Abstract
Background:
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is associated with a higher prevalence of osteoporosis. However, the underlying mechanisms linking OSAHS with bone loss are still unclear. The aim of this study was to investigate the changes of receptor activator of nuclear factor-κB ligand (RANKL, an osteoclastogenesis-promoting factor) and osteoprotegerin (OPG, the decoy receptor for RANKL), oxidative stress and bone metabolism markers in OSAHS, in order to understand the potential mechanisms underlying bone loss in OSAHS patients.
Methods:
Forty-eight male patients with OSAHS, confirmed by polysomnography (PSG) study, were enrolled. Twenty male subjects who were confirmed as not having OSAHS served as the controls. The subjects’ bone mineral density (BMD) was assessed in lumbar spine and femoral neck using dual-energy X-ray absorptiometry (DXA). Blood samples were collected from all subjects for measurement of RANKL, OPG, the bone formation marker bone-specific alkaline phosphatase (BAP), the bone resorption marker tartrate-resistant acid phosphatase 5b (TRAP-5b), and total antioxidant capacity (TAOC).
Results:
The BMD and the T-score of the femoral neck and the lumbar spine were significantly lower in OSAHS patients as compared to the control group (P < 0.05). The serum level of BAP was significantly decreased in the OSAHS group (15.62 ± 5.20 μg/L) as compared to the control group (18.83 ± 5.50 μg/L, t = −2.235, P < 0.05), while the levels of TRAP-5b did not differ between the two groups (t = −1.447, P > 0.05). The serum level of OPG and the OPG/RANKL ratio were lower in the OSAHS group compared to the control group (both P < 0.05). TAOC level was also decreased significantly in the OSAHS group (P < 0.05). Correlation analysis showed that the TAOC level was positively correlated with BAP in the OSAHS group (r = 0.248, P = 0.04), but there were no correlations between TAOC and the BMD or the T-scores. The correlations between the level of OPG (or the OPG/RANKL ratio) and BMD or TAOC did not reach significance.
Conclusion:
In OSAHS patients, lower levels of TAOC were associated with decreased bone formation, suggesting a role of oxidative stress in bone loss, while the role of OPG/RANKL imbalance in bone metabolism in OSAHS needs further evaluation.
Keywords: Obstructive sleep apnea-hypopnea syndrome, Osteoporosis, Receptor activator of nuclear factor-κB ligand, Oxidative stress
Introduction
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a sleep disorder characterized by apneas or hypopneas due to upper airway narrowing, leading to repetitive hypoxemia and hypercapnia, with chronic intermittent hypoxia (CIH) as its salient pathophysiological manifestation. CIH resembles the ischemia-reperfusion process in that excess oxygen free radicals are generated by repeated hypoxia and reoxygenation, which causes pro-oxidative and anti-oxidative imbalance and oxidative stress,[1] a key mechanism underlying OSAHS-induced damages. As such, total antioxidant capacity (TAOC) is considered as an indicator reflecting the level of oxidative stress.
OSAHS is associated with a variety of systemic diseases, such as cardiovascular diseases, diabetes, and osteoporosis. It has been generally recognized that OSAHS is associated with a higher incidence of osteoporosis.[2–4] However, the molecular mechanisms underlying the association between OSAHS and bone loss remains speculative, although it is believed that oxidative stress plays an important role in this pathophysiological process, and that hypoxia per se can influence bone metabolism by disrupting the osteoblast/osteoclast balance.[5]
Receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin (OPG) have been identified as two principal cytokines controlling osteoclastic differentiation and activation. The predominant role of RANKL in bone physiology is the stimulation of osteoclastic differentiation and activation. Binding of RANKL to its receptor RANK stimulates differentiation of osteoclastic precursors into mature osteoclasts, and activation of mature osteoclasts. OPG act as a decoy receptor for RANKL and thus down-regulates RANKL signaling through RANK, acting as an inhibitor of bone resorption. In fact, bone remodeling appears to be mainly controlled by the balance of OPG/RANKL.[6] In our previous study,[7] we found an association between OPG/RANKL imbalance and bone loss in patients with chronic obstructive pulmonary disease (COPD), a lung disease with significant systemic effects. Here we investigated whether there was an imbalance of OPG/RANKL in OSAHS with abnormal bone metabolism. OSAHS is associated with a higher level of oxidative stress, which further affects bone metabolism in various ways according to recent studies[8,9] However, whether oxidative stress is associated with OPG/RANKL expression in OSAHS has not been described. Therefore, we also examined the level of oxidative stress in order to understand the potential mechanisms underlying bone loss and OPG/RANKL imbalance in this disease.
Methods
Ethical approval
The study was approved by the Ethics Committee of Beijing Shijitan Hospital Affiliated to Capital Medical University. Informed consent was signed by all participants.
Subjects
From September 2017 to June 2018, 48 male patients with OSAHS (aged 18–60 years) were enrolled based on polysomnography (PSG) results at Sleep Center of Beijing Shijitan Hospital. In the meantime, 20 male controls without OSAHS were included. The two groups were matched for age and body mass index (BMI). There were 20 and 8 current or former smokers in the OSAHS group and the control group respectively.
The exclusion criteria included (1) Past history of skeletal diseases, such as fragility fracture, especially hip fracture, distal ulna and radius fracture and vertebral fracture; (2) Current or past use of inhaled or systemic glucocorticoids; (3) Having systemic diseases that might affect bone metabolism within 1 year prior to recruitment, such as renal insufficiency and thyroid diseases; (4) Having taken medicines that might affect bone metabolism within 1 year prior to recruitment; (5) Low BMI (< 19 cm2/kg); (6) COPD, rheumatoid arthritis, chronic liver disease, inflammatory bowel disease, multiple myeloma, excessive drinking, and cerebrovascular accidents.
Polysomnographic study
Embla N7000 Polysomnography System (Dock Lane, Melton, Woodbridge, Suffolk IP12 1PE, United Kingdom) was used to detect apnea-hyponea index (AHI), lowest pulse oxygen saturation (LSpO2) and mean pulse oxygen saturation (MSpO2). Obstructive apnea was defined as absence or apparent decrease of airflow (by ≥ 90% as compared with the baseline) for ≥ 10 s, with thoracoabdominal respiratory efforts. Hypopnea was defined as airflow reduction by ≥ 30% as compared with the baseline with SpO2 reduction equal to or more than 4% for ≥ 10 s, or as airflow reduction by ≥ 50% with SpO2 reduction by above 3% for ≥ 10 s. Based on AHI and SpO2, the degree of apnea-hyponea was graded as mild (AHI 5–15 times/h, LSpO2 85–90%), moderate (AHI 15–30 times/h, LSpO2 80–84%) and severe (AHI > 30 times/h, LSpO2 < 80%).[10]
Bone densitometry assessment
Bone mineral density (BMD) at lumbar spine and femoral neck was assessed by dual-energy X-ray absorptiometry scanner (Hologic Inc, Bedford, MA, USA). BMD was reported as an absolute value (g/cm2) and a T score, which represents the number of standard deviations from a young, gender- and ethnic group-specific reference mean.[7]
Blood sampling and assays
Blood samples were collected for measurement of serum RANKL, OPG, the bone resorption marker TRAP-5b by enzyme-linked immunosorbent assay (ELISA), and the bone formation marker BAP by EIA, and TAOC by ELISA. RANKL and OPG kits were purchased from Biomedica Medizinprodukte GmbH & Co KG (Divischgasses 4, A-1210 Vienna, Austria), BAP and TRAP-5b kits from Immunodiagnostic Systems Limited (10 Didcot Way, Boldon Business Park, Boldon, Tyne &Wear, NE 35 9PD, United Kingdom), TAOC kit from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All procedures were performed according to instruction manuals.
Statistical analysis
SPSS 22.0 software (SPSS Inc., USA) was used for statistical analyses. Continuous variables were shown as mean ± standard deviation (SD) and compared between the two groups using independent 2-sample t-test. Chi-square test was used for categorical variables. Pearson correlation coefficient was calculated, with P < 0.05 indicating significant difference.
Results
Demographic data and characteristics of the study subjects
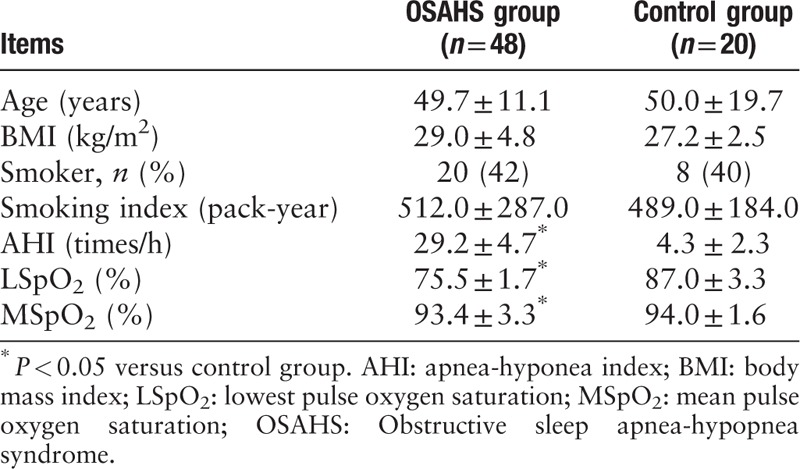
Forty-eight male patients with OSAHS, 19 mild-moderate and 29 severe, were enrolled. The patients aged 49.7 ± 11.1 years, with a mean BMI of 29.0 ± 4.8 kg/m2. Twenty normal controls were all males and aged 50.0 ± 19.7 years, with a mean BMI of 27.2 ± 2.5 kg/m2. No significant differences in age and BMI were observed between the two groups (t = 0.275, 1.880, P = 0.309, 0.064, respectively). There were 20 (42%) and 8 (40%) smokers in the two groups (t = 0.082, P = 0.774), and the smoking index was 512.0 ± 287.0 and 489.0 ± 184.0 pack-years, respectively (t = 0.673, P = 0.347), which showed no significant difference. For the OSAHS group, AHI, LSpO2 and MSpO2 were 29.2 ± 4.7 times/h, 75.5 ± 1.7%, and 92.0 ± 3.1%, respectively (Table 1).
Table 1.
Characteristics of the male patients with OSAHS and male controls without OSAHS
Increased bone loss in patients with OSAHS
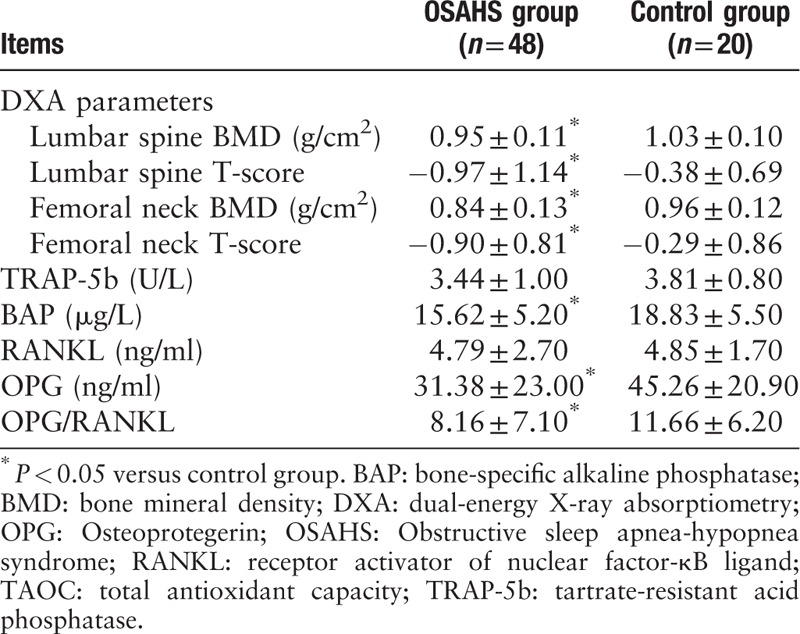
The BMDs and the T-scores of the femoral neck (t = −3.506, −4.246, all P < 0.05), and those of the lumbar vertebrae (t = −2.738, −4.125, all P < 0.05) were all significantly lower in the OSAHS group as compared to the control group (Table 2), indicating increased bone loss in patients with OSAHS.
Table 2.
Comparison of DXA parameters and serum markers between the two groups
Decreased bone formation markers and OPG/RANKL imbalance in OSAHS
The serum level of BAP, a bone formation marker, was lower in the OSAHS group than that in the control group (t = −2.235, P = 0.032), but that of TRAP-5b, a bone resorption marker, was not different between the two groups (t = −1.447, P = 0.153), indicating decreased bone formation in OSAHS. Although the level of RANKL did not differ between groups (t = −0.110, P = 0.913), the level of OPG and the OPG/RANKL ratio were significantly decreased in the OSAHS group as compared to those in the control group (t = −2.329, P = 0.023), suggesting an imbalance towards osteoclastogenesis in OSAHS (Table 2).
Decreased anti-oxidative activity in OSAHS
The level of TAOC (an anti-oxidative marker) was significantly decreased in the OSAHS group compared to the control group (t = −3.048, P = 0.003, Table 2), suggesting an imbalance between oxidative stress and anti-oxidative capacity in OSAHS.
Correlation between oxidative stress and bone metabolism
Correlation analysis showed that the TAOC level was positively correlated with the bone formation marker BAP in the OSAHS group (r = 0.248, P = 0.04, Figure 1). However, no significant correlation was found between TAOC and OPG or OPG/RANKL ratio.
Figure 1.
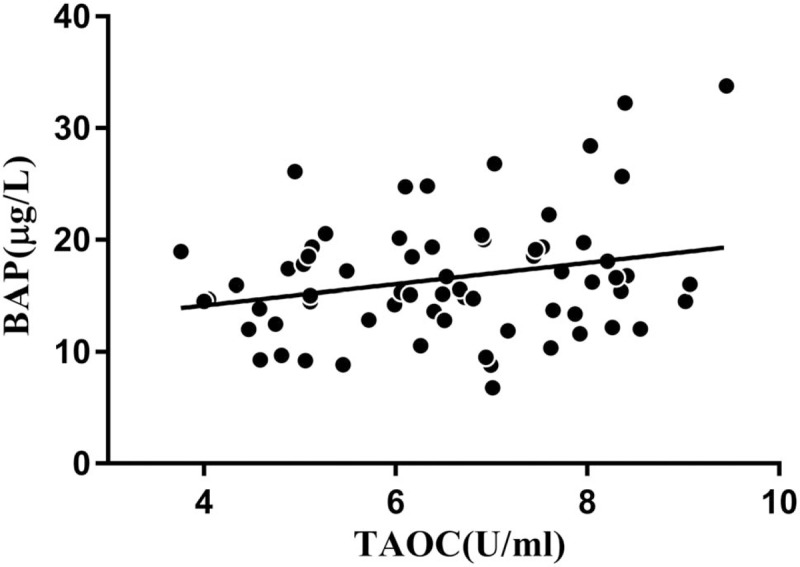
Correlation between the serum levels of total antioxidant capacity (TAOC) and bone-specific alkaline phosphatase (BAP) (r = 0.248, n = 48).
Discussion
In this study, we showed that there was an imbalance of OPG/RANKL, together with decreased bone formation marker BAP in OSAHS patients complicated with lower BMD, suggesting that disturbance of the OPG/RANKL system may be involved in abnormal bone metabolism in this disease.
The osteoporosis-related proteins OPG/RANKL are important regulators of bone metabolism and remodeling, and a delicate balance between the two is required to maintain normal bone metabolism.[6,7] Although OSAHS was associated with an increased risk of osteoporosis, whether there was up-regulation of RANKL production or imbalance of OPG/RANKL had not been reported. In the current study, we found a lower level of OPG and OPG/RANKL ratio in OSAHS patients as compared to a group of gender-and smoking-matched normal controls, suggesting an imbalance towards osteoclastogenesis in OSAHS.
The mechanisms driving an imbalance of OPG/RANKL may be associated with oxidative stress and systemic inflammation, two major pathophysiological features of OSAHS. Evidence indicates that oxidative stress, resulting from an imbalance of oxidants and antioxidants in favor of the former, is implicated in the development and progression of osteoporosis.[11,12] An earlier study[13] found that compared to subjects of the similar age without osteoporosis, patients with postmenopausal osteoporosis showed increased levels of total oxidation state (TOS) and oxidative stress index (OSI), but decreased TAOC. Molecules from oxidative stress may have an impact on the OPG/RANKL system. Xu et al[14] found that CIH directly activated the regulatory signaling pathway related to bone formation, by an effect on the OPG/RANKL system. An in vitro study showed that hydrogen molecules prevented RANKL-induced osteoclast differentiation by inhibition of reactive oxygen species formation, suggesting an interaction between oxidative stress and RANKL production.[15]
Consistent with lower BMD in the patients with OSAHS, the bone formation marker BAP was decreased, and positively correlated with TAOC, again indicating the role of oxidative stress in bone loss in this disease. Biochemical markers of bone turnover reflect bone homeostasis, that is, the activity of osteoblasts and osteoclasts in both physiological and pathophysiological conditions. The level of BAP is associated with osteoblasts and preosteoblasts, and considered to be the most accurate marker of bone formation. The level of BAP showed good correlation with fracture risk in patients with chronic kidney dysfunction.[16] Abnormality of bone metabolism markers precedes development of morphological osteoporosis, and is important for assessment of fracture risk.[17] In this study, the lower level of BAP and its association with TAOC in OSAHS suggests that CIH may lead to increased OSI but decreased anti-oxidative capacity, which then exert effects on bone metabolism via different pathways including the OPG/RANKL system.
In addition to the above-mentioned mechanisms, hypoxia per se might be a potential cause or contributing factor for abnormal bone metabolism in patients with OSAHS. Studies have shown that hypoxia enhances the activity of osteoclasts and increases bone reabsorption. Utting et al[18] investigated the effect of hypoxia on rat osteoblast function in a primary culture, and found that the inhibitory effect of hypoxia on bone formation was partly due to decreased osteoblast proliferation. Another study examined the effect of oxygen tension on the formation and function of osteoclasts by cultures of mouse marrow.[19] The result showed that progressive increase in formation of multinucleated osteoclasts and resorption pits could be induced by reducing oxygen tension from the ambient atmospheric level of 20% by increasing the proportion of nitrogen, suggesting a possible association between systemic hypoxia and bone loss. In addition, hypoxia also markedly reduced osteoblast alkaline phosphatase (ALP) activity and expression of mRNAs for ALP and osteocalcin, suggesting inhibition of differentiation to the osteogenic phenotype.
There are several limitations to our study. Firstly, significant correlations were not observed between the OPG/RANKL ratio and the level of TAOC in patients with OSAHS, and therefore an association between oxidative stress and disturbed OPG/RANKL balance cannot be demonstrated from this study. Secondly, because of the cross-sectional nature of the study, the changes of OPG/RANKL, TAOC and bone metabolism markers in OSAHS after therapy are not available. Further studies are warranted to answer this interesting question. Lastly, our results may be biased by the male gender only- and smoker only- enrollment of subjects. Because of the small size of the study, we did so to exclude confounding by the effect of gender and cigarette smoking. Therefore, larger-sized studies including male and female, smoking and non-smoking patients with OSAHS are needed to elucidate the bone metabolism status and the potential mechanisms of enhanced osteoporosis in this disease.
Acknowledgements
None.
Funding
None.
Conflicts of interest
There are no conflicts of interest.
Footnotes
How to cite this article: Ma XR, Wang Y, Sun YC. Imbalance of osteoprotegerin/receptor activator of nuclear factor-κB ligand and oxidative stress in patients with obstructive sleep apnea-hypopnea syndrome. Chin Med J 2019;000. doi: 10.1097/CM9.0000000000000046
References
- 1.Katsoulis K, Kontakiotis T, Spanogiannis D, Vlachogiannis E, Kougioulis M, Gerou S. Total antioxidant status in patients with obstructive sleep apnea without comorbidities: the role of the severity of the disease. Sleep Breath 2011; 15:861–866. doi: 10.1007/s11325-010-0456-y. [DOI] [PubMed] [Google Scholar]
- 2.Uzkeser H, Yildirim K, Aktan B, Karatay S, Kaynar H, Araz O, et al. Bonemineral density in patients with obstructive sleep apnea syndrome. Sleep Breath 2013; 17:339–342. doi: 10.1007/s11325-012-0698-y. [DOI] [PubMed] [Google Scholar]
- 3.Chen YL, Weng SF, Shen YC, Chou CW, Yang CY, Wang JJ, et al. Obstructive sleep apnea and risk of osteoporosis: a population-based cohort study in Taiwan. J Clin Endocrinol Metab 2014; 99:2441–2447. doi: 10.1210/jc.2014-1718. [DOI] [PubMed] [Google Scholar]
- 4.Choi SB, Lyu IS, Lee W, Kim DW. Increased fragility fracture risk in Korean women who snore: a 10-year population-based prospective cohort study. BMC Musculoskelet Disord 2017; 18:236.doi: 10.1186/s12891-017-1587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atashi F, Modarressi A, Pepper MS. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev 2015; 24:1150–1163. doi:10.1089/scd.2014.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 2001; 142:5050–5055. [DOI] [PubMed] [Google Scholar]
- 7.Bai P, Sun Y, Jin J, Hou J, Li R, Zhang Q, Wang Y. Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir Res 2011; 12:157.doi:10.1186/1465-9921-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 2007; 282:27285–27297. doi:10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baek KH, Oh kw, Lee WY, Lee SS, Kim MK, Kwon HS, et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int 2010; 87:226–235. doi:10.1007/s00223-010-9393-9. [DOI] [PubMed] [Google Scholar]
- 10.Chinese Thoracic Society Obstructive sleep apnea hypopnea syndrome diagnosis and treatment guidelines (revised in 2011) (in Chinese). Chin J Tuberc Respir Dis 2012; 35:9–12. doi:10.3760/cma.j.issn.1001-0939.2012.01.007. [Google Scholar]
- 11.Zhou Q, Zhu L, Zhang D, Li N, Li Q, Dai P, et al. Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis Markers 2016; 2016:7067984.doi: 10.1155/2016/7067984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 2010; 31:266–300. doi:10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinbeck MJ, Appel WH, Jr, Verhoeven AJ, Karnovsky MJ. NADPH-oxidase expression and in situ production of superoxide by osteoclasts actively resorbing bone. J Cell Biol 1994; 126:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu N, Liu H, Qu F, Fan J, Mao K, Yin Y, et al. Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp Mol Pathol 2013; 94:33–39. doi:10.1016/j.yexmp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Li DZ, Zhang QX, Dong XX, Li HD, Ma X. Treatment with hydrogen molecules prevents RANKL-induced osteoclast differentiation associated with inhibition of ROS formation and inactivation of MAPK, AKT and NF-kappa B pathways in murine RAW264.7 cells. J Bone Miner Metab 2014; 32:494–504. doi: 10.1007/s00774-013-0530-1. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama Y, Taniguchi M, Kazama JJ, Yokoyama K, Hosoya T, Yokoo T, et al. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol Dial Transplant 2014; 29:1532–1538. doi: 10.1093/ndt/gfu055. [DOI] [PubMed] [Google Scholar]
- 17.Bhattoa HP. Laboratory aspects and clinical utility of bone turnover markers. EJIFCC 2018; 292:117–128. eCollection 2018 Jul. [PMC free article] [PubMed] [Google Scholar]
- 18.Utting JC, Robins SP, Brandao-Burch A, Orriss IR, Behar J, Arnett TR. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res 2006; 312:1693–1702. dol:10.1016/j.yexcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Arnett TR, Gibbons DC, Utting JC, Orriss IR, Hoebertz A, Rosendaal M, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol 2003; 196:2–8. [DOI] [PubMed] [Google Scholar]


