Abstract
Background/Aims
Acute appendicitis is the most frequent cause of acute abdomen emergency surgery. It continues to be a problem today due to delayed diagnosis and its high perforation rate. For this reason, diagnostic tests continue to be developed. In this experimental study, the diagnostic significance of blood procalcitonin (PCT), interleukin (IL)-6, IL-2, and D-dimer levels in an acute appendicitis model in rabbits was investigated.
Materials and Methods
A total of five groups were included: control group, sham group, and three different acute appendicitis groups. In the appendicitis groups, the appendix was ligated by laparotomy, and the blood PCT, IL-6, IL-2, and D-dimer levels were measured at 12 (group 3), 24 (group 4), and 48 h (group 5). Then, an appendectomy was performed.
Results
In the present study, PCT and IL-6 levels increased in parallel with the inflammation of the appendix in all groups and were found to be statistically significant. IL-2 and D-dimer values were higher in the groups diagnosed with appendicitis but were not statistically significant.
Conclusion
In our experimental study, PCT and IL-6 levels were determined to be important in the early diagnosis of acute appendicitis, especially IL-6, and that these two parameters are more important markers than IL-2 and D-dimer.
Keywords: Acute appendicitis, procalcitonin, IL-6, IL-2, D-dimer
INTRODUCTION
Acute appendicitis is the most common cause of acute abdominal pain. It has common symptoms with other abdominal pains and may be confused with many diseases. In 1711, Lorenz Heister first showed the importance of appendicitis (1). The first known appendectomy was performed in 1735 by Claudius Amyand (1). Currently, there are an estimated 50,000 books and publications about the appendix (1).
Today, appendectomy is the most common intra-abdominal surgical operation (2). Although there has been a significant reduction in deaths due to acute appendicitis with evolving technology and increasing diagnostic methods, there has been no reduction in perforation and negative appendectomy (NA) (2). The rate of NA is 20%–30%, and this rate can be up to 35% in women, especially in children and in the elderly (1,2). Perforated acute appendicitis is seen in 25% of laparotomies, increasing the morbidity and mortality rates (2).
Many parameters have been examined to support the diagnosis of acute appendicitis until today (2). The main purpose of the diagnostic methods to support the diagnosis of acute appendicitis is to decrease the rate of negative laparotomy, which reaches up to 5%–35%, and perforated appendicitis, thereby decreasing hospital costs and workforce loss (3). An increase in sensitivity rates of diagnostic tests and imaging methods also increases costs and therefore cannot be used commonly (1,3).
Currently, there is still no ideal diagnostic tool that can be used alone to make a definite diagnosis before surgery. Research to find non-invasive, inexpensive, and practically usable laboratory methodologies that do not depend on the user’s experience in the diagnosis of acute appendicitis is still ongoing (4).
Procalcitonin (PCT) and interleukin (IL)-6 were acute phase reactants and correlated between the acute course of infections and their plasma levels (4). IL-2 levels, which induce the immune response and activate lymphocytes and monocytes, are elevated in inflammation. Infection and activation of the sepsis coagulation cascade are common and early events, and many of the molecules involved in this process, such as D-dimer, are also important amplifiers of the inflammatory response. PCT, IL-6, IL-2, and D-dimer plasma levels are elevated at different levels in the course of acute appendicitis (5). Measurement of the levels of these molecules, which can now be performed easily and relatively inexpensively, may be useful in diagnosing acute appendicitis and in determining prognosis.
The aim of this experimental study was to investigate the diagnostic contributions of blood PCT, D-dimer, IL-2, and IL-6 levels in the early or late phase of patients with a preliminary diagnosis of acute appendicitis.
MATERIALS AND METHODS
The study was conducted in accordance with the principles related to the animal experiments. The animal research ethics committee approved the study (protocol no. KS-08/19 K).
Subjects
A total of 35 New Zealand male rabbits weighing 3000 g were used in the study. The animals were kept in the same laboratory conditions throughout the study and fed with commercial rabbit food and normal tap water. All animals were starved for 12 h prior to surgery providing only water. A total of five groups with seven rabbits in each experimental group were studied depending on power analysis calculation.
Group 1 (control group)
Blood was obtained from the ear of the rabbits to determine the IL-2, IL-6, PCT, and D-dimer levels without surgery.
Group 2 (sham group)
The rabbits were laparotomized, and a waiting period of 45 min was passed. Then, the abdomen was closed, and blood was obtained from an ear vein to determine the IL-2, IL-6, PCT, and D-dimer levels.
Group 3 (12-hour group)
The appendix of the rabbits was ligated with laparotomy, and blood was obtained from an ear vein to determine the IL-2, IL-6, PCT, and D-dimer levels at 12 h. Appendectomy was performed after the completion of the experimental procedures.
Group 4 (24-hour group)
The appendix of the rabbits was ligated with laparotomy, and blood was obtained from an ear vein to determine the IL-2, IL-6, PCT, and D-dimer levels at 24 h. Appendectomy was performed after the completion of the experimental procedures.
Group 5 (48-hour group)
The appendix of the rabbits was ligated with laparotomy, and blood was obtained from an ear vein to determine the IL-2, IL-6, PCT, and D-dimer levels at 48 h. Appendectomy was performed after the completion of the experimental procedures.
Surgical method
Subjects that were starved for 12 h prior to surgery providing only water were injected intramuscularly with 50 mg/kg ketamine sodium and 25 mg/kg xylazine HCl for general anesthesia. After anesthesia, the frontal abdominal walls of the rabbits in groups 2, 3, 4, and 5 were shaved and cleaned with 10% povidone iodine. Then, the abdomen was opened with a 4 cm median incision, and the appendix was located. In groups 3, 4, and 5, the appendices were ligated at approximately 10 cm distal part with a 2.0 vicryl suture to preserve mesenteric blood flow, and an experimental appendicitis model was established. Then, the abdomen was closed with a 2.0 silk suture.
Laboratory analysis
After the first operation, a blood sample was obtained from an ear vein at 12 (group 3), 24 (group 4), and 48 h (group 5) to evaluate the IL-2, IL-6, cytokine, PCT, and D-dimer levels.
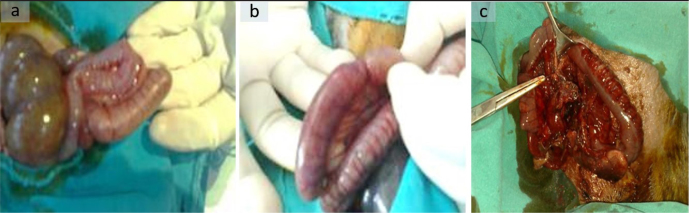
After the blood samples were obtained, the previous incision site of all rabbits in groups 3, 4, and 5 was reopened, and appendectomy was performed. The incision sites were closed individually with 2.0 silk sutures. During these procedures, the rabbits were protected from hypothermia using a light source (Figure 1).
Figure 1. a–c.
Experimental models of acute appendicitis. Group 3–12th hour (a); Group 4–24th hour (b); Group 5–48th hour (c)
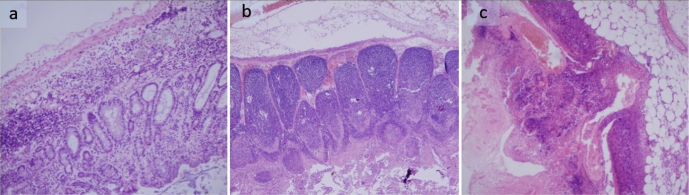
For pathological evaluation, the removed appendix material was fixed in 10% formalin solution for 12 h. Then, the horizontal sections were removed, and the tissue was sampled. After the routine tissue procedures, serial sections of 5 μm thickness were obtained from the samples embedded in paraffin blocks. The samples were deparaffinized, stained with hematoxylin and eosin, and closed with a lamel. All preparations were evaluated under a light microscope (Figure 2).
Figure 2. a–c.
Acute appendicitis histopathological findings. Group 3–12th hour (a); Group 4–24th hour (b); Group 5–48th hour (c) (H–E)
Subsequently, 3 mL blood samples obtained from an ear vein of each rabbit for IL-2, IL-6, PCT, and D-dimer level measurements were centrifuged at 3000 rpm for 10 min, and sera were separated and stored at −70 °C. The sera were dissolved at room temperature on the day of the enzyme-linked immunosorbent assay (ELISA) study. ELISA kits for IL, PCT, and D-dimer (BioSource International Inc., Camarillo, CA, USA) were used. ELISA plates were evaluated using an EL800x microplate reader, and IL-2, IL-6, PCT, and D-dimer measurements were made.
Statistical analysis
Data were analyzed by the Statistical Package for Social Sciences Statistics version 12.2 package program (SPSS Inc.; Chicago, IL, USA). Normality tests for the variables were performed by the Shapiro-Wilk test. The Student’s t-test was used for comparison of two groups, and the analysis of variance (ANOVA) test was used for comparison of five groups for variables that are normally distributed in tests for normality. For variables that are not normally distributed, the Mann-Whitney U test was used for comparison of two groups, and the Kruskal-Wallis H test was used for comparison of five groups.
RESULTS
All rabbits in the experimental study completed the study period, and no mortality was observed. According to multiple comparisons conducted to determine the differences between the groups in the measurements of PCT, IL-6, IL-2, and D-dimer levels, PCT values significantly differ between the groups. IL-6 levels did not differ between groups 3 and 4 and groups 3 and 5, but there were significant differences between the other groups. IL-2 levels differed only between groups 1 and 3 and groups 1 and 5. There was no significant difference between the other groups. Evaluation of the difference between the groups in the D-dimer variable demonstrated that the value in group 1 was lower than that in groups 4 and 5, and the value in group 2 was lower than that in groups 3, 4, and 5. The ANOVA test revealed p=0.0001 for PCT, p=0.0001 for IL-6, and p=0.007 for IL-2. There were significant differences between the groups in PCT, IL-6, and IL-2 variables (Table 1).
Table 1.
The mean values for the groups of parameters studied in the blood
| Groups | Procalcitonin (pg/mL) Mean±SD |
IL-6 (pg/mL) Mean±SD |
IL-2 (pg/mL) Mean±SD |
D-dimer (ug/mL) Mean±SD |
|---|---|---|---|---|
| Group 1 | 14.3±1.2 | 1.9±4 | 49.9±26.6 | 0.096±0.053 |
| Group 2 | 17.8±1.2 | 2.4±3.9 | 77.6±46.7 | 0.074±0.085 |
| Group 3 | 22.3±1.5 | 46.8±7.7 | 227.1±74.7 | 0.232±0.055 |
| Group 4 | 27.7±2.3 | 45.7±4.9 | 198.5±38.6 | 0.300±0.127 |
| Group 5 | 40.6±5.4 | 43.2±6.4 | 247.8±48.6 | 0.220±0.034 |
Appendectomy specimens removed in the appendectomy groups were evaluated histopathologically. In group 3, appendectomy specimens showed submucosal edema and mild inflammatory cellular infiltrates, including eosinophilic leukocytes in the lamina propria and submucosal area. In group 4, appendectomy specimens showed focal mucosal necrosis, ulceration, significant lymphoid hyperplasia, marked congestion at the submucosal area, edema, and moderate to severe neutrophil infiltration in the submucosa and mucosa layers. In group 5, appendectomy specimens showed diffuse transmural necrosis and mucosal ulceration, focal perforation, and marked neutrophilic infiltration (peritonitis) in the periappendiceal fat tissue (Figure 2).
The distribution of PCT, IL-6, IL-2, and D-dimer values in the groups was examined for pathological correlation. PCT and D-dimer values were found to be significantly higher in the groups histopathologically diagnosed with acute appendicitis (p<0.05). IL-6 and IL-2 levels were also significantly higher in the groups histopathologically diagnosed with acute appendicitis (p<0.05). In conclusion, the evaluated blood markers were correlated with histopathological findings of appendix inflammation (Table 2).
Table 2.
Correlation between blood markers and pathology results
| Blood Parameters | Pathology Results | n | Mean | Median | Min. | Max. | p |
|---|---|---|---|---|---|---|---|
| Procalcitonin (pg/mL) | Not Appendicitis | 14 | 19.3 | 17.1 | 12.6 | 41.8 | 0.000* |
| Appendicitis | 21 | 28.4 | 25.9 | 20.4 | 48.2 | ||
| D-dimer (ug/mL) | Not Appendicitis | 14 | 0.01 | 0.021 | 0.0 | 0.3 | 0.000* |
| Appendicitis | 21 | 0.2 | 0.2 | 0.1 | 0.5 | ||
| IL-6 (pg/mL) | Not Appendicitis | 14 | 2,1 | 7,8 | 1,4 | 16,2 | 0,0001† |
| Appendicitis | 21 | 45,8 | 46,5 | 14,7 | 61,8 | ||
| IL-2 (pg/mL) | Not Appendicitis | 14 | 63,8 | 164,8 | 104,7 | 228,9 | 0,002† |
| Appendicitis | 21 | 224,5 | 220,5 | 133,9 | 356,1 |
Mann Whitney U;
Student t test
Cut-off, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and odds ratio were calculated for blood tests. Sensitivity values of PCT and IL-6 were >90%, and sensitivity of D-dimer was close to 90%. This rate was 76% for IL-2. Similarly, PPV values of PCT and IL-6 were found to be approximately 90%. The PPV value of D-dimer was 82%, whereas the PPV value of IL-2 was 80%. Multiple comparisons and Kruskal-Wallis tests also support that PCT and IL-6 are more important determinants of appendicitis than IL-2 and D-dimer (Table 3).
Table 3.
Cut-off, sensitivity, specificity, PPV and NPV values for blood tests
| Blood Parameters | Cut-Off | Sensitivity | Specificity | PPV£ | NPVa | OR | Lb | Ub |
|---|---|---|---|---|---|---|---|---|
| Procalcitonin (pg/mL) | 21.24 | 90.47 | 85.71 | 90.47 | 85.71 | 57 | 7.05 | 460.36 |
| IL-6 (pg/mL) | 33.73 | 90.476 | 78.57 | 86.363 | 84.615 | 34.83 | 5.02 | 241.71 |
| IL-2 (pg/mL) | 175.35 | 76.19 | 71.42 | 80 | 66.66 | 8 | 1.72 | 37.09 |
| D-dimer (ug/mL) | 0.105 | 89.47 | 85.69 | 82.74 | 87.51 | 57 | 7.501 | 406.326 |
Positive Predictive Value;
Negative Predictive Value;
95% CI for OR
In a receiver operating characteristic analysis, curves to the left and above the reference line represent higher accuracy, i.e., clinical adequacy. Therefore, it may be observed that PCT and IL-6 tests provide higher accuracy (Figure 3).
Figure 3.
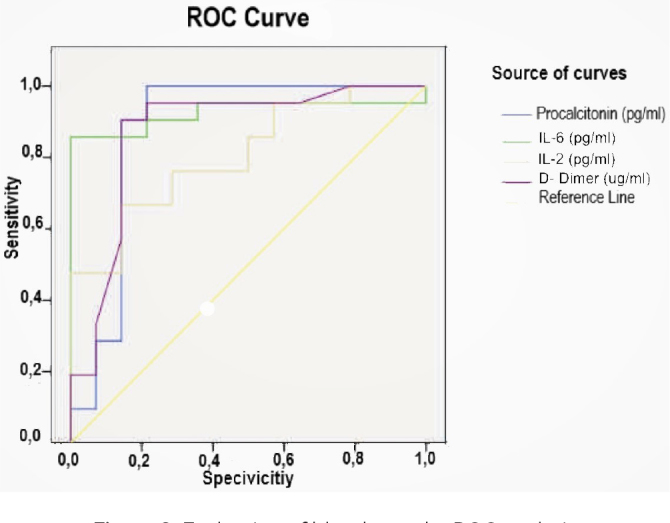
Evaluation of blood tests by ROC analysis
DISCUSSION
Acute appendicitis is among the most common surgical emergencies worldwide and has an estimated lifetime risk of 7%–8% (4). It occurs in approximately 90–100/100,000 individuals per year in developed countries. It is usually seen in the second or third decade of life and is usually more common in men (1). Although it is known that the factors that cause direct luminal occlusions, such as fecaliths, lymphoid hyperplasia, tumor, and several infectious agents, such as Fusobacterium and Escherichia coli, cause appendicitis, the exact etiology is still unknown (4,5). Studies have reported that a positive family history increases the risk of acute appendicitis three times (6). Today, research on its etiology focuses on genetic factors, environmental effects, and infections (2,3).
The risk of acute appendicitis is high in any patients presenting with acute abdomen; although various biomarkers and imaging methods, such as sonography and tomography, are helpful in the diagnosis, definitive diagnosis before surgery is still difficult (4,7). Ultrasonography depends on the experience of the sonographer and the dependent variables, such as the quality and resolution of the devices; therefore, tomography is the best radiological method for detecting appendicitis, but radiation exposure and long-term cancer risks are a cause of great concern (5,7). The Alvarado, modified Alvarado, Fenyo-Lindberg, Lintula, Eskelinen, Teicher, and Christian scoring systems, which are based on symptoms, clinical findings, and laboratory results and applied during the decision making of acute appendicitis surgery, also helped the diagnosis (4,8).
The rate of diagnosis of non-perforated appendicitis has been increased, but the perforation rate has remained constant regardless of the diagnosis and treatment approach (3). Today, mortality in acute appendicitis is 0.09%–0.24% in developed countries and 1%–4% in developing countries (1). When perforation develops in the elderly, the mortality rate reaches 15%–20% (1,4). On the other hand, the proportion of NA in children and the elderly is 20%–30%, which can be up to 35% in women (7,9). Unfortunately, the decline in acute appendicitis mortality over the last 30 years is not accompanied by a significant increase in diagnostic accuracy (4,7). For this reason, NAs cause a significant medical and financial burden (9). NA results in important morbidities, such as wound infections, intestinal obstruction, and infertility (2,9).
Numerous parameters have been examined to support the diagnosis of acute appendicitis until today (5). Biomarkers are used to support anamnesis and physical examination, especially in children, women of childbearing age, and elderly patients where the diagnosis is difficult (1,4,7). However, in some cases, none of the new inflammatory markers, including leukocyte, C-reactive protein (CRP), or PCT, can identify acute appendicitis with high specificity and sensitivity (7,10).
CRP, IL-6, tumor necrosis factor, PCT, granulocyte colony-stimulating factor, calprotectin, chemokine ligand-8, serum amyloid A, matrix metalloproteinase 9, and myeloperoxidase are among the inflammatory markers investigated (7,11). In the present study, we examined the correlation between PCT, IL-6, IL-2, and D-dimer levels and histopathologically evaluated appendix inflammation.
Procalcitonin is a precursor of calcitonin (10). It is rapidly produced by the C cells of the thyroid gland, K cells of the lungs, and many parenchymal tissues that are stimulated by endotoxin or inflammatory cytokines. Bacterial lipopolysaccharides and proinflammatory cytokines are the most potent inducers of PCT production (12). Unlike CRP, PCT levels do not increase in patients with sterile inflammation or viral infection. For this reason, it is a good biomarker in many inflammatory conditions, such as acute appendicitis, sepsis, and meningitis (13).
In some studies, it has been suggested that PCT can be used not only as a diagnostic marker for acute appendicitis in both children and adults but also as a prognostic indicator of complications (14). PCT has also been reported to be a better diagnostic test than CRP since it allows patients with real acute appendicitis to be distinguished from patients with abdominal pain with a normal appendix (14). Some studies have reported that PCT is not effective at the early stages of acute appendicitis but is a good test for acute necrotizing appendicitis or perforated appendicitis (15). In contrast, some investigators have reported that PCT has a low sensitivity for the diagnosis of acute appendicitis; its diagnostic accuracy is less than CRP and leukocyte, and it should not be used routinely (16).
In the present study, the highest sensitivity and positive predictive rates were observed in the PCT group when the parameters we investigated for correlation were considered. More importantly, the PCT levels showed an increasing trend in all groups, parallel to the inflammation in the clinical picture of appendicitis. In the experimental appendicitis model in rabbits, the results of blood samples obtained at 12, 24, and 48 h showed that the sensitivity of PCT was >90%, the specificity value was 85%, and the cut-off value was 21.24 pg/mL. PCT was found to be diagnostic for acute appendicitis. Studies on the efficacy of PCT in other acute peritonitis models are still needed.
Studies have reported that IL-6 levels show significant increases in the presence of acute appendicitis, especially when perforated appendicitis develops, and may be useful in the diagnosis (17). However, some studies showed that IL-6 levels are not a predictor of acute appendicitis (18), but there was no difference in the appendicitis groups. We believe that IL-6 has reached the highest levels in early appendicitis in our study because it is an early indicator of inflammation. Therefore, we believe that it can be used for diagnosis at an early period.
IL-2 is a growth factor for T cells, and it was also shown to stimulate natural killer and B cells. It plays an important role especially in autoimmune inflammatory diseases (19). In some studies, it has been reported that IL-2 levels increase in acute focal and suppurative appendicitis (19). However, other studies have noted that IL-2 levels are not a predictor of acute appendicitis, whereas increased levels of IL-6 may be more helpful in the diagnosis (18,19). In our study, although the correlation between IL-2 serum levels and histopathological findings was high, it was not found to be statistically significant. In addition, the sensitivity (76.19%) and specificity (71.42%) of IL-2 were the lowest among the parameters studied.
D-dimer is a fibrin degradation product and is increased in cases of venous stasis, thrombus, and ischemia (19,20). In acute appendicitis, ischemia occurs with increased obstruction and intraluminal pressure in the appendix lumen (4). Studies have reported that D-dimer can be used especially in the diagnosis of perforated appendicitis and to follow its prognosis (20). However, some studies have shown that D-dimer levels are not statistically significant in the diagnosis of acute appendicitis (20,21).
In the present study, it was seen that the sensitivity of D-dimer was close to 90%. Statistical evaluation of D-dimer values revealed that the groups diagnosed with appendicitis had significantly higher levels than the control and sham groups, but there was no statistically significant difference between the appendicitis groups.
When we considered sensitivity, specificity, PPV, and NPV in our study, the highest values were found for calcitonin. IL-6, D-dimer, and IL-2 followed PCT in order. It was concluded that PCT and IL-6 were more important determinants of appendicitis than IL-2 and D-dimer.
In conclusion, our study has shown that PCT and IL-6 may be of particular value in the diagnosis of acute appendicitis. We believe that PCT and IL-6 may be used as adjunctive laboratory tests in clinically difficult cases. They may help decrease the rates of negative laparotomy. Since they show the stages of necrotizing and perforating appendicitis, better time, cost, and loss of workload associated with them can be avoided. While our results need to be supported by further clinical investigations, we believe that these two determinants can be used as adjunctive laboratory tests in clinically difficult cases.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the animal research ethics committee (Protocol No: KS-08/19 K).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.O.G.; Design - V.O.G., M.Ö.M.; Supervision - V.O.G., M.O.M.; Resources - V.O.G.; Materials - V.O.G., A.F.Ç.; Data Collection and/or Processing - V.O.G.; Analysis and/or Interpretation - V.O.G., A.F.Ç., S.D.; Literature Search - S.D., V.O.G.; Writing Manuscript - S.D.; Critical Review - S.D., V.O.G., M.Ö.M., A.F.Ç.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Collins CM, Davenport DL, Talley CL, Bernard AC. Appendicitis Grade, Operative Duration and Hospital Cost. J Am Coll Surg. 2018;226:578–83. doi: 10.1016/j.jamcollsurg.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 2.Humes DJ, Simpson J. Acute appendicitis. BMJ. 2006;333:530–4. doi: 10.1136/bmj.38940.664363.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart B, Khanduri P, McCord C, et al. Global disease burden of conditions requiring emergency surgery. Br J Surg. 2014;101:9–22. doi: 10.1002/bjs.9329. [DOI] [PubMed] [Google Scholar]
- 4.Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT. Acute appendicitis: modern understanding of pathogenesis, diagnosis and management. Lancet. 2015;386:1278–87. doi: 10.1016/S0140-6736(15)00275-5. [DOI] [PubMed] [Google Scholar]
- 5.Lamps LW. Infectious causes of appendicitis. Infect Dis Clin North Am. 2010;24:995–1018. doi: 10.1016/j.idc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Ergul E. Heredity and familial tendency of acute appendicitis. Scand J Surg. 2007;96:290–2. doi: 10.1177/145749690709600405. [DOI] [PubMed] [Google Scholar]
- 7.Shogilev DJ, Duus N, Odom SR, Shapiro NI. Diagnosing appendicitis: evidence-based review of the diagnostic approach in 2014. West J Emerg Med. 2014;15:859–71. doi: 10.5811/westjem.2014.9.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xingye W, Yuqiang L, Rong W, Hongyu Z. Evaluation of Diagnostic Scores for Acute Appendicitis. J Coll Physicians Surg Pak. 2018;28:110–4. doi: 10.29271/jcpsp.2018.02.110. [DOI] [PubMed] [Google Scholar]
- 9.Jeon BG. Predictive factors and outcomes of negative appendectomy. Am J Surg. 2017;213:731–8. doi: 10.1016/j.amjsurg.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Yu CW, Juan LI, Wu MH, Shen CJ, Wu JY, Lee CC. Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br J Surg. 2013;100:322–9. doi: 10.1002/bjs.9008. [DOI] [PubMed] [Google Scholar]
- 11.Andersson M, Ruber M, Ekerfelt C, Hallgren HB, Olaison G, Andersson RE. Can new inflammatory markers improve the diagnosis of acute appendicitis? World J Surg. 2014;38:2777–83. doi: 10.1007/s00268-014-2708-7. [DOI] [PubMed] [Google Scholar]
- 12.Becker KL, Nylén ES, White JC, Müller B, Snider RH., Jr Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–25. doi: 10.1210/jc.2002-021444. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita H, Yuasa N, Takeuchi E, et al. Diagnostic value of procalcitonin for acute complicated appendicitis. Nagoya J Med Sci. 2016;78:79–88. [PMC free article] [PubMed] [Google Scholar]
- 14.Chandel V, Batt SH, Bhat MY, Kawoosa NU, Yousuf A, Zargar BR. Procalcitonin as the biomarker of inflammation in diagnosis of appendicitis in pediatric patients and prevention of unnecessary appendectomies. Indian J Surg. 2011;73:136–41. doi: 10.1007/s12262-010-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu CW, Juan LI, Wu MH, Shen CJ, Wu JY, Lee CC. Systematic review and meta-analysis of the diagnostic accuracy of procalcitonin, C-reactive protein and white blood cell count for suspected acute appendicitis. Br J Surg. 2013;100:322–9. doi: 10.1002/bjs.9008. [DOI] [PubMed] [Google Scholar]
- 16.Kaya B, Sana B, Eris C, Karabulut K, Bat O, Kutanis R. The diagnostic value of D-dimer, procalcitonin and CRP in acute appendicitis. Int J Med Sci. 2012;9:909–15. doi: 10.7150/ijms.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acharya A, Markar SR, Ni M, Hanna GB. Biomarkers of acute appendicitis: systematic review and cost-benefit trade-off analysis. Surg Endosc. 2017;31:1022–31. doi: 10.1007/s00464-016-5109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serour F, Herman A, Babai I, et al. Evaluation of a possible inflammatory response after appendectomy for non-perforated appendicitis in children. Eur J Pediatr Surg. 2010;20:29–34. doi: 10.1055/s-0029-1241875. [DOI] [PubMed] [Google Scholar]
- 19.Sack U, Biereder B, Elouahidi T, Bauer K, Keller T, Tröbs R-B. Diagnostic value of blood inflammatory markers for detection of acute appendicitis in children. BMC Surg. 2006;6:15. doi: 10.1186/1471-2482-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cayrol J, Miguez MC, Guerrero G, Tomatis C, Simal I, Mara-ón R. Diagnostic accuracy and prognostic utility of D Dimer in acute appendicitis in children. Eur J Pediatr. 2016;175:313–20. doi: 10.1007/s00431-015-2632-3. [DOI] [PubMed] [Google Scholar]
- 21.Mentes O, Eryilmaz M, Harlak A, et al. Can D-dimer become a new diagnostic parameter for acute appendicitis? Am J Emerg Med. 2009;27:765–9. doi: 10.1016/j.ajem.2008.06.001. [DOI] [PubMed] [Google Scholar]