Abstract
Background:
Owing to the multifactorial nature of the pathogenesis of diabetic peripheral neuropathy (DPN), conventional drug therapies have not been effective. The application of stem cells transplantation may be useful for the treatment of DPN. This study was designed to assess the safety and therapeutic effects of autologous bone marrow mononuclear cells (BMMNCs) transplantation on the treatment of refractory DPN.
Methods:
One hundred and sixty-eight patients with refractory DPN were recruited and enrolled in the study. They received intramuscular injection of BMMNCs and followed at 1, 3, 6, 12, 18, 24, and 36 months after the transplantation. Clinical data, Toronto Clinical Scoring System (TCSS), and nerve conduction studies (NCSs) were compared before and after the transplantation.
Results:
The signs and symptoms of neuropathy were significantly improved after BMMNCs transplantation. The values of the TCSS scores at 1 month (9.68 ± 2.49 vs. 12.55 ± 2.19, P < 0.001) and 3 months (8.47 ± 2.39 vs. 12.55 ± 2.19, P < 0.001) after the treatment reduced significantly compared with the baseline value. This decrement remained persistent until the end of the study. The conduction velocity and action potential and sensory nerves were significantly improved after transplantation (3 and 12 months after the treatment vs. the baseline: motor nerve conduction velocity, 40.24 ± 2.80 and 41.00 ± 2.22 m/s vs. 38.21 ± 2.28 m/s, P < 0.001; sensory nerve conduction velocity, 36.96 ± 2.26 and 39.15 ± 2.61 m/s vs. 40.41 ± 2.22 m/s, P < 0.001; compound muscle action potential, 4.67 ± 1.05 and 5.50 ± 1.20 μV vs. 5.68 ± 1.08 μV, P < 0.001; sensory nerve action potential, 4.29 ± 0.99 and 5.14 ± 1.26 μV vs. 5.41 ± 1.14 μV, P < 0.001). No adverse event associated with the treatment was observed during the follow-up period.
Conclusions:
Autologous transplantation of BMMNCs may be an effective and promising therapeutic strategy for the treatment of refractory DPN.
Keywords: Bone marrow mononuclear cells, diabetic peripheral neuropathy, autologous transplantation, Toronto clinical scoring system, nerve conduction
Introduction
Diabetic peripheral neuropathy (DPN) is one of the most common and severe chronic complications in patients with type 2 diabetic mellitus (T2DM). It affects approximately half of the patients during the course of the disease. It is characterized by the symmetrical loss of sensory perception in later stage, resulting in an increased risk of foot ulceration and lower extremity amputation.[1] It contributes to the increased risk of morbidity and mortality in patients with diabetes, impairing the quality of life and causing societal burden.[2]
Currently, only a few treatments for DPN are available, in which the enhanced glucose control is a recommended strategy to attenuate symptoms.[1] However, this treatment strategy has shown a marginal effect on preventing the progression of diabetic neuropathy. Other therapies aimed at the relief of the neuropathic symptoms include aldose reductase inhibitors, α-lipoic acid, and transketolase activators.[3] It has been accepted that the pathologic alteration of DPN involves multiple factors. However, most treatments have focused only on one factor, which limits the therapeutic efficacy. Therefore, it is suggested that the stem cell-based therapies could be a promising strategy to treat DPN.
In response to the stimulation of exogenous cytokines, the bone marrow-derived stem cells (BMCs) can be mobilized to perform the repair function. Recent studies have proven that the transplanted BMCs produce angiogenic and neuroprotective factors to ameliorate neuropathy symptoms through paracrine mechanisms in animal models.[4,5] Multiple studies have shown that the administration of BMCs inhibits pro-inflammatory cytokines signaling pathways in streptozotocin (STZ)-induced diabetic rats models.[6,7] Among different types of BMCs, bone marrow mononuclear cells (BMMNCs) have been the most commonly used therapeutic cells for regenerative purposes.[8]
Some small-scale clinical trials have already investigated the efficacy of applying BMMNCs to treat critical limb ischemia,[9] peripheral artery disease,[10] heart failure, and acute myocardial infarction.[11] However, there was no clinical application of stem cells in treating patients with DPN till date. Therefore, we conducted a study to evaluate the efficacy and safety of applying autologous BMMNCs transplantation therapy in patients with DPN.
Methods
Ethical approval
The patients were briefed about this study. All participants had signed the informed consent form before the study enrollment. This clinical study was approved by the Ethics Committee of The Central Hospital of Wuhan, Wuhan, China.
Patients
One hundred and sixty-eight patients were enrolled in this clinical trial from March 2014 to December 2017. The inclusion criteria were: 1) T2DM according to 2013 American Diabetes Association standards[12]; 2) diagnosed with refractory DPN (defined in the next section); and 3) age 30–80 years old. The exclusion criteria were as follows: 1) severe hepatic and renal dysfunction; 2) hypercoagulable states or with hematological diseases; 3) other causes of peripheral neuropathy (including genetic or metabolic diseases, mental disorder, pharmacological, surgical, alcohol abuse, toxin exposure, etc.); 4) foot ulcers and limb deformity; 5) pregnancy; and 6) history of malignancy <5 years before the study start.
Definitions of refractory DPN
-
1.
DPN was defined as the presence of an abnormality of nerve conduction and a symptom/symptoms or a sign/signs of neuropathy.[1]
-
2.
Refractory DPN was defined as the duration of DPN longer than 2 years, with insufficient relief of the neuropathic symptoms or signs when combined with the uses of conventional drugs for at least 1 year. The conventional drug therapies include the antioxidant (α-lipoic acid), transketolase activators (thiamines and allithiamines), and aldose reductase inhibitors.
Clinical assessment before and after transplantation
All patients received the autologous BMMNCs transplantation. Clinical data, medication, and laboratory data were collected before the procedure and at every follow-up visit, which was scheduled at 1, 3, 6, 12, 18, 24, and 36 months after transplantation. All neurological examinations were performed at every visit, and patients were requested to finish the Toronto Clinical Scoring System (TCSS). Nerve conduction studies (NCSs) were performed pre-transplantation, and 3 and 12 months post-transplantation. Attention was paid during the follow-up visits specifically to any potential adverse effects due to the transplantation.
All patients received similar routine treatments throughout the course of this trial, including intensive control of blood glucose, blood lipids, and blood pressure.
Toronto Clinical Scoring System
All participants had undergone a thorough neurological examination and finished TCSS before and at every follow-up visit after transplantation, including symptoms, lower limb reflex, and sensory tests (pinprick, temperature, light touch, vibration, and position sensation). The maximum score of TCSS is 19. The severity of neuropathy was graded according to Perkins et al[13]: 1 to 5 points for no neuropathy; 6 to 8 points for mild neuropathy; 9 to 11 points for moderate neuropathy; and 12 to 19 points for severe neuropathy.
Measurements of NCS
NCS was performed with Viking Quest® (Nicolet Biomedical Inc, WI, USA) by the same experienced technician who was blinded to the patients’ clinical information. Routine NCS included sensory nerve conduction velocity (sNCV), sensory nerve action potential (SNAP) in superficial peroneal and sural nerves, motor nerve conduction velocity (mNCV), and compound muscle action potential (CMAP) in peroneal and posterior tibial nerves. We also evaluated F responses and H reflexes. The test was standardized for temperature, stimulation protocol, and side of testing.
Preparation of BMMNCs and transplantation procedures
To mobilize the stem cells in bone marrow, patients received treatment with 5 μg·kg−1·d−1 recombinant human granulocyte colony-stimulating factor (G-CSF, Qilu Pharmaceutical, China) by subcutaneous injection for 3 days. After G-CSF mobilization, 200 mL bone marrow was aspirated from the posterior superior iliac crest under aseptic and anesthetic conditions. The preparation of BMMNCs was processed in the laminar flow laboratory. Mononuclear stem cells were separated by Ficoll-Hypaque density–gradient centrifugation. Then, the mononuclear cell layer was harvested and washed 3 times with normal saline and resuspended in normal saline.
Subsequently, the prepared BMMNCs suspensions were injected intramuscularly to both thighs and legs (50 sites, 2 cm × 2 cm in intervals, 1–5 cm in depth, 1 mL BMMNCs per site).
Statistical analysis
All analyses were performed using SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA). All data were expressed as mean ± standard deviation (SD) for continuous variables and in percentages for discrete variables. Differences between before and after transplantation were tested by paired sample t-test for continuous variables and the χ2 test for categorical variables. Multiple group comparisons were performed by analysis of variance (ANOVA). A value of P < 0.05 was considered as statistically significant.
Results
Patients’ characteristics
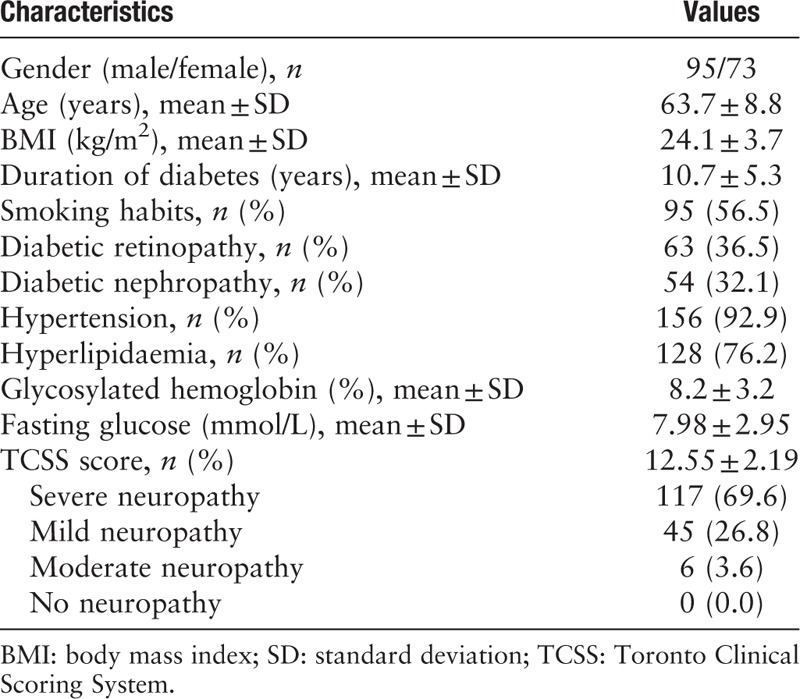
The characteristics of patients are summarized in Table 1. A total of 168 patients with a mean age of 63.7 ± 8.8 years and a mean diabetes duration of 10.7 ± 5.3 years were included in this study. One patient died during the follow-up (30 months after the transplantation) because of cardiovascular events, and 6 patients lost contact and did not show in the follow-ups due to various reasons.
Table 1.
Baseline features and clinical characteristics of the patients enrolled
Clinical efficacy of transplantation
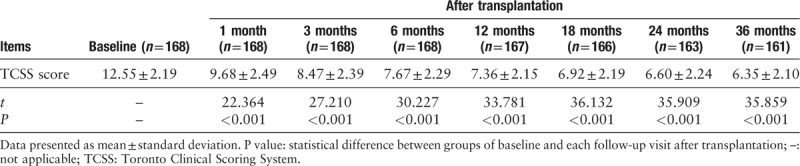
The signs and symptoms of neuropathy relief were defined as the primary outcome of our study, which were assessed using TCSS. The TCSS scores significantly decreased at 1 and 3 months after the transplantation compared to the baseline values as shown in Table 2. This decrement persisted until 36 months after transplantation. We observed a significant improvement of the main neuropathy manifestations, such as lower extremity pain, numbness, tingling, and weakness during the first 3 months after injection of BMMNCs.
Table 2.
TCSS Scores before and after transplantation treatment
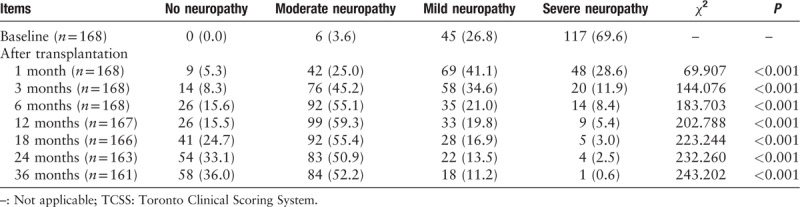
Furthermore, the severity of TCSS significantly improved at the first month after transplantation, at which time the proportion of severe neuropathy decreased from 69.6% to 28.6%. This proportion continued to decrease to only 0.6% at 36 months after treatment, whereas the proportion of no neuropathy gradually increased from 0.0% to 36.0% at the end of this study as shown in Table 3.
Table 3.
Severity of TCSS scores before and after transplantation treatment, n (%)
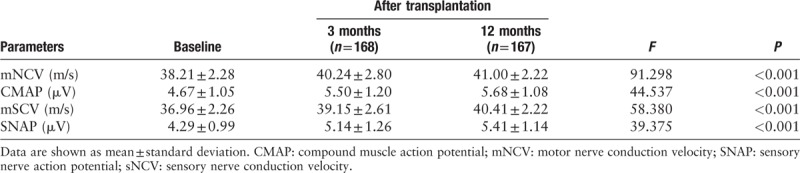
Analysis of the NCS results revealed a significant improvement of conduction velocity and action potential of sensory and motor nerves after the transplantation of BMMNCs [Table 4]. Three months after the transplantation, mNCV and sNCV increased from 38.21 ± 2.28 m/s to 40.24 ± 2.80 m/s and 36.96 ± 2.26 m/s to 39.15 ± 2.61 m/s, respectively. Then, mNCV and sNCV continued to increase to 41.00 ± 2.80 m/s and 40.41 ± 2.22 m/s, respectively, at 12 months after the treatment. CMAP improved from 4.67 ± 1.05 μV at baseline to 5.50 ± 1.20 μV at 3 months, and then further increased to a level of 5.68 ± 1.08 μV at 12 months. SNAP gradually increased from 4.29 ± 0.99 μV at baseline to 5.14 ± 1.26 μV at 3 months, and to 5.41 ± 1.14 μV at 12 months.
Table 4.
Nerve conduction studies before and after the transplantation treatment
Procedural safety
No adverse events regarding bone marrow aspirations were observed. No cases of infection, bleeding, and allergic reactions were detected immediately after transplantation. No cases of rejection and malignancy were detected during the follow-up visits.
Only 19 patients (11.31%) experienced slightly mild discomforts, such as slight pain or swelling feelings at the injection sites several hours after the transplantation. Such uncomfortable symptoms were well tolerated and disappeared in 3 days.
Discussion
In this pilot clinical study, we observed that autologous transplantation of BMMNCs effectively improved the clinical manifestations of neuropathy and restored the functions of peripheral nerves. No reports of immediate and long-term adverse events related to the therapy were observed, demonstrating the safety of BMMNCs transplantation. These results indicated that autologous transplantation of BMMNCs could be a promising treatment for refractory DPN.
The pathogenic mechanism of DPN is not completely understood. Growing evidence suggests that the infiltration of inflammation factors and deficiency of local neurotrophic and angiogenic factors contribute to the onset and progression of diabetic neuropathy.[14,15] The efforts to design different experimental therapies targeting to block the mechanisms involved in DPN's progression were made. Experiments show that the administrations of antioxidants,[16] neuroprotective factors,[17] anti-inflammatory factors,[18] and growth factors[19] contributed to an increase in nerve conduction velocity. However, the transformation of these experiments to clinical studies has been unsuccessful.[20,21] It seems that the pathogenesis of DPN is multifactorial and complicated, and a single pathway-based therapy can hardly be effective. The application of stem cells-based therapy simultaneously promoting angiogenesis and neuron regeneration may have great value in treating DPN.
Although several different stem cell types have been tested to treat DPN in animal models, the most commonly used cells are BMMNCs, which can easily be isolated from bone marrow, umbilical cord blood, and other adult tissues. Stimulation with G-CSF is a common and effective method to collect a large number of BMMNCs for autologous transplantation.[22]
BMCs were found to improve nerve conduction velocities in STZ-induced diabetic rats.[9] By producing neurotrophic and angiogenic factors through the paracrine mechanism, the BMCs were able to ameliorate the injury of peripheral nerve,[23] although BMMNCs transplantation was used for a wide variety of diseases, such as heart failure,[11] critical limb ischemia,[9] and foot ulcer.[10] We only found out 1 clinical study using mesenchymal stem cells for DPN in the ClinicalTrials.gov database (NCT02387749). Currently, no results of clinical trial like ours have been published yet. So far as we know, this is the first clinical study to apply autologous transplantation of BMMNCs to treat refractory DPN.
There were no serious adverse events related to bone marrow aspirations and BMMNCs injection during our follow-up period; few of our patients were followed even longer with no reports of side effects. Similarly, many other trails of BMMNCs transplantation have also indicated it as a safe therapeutic approach, as no complications occurred during long-term follow-ups.[9,24] These results demonstrated the safe use of autologous BMMNCs transplantation in patients with DPN.
There was a small portion of patients with a limited relief of the neuropathy symptoms in our study. The low-yielding cell numbers of BMMNCs after G-CSF mobilized may contribute to the poor therapeutic effect. Fadini et al[25] reported that the mobilization ability of stem cells in response to G-CSF was impaired in patients with diabetes. And the occurrence of diabetes might result in the defective mobilization of BMCs by changing the microanatomy and physiology of bone marrow.[26] All the results of these studies indicated that diabetes may adversely affect stem cell-based therapies by jeopardizing the microenvironment of bone marrow niches, which are crucial for the maintenance and expansion of stem cells. Efforts were made to improve the therapeutic effectiveness of stem cells transplantation. The results of in-vitro studies showed that when G-CSF and plerixafor were used in combination, the stem cells were able to restore the mobilization ability.[25,27] Several studies have proven that the functions of stem cells could be restored by administration of hydrogen sulfide.[28,29] More efforts are needed to transform these experimental findings into clinical applications to improve the therapeutic efficacy of stem cells in patients with DPN in the future.
There were several limitations in our research. First, we did not set up the control group and only analyzed the differences before and after BMMNCs transplantation. Our study only included patients with refractory DPN, as these patients did not respond to conventional therapies and autologous BMMNCs transplantation may be one of the very few promising treatment options. To determine the therapeutic efficacy of BMMNCs in patients with DPN, randomized double-blind controlled trials may be necessary in the future. Second, without measurements of inflammation and anti-inflammation cytokines, neurotrophic, and angiogenic factors, we were unable to determine the potential mechanisms of BMMNCs in treating neuropathy symptoms. This study could be strengthened if more analyses were performed. Although further studies are required to reveal the mechanism of BMMNCs in the treatment of DPN, we believed that this method provides a promising option for patients with DPN.
In conclusion, our 36-month follow-up study proves that transplantation therapy using BMMNCs mobilized by G-CSF stimulation is a safe and effective treatment for refractory DPN.
Acknowledgements
None.
Funding
This work was supported by a grant from the Research Fund for projects of Hubei provincial Health Department (No. JX4B56) and the National Natural Science Foundation of China (No. 81370942).
Conflicts of interest
The authors declare that they have no conflict of interest.
Author contributions
All authors participated in the study design. Mao H contributed to funding acquisition. Wei W and Mao H acquired and analyzed the data. Wei W wrote and revised the manuscript and contributed to the discussion. Mao H and Zhao S reviewed and edited the manuscript and contributed to the discussion. Tang Y performed statistical analyses. Dong JJ performed the nerve conduction study. Jia T, Fu XL, and Lyu XY performed the preparation of BMMNCs and transplantation Procedures.
Footnotes
How to cite this article: Mao H, Wei W, Fu XL, Dong JJ, Lyu XY, Jia T, Tang Y, Zhao S. Efficacy of autologous bone marrow mononuclear cell transplantation therapy in patients with refractory diabetic peripheral neuropathy. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000009
References
- 1.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 2013; 11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith SC, Lamping DL, Maclaine GD. Measuring health related quality of life in diabetic peripheral neuropathy: a systematic review. Diabetes Res Clin Pract 2012; 96:261–270. doi: 10.1016/j.diabres.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Barrett EJ, Liu Z, Khamaisi M, King GL, Klein R, Klein BEK, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab 2017; 102:1–68. doi: 10.1210/jc.2017-01922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han JW, Choi D, Lee MY, Huh YH, Yoon YS. Bone marrow-derived mesenchymal stem cells improve diabetic neuropathy by direct modulation of both angiogenesis and myelination in peripheral nerves. Cell Transplant 2016; 25:313–326. doi: 10.3727/096368915X688209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naruse K, Sato J, Funakubo M, Hata M, Nakamura N, Kobayashi Y, et al. Transplantation of bone marrow-derived mononuclear cells improves mechanical hyperalgesia, cold allodynia and nerve function in diabetic neuropathy. PLoS ONE 2011; 6:e27458.doi: 10.1371/journal.pone.0027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evangelista AF, Vannier-Santos MA, de Assis Silva GS, Silva DN, Juiz PJL, Nonaka CKV, et al. Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J Neuroinflammation 2018; 22:189.doi: 10.1186/s12974-018-1224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monfrini M, Donzelli E, Rodriguez-Menendez V, Ballarini E, Carozzi VA, Chiorazzi A, et al. Therapeutic potential of mesenchymal stem cells for the treatment of diabetic peripheral neuropathy. Exp Neurol 2017; 288:75–84. doi: 10.1016/j.expneurol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Park JS, Choi YJ, Kim MO, Huh YH, Kim SW, et al. Bone marrow mononuclear cells have neurovascular tropism and improve diabetic neuropathy. Stem cells 2009; 27:1686–1696. doi: 10.1002/stem.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 2005; 28:2155–2160. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Dardik A, Fang K, Huang R, Gu Y. Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells. Stem Cell Res Ther 2017; 8:228.doi: 10.1186/s13287-017-0683-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gyöngyösi M, Haller PM, Blake DJ, Martin Rendon E. Meta-analysis of cell therapy studies in heart failure and acute myocardial infarction. Circ Res 2018; 123:301–308. doi: 10.1161/CIRCRESAHA.117.311302. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association Standards of medical care in diabetes––2013. Diabetes Care 2013; 36 Suppl1:1-66. [Google Scholar]
- 13.Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001; 24:250–256. [DOI] [PubMed] [Google Scholar]
- 14.Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a therapeutic target for diabetic neuropathies. Curr Diab Rep 2016; 16:1–10. doi: 10.1007/s11892-016-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Premkumar LS, Pabbidi RM. Diabetic peripheral neuropathy: role of reactive oxygen and nitrogen species. Cell Biochem Biophys 2013; 67:373–383. doi: 10.1007/s12013-013-9609-5. [DOI] [PubMed] [Google Scholar]
- 16.Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 2000; 49:1006–1015. [DOI] [PubMed] [Google Scholar]
- 17.Mizisin AP, Vu Y, Shuff M, Calcutt NA. Ciliary neurotrophic factor improves nerve conduction and ameliorates regeneration deficits in diabetic rats. Diabetes 2004; 53:1807–1812. [DOI] [PubMed] [Google Scholar]
- 18.Shi X, Chen Y, Nadeem L, Xu G. Beneficial effect of TNF-α inhibition on diabetic peripheral neuropathy. J Neuroinflammation 2013; 10:69.doi: 10.1186/1742-2094-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, et al. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest 2001; 107:1083–1092. doi: 10.1172/JCI12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol 2002; 50:393–413. [DOI] [PubMed] [Google Scholar]
- 21.Veves A, King GL. Can VEGF reverse diabetic neuropathy in human subjects? J Clin Invest 2001; 107:1215–1218. doi: 10.1172/JCI13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon PR, Leimig T, Babarin-Dorner A, Houston J, Holladay M, Mueller I, et al. Large-scale isolation of CD133+ progenitor cells from G-CSF mobilized peripheral blood stem cells. Bone Marrow Transplant 2003; 31:17–22. doi: 10.1038/sj.bmt.1703792. [DOI] [PubMed] [Google Scholar]
- 23.Han JW, Sin MY, Yoon YS. Cell therapy for diabetic neuropathy using adult stem or progenitor cells. Diabetes Metab J 2013; 37:91–105. doi: 10.4093/dmj.2013.37.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011; 92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Fadini GP, Albiero M, Vigili de Kreutzenberg S, Boscaro E, Cappellari R, Marescotti M, et al. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care 2013; 36:943–949. doi: 10.2337/dc12-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferraro F, Lymperi S, Méndez-Ferrer S, Saez B, Spencer JA, Yeap BY, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med 2011; 3: doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fadini GP, Fiala M, Cappellari R, Danna M, Park S, Poncina N, et al. Diabetes limits stem cell mobilization following G-CSF but not Plerixafor. Diabetes 2015; 64:2969–2977. doi: 10.2337/db15-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, et al. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 2014; 63:1763–1778. doi: 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Z, Garikipati VN, Nickoloff E, Wang C, Polhemus DJ, Zhou J, et al. Restoration of hydrogen sulfide production in diabetic mice improves reparative function of bone marrow cells. Circulation 2016; 134:1467–1483. doi: 10.1161/CIRCULATIONAHA.116.022967. [DOI] [PMC free article] [PubMed] [Google Scholar]


