Abstract
Background
This study was designed to evaluate the clinical and radiographic outcomes of patients with nutcracker syndrome (NCS) who were treated with three-dimensional printing (3DP) extravascular titanium stents (EVTSs). The 3DP EVTS was expected to release the hypertension of the left renal vein (LRV) produced by its compression between the superior mesenteric artery (SMA) and the aorta without causing any complications.
Method
The pre-operative kidney model of each patient was printed out to enable surgical planning. After that, the EVTS was designed based on the LRV's primitive physiologic structure using computer-aided design software, and each stent was printed out with a precision setting of 20 μm. Seventeen patients who had been suffering from NCS underwent laparoscopic 3DP EVTS placement. The surgical procedure was designed for the placement of EVTS, taking great care in positioning and fixing the stent. Surgical data, which included patient demographic characteristics as well as pre- and post-operative test results, were collected and analyzed.
Results
The mean duration of surgery was 75 ± 9 min, and the mean blood loss was 20 ± 5 mL. Computed tomography examinations revealed that the pre- and post-operative angle between the SMA and the aorta ranged from 18.7° ± 4.3° to 48.0° ± 8.8° (P < 0.05); in patients with left varicocele, the mean diameter of the left spermatic vein ranged from 3.7 ± 0.5 to 1.3 ± 0.2 mm (P < 0.05). Moreover, Doppler ultrasound examinations showed that the peak velocity of blood flow at the hilar area ranged from 12.4 ± 3.3 to 18.5 ± 3.4 cm/s (P < 0.05). No side effects were observed in the 24 to 42 months following surgery.
Conclusion
The findings after 2 years of follow-up suggest that the 3DP EVTS is a safe and effective minimally invasive alternative for the treatment of NCS.
Keywords: Nutcracker syndrome, Extravascular stent, Three-dimensional printing, Titanium, Minimally invasive, Laparoscopy
Introduction
Nutcracker syndrome (NCS), also known as left renal vein (LRV) hypertension, is a rare syndrome caused by extrinsic compression of the LRV between the superior mesenteric artery (SMA) and the aorta.[1–3] The clinical manifestations generally found in these patients include hematuria, proteinuria, left flank pain, and pelvic congestion syndrome in females and left varicocele in males.[4,5] In cases presenting with tolerable symptoms and mild hematuria, conservative treatment is recommended.[6] However, surgery may be considered for severe symptoms, such as persistent orthostatic proteinuria and varicocele formation; it may also be appropriate when conservative management has proved ineffective in patients below 18 years of age after 2 years and in adults after 6 months.[1,7]
Various approaches to surgical management for NCS have been reported since 1974.[8] Currently, the most widely employed options include open vascular approaches, laparoscopic techniques, and the placement of endovascular stents (EVSs).[9–11] Compared with open surgeries, laparoscopic techniques are less invasive and associated with reduced post-operative morbidity.[12,13] After the placement of EVSs, improvements were noted in LRV diameter as well as in the peak velocity ratio and renocaval pressure gradient. The EVS approach is; however, not without risks, with complications including incorrect stent placement and stent migration requiring surgical intervention.[9,14–16]
In 2016, Li et al[17] reported the surgical nursing method for a case involving laparoscopic three-dimensional printed (3DP) extravascular titanium stent (EVTS) placement at Tangdu Hospital, The Fourth Military Medical University. The follow-up result showed that the patient recovered quickly, without complications. Our team recently reported the case of a 29-year-old female patient with posterior NCS who was treated using 3DP individualized EVTS; the 2-year follow-up, in this case, revealed a satisfactory outcome.[18] As far as we know, there has been no clinical research regarding the application of 3DP individualized EVTS in the treatment of patients with NCS. The purpose of the present retrospective study was to evaluate the clinical and radiographic outcomes of selected patients with NCS treated with 3DP EVTSs. We hypothesized that the individualized 3DP EVTSs could provide a minimally invasive, effective, and safe form of management in these patients leading to improved radiographic and clinical outcomes.
Methods
Ethical approval
The experimental protocol and informed consent for this study, which followed the principles outlined in the Declaration of Helsinki, were approved by the Institutional Ethics Committee of the Fourth Military Medical University (No. 2015009). The patients and/or their families were informed that data from the case would be submitted for publication.
Study population
From August 2015 to December 2016, a total of 17 patients (two females and 15 males) diagnosed with NCS were referred to the Department of Urology, Tangdu Hospital. The inclusion criteria for our study were as follows: (1) one or more presenting syndromes including gross hematuria, proteinuria, gonadal varices (varicocele or ovarian vein syndrome), pelvic pain, flank pain, and/or abdominal pain[7]; (2) LRV diameter ratio (hilum-to-aorta mesenteric ratio) equal to or more than 4.9[7,19]; (3) angle between SMA and aorta less than 41°[7,19]; (4) no primary or other secondary kidney disease and no history of hypertension; and (5) no previous surgical management. Patients were followed for 2 years through periodic clinical and radiologic examinations.
Surgical planning and 3DP
Using a 64-detector computed tomography (CT) scanner (GE Light Speed VCT, Boston, MA, USA), CT images with a slice thickness set at 1.0 mm were obtained from all patients. CT image output in the DICOM format was exported to Mimics (Version 19.0, Materialise NV, Belgium), and 3D-DOCTOR software (Version 4.0, Able Software Co., Lexington, MA, USA) was adopted for kidney modeling. After that, the data were transferred to a fused deposition modeling (FDM) 3D printer (TD-III, 3D Printing Research Center of the Fourth Military Medical University, China) in the standard tessellation language format. As shown in Figure 1A, each pre-operative kidney model, printed in melted polylactic acid, was used to enable surgical planning.
Figure 1.
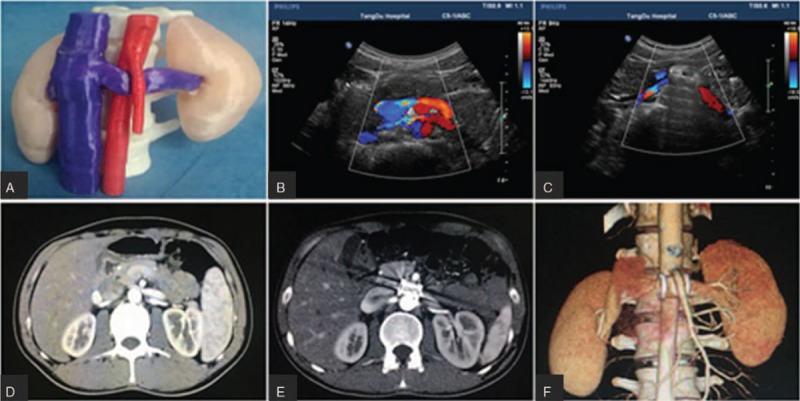
Representative image of the patient. NCS model was 3D printed for surgical planning (A). Pre-operative and DUS and CT examinations showed compression and stricture of the LRV with high blood flow acceleration in the stenotic area (B, D). Post-operative DUS and CT examination and 3D imaging revealed no patent high blood flow in the LRV and confirmed the stability of the extravascular stent (C, E, and F). 3D: Three-dimensional; CT: Computed tomography; DUS: Doppler ultrasound; LRV: Left renal vein; NCS: Nutcracker syndrome.
Each 3DP EVTS was designed according to the LRV physiologic structure of each patient using Siemens NX software (Version 10.0, Plano, Texas, USA). The stent was designed so that (1) the curvature of the surface and the raised edges matched to the shape of the aorta and (2) a porous mesh was used in order to improve adherence of the surrounding tissue [Figure 2]. The length of the 3DP stent was consistent with the width of the aorta. The stent was designed in the form of two hinged halves so that it could be placed around the LRV [Figure 2B]. The modeling data were put into an FDM 3D printer (SLM-250S; EOS, Krailling, Bayern, Germany), and the stent was printed out using medical titanium alloy powder with the printing precision setting as 200 μm. In addition, the implant was processed in surgical grade equipment, including ultrasonic cleaning, ethylene oxide sterilization, and monitoring of disinfection. Finally, the EVTS weighed in the range of 3.1 to 4.3 g and was about 16.1 to 19.3 mm in length. Moreover, in the transverse section, its long and short inner shafts ranged in length from 14.0 to 16.0 mm and 7.0 to 8.0 mm, respectively [Figure 2].
Figure 2.
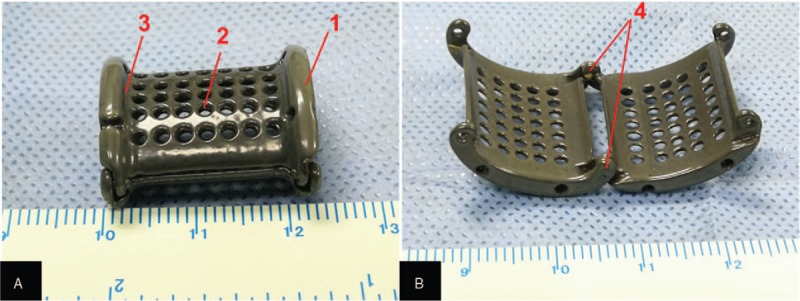
Representative image of the 3D printed titanium extravascular stent. The patient-specific extravascular stent was designed properly (A, B): 1, the raised edges on both ends of the stent; 2, the porous design; 3, the shallow groove for accommodating the aorta/SMA, and 4, the hinges of the stent. 3D: Three-dimensional; SMA: Superior mesenteric artery.
Surgical technique
In order to make sure that the 3DP EVTS would be placed and fixed properly, the surgical procedure for placement of the stent was carefully designed and conducted. The patient underwent general anesthesia, and a urethral catheter and nasogastric tube were inserted. The patient was placed in the right lateral position with care to pad pressure points. After the patient had been appropriately prepped, a 10-mm supraumbilical camera port was placed on the left side of the rectus abdominis at the level of the umbilicus, and a pneumoperitoneum was established. Under direct vision, two ports were placed 8 and 16 cm to the left of the camera port. A fourth port was placed 2 cm below the intersection point of the left midaxillary line and costal arch.
Sutures were fixed to the four corners of the stent in order to facilitate traction and placement, and the stent was introduced through the 10-mm supraumbilical camera port into the abdominal cavity. The peritoneum was cut along the left side of the line, from the upper edge of the spleen to the lower edge of the kidney. The descending colon was isolated to reveal the ventral Gerota fascia, which was then opened at the renal hilum to expose the LRV, left adrenal central vein, and left genital vein [Figure 3A]. Then the LRV was exposed completely up to the inferior vena cava, and the preaortic fibrous tissue around the LRV between the aorta and the SMA was released and resected [Figure 3B]. The left adrenal central vein and genital vein were routinely preserved; the left genital vein was ligated at the renal hilum in those patients with severe left varicocele. With gentle traction applied to the LRV, the opened extravascular stent was placed behind the LRV by pulling on the sutures that had been fixed on it [Figure 3C]. The stent was then closed and gently pushed into the compressed area between the SMA and aorta [Figure 3D]. The position of the stent was adjusted, and the recovery of blood flow in the LRV was confirmed. Finally, the fibrous sheath around the SMA and left connective tissue of the aorta were sutured. At the end of the procedure, the SMA was securely fixed on the arched surface of the extravascular stent in order to prevent migration [Figure 3D].
Figure 3.
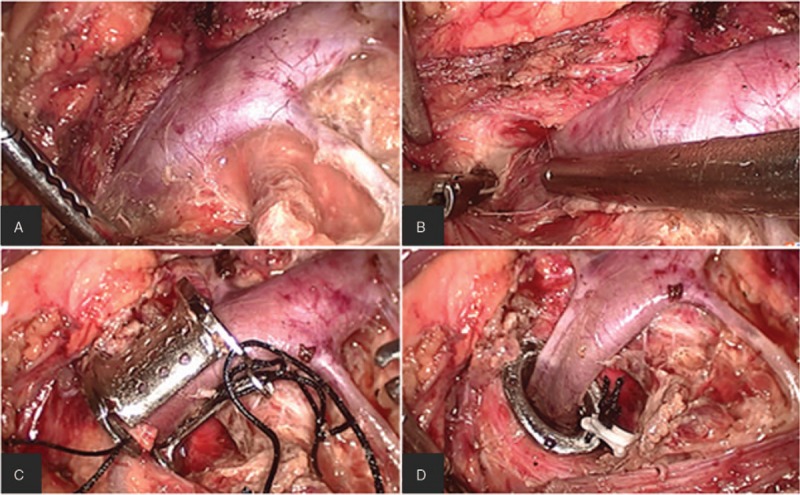
The stent was placed and fixed step by step. The LRV, the LACV, and the left reproductive vein were exposed (A); the compressed LRV between SMA and aortaventralis was freed by cutting off the surrounding fibrous tissue, and the IVC was also exposed (B); the stent was placed around the LRV (C); the stent was pushed between the SMA and aortaventralis, and the dialated LRV collapsed (D). IVC: Inferior vena cava; LACV: Left adrenal central vein; LRV: Left renal vein; SMA: Superior mesenteric aorta.
Post-operative course and follow-up
A standard post-operative protocol was followed in all patients. Pain control was achieved using intravenous nonopioid analgesics as necessary. Pre-operative antibiotic prophylaxis was continued until the day after the surgery. The urethral catheter was removed on the second post-operative day, and abdominal drainage tubes were removed 2 to 4 days after surgery. Oral intake was initiated on the first post-operative day with clear liquids, and a normal diet was gradually reintroduced. Patients were encouraged to ambulate 3 days after the surgery and were discharged with advice to avoid strenuous activities for 3 months.
Clinical, Doppler ultrasound (DUS), and CT examinations were scheduled at 1, 3, 6, and 12 months and then annually or if symptoms recurred. The restenosis was defined as more than one symptom associated with NCS.
Statistical analysis
All statistical calculations were performed with SPSS software, version 16.0 for personal computer (SPSS Inc., Chicago, IL, USA). The paired samples t test was performed to compare the patients’ data before and 3 months after treatment. A value of P < 0.05 was regarded as statistically significant.
Results
Seventeen patients with a mean age of 20.2 years (range: 10–29), including 15 males and two females, were consecutively selected for this study. Table 1 shows their general characteristics and clinical features. The body mass indice of both male and female patients were below normal. More than half of the males had left varicoceles, and the mean diameter of their left spermatic veins was 3.71 ± 0.49 mm. In addition, eight patients had gross hematuria and two had proteinuria. Over half of the male patients suffered from left abdominal pain, left flank pain, and a left scrotal bulge. The mean length of surgery was 75 ± 9 min, and the mean blood loss was 20 ± 5 mL. Gross hematuria and flank pain were relieved within 5 days after surgery, and microhematuria, proteinuria, and left varicocele disappeared within 2 weeks. Three patients complained of abdominal pain after surgery, but the pain resolved with symptomatic treatment. The overall post-operative length of stay in hospital was 5 to 7 days after surgery. All patients were discharged without complications.
Table 1.
Patient information and basline clinical characteristics.
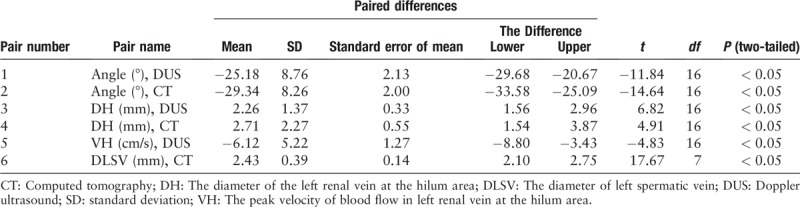
To investigate the effects of 3DP EVTS on the relieve of patients symptoms, pre-operative (1 week before surgery) and post-operative (3 months after surgery) test results – including the angle between SMA and the aorta, the diameter of the LRV (indicated as DH), and the peak blood flow velocity in the LRV (indicated as VH) at the hilar area, as well as the diameter of the LV (indicated as DLV) – were compared [Figure 1B−1E] [Table 2]. Results showed that the pre-operative and post-operative mean angles obtained from DUS examination were 21.44° ± 4.12° and 46.62° ± 8.55°, respectively, whereas the pre- and post-operative values obtained from CT examinations were 18.68° ± 4.25° and 48.02° ± 8.82°, respectively. In addition, the pre-operative DH means were 10.6 ± 1.3 mm for DUS and 11.3 ± 2.2 mm for CT, whereas in post-operative tests, the DH means were 8.4 ± 1.6 and 8.6 ± 1.2 mm, respectively. Moreover, the mean value of VH before surgery was 12.4 ± 3.3 cm/s, and it increased to 18.5 ± 3.4 cm/s post-operatively. Finally, the mean value of DLV was 3.7 ± 0.5 mm before surgery, and it was reduced to 1.3 ± 0.2 mm after surgery [Table 2]. The pre- and post-operative differences were statistically significant for all the comparisons (P < 0.05) [Table 3]. Very importantly, during the follow-up period, the stent between the LRV and the SMA remained stable, and no migration of any implanted stent was found [Figure 1F], which confirmed the stability of the 3DP EVTS installation.
Table 2.
Statistics of paired samples t test for pre-operative and post-operative.

Table 3.
Paired samples t test for pre-operative and post-operative.
The follow-up of each patient lasted for at least 24 months, during which period radiologic and clinical examinations showed that there were no complications and no side effects or restenosis of the LRV after surgery.
Discussion
With recent improvements in diagnostic technology, increasing numbers of patients complaining of hematuria, proteinuria, flank pain, and varicose veins are being diagnosed with NCS,[3] and the standard of care for the surgical treatment of NCS has been LRV transposition.[20] This procedure can be conducted either with open surgery or laparoscopy.[21] Ahmed et al[22] concluded that LRV transplantation is the optimal management option for NCS. However, it is carried out through a midline transperitoneal approach, which entails the risks of LRV thrombosis (about 12.5%) and intractable hematuria (about 12.5%–14.3%), eventually leading to nephrectomy.[10] SMA transposition is an alternative aggressive option for the treatment of NCS.[23] Although this approach entails a reduced risk of renal vein thrombosis, as the renal vein is left untouched, there is a significant risk of mesenteric ischemia, which may explain why only a few cases of SMA transposition have been reported.[24]
As far as we know, vascular stent treatment including the placement of both internal and external vascular stents has become an attractive, simple, and minimally invasive option with good results. Chen et al[14] have reported on their large series comprising endovascular stenting resulting in minimal complications and the resolution of symptoms in 59 of 61 patients. However, these stents were prone to migrate to the inferior vena cava or the right atrium, with a potential for occlusion due to thrombosis.[25] Another larger series involving endovascular stenting reported a 6.7% rate of migration after stent placement.[26] In addition, anti-coagulant drugs should be taken after EVS treatment for at least 3 months while the stent becomes endothelialized.[3] Wang et al[27] reported 13 cases of external vascular stenting treatment for NCS. The results showed that post-operatively, ten patients were symptom-free, two experienced a mild improvement, and one patient had a migrated stent, which may have been caused by an unstable suture. Another case of external stenting was recently reported by Igor et al, whose team conducted a robot-assisted laparoscopic extravascular stent surgery for a female patient. The result showed that stent placement successfully shielded the SMA from compression by the LRV, thus resolving the patient's symptoms of pelvic congestion; thereafter, no further procedure involving the gonadal vein was required.[28]
Over the last two decades, 3DP technology has been developing rapidly, especially in its application to the medical field. The combination of 3DP technique and medical treatment has solved many obstinate medical problems in a novel way. We designed the 3DP EVTS model based on each patient's CT data, adjusting the size of the stent in order to make it fit the LRV structure of each one as appropriately as possible. The anti-migration design is of paramount importance to the success of the EVTS treatment. According to the published literature, the problem of post-surgical shifting of both internal (about 7.3%) and external (about 7.6%) vascular stents has, up to now, been unavoidable.[29,30] In our research, thanks to the design comprising raised sides and a porous layer (which help to hold the stent in position), we found no such problem in the course of our follow-up period.
Furthermore, stent collapse due to insufficient stiffness is another common consequence of internal and external vascular stent placement.[25,31] Since the 1980s, titanium has been seen as the ideal raw material for implants because of its excellent biocompatibility, pronounced compressive strength, lightweight, and low cost.[32,33] We used titanium to make our EVTSs, and their compressive strength fully met the demand for a compressive force against the SMA and abdominal aorta, thus avoiding stent collapse. In fact, we observed that during surgery, the distal LRV relaxed immediately after placement of the EVTS, and the blood flow quickly became unobstructed, as demonstrated by post-operative CT and DUS examinations [Figure 1B and 1E].
Laparoscopic renovascular surgery is a minimally invasive technique that ensures a more rapid patient recovery and provides results nearly equivalent to those of open surgery.[13,34] In 2010, Hartung et al[35] first reported laparoscopic reimplantation of the LRV into the IVC for patients with NCS. Since then, increasing numbers of cases involving NCS have been treated via a laparoscopic approach. However, laparoscopic transposition or venous bypass is a technically demanding procedure posing the risk of bleeding complications.[34,35] In our study, compared with the traditional laparoscopic approach, placement of the 3DP EVTS was less difficult, requiring only dissociation of the LRV. Key to the success of this operation is complete removal of the fibrous tissue surrounding the LRV to make room for 3DP EVTS placement. Moreover, the SMA sheath was sutured carefully to the sheath of the abdominal aorta, so that the SMA was fixed to the ventral groove of the stent, thus ensuring the stability not only of the SMA but also that of the stent.
Several limitations warrant discussion. First, this study was conducted in patients with NCS using 3DP EVTSs only, without resort to comparative data from traditional surgical treatment. Thus, although follow-up examinations showed that 3DP EVTS was an effective and safe alternative for NCS treatment, there were no robust conclusions regarding 3DP EVTS as compared with traditional management. Second, only 17 patients were included in our study; a larger sample size might have given us more information. Third, it must be noted that the follow-up in this study ranged from 24 to 42 months. A longer-term therapeutic and side-effect follow-up is suggested for future studies.
Conflicts of interest
None.
Footnotes
How to cite this article: Wang H, Guo YT, Jiao Y, He DL, Wu B, Yuan LJ, Li YY, Yang Y, Cao TS, Zhang B. A minimally invasive alternative for the treatment of nutcracker syndrome using individualized three-dimensional printed extravascular titanium stents. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000255
He Wang and Yi-Tong Guo contributed equally to this work.
References
- 1.Zhang H, Li M, Jin W, San P, Xu P, Pan S. The left renal entrapment syndrome: diagnosis and treatment. Ann Vasc Surg 2007; 21:198–203. doi: 10.1016/j.avsg.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc 2010; 85:552–559. doi: 10.4065/mcp.2009.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Y, Wu Z, Chen S, Tian L, Li D, Li M, et al. Nutcracker syndrome – how well do we know it? Urology 2014; 83:12–17. doi: 10.1016/j.urology.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam S, Bumpus K, Kapadia SR, Gray B, Lyden S, Shishehbor MH. The nutcracker syndrome. Ann Vasc Surg 2011; 25:1154–1164. doi: 10.1016/j.avsg.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Shin JI, Lee JS, Kim MJ. The prevalence, physical characteristics and diagnosis of nutcracker syndrome. Eur J Vasc Endovasc Surg 2006; 32:335–336. doi: 10.1016/j.ejvs.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Shin JI, Baek SY, Lee JS, Kim MJ. Follow-up and treatment of nutcracker syndrome. Ann Vasc Surg 2007; 21:402.doi: 10.1016/j.avsg.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Ananthan K, Onida S, Davies AH. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg 2017; 53:886–894. doi: 10.1016/j.ejvs.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Pastershank SP. Left renal vein obstruction by a superior mesenteric artery. J Can Assoc Radiol 1974; 25:52–54. [PubMed] [Google Scholar]
- 9.Neste MG, Narasimham DL, Belcher KK. Endovascular stent placement as a treatment for renal venous hypertension. J Vasc Interv Radiol 1996; 7:859–861. doi: 10.1016/S1051-0443(96)70861-8. [DOI] [PubMed] [Google Scholar]
- 10.Reed NR, Kalra M, Bower TC, Vrtiska TJ, Ricotta JJ, 2nd, Gloviczki P. Left renal vein transposition for nutcracker syndrome. J Vasc Surg 2009; 49:386–393. doi: 10.1016/j.jvs.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Hohenfellner M, D’Elia G, Hampel C, Dahms S, Thuroff JW. Transposition of the left renal vein for treatment of the nutcracker phenomenon: long-term follow-up. Urology 2002; 59:354–357. doi: 10.1016/S0090-4295(01)01537-0. [DOI] [PubMed] [Google Scholar]
- 12.Xu D, Liu Y, Gao Y, Zhang L, Wang J, Che J, et al. Management of renal nutcracker syndrome by retroperitoneal laparoscopic nephrectomy with ex vivo autograft repair and autotransplantation: a case report and review of the literature. J Med Case Rep 2009; 3:82.doi: 10.1186/1752-1947-3-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu D, Gao Y, Chen J, Wang J, Ye J, Liu Y. Laparoscopic inferior mesenteric-gonadal vein bypass for the treatment of nutcracker syndrome. J Vasc Surg 2013; 57:1429–1431. doi: 10.1016/j.jvs.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Zhang H, Shi H, Tian L, Jin W, Li M. Endovascular stenting for treatment of nutcracker syndrome: report of 61 cases with long-term followup. J Urol 2011; 186:570–575. doi: 10.1016/j.juro.2011.03.135. [DOI] [PubMed] [Google Scholar]
- 15.Rana MA, Oderich GS, Bjarnason H. Endovenous removal of dislodged left renal vein stent in a patient with nutcracker syndrome. Semin Vasc Surg 2013; 26:43–47. doi: 10.1053/j.semvascsurg.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Mou Y, Cheng Y, Wang H, Zheng Z. Late stent migration into the right ventricle in a patient with nutcracker syndrome. Ann Vasc Surg 2015; 29:839.e1–839.e4. doi: 10.1016/j.avsg.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Shi W. The nursing method for a case of laparoscopic treatment of nutcracker syndrome using 3D printing technique (in Chinese). Chin Nurs Res 2016; 30:2430–2431. doi: 10.3969/j.issn.1009-6493.2016.19.039. [Google Scholar]
- 18.Guo YT, Wang H, Wang JP, Zhang B. Two-year follow-up on laparoscopic three-dimensional printed extravascular stent placement for posterior nutcracker syndrome. Chin Med J 2018; 131:2895–2896. doi: 10.4103/0366-6999.246075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KW, Cho JY, Kim SH, Yoon JH, Kim DS, Chung JW, et al. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. Eur J Radiol 2011; 80:648–654. doi: 10.1016/j.ejrad.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Avgerinos ED, McEnaney R, Chaer RA. Surgical and endovascular interventions for nutcracker syndrome. Semin Vasc Surg 2013; 26:170–177. doi: 10.1053/j.semvascsurg.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Gunka I, Navratil P, Lesko M, Jiska S, Raupach J. Laparoscopic left renal vein transposition for nutcracker syndrome. Ann Vasc Surg 2016; 31:209.e1–209.e5. doi: 10.1016/j.avsg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed K, Sampath R, Khan MS. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg 2006; 31:410–416. doi: 10.1016/j.ejvs.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 23.Yang BZ, Li Z, Wang ZG. Nutcracker syndrome due to left-sided inferior vena cava compression and treated with superior mesenteric artery transposition. J Vasc Surg 2012; 56:816–818. doi: 10.1016/j.jvs.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PN, Darling RC, 3rd, Chang BB, Shah DM, Leather RP. A case of nutcracker syndrome: treatment by mesoaortic transposition. J Vasc Surg 1992; 16:663–665. doi: 10.1016/0741-5214(92)90176-9. [DOI] [PubMed] [Google Scholar]
- 25.Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg 2001; 34:812–819. doi: 10.1067/mva.2001.118802. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z, Zheng X, He Y, Fang X, Li D, Tian L, et al. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord 2016; 4:193–199. doi: 10.1016/j.jvsv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang SZ, Zhang WX, Meng QJ, Zhang XP, Wei JX, Qiao BP. Laparoscopic extravascular stent placement for nutcracker syndrome: a report of 13 cases. J Endourol 2015; 29:1025–1029. doi: 10.1089/end.2014.0411. [DOI] [PubMed] [Google Scholar]
- 28.Sorokin I, Nelson J, Rectenwald JE, Cadeddu JA. Robot-assisted laparoscopic extravascular stent for nutcracker syndrome. J Robot Surg 2018; 12:561–565. doi: 10.1007/s11701-017-0744-7. [DOI] [PubMed] [Google Scholar]
- 29.Policha A, Lamparello P, Sadek M, Berland T, Maldonado T. Endovascular treatment of nutcracker syndrome. Ann Vasc Surg 2016; 36:295.e1–295.e7. doi: 10.1016/j.avsg.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Hartung O, Grisoli D, Boufi M, Marani I, Hakam Z, Barthelemy P, et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vacs Surg 2005; 42:275–280. doi: 10.1016/j.jvs.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 31.Tian L, Chen S, Zhang G, Zhang H, Jin W, Li M. Extravascular stent management for migration of left renal vein endovascular stent in nutcracker syndrome. BMC Urol 2015; 15:73.doi: 10.1186/s12894-015-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. J R Soc Interface 2008; 5:1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neoh KG, Hu X, Zheng D, Kang ET. Balancing osteoblast functions and bacterial adhesion on functionalized titanium surfaces. Biomaterials 2012; 33:2813–2822. doi: 10.1016/j.biomaterials.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Nogueira JM, Cangro CB, Fink JC, Schweitzer E, Wiland A, Klassen DK, et al. A comparison of recipient renal outcomes with laparoscopic versus open live donor nephrectomy. Transplantation 1999; 67:722–728. doi: 10.1097/00005392-199909010-00111. [DOI] [PubMed] [Google Scholar]
- 35.Hartung O, Azghari A, Barthelemy P, Boufi M, Alimi YS. Laparoscopic transposition of the left renal vein into the inferior vena cava for nutcracker syndrome. J Vasc Surg 2010; 52:738–741. doi: 10.1016/j.jvs.2010.04.018. [DOI] [PubMed] [Google Scholar]


