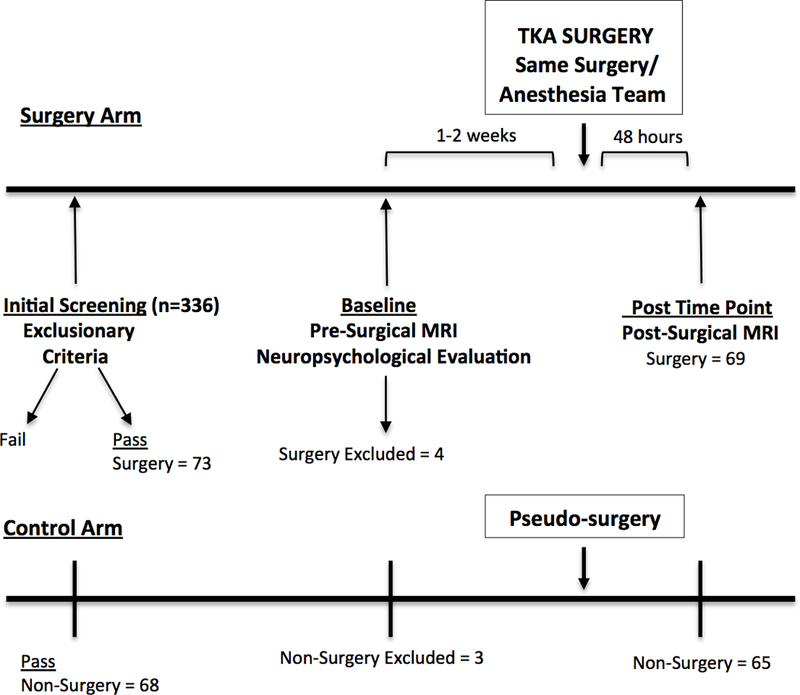
Schematic design of parallel surgery and non-surgery participant timelines.
TKA = total knee arthroplasty.
We determined MCI classification based on the comprehensive criteria discussed in Jak, et al. [19]. According to these criteria, an individual meets classification for MCI if they fall below one standard deviation in at least two measures within any one domain. This comprehensive criteria has association with Alzheimer’s disease biomarkers (apolipoprotein E - APOEs4 allele, cerebrospinal fluid amyloid-beta and tau) and ability to detect individuals who progress to dementia [19, 20]. Based on the guidelines, MCI was classified for patients with impairment in any of the following domains: Attention/Processing Speed Domain: Part A of the Trail Making Test (total time) [21], and Wechsler Adult Intelligence Scale, 3rd Edition, subtests Letter Number Sequencing (total score) and Digit Span (total correct forward [22]); Inhibitory/Executive Domain: Part B of the Trail Making Test (total time) [21], Stroop Color Word Test (total correct) [23], Delis-Kaplan Executive Function System (D-KEFS) test, subtest Tower Test (total achievement score) [24], and the Language Domain: Controlled Oral Word Association (total words) [25, 26] and the Boston Naming Task (BNT; total) [27]; Visuospatial Domain: Judgment of Line Orientation (JLO; total score) [28], the Rey-Osterrieth Complex Figure Test - copy (Denman score) [29, 30], and Matrix-Reasoning (Wechsler Abbreviated Scale of Intelligence – III) (total score), Memory Domain: Hopkins Verbal Learning Test - delay (HVLT-delay; total recall sore)[31], the Logical Memory Test - delay (total score; Wechsler Memory Scale – third edition [22]), the Rey-Osterrieth Complex Figure Test - delay [29, 30]. For further information about these neuropsychological measures, please refer to Lezak, et al. [25].
Anesthesia and Surgery Protocol
Protocols were standardized, with surgery participants receiving intravenous midazolam (1–4 mg) followed by continuous femoral nerve block (CFNB) and single-injection subgluteal sciatic nerve block with 20 mL and 30 mL, respectively, of 0.5% ropivacaine as a bolus injection. The CFNB was continued with ropivacaine 0.2% at an infusion rate of 10 mL per hour. No opioids were added. Propofol (100–750mL), fentanyl (induction:0–150mcg; maintenance: 0–225mcg), and rocuronium (0–50mg) were used for anesthesia induction and intubation. Patients were ventilated with an air oxygen mixture to maintain an end tidal carbon dioxide at 35 ± 5 mm, FiO2 between 0.5 and 0.7; anesthesia was maintained with inhaled sevoflurane and intravenous fentanyl and rocuronium. Propofol boluses were administered as needed to maintain desirable target BIS range between 40 and 60. Total knee replacement surgery was done in a standard manner for all patients by the same surgeon. A tourniquet was used for all cases set to 250 mm Hg and inflated prior to incision and deflated just prior to closure. Bony preparation was done by intramedullary instrumentation for the femoral side and extramedullary for the tibial side. The anterior and posterior cruciate ligaments were sacrificed for all patients and implants were fixed to the bone using bone cement. Perioperative information, including surgery events (e.g., induction, intubation, incision, tourniquet inflation and release, etc.), anesthetic drugs, and intraoperative medications, were recorded on a standardized study data collection sheet and confirmed with the official anesthesia record.
Neuroimaging
Structural and resting state functional MRI was conducted both pre- and 48 hours/post-surgery or post pseudo surgery date. Delirium was screened twice per inpatient day using the Confusion Assessment Method [32]. Participants did not show signs of delirium at the time of MRI acquisition.
MRI Acquisition
A 3T Siemens Verio scanner with an eight-channel head coil ran T1-weighted and resting state fMRI sequences for each participant and time-point. T1-weighted data were acquired with the following parameters: TR: 2500ms; TE: 3.77ms; 176 sagittal 1mm3 slices, 1 mm isotropic resolution; 256×256×176 matrix, 7/8 phase partial Fourier, total acquisition time: 9:22. Resting state fMRI data were acquired with participants’ eyes closed and with the following parameters: TR: 2000ms; TE: 30ms; 36 transverse slices; 3.5 mm3 isotropic voxel size, 225×225×126 matrix, GRAPPA, total acquisition time: 7:38. Participants provided a rating on pain from zero to 100 (100 = max pain) immediately prior to beginning the resting state scan with this information included in the statistical model as a covariate (see below).
Functional MRI Data Preprocessing
We preprocessed the resting state fMRI of all participants in the subsequent steps. We removed the first six functional scans for each participant to eliminate transients. The remaining fMRI images were preprocessed using Statistical Parametric Mapping (SPM; www.fil.ion.ucl.ac.uk/spm). Slice timing correction was conducted to compensate for acquisition delays across slices. Motion artifacts were corrected by realigning all timing corrected functional images to the first image. Motion scrubbing was further applied to minimize the negative impact of motion on functional connectivity [33]. Following the motion correction, all the functional images were co-registered to the T1 structural image, which were then normalized to the standard Montreal Neurological Institute (MNI)152 T1 template[33]. These slices were resampled at the resolution of 3mm×3mm×3mm resolution. We smoothed functional images in the MNI template space with an 8mm full width at half-maximum isotropic Gaussian kernel.
fMRI Regions of Interest Selection
Four resting state networks (RSNs): default mode network (DMN), central executive network (CEN), salience network (SN), and visual network (VN) were chosen according to the standard coordinates [34, 35]. The visual network is a resting state network that was not expected to be affected by surgery, and therefore was used as a control network. The regions of interest (ROIs) for the RSNs included the following: DMN - medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), bilateral angular gyrus (AG), and bilateral lateral temporal lobe (TL); CEN - bilateral dorsolateral prefrontal cortex (DLPFC) and bilateral inferior parietal lobule (IPL); SN - dorsal anterior cingulate cortex (ACC) and bilateral anterior insula (IN); and the VN - bilateral central visual cortex (V1C), bilateral peripheral visual cortex (V1P), bilateral extrastriate visual cortex in the central fields (ExC), and bilateral extrastriate visual cortex in the peripheral fields (ExP). These ROIs, representing different brain regions (heretofore called network nodes), were defined using a five radius mm sphere, centered at the coordinates of that region. Table 1 shows ROI coordinates.
Table 1.
Montreal Neurological Institute (MNI) Coordinates of Regions of Interest
| ROI | X | MNI Y |
Z | BA |
|---|---|---|---|---|
| DMN | ||||
| PCC | 1 | −51 | 29 | 23 |
| mPFC | −1 | 61 | 22 | 10 |
| 1AG | −48 | −66 | 34 | 39 |
| 1LT | −65 | −23 | −9 | 21 |
| rAG | 53 | −61 | 35 | 39 |
| rLT | 61 | −21 | −12 | 21 |
| SN | ||||
| ACC | −1 | 10 | 46 | 6 |
| 1IN | −38 | 14 | 5 | 13 |
| rIN | 37 | 18 | 5 | 13 |
| CEN | ||||
| 1DLPFC | −44 | 27 | 33 | 9 |
| 1IPL | −53 | −50 | 39 | 39 |
| rDLPFC | 46 | 28 | 31 | 9 |
| rIPL | 54 | −44 | 43 | 40 |
| VN | ||||
| 1V1C | −1 3 | −100 | −8 | 18 |
| 1V1P | −16 | −74 | 7 | 17 |
| 1ExC | −32 | −89 | −1 | 18 |
| 1ExP | −3 | −74 | 23 | 18 |
| rV1C | 13 | −100 | −8 | 17 |
| rV1P | 16 | −74 | 7 | 17 |
| rExC | 32 | −89 | −1 | 18 |
| rExP | 3 | −74 | 23 | 18 |
BA = Broadman’s Area; MNI = Montreal Neurological Institute; R0I=Region of Interest
Covariates of Interest
Based on prior research, we considered five covariates of interest when processing the resting state differences. These covariates are summarized below with rationale for their inclusion.
Age –
Research shows an interaction of age in resting state networks such that increased age is associated with decreased connectivity in the DMN, and increased internetwork connectivity between the DMN and the anterior cingulate cortex [36].
Education –
Individuals with more education show higher functional connectivity in the DMN [37]. Education is widely used a proxy for cognitive reserve [38, 39].
Sex –
There is an interaction of sex in resting state networks in an older population (over the age of 65), in that male participants have more increase in internetwork connectivity of the DMN and anterior cingulate cortex, at rest, compared to females [36].
Morphine –
Morphine can significantly decrease resting state functional connectivity in the DMN and SN when compared to a placebo [40]. We used a published conversion algorithm to calculate morphine equivalent dosages (MED) [41]. Due to half-life aspects of the medication, MED was considered potentially active if the most recent dose was within six hours prior to the post-surgery MRI [42].
Pain –
Degree of pain impacts resting functional connectivity in differing ways [43] [44]. Previous research has shown there to be an increase in resting functional connectivity in patients with knee osteoarthritis in the insular cortex that is related to intensity of pain, but a decrease in resting functional connectivity from the medial prefrontal cortex to posterior parts of the DMN [45]. Pain assessment ratings (0–100; 100=worst) were acquired before the resting state functional MRI. If a participant was missing a pain assessment rating, a pain rating was imputed for him/her based on taking the average of 10 imputed scores from participants belonging to the same group (surgery/non-surgery). Imputations were conducted in a statistical software program using a regression approach.
Functional Connectivity Analyses
We extracted resting state functional MRI time series from all the voxels in each spherical regions of interest (ROI). Nine nuisance signals were regressed out including six movement variables and three averaged signals representing white matter, cerebrospinal fluid, and global signal. The time series were then filtered with a finite impulse response bandpass filter (between 0.01 and 0.1 Hz). The filtered signals were averaged across all voxels within a ROI to obtain one signal per ROI. Motion scrubbing, a motion censoring procedure [34], was conducted on the blood oxygen level dependent signal to minimize the potential adverse effects of movement on functional connectivity. We quantified the functional connectivity between each pair of ROIs within each resting state network using the Pearson cross correlation between each pair of blood oxygenation level dependent (BOLD) signals. To reduce effects of potentially confounding variables on functional connectivity values, we regressed out covariates (age, education, sex, morphine, pain) from pre-processed functional connectivity Pearson correlation values That is - in a regression model - all covariates were IVs and functional connectivity (correlation coefficient) was the DV. Residuals plus the mean from this model were saved and used for further analysis.
Two variables were calculated at pre- and post-surgical time point for each participant: 1) Mean connectivity: average of cross correlation values of all possible pairs of ROIs within each RSN for each participant and 2) node connectivity: the sum of all correlations of a given node to all other nodes in the network. This was calculated for each ROI in each RSN.
Statistical Analyses
An independent samples t-test was conducted to compare all surgery to all non-surgery participants on demographic variables. Additionally, a two-way analysis of variance (ANOVA) was conducted to examine demographic differences between groups (MCI surgery, non-MCI surgery, MCI non-surgery, non-MCI non-surgery). Post hoc analyses assessed significant interactions between groups to find specific group differences.
Mixed-repeated measures ANOVA assessed group (surgery vs. non-surgery) by time-point (pre-post) differences. All significant interactions were explored using pairwise comparisons. Paired samples t-test assessed change in node connectivity within surgery group, Bonferroni corrected for multiple comparisons. A Cohen’s d statistic was calculated for each group using G*power [46, 47] to quantify the magnitude of change.
A paired samples t-test was conducted on mean connectivity pairing pre and post time points for MCI surgery, non-MCI surgery, MCI non-surgery, non-MCI non-surgery. A Cohen’s d statistic was calculated for each group using G*power [46, 47] to quantify the magnitude of change. Paired samples t-test was chosen instead of an ANOVA because of differences in sample size between MCI and non-MCI groups.
Results
Surgery and Non-Surgery Group Participants.
A total of 232 surgery patients were referred by the study surgeon (HP) and contacted for study inclusion. Of these, 116 agreed to consider the study with 73 meeting inclusion and exclusion criteria and completing baseline neuropsychological assessment and MRI. Data from four surgery participants were excluded due to presence of pre-existing silent strokes (2 participant) and MRI post-surgery scanner complications (2 participants). A total of 69 completed baseline assessment and pre-/post-surgery imaging sessions. One surgery participant required an imputed pain score at the post time-point. For non-surgery orthopedic peers, 104 participants were screened with 68 enrolled. Of the 68, two non-surgery peers were withdrawn due to concerns for a learning disorder identified during neuropsychological testing and one was excluded for a missing RS-fMRI sequence. A total of 65 completed the baseline assessment and pre-post pseudo-surgery imaging sessions.
Participant Characteristics.
See Table 2. Surgery and non-surgery groups did not differ on age, education, gender, race, pre-surgery MRI pain at time of resting state MRI scan, and days between baseline/pre-surgery MRI and post-surgery MRI. Although non-surgery participants were selected to match surgery participants demographically and via a thorough screening process, the two groups did differ on a telephone cognitive screener (Telephone Interview for Cognitive Status) [14] where the surgery group scored on average 1.84 points lower than non-surgery group (p = .006). After surgery, the surgery group reported significantly higher pain at time of the resting state scan relative to non-surgery peers (p = <.001). Although four surgery participants were identified with delirium lasting less than one day, no participants had evidence of delirium at the time of the post-surgery MRI; all participants were included in the analyses.
Table 2.
Participant Characteristics Separated by Surgery Group.
| Demographic | Surgery (n = 69) Mean ± SD |
Non-surgery (n = 65) Mean ± SD |
P Value |
|---|---|---|---|
| Age | 69.35±7.12 (range: 60–85) | 68.37±5.50 (range: 60–83) | 0.377 |
| Education | 15.23±2.83 (range: 10–23) | 16.11±2.64 (range: 12–24) | 0.067 |
| Sex (M:F) | 33:36 | 28:37 | 0.435 |
| Race (W:NW) | 61:8 | 61:4 | 0.119 |
| TICS | 36.71±4.23 (range: 26–47)* | 38.55±3.25 (range: 30–44) | 0.006 |
| Pre MRI Pain | 12.57±19.87 (range: 0–75) | 7.67±14.72 (range: 0–70) | 0.110 |
| Post MRI Pain | 40.10±22.98 (range: 0–100)* | 7.05±10.61 (range: 0–40) | <0.001 |
| Pre to Post MRI day span | 8.77±5.91 (range: 3-41) | 7.36±3.16 (range: 2–21) | 0.093 |
| MED | 11.57±10.87 (range: 0–37.50) | -------------------------------- | |
MED = morphine equivalent medication dose; MRI=Magnetic Resonance Imaging; TICS = Telephone Interview Cognitive Status
Resting State Network Change in Surgery Group (Mixed repeated-measures ANOVA (surgery n=69 and non-surgery n=65).
See Table 3. For the DMN, there was a significant interaction of group and time point (pre and post) [F(1, 132) = 20.856, p<.001)], where the surgery group’s functional connectivity declined more from pre to post-surgery (mean difference = −.089, p<.001). There was a significant similar interaction for the CEN [F(1, 132) = 6.851, p=.010], where the surgery group declined more (mean difference = −.052, p = .020), and the SN [F(1, 132) = 15.300, p<.001], where surgery group also declined more (mean difference = −.134, p<.001). There was no significant interaction for the VN. Partial eta squared values were calculated to assess magnitude of interaction.
Table 3.
Repeated Analysis of Variance for Mean Connectivity By Surgery and Non-Surgery Group
| Network | Effect/ Interaction | df | F | p | partial η2 |
|---|---|---|---|---|---|
| DMN | |||||
| Time point | 1, 132 | 9.288 | 0.003** | 0.066 | |
| Group* Time point | 1, 132 | 20.856 | <0.001*** | 0.136 | |
| CEN | |||||
| Time point | 1, 132 | 1.146 | 0.286 | 0.009 | |
| Group* Time point | 1, 132 | 6.851 | 0.010* | 0.049 | |
| SN | |||||
| Time point | 1, 132 | 6.906 | 0.010* | 0.05 | |
| Group* Time point | 1, 132 | 15.3 | <0.001*** | 0.104 | |
| VN | |||||
| Time point | 1, 132 | 1.298 | 0.257 | 0.01 | |
| Group* Time point | 1, 132 | 0.183 | 0.67 | 0.001 | |
= p<.05
= p<.01
= p<.001
CEN = Central Executive Network; DMN = Default Mode Network
SN = Salience Network; VN = Visual Network
Pre-Post-surgery Node Connectivity Change for Surgery.
See Table 4, Figure 2, and Supplemental Figure demonstrating magnitude of node changes from pre- to post-surgery. All comparisons were Bonferroni corrected.
Table 4.
Node Connectivity Paired Correlation Coefficients in the Surgery Group
| Mean Sum Correlation (SD) | |||||||
|---|---|---|---|---|---|---|---|
| RSN | ROI | Pre | Post | t(df) | P | CI | d |
| DMN | |||||||
| PCC | 1.587(.626) | 1.142(0.722) | 4.442(68) | <0.000*** | 0.25–0.65 | 0.54 | |
| rAG | 1.530(.662) | 1.187( 0657) | 3.912(68) | <0.000*** | 0.17–0.52 | 0.47 | |
| 1AG | 1.790(.549) | 1.323(0.641) | 5.551(68) | <0.000*** | 0.30–0.64 | 0.67 | |
| mPFC | 1.056(.878) | 0.547(0.814) | 4.440(68) | <0.000*** | 0.28–0.74 | 0.52 | |
| rLT | 1.031(619) | 0.702(0.677) | 3.608(68) | 0.001** | 0.09–0.15 | 0.44 | |
| 1LT | 1.034(.579) | 0.797(0.661) | 2.929(68) | 0.005** | 0.08–0.08 | 0.35 | |
| CEN | |||||||
| 1DLPFC | 0.753(0.443) | 0.663(0.411) | 1.645(68) | 0.105 | −0.02–0.20 | 0.20 | |
| rDLPFC | 0.844(0.446) | 0.716(0.416) | 2.251(68) | 0.028 | 0.01–0.24 | 0.29 | |
| 1IPL | 0.822(0.438) | 0.693(0.421) | 2.479(68) | 0.016 | 0.03–0.23 | 0.27 | |
| rIPL | 0.934(0.402) | 0.794(0.445) | 2.340(68) | 0.022 | 0.02–0.26 | 0.28 | |
| SN | |||||||
| ACC | 0.587(0.406) | 0.347(0.341) | 4.647(68) | <0.000*** | 0.14–0.34 | 0.56 | |
| 1AI | 0.776(0.291) | 0.639(0.277) | 3.540(68) | 0.001*** | 0.06–0.10 | 0.43 | |
| rAI | 0.789(0.283) | 0.648(0.273) | 3.444(68) | 0.001*** | 0.06–0.22 | 0.42 | |
Note.
= p<.01
= p<.001, d=Cohen’s D, CI = Confidence Interval
CEN = Central Executive Network; DMN = Default Mode Network; SN = Salience Network VN = Visual Network Please see acronym table for other abbreviations
Figure 2.
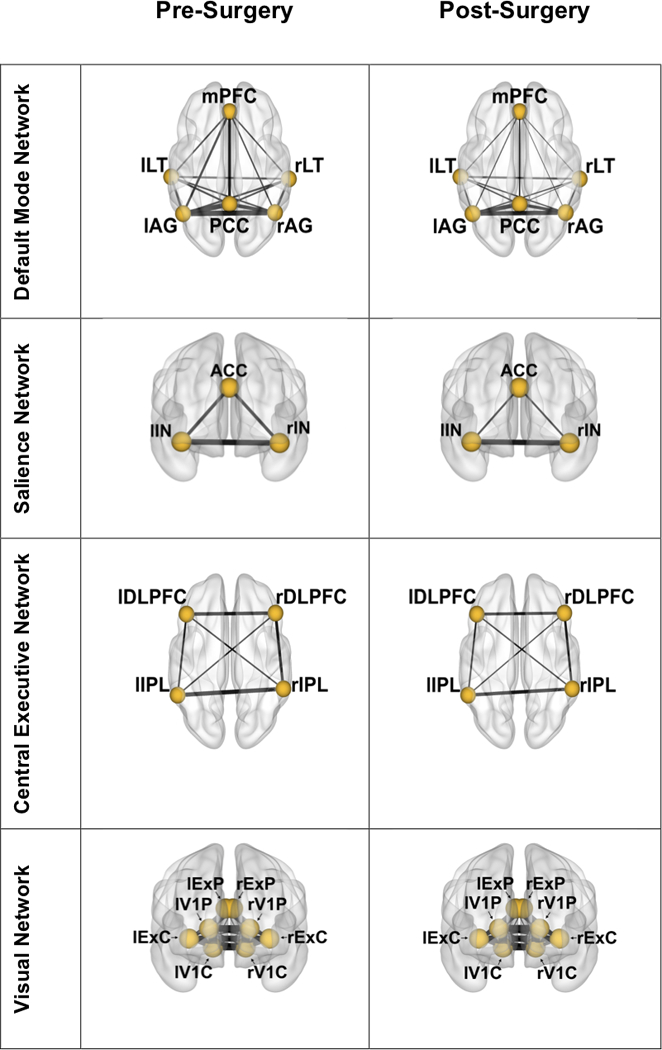
Surgery group mean node connectivity changes from pre to post time points. Line thickness between nodes is weighted by node-to-node correlation. Lowercase “r” and “l” denote right and left brain hemispheres, respectively. Node abbreviation is as follows:
DMN: mPFC – medial prefrontal cortex; LT – lateral temporal; AG – angular gyrus; PCC – posterior cingulate cortex; SN: ACC – anterior cingulate cortex; IN – insula; CEN: DLPFC – dorsolateral prefrontal cortex; IPL – inferior parietal lobe; VN: ExP – extrastriate peripheral fields; V1P – peripheral visual cortex; ExC – extrastriate central fields; V1C – central visual cortex
DMN:
After correcting for multiple comparisons (alpha = .008), there was a significant decline from pre to post time point in sum correlation for all nodes (p≤.005). The left angular gyrus had the greatest magnitude of decline (Cohen’s d = .67) (Supplemental Figure).
CEN:
After correcting for multiple comparisons, there were no significant differences in node connectivity in any ROIs from pre to post time point.
SN:
After correcting for multiple comparisons (alpha = .016), there was a significant decline from pre to post time point in sum correlation for all nodes (p≤.001). The dorsal anterior cingulate cortex had the greatest magnitude of decline (Cohen’s d = .56).
VN:
There was no significant change in node connectivity from pre to post time point.
Participants Classified with Mild Cognitive Impairment
Participant Characteristics.
See Table 5. The final groups of participants consisted of 69 surgery patients (MCI surgery = 13, non-MCI surgery = 56) and 65 non-surgery patients (MCI non-surgery = 10, non-MCI non-surgery = 55). Neuropsychological assessment scores for each group are listed in Table 6.
Table 5.
Participant Characteristics Defined by Surgery and Mild Cognitive Impairment (MCI) Status.
| Demographic | MCI Surgery (n = 13) Mean ± SD |
Non-MCI Surgery (n = 56) Mean ± SD |
MCI Non-Surgery (n = 10) Mean ± SD |
Non-MCI Non-Surgery (n = 55) Mean ± SD |
p value |
|---|---|---|---|---|---|
| Age | 72.38±8.22 (range: 60–85) | 68.64±6.73 (range: 60–85) | 66.6±5.72 (range: 61–81) | 68.9±5.61 (range: 60–83) | 0.149 |
| Education | 13.08±2.36* (range: 10–17) | 15.76±2.73 (range: 12–23) | 14.85±3.06 (range:12–22) | 16.30±2.55 (range: 9–24) | 0.001 |
| Sex (M:F) | 7:6 | 26:30 | 5:5 | 22:33 | 0.862 |
| Race (W:NW) | 12:1 | 49:7 | 8:2 | 53:2 | 0.236 |
| TICS | 33.38±3.57* (range: 26–38) | 37.48±4.01 (range: 27–47) | 35.90±2.38 (range: 32–40) | 38.84±3.13 (range: 30–44) | <0.001 |
| PreMRI Pain | 14.62±22.50 (range: 0–75) | 12.09±19.40 (range: 0–75) | 8.30±11.84 (range: 0–30) | 7.56±15.27 (range: 0–70) | 0.428 |
| PostMRI Pain | 46.69±23.09* (range: 0–80) | 38.57±22.89 (range: 2-100) | 10.90±13.76 (range: 0–40) | 6.34±9.2 (range: 0–40) | <0.001 |
| PrePost MRI day span | 8.54±5.38 (range: 3-21) | 8.82±6.07 (range: 3–41) | 6.90±2.38 (range: 3-11) | 7.45±3.29 (range: 2–21) | 0.399 |
| MED | 14.23±12.05 (range: 0–30) | 10.96±10.60 (range: 0–37.50) | -------------------------------- | -------------------------------- | 0.331 |
denotes significant differences, where MCI-surgery group is significantly lower than all other groups on education and Telephone Interview for Cognitive Status (TICS) and higher on PostMRI pain. Please see acronym table for abbreviations.
Table 6.
Neuropsychological Assessments Separated by Surgery and Mild Cognitive Impairment (MCI) Status.
| Domain | Measures | MCI Surgery (n = 13) Mean±SD |
Non-MCI Surgery ( n = 56) Mean±SD |
MCI Non- Surgery (n = 10) Mean±SD |
Non-MCI Non-Surgery (n = 55) Mean±SD |
|---|---|---|---|---|---|
| Attention | |||||
| TMT Part A | −0.68 (1.20) | −0.09(0.80) | −0.14 (0.92) | 0.30(0.96) | |
| LNS | −0.64 (0.78) | 0.55(0.85) | −0.33 (0.83) | 0.80(0.89) | |
| Digit Span, Forward | 0.01 (0.64) | 0.38(1.02) | −0.27 (0.94) | 0.57(0.90) | |
| Inhibitory/Executive | TMT Part B | −0.31(0.97) | 0.12(0.72) | −0.60(1.11) | 0.35(0.81) |
| D-KEFS Tower | 0.05(0.73) | 0.80(0.75) | 0.43(0.57) | 0.68(0.89) | |
| SCWT, color-word | −0.53(0.71) | 0.31(0.87) | −0.64(0.74) | 0.38(0.76) | |
| Language | |||||
| Letter Fluency | −0.85(0.80) | −0.11(0.90) | −0.22(0.82) | 0.13(1.07) | |
| Animal Fluency | −0.72(0.88) | 0.51(0.67) | 0.02 (0.39) | 0.59(0.83) | |
| BNT | 0.25(0.79) | 0.52(1.02) | 0.83 (1.21) | 1.32(0.83) | |
| Visuospatial | |||||
| Rey-O copy | −1.15(1.29) | −0.11(0.61) | −1.23(1.31) | −0.07(0.56) | |
| Matrix Reasoning | −0.14(1.17) | 0.97(0.98) | 0.09(1.16) | 1.45(0.67) | |
| JLO | 0.16(1.04) | 0.63(0.65) | −0.38(1.37) | 0.55(0.74) | |
| Memory | |||||
| HVLT- delay | −1.42(0.86) | −0.23(0.99) | 0.12 (0.85) | 0.46(0.98) | |
| Logical Memory -delay | 0.13(1.01) | 1.30(0.77) | 0.73(1.25) | 1.80(0.78) | |
| Rey-O - delay | −0.62(0.80) | 0.04(0.73) | −0.53(0.86) | 0.31(0.83) | |
All scores are Z-scores based on standardized normative references correcting for age; please see acronym table for abbreviations
Groups did not differ on age, sex, race, baseline pre-surgery pain level at the time of MRI scan, and days between baseline/pre-surgery MRI and post-surgery MRI. The MCI surgery group had significantly fewer years of education (p = .001), scored lower on the TICS (p= <.001), and had higher post-surgery MRI pain (p = <.001) than the non-MCI surgery and non-MCI non-surgery groups. There were no morphine equivalent dose differences in MCI surgery versus non-MCI surgery group after surgery (p=.331).
Within Group MCI Surgery and MCI Non-Surgery Resting State Network Change See Tables 7 and 8, and Figure 3. MCI surgery and non-MCI surgery groups showed a significant decline in DMN mean connectivity (MCI: t(12) = 2.902, p = .013, d=.802; non-MCI: t(55) = 4.358, p<.001, d=.579) and SN mean connectivity (MCI: t(12) = 3.871, p = .002, d= 1.078; non-MCI: t(55) = 3.327, p=.002, d=.446). For both DMN and SN, based on effect size, the functional connectivity decline was more severe for the MCI group. There were no significant changes for the CEN or VN in either group. Node functional connectivity changes were not assessed due to the small sample size for the MCI groups. There were no significant pre to post-surgery changes in the MCI non-surgery or the non-MCI non-surgery groups.
Table 7.
Pre and Post-Surgery Resting State Network Paired Pearson Correlation Coefficients by Surgery Mild Cognitive Impairment (MCI) Status
| MCI (n=13) | Non-MCI (n=56) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Correlation (SD) | Mean Correlation (SD) | |||||||||||
| Pre-surgery | Post-surgery | t(df) | p | CI | d | Pre-surgery | Post-surgery | t(df) | p | CI | d | |
| DMN | 0.286(0.136) | 0.165(0.100) | 2.902(12) | 0.013* | 0.03–0.21 | 0.802 | 0.263(0.095) | 0.195(0.118) | 4.358(55) | <001** | 0.04–0.10 | 0.579 |
| CEN | 0.310(0.145) | 0.234(0.133) | 2.155(12) | 0.052 | −0.00–0.15 | 0.594 | 0.272(0.121) | 0.240(0.125) | 1.869(55) | 0.067 | −0.0–0.07 | 0.248 |
| SN | 0.384(0.147) | 0.249(0.110) | 3.871(12) | 0.002** | 0.06–0.21 | 1.078 | 0.353(0.153) | 0.278(0.140) | 3.227(55) | 0.002** | 0.03–0.12 | 0.446 |
| VN | 0.263(0.094) | 0.196(0.083) | 1.924(12) | 0.078 | −0.01–0.14 | 0.537 | 0.254(0.124) | 0.249(0.094) | 0.309(55) | 0.758 | −0.03–0.04 | 0.042 |
= p<.05
= p<.01
= p<.001, d=Cohen’s D, CI = Confidence Interval
CEN = Central Executive Network; DMN = Default Mode Network; SN = Salience Network; VN = Visual Network
Table 8.
Pre and Post-Surgery Resting State Network Paired Pearson Correlation Coefficients by Non-Surgery Mild Cognitive Impairment Status
| MCI (n=10) | Non-MCI (n=55) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Correlation (SD) | Mean Correlation (SD) | |||||||||
| Pre-surgery | Post-surgery | t(df) | p | CI | Pre | Post | t(df) | p | CI | |
| DMN | 0.217(0.095) | 0.241(0.114) | −0.579(9) | 0.467 | −0.10–0.05 | 0.271(0.110) | 0.285(0.083) | −0.904(54) | 0.370 | −0.04–0.02 |
| CEN | 0.267(0.111) | 0.287(0.099) | −0.537(9) | 0.604 | −0.10–0.06 | 0.275(0.124) | 0.292(0.109) | −0.956(54) | 0.343 | −0.05–0.02 |
| SN | 0.363(0.127) | 0.346(0.136) | 0.0599(9) | 0.590 | −0.05–0.09 | 0.394(0.133) | 0.417(0.133) | −1.155(54) | 0.253 | −0.06–0.02 |
| VN | 0.369(0.143) | 0.328(0.129) | 0.702(9) | 0.501 | −0.09–0.17 | 0.278(0.102) | 0.277(0.111) | 0.101(54) | 0.920 | −0.02–0.03 |
= p<.05
= p<.01
= p<.001, d=Cohen’s D, CI = Confidence Interval
CEN = Central Executive Network; DMN = Default Mode Network; SN = Salience Network; VN = Visual Network
Figure 3.
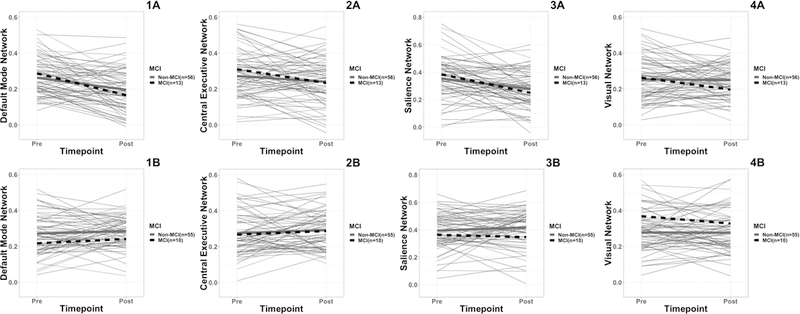
Plot of mean pre and post functional network connectivity values for MCI and non-MCI surgery (A) and non-surgery (B) groups. Thinner lines are individual participants (grey are non-MCI and dashed black are MCI). Thicker lines represent group means (grey are non-MCI and dashed black are MCI). Y-Axis is correlation coefficient. 1A = DMN connectivity surgery group; 1B = DMN connectivity non-surgery group; 2A = CEN connectivity surgery group; 2B = CEN connectivity non-surgery group 3A = SN connectivity surgery group; 3B = SN connectivity non-surgery group; 4A = VN connectivity surgery group; 4B = VN connectivity non-surgery group.
Discussion
This study expanded upon a recent publication by Huang, et al. [3]. With a larger sample participant size, we show that within 48 hours following elective total knee arthroplasty there is an acute decline in connectivity in three major resting state networks: default mode network, salience network, and central executive network. We demonstrate that the neural injury caused by surgery is selective to cognitive networks; while the DMN and SN changed, the visual network (a sensory network) did not significantly change from pre to post-surgery scans. Pre-/post-surgery node-level functional connectivity differences within resting state networks reveal further insights into the nature of network change. Within the DMN, posterior nodes had a greater decline relative to anterior nodes (See Table 5 and Supplemental Figure). Within the SN, the anterior cingulate cortex had the greatest decline. There were no node connectivity differences between nodes in the CEN after multiple comparison correction. Also expanding on Huang, et al. [3] we show that perioperative connectivity changes appear more pronounced for individuals meeting criteria for mild cognitive impairment (MCI). The magnitude of mean functional connectivity decline was nearly one and a half times larger in the DMN and nearly two and a half times larger in the SN post-surgery for the MCI surgery group relative to the non-MCI surgery group.
Surgery Related Resting State Network Change and Within Network Node Change
Within our surgery participant group, a large post-surgery functional connectivity decline occurred for the DMN, SN and CEN. This is consistent with prior literature showing DMN and SN connectivity disruption with general anesthesia [3, 48, 49]. Broadly, the DMN is involved in self-referential processes and stimulus-independent thought, typically active during internal and/or imagined thought [50, 51]. The DMN can be divided into anterior and posterior subsystems, with the mPFC acting as the center of the anterior subsystem, and PCC acting as the center of the posterior subsystem [52, 53]. The SN is primarily involved in detecting and filtering relevant stimuli as requiring external or internal attention, subsequently determining and guiding behavior [54]. CEN activation relates to working memory, problem solving, and goal directed decision-making [55–57]. Emerging evidence has suggested that the SN is heavily involved in modulating dynamic activity and interactions of the DMN and CEN [56, 58].
Within our surgery participant group, we identified particular nodes only within the DMN and SM showing greater resting state network decline following TKA. For the DNM, the PCC and bilateral AG, both of which are included in the posterior subsystem, showed greater decline in connectivity than other nodes in the DMN following surgery. Surgical level sedation from anesthesia is associated with decreased blood flow in the PCC, a region known to be involved in internally-directed thoughts [59]. Disruption of connectivity between the subcortical and cortical systems of the brain have been associated with loss of consciousness [5, 6]. Perhaps, even after arousal, some patients continue to have lingering disruption to these connections. For the SN, the anterior cingulate cortex showed a greater magnitude of connectivity decline than other nodes in the SN. The ACC is a hub for networks involved in attention and cognitive control [54]. Future research now needs to understand if acute reduced connectivity of these DMN and SN nodes, brain areas linked to numerous brain areas and cognitive functions (e.g., language, visual-naming integration, awareness, memory), has implications for longer-term post-surgery cognitive outcome.
In contrast to the DMN and SN, there were no node changes within the CEN. The CEN is composed of bilateral DLPFC and bilateral inferior parietal lobes. These areas are largely involved in working memory and visuospatial attention [60, 61]. Remarkably, although the surgery group had an overall decline in the CEN functional connectivity, at the node level, the connectivity change was not sufficiently large to pass the multiple comparison control. We now need investigation into why CEN nodes uniformly change (and change in a smaller magnitude) relative to other networks at least in non-demented older adults after TKA.
Network Change for Surgery Participants Classified with Mild Cognitive Impairment
Within our participant sample, the magnitude of pre to post-surgery mean connectivity decline was larger in those with MCI relative to non-MCI surgery participants. DMN and SN had largest decline in those with MCI. MCI mean connectivity decline in SN was twice as large compared to those without MCI. These findings speak to the potential vulnerability of DMN resting state networks in MCI [8–11], abnormal connectivity in the SN in MCI and AD patients [62–64], and these patients’ neuronal susceptibility to surgically related insults.
Due to MCI sample size limitations we did not examine within network node changes for MCI relative to non-MCI peers. We speculate specific brain regions for MCI patients are particular vulnerable to surgical insults. DMN and SN anatomical regions of interest include the medial temporal cortical areas, anterior cingulate, and insular regions – area vulnerable to early onset AD [65, 66], as well as small vessel vascular disease [67, 68]. Future research needs to examine node network changes for a larger sample of participant with MCI or AD electing surgical procedures with anesthesia.
There is a growing body of research suggesting a breakdown or fragmentation of neural networks with general anesthesia [69, 70]. For example, using measurements of local field potentials and intracranial electrocorticograms, Lewis, et al. [70] showed a shift in cortical dynamics with the onset of propofol-induced unconsciousness. The authors found that slow-wave oscillations occurred asynchronously throughout the cortex, while spatially close (<4mm) neuronal populations were able to maintain spike rates during certain oscillation phase windows. Boveroux, et al. [69] shows a breakdown in connectivity of the DMN in individuals under general anesthesia (Propofol) during an fMRI scan. These investigations suggest general anesthesia is not benign. For our study, we cannot rule out the possibility that anesthesia is a contributing factor to our functional connectivity findings.
We recognize study limitations. First, our study used a functional MRI procedure with eyes closed. This procedure was employed to avoid potential difficulties with maintaining focus on a fixation point. We recognize an eyes-closed position may not be ideal; participants might fall asleep during the resting state sequence acquisition. To minimize this, however, we started the resting state sequence within the first three minutes of the scan session and participants were spoken to before and after the sequence. We also note that some research suggests light sleep does not disrupt resting state networks [71]. Second, we also recognize limitations to using a resting state scan. Although previous literature has linked connectivity of CEN at rest to working memory performance [72], perhaps CEN node vulnerability would have been identified with a task-based functional approach. Third, as noted above, we had a small participant sample within the MCI groups and this limited power. These groups are well characterized, however, from a comprehensive neuropsychological assessment and careful baseline screening. While there was not a significant interaction of MCI group by surgery group, substantial differences in the magnitude of the connectivity decline were found comparing MCI and non-MCI groups in two of three RSNs using a paired samples t-test. It is possible our MCI group analysis was too underpowered to detect an interaction of MCI group and time point. Other potential limitations include the MCI group had significantly lower education than non-MCI surgery and non-surgery peers. Fewer years of education, but not age, is a critical group distinction that likely indicates differences in cognitive reserve between MCI and non-MCI groups [73]. We attempted to control for these differences by regressing education out of the fMRI signal. Nevertheless, it is possible that education/cognitive reserve had additional effects on post-surgical functional connectivity declines that regression could not address. Previous research suggests pain medication affects resting state connectivity [43, 44]. While we controlled for morphine equivalent medication dose in our analyses, it is possible that the myriad of pain medications have heterogeneous effects on resting state connectivity and could have affected connectivity beyond our statistical covariation. There were also no statistical differences in anticholinergic burden, quantified as a Magellan Score in our surgery or non-surgery group at either time point [74].
While this study provides evidence of RSN changes after TKA under general anesthesia, there are many directions for future research. Future studies now need to examine if type of preoperative cognitive profiles predict type of mean functional connectivity change following surgery with general anesthesia. In addition, we do not know if acute resting state network change heralds long-term cognitive outcomes after major orthopedic surgery, or if RSN changes and specific node vulnerability remains after a period of three months or one year. Finally, given that previous research shows anterior/posterior connectivity differences for MCI [64], a next step is to examine node functional connectivity for MCI versus non-MCI within a larger participant sample.
Conclusion:
Surgery with general anesthesia selectively alters resting state networks and particularly specific nodes within the DMN and SN. Participants with MCI appear to be particularly vulnerable to these changes.
Supplementary Material
Magnitude of Node Change from Pre- to Post-Surgery (Cohen’s d Value). Pre—Post-surgery functional connectivity differences were not observed with the Visual Network (VN) and so this network is not shown in the figure.
Acknowledgements
Research reported in this publication was supported by National Institute of Nursing Research of the National Institutes of Health under award number R01NR014181. Research was also partially supported by the National Institutes of Health’s Clinical and Translational Science Awards program through UL1TR001427, KL2TR001429, and TL1TR001428, and the National Institute of Neurological Disorders and Stroke training grant T32NS082168.
We wish to acknowledge the very valuable time and efforts of the research participants, as well as staff Donna Weber and Kristi Ayers, associated graduate students Nadine Schwab, Loren Hizel, and Elle Wiggins, and research assistant Allison Choi. We also acknowledge the flexibility of the radiology technology staff who provided us early am time periods to complete pre and postoperative scans on the 3T clinical scanner.
Acronyms
- (f)MRI
(functional) Magnetic Resonance Imaging
- ACC
anterior cingulate cortex
- AD
Alzheimer’s Disease
- AG
angular gyrus
- ANOVA
Analysis of Variance
- BNT
Boston Naming Test
- CEN
Central Executive Network
- Digit Span
Digit Span subtest
- D-KEFs
Delis-Kaplan Executive Function System
- DLPFC
dorsolateral prefrontal cortex
- DMN
Default Mode Network
- ExC
extrastriate central fields
- ExP
extrastriate peripheral fields
- IN
insula
- IPL
inferior parietal lobe
- JLO
Judgment of Line Orientation
- LNS
Letter Number Sequencing
- LT
lateral temporal
- MCI
Mild Cognitive Impairment
- MED
Morphine Equivalent Dosage
- MNI
Montreal Neurological Institute
- mPFC
medial prefrontal cortex
- PCC
posterior cingulate cortex
- Rey-O
Rey Osterrieth Complex Figure
- ROI
Region of Interest
- RSN
Resting State Network
- SCWT
Stroop Color Word Test
- SN
Salience Network
- TICS
Telephone Interiew for Cognitive Status
- TKA
Total Knee Arthroplasty
- TMT
Trail Making Test
- V1C
central visual cortex
- V1P
peripheral visual cortex
Footnotes
The authors have no conflict of interest to report.
References
- [1].Abildstrom H, Rasmussen LS, Rentowl P, Hanning CD, Rasmussen H, Kristensen PA, Moller JT (2000) Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly Acta Anesthesiologia Scandanavia 44, 1246–1251. [DOI] [PubMed] [Google Scholar]
- [2].Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Kortilla K, Munoz L, Dodds C, Hanning CD, Moller JT (2003) Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiologica Scandinavica 47, 260–266. [DOI] [PubMed] [Google Scholar]
- [3].Huang H, Tanner J, Parvataneni H, Rice M, Horgas A, Ding M, Price C (2018) Impact of Total Knee Arthroplasty with General Anesthesia on Brain Networks: Cognitive Efficiency and Ventricular Volume Predict Functional Connectivity Decline in Older Adults. J Alzheimers Dis 62, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG (2012) Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp 33, 2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xie G, Deschamps A, Backman SB, Fiset P, Chartrand D, Dagher A, Plourde G (2011) Critical involvement of the thalamus and precuneus during restoration of consciousness with physostigmine in humans during propofol anaesthesia: a positron emission tomography study. Br J Anaesth 106, 548–557. [DOI] [PubMed] [Google Scholar]
- [6].Ramani R (2017) Neuronal Connectivity, General Anesthesia, and the Elderly. Current Anesthesiology Reports 7, 333–339. [Google Scholar]
- [7].White NS, Alkire MT (2003) Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. NeuroImage 19, 402–411. [DOI] [PubMed] [Google Scholar]
- [8].Lee ES, Yoo K, Lee YB, Chung J, Lim JE, Yoon B, Jeong Y (2016) Default Mode Network Functional Connectivity in Early and Late Mild Cognitive Impairment Results From the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer Disease and Associated Disorders 30, 289–296. [DOI] [PubMed] [Google Scholar]
- [9].Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P (2005) Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum Brain Mapp 26, 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gardini S, Venneri A, Sambataro F, Cuetos F, Fasano F, Marchi M, Crisi G, Caffarra P (2015) Increased functional connectivity in the default mode network in mild cognitive impairment: a maladaptive compensatory mechanism associated with poor semantic memory performance. J Alzheimers Dis 45, 457–470. [DOI] [PubMed] [Google Scholar]
- [11].Li M, Zheng G, Zheng Y, Xiong Z, Xia R, Zhou W, Wang Q, Liang S, Tao J, Chen L (2017) Alterations in resting-state functional connectivity of the default mode network in amnestic mild cognitive impairment: an fMRI study. BMC Med Imaging 17, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Satz P (1993) Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology 7, 273–295. [Google Scholar]
- [13].Welsh K, Breitner JSC, Magruder-Habib K (1993) Detection of dementia in the elderly using telephone screening of cogntiive status. Neuropsychiatry, Neuropsychology & Behavioral Neurology 2, 103–110. [Google Scholar]
- [14].Cook SE, Marsiske M, McCoy KJ (2009) The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol 22, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Association AP ed. (2013) Diagnostic and statistical manual of mental disorders, Washington, D.C. [Google Scholar]
- [16].Hizel LP, Warner ED, Wiggins ME, Tanner J, Parvataneni H, Davis R, Penney DL, Libon DJ, Tighe P, Garvan CW, Price CC (2018) Clock drawing performance slows for older adults after total knee replacement surgery. Anesthesiology-Analgesia in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases 40, 373–383. [DOI] [PubMed] [Google Scholar]
- [18].Lawton MP, Brody EM (1969) Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist 9, 179–186. [PubMed] [Google Scholar]
- [19].Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC (2009) Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 17, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP (2014) Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis 42, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Corrigan JD, Hinkeledy NS (1987) Relationships between parts A and B of the Trail Making Test. Journal of Clinical Psychology 43, 402–409. [DOI] [PubMed] [Google Scholar]
- [22].Holdnack HA ed. (1997) Weschler Memory Scale, third edition (WMS-III), The Psychological Corporation, San Antonio, TX. [Google Scholar]
- [23].Stroop JR (1935) Studies of interference in serial verbal reaction. Journal of Experimental Psychology 18, 643–662. [Google Scholar]
- [24].Delis DC, Kaplan E, Kramer JH eds. (2001) Delis-Kaplan Executive Function System (D-KEFS), The Psychological Corporation; San Antonio, TX. [Google Scholar]
- [25].Lezak MD, Howieson DB, Bigler ED, Tranel D eds. (2012) Neuropsychological assessment, Oxford University Press, New York. [Google Scholar]
- [26].Spreen O, Strauss E eds. (1998) A Compendium of neuropsychological tests: Administration, norms, and commentary., Oxford University Press, New York, NY. [Google Scholar]
- [27].Kaplan E, Goodglass H, Weintraub S eds. (1983) Boston Naming Test, Lea & Febiger, Philadelphia [Google Scholar]
- [28].Benton AL, Hamsher K, Varney NR, Spreen O eds. (1983) Contributions to neuropsychological assessment, Oxford University Press, Ney York, NY. [Google Scholar]
- [29].Rey A (1941) L’examen psychologique dans les cas d’encephalopathie traumatque. Archives de Psychologie 28, 215–285. [Google Scholar]
- [30].Osterrieth PA (1944) Filetest de copie d’une figure complex: Contribution a l’etude de la perception et de la memoire. Archives de Psychologie 30, 286–356. [Google Scholar]
- [31].Brandt J (1991) The hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clinical Neuropsychologist 5, 125–142. [Google Scholar]
- [32].Inouye SK, Vvan Dyck CH, Alessi CA, Balkin S, Seigal AP, Horwitz RI (1990) Clarifying confusion: The confusion assessment method. Annals of Internal Medicine 113, 941–948. [DOI] [PubMed] [Google Scholar]
- [33].Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM (1993) in Proceedings of IEEE-Nuclear Science Symposium and Medical Imaging Conference, pp. 1813–1817. [Google Scholar]
- [34].Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011) Functional network organization of the human brain. Neuron 72, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Goldstone A, Mayhew SD, Przezdzik I, Wilson RS, Hale JR, Bagshaw AP (2016) Gender Specific Reorganization of Resting-State Networks in Older Age. Front Aging Neurosci 8, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bozzali M, Dowling C, Serra L, Spano B, Torso M, Marra C, Castelli D, Dowell NG, Koch G, Caltagirone C, Cercignani M (2015) The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. J Alzheimers Dis 44, 243–250. [DOI] [PubMed] [Google Scholar]
- [38].Valenzuela MJ, Sachdev P (2006) Brain reserve and dementia: a systematic review. Psychol Med 36, 441–454. [DOI] [PubMed] [Google Scholar]
- [39].Valenzuela MJ, Sachdev P (2006) Brain reserve and cognitive decline: a non-parametric systematic review. Psychological Medicine 36. [DOI] [PubMed] [Google Scholar]
- [40].Khalili-Mahani N, Zoethout RM, Beckmann CF, Baerends E, de Kam ML, Soeter RP, Dahan A, van Buchem MA, van Gerven JM, Rombouts SA (2012) Effects of morphine and alcohol on functional brain connectivity during “resting state”: a placebo-controlled crossover study in healthy young men. Hum Brain Mapp 33, 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dowell D, Haegerich TM, Chou R (2016) CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA 315, 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ordonez Gallego A, Gonzalez Baron M, Espinosa Arranz E (2007) Oxycodone: a pharmacological and clinical review. Clin Transl Oncol 9, 298–307. [DOI] [PubMed] [Google Scholar]
- [43].Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V (2013) Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain 154, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kuner R, Flor H (2016) Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 18, 20–30. [DOI] [PubMed] [Google Scholar]
- [45].Baliki MN, Mansour AR, Baria AT, & Apkarian AV (2014). Functional reorganization of the default mode network across chronic pain conditions. PloS one, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Faul F, Erdfedler E, Lang AG, Buchner A (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39, 175–191. [DOI] [PubMed] [Google Scholar]
- [47].Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41, 1149–1160. [DOI] [PubMed] [Google Scholar]
- [48].Browndyke JN, Berger M, Harshbarger TB, Smith PJ, White W, Bisanar TL, Alexander JH, Gaca JG, Welsh-Bohmer K, Newman MF, Mathew JP (2017) Resting-State Functional Connectivity and Cognition After Major Cardiac Surgery in Older Adults without Preoperative Cognitive Impairment: Preliminary Findings. J Am Geriatr Soc 65, e6–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Palanca BJ, Mitra A, Larson-Prior L, Snyder AZ, Avidan MS, Raichle ME (2015) Resting-state Functional Magnetic Resonance Imaging Correlates of Sevoflurane-induced Unconsciousness. Anesthesiology 123, 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007) Wandering minds: The Default Mode Network and stimulus-independent thought. Science 315, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Spreng RN, Mar RA, Kim ASN (2008) The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the Default Mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience 21, 489–510. [DOI] [PubMed] [Google Scholar]
- [52].Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA (2008) Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18, 1856–1864. [DOI] [PubMed] [Google Scholar]
- [53].Xu X, Yuan H, Lei X (2016) Activation and Connectivity within the Default Mode Network Contribute Independently to Future-Oriented Thought. Sci Rep 6, 21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214, 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006) A core system for the implementation of task sets. Neuron 50, 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- [57].Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Science 15, 483–506. [DOI] [PubMed] [Google Scholar]
- [58].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kaisti KK, Metsahonkala L, Teras M, Oikonen V, Aalto S, Jaaskelainen S, Hinkka S, Scheinin H (2002) Effects of surgical levels of propofol and sevoflurance anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography Anesthesiology 96, 1358–1370. [DOI] [PubMed] [Google Scholar]
- [60].Petrides M (2000) The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res 133, 44–54. [DOI] [PubMed] [Google Scholar]
- [61].Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3, 201–215. [DOI] [PubMed] [Google Scholar]
- [62].Badhwar A, Tam A, Dansereau C, Orban P, Hoffstaedter F, Bellec P (2017) Resting-state network dysfunction in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimers Dement (Amst) 8, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].He X, Qin W, Liu Y, Zhang X, Duan Y, Song J, Li K, Jiang T, Yu C (2014) Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp 35, 3446–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Krajcovicova L, Marecek R, Mikl M, Rektorova I (2014) Disruption of resting functional connectivity in Alzheimer’s patients and at-risk subjects. Curr Neurol Neurosci Rep 14, 491. [DOI] [PubMed] [Google Scholar]
- [65].Ye BS, Seo SW, Yang JJ, Kim HJ, Kim YJ, Yoon CW, Cho H, Noh Y, Kim GH, Chin J, Kim JH, Jeon S, Lee JM, Na DL (2014) Comparison of cortical thickness in patients with early-stage versus late-stage amnestic mild cognitive impairment. Eur J Neurol 21, 86–92. [DOI] [PubMed] [Google Scholar]
- [66].Wu L, Rowley J, Mohades S, Leuzy A, Dauar MT, Shin M, Fonov V, Jia J, Gauthier S, Rosa-Neto P, Alzheimer’s Disease Neuroimaging I (2012) Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS One 7, e47905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Spence JD (2019) Blood Pressure Gradients in the Brain: Their Importance to Understanding Pathogenesis of Cerebral Small Vessel Disease. Brain Sci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Spence JD (2019) The Importance of Blood Pressure Gradients in the Brain: Cerebral Small Vessel Disease. JAMA Neurol. [DOI] [PubMed] [Google Scholar]
- [69].Boveroux P, Vanhaudenhuyse A, Bruno M, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenavaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M (2010) Breakdown of within- and between-network Resting State Functional Magnetic Resonance Imaging Connectivity during Propofol-induced Loss of Consciousness. Anesthesiology 113, 1038–1053. [DOI] [PubMed] [Google Scholar]
- [70].Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, Purdon PL (2012) Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A 109, E3377–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Larson-Prior L, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME (2008) Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences of the United States of America 106, 4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fang X, Zhang Y, Zhou Y, Cheng L, Li J, Wang Y, Friston KJ, Jiang T (2016) Resting-State Coupling between Core Regions within the Central-Executive and Salience Networks Contributes to Working Memory Performance. Front Behav Neurosci 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society 8, 448–460. [PubMed] [Google Scholar]
- [74].Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE (2008) The anticholinergic risk scale and anticholinergic adverse effects in older persons. The Journal of the American Medical Association Internal Medicine 168, 508–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Magnitude of Node Change from Pre- to Post-Surgery (Cohen’s d Value). Pre—Post-surgery functional connectivity differences were not observed with the Visual Network (VN) and so this network is not shown in the figure.


