Abstract
Background:
Mental stress-induced myocardial ischemia (MSIMI) is closely associated with adverse cardiac events in patients with coronary artery disease (CAD) and we aimed to determine whether biomarkers and blood pressure could be potential predictors of MSIMI.
Methods:
This study enrolled 82 patients with documented CAD between June 1, 2017 and November 9, 2017. Patient blood samples were obtained at resting period and at the end of mental arithmetic. Then, patients were assigned to MSIMI positive group and MSIMI negative group. The main statistical methods included linear regression, receiver operating characteristic (ROC) curves, and logistic regression.
Results:
Patients with CAD with MSIMI had significantly greater median resting N-terminal pro-brain natriuretic peptide (NT-proBNP, 141.02 [45.85–202.76] pg/mL vs. 57.95 [27.06–117.64] pg/mL; Z = −2.23, P = 0.03) and mean systolic blood pressure (SBP) (145.56 ± 16.87 mmHg vs. 134.92 ± 18.16 mmHg, Z = −2.13, P = 0.04) when compared with those without MSIMI. After 5-min mental stress task, those who developed MSIMI presented higher elevation of median post-stressor high sensitivity cardiac troponin I (hs-cTnI, 0.020 [0.009–0.100] ng/mL vs. 0.009 [0.009–0.010] ng/mL; Z = −2.45, P = 0.01), post-stressor NT-proBNP (138.96 [39.93–201.56] pg/mL vs. 61.55 [25.66–86.50] pg/mL; Z = −2.15, P = 0.03) compared with those without MSIMI. Using the ROC curves, and after the adjustment for basic characteristics, the multiple logistic regression analysis showed that patients presenting a post-stressor hs-cTnI ≥ 0.015 ng/mL had seven-fold increase in the risk of developing MSIMI (odds ratio [OR]: 7.09; 95% confidence interval [CI]: 1.65–30.48; P = 0.009), a rest NT-proBNP ≥ 80.51 pg/mL had nearly eight-fold increase (OR: 7.85; 95% CI: 1.51–40.82; P = 0.014), a post-stressor NT-proBNP ≥ 98.80 pg/mL had 35-fold increase (OR: 34.96; 95% CI: 3.72–328.50; P = 0.002), a rest SBP ≥ 129.50 mmHg had 11-fold increase (OR: 11.42; 95% CI: 1.21–108.17; P = 0.034).
Conclusions:
The present study shows that CAD patients with higher hs-cTnI level, and/or greater NT-proBNP and/or SBP are at higher risk of suffering from MSIMI when compared with those without MSIMI, indicating that hs-cTnI, NT-proBNP, SBP might be potential predictors of MSIMI.
Keywords: Coronary artery disease, Depression, Anxiety, Blood pressure, Biomarkers
Introduction
Coronary artery disease (CAD) remains the leading cause of death around the world. About 30% to 60% of patients with CAD could present with myocardial ischemia induced by mental stress as mentioned by recent studies.[1,2] Mental stress-induced myocardial ischemia (MSIMI) is hypothesized to be associated with the following factors: inflammatory response,[3] hyperactivation of the sympathetic nervous system,[4] increased catecholamines,[5] anger,[6] and a diagnosis of depression[7] and anxiety.[8] Furthermore, some related studies have shown that MSIMI triggered in the laboratory is closely associated with adverse cardiac events in patients with CAD.[9–11] Wei et al[9] conducted a meta-analysis including five studies, with a total number of 555 patients with CAD and 117 subsequent cardiac events, demonstrating a two-fold increased risk of adverse outcomes in patients with MSIMI. In Sun et al's[10] research on the relationship between mental stress-induced left ventricular dysfunction and adverse outcome in 310 patients with ischemia heart disease, after a median 4-year follow-up, they elucidated that the reduction of left ventricular ejection fraction induced by mental stress could predict the increased risk of adverse cardiac outcomes. In another study, myocardial annular velocity changes during mental stress were proved to be predictors of adverse cardiovascular outcomes; moreover, the greater decrease of diastolic early (e′) and diastolic late (a′) was associated with the higher possibility of major adverse cardiac events (MACE).[11] Therefore, it is of vital importance to identify patients with CAD susceptible to MSIMI. Until recently, MSIMI could be diagnosed via echocardiography or electrocardiography (ECG),[12] sestamibi single-photon emission computed tomography (SPECT) imaging,[13] or peripheral arterial tonometry technique.[14] In our recent study, we have not found any difference in vascular severity between the MSIMI negative group and the positive group in patients with CAD.[15] Regarding the convenience and important role of biomarkers (cardiac troponin I [cTnI],[16] C-reactive protein [CRP][17]) and blood pressure[18] in cardiovascular diseases and MSIMI, we aimed to determine whether biomarkers and blood pressure could be potential predictors of MSIMI in this study.
Materials and methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Anzhen Hospital (No: 2014015X). All patients provided written informed consent.
Sample size
According to the sample equation of receiver operating characteristic (ROC) curve,[19,20] we computed the sample size by the following equation and using Power Analysis and Sample Size (PASS) software 15.0 (NCSS, LLC; Kaysville, UT, USA).
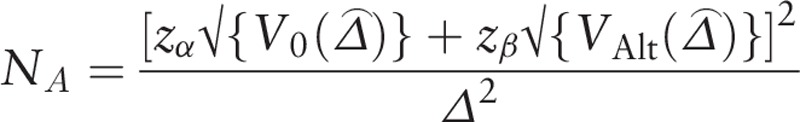 |
V0 (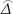 ) = NA var0 (
) = NA var0 (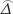 ), VAlt (
), VAlt (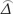 ) = NA varAlt (
) = NA varAlt (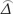 ),
),  was as the difference between the accuracies of two diagnostic tests,
was as the difference between the accuracies of two diagnostic tests, 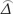 was as maximum likelihood estimate of
was as maximum likelihood estimate of  , NA was the number of MSIMI negative patients, varAlt (
, NA was the number of MSIMI negative patients, varAlt (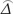 ) was the variance of
) was the variance of 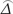 under the alternative hypothesis, Zα and Zβ were the upper α and β percentiles of the standard normal distribution, α = 0.05, Zα=1.96 (two-sided test), Zβ = 1.282.
under the alternative hypothesis, Zα and Zβ were the upper α and β percentiles of the standard normal distribution, α = 0.05, Zα=1.96 (two-sided test), Zβ = 1.282.
The sample size computed by PASS 15.0 was 72, therefore the sample size in our study 82 was sufficient for this statistical analysis.
Patients enrollment
This was a prospective cohort study. This study enrolled 82 patients with documented CAD including 54 males and 28 females, at an average age of 60.1 ± 9.8 years. It was conducted between June 1, 2017 and November 9, 2017. Inclusion criteria were the following: (1) male or female; (2) >18 years of age; (3) documented CAD; and (4) signed informed consent. The exclusion criteria were the following: (1) severe major organ dysfunction; (2) severe psychiatric disorders; (3) acute coronary syndrome in recent 2 months; (4) pregnant; or (5) cognitive impairment [Figure 1].
Figure 1.
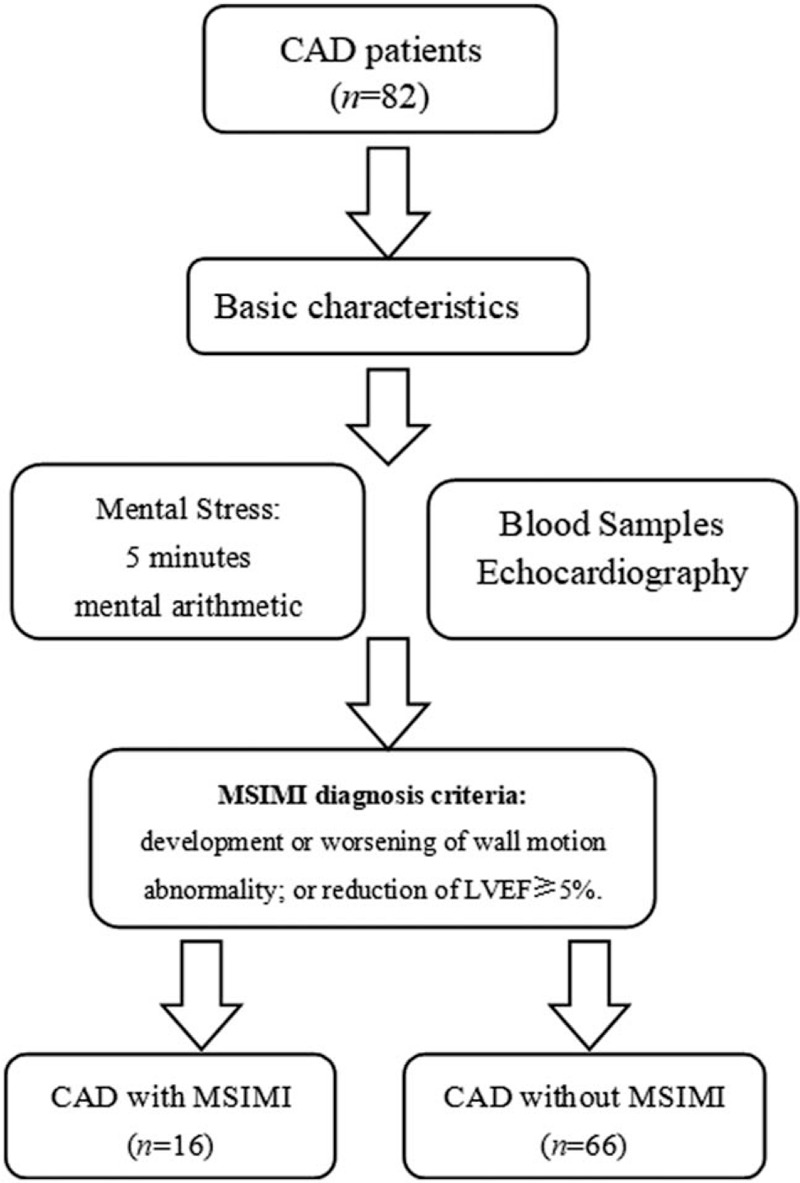
Flow chart of patient enrollment and diagnosis. CAD: Coronary artery disease; MSIMI: Mental stress-induced myocardial ischemia; LVEF: Left ventricular ejection fraction.
Study design
All the patients received blood withdrawn and echocardiography before and after a 5-min mental stress task. MSIMI was diagnosed as follows. Then we analyzed the relationship between the biomarkers, blood pressure, heart rate, and MSIMI by statistical analysis.
Basic characteristics
After enrollment, patients were asked to complete a standardized questionnaire including sex, age, height, weight, history of hypertension, hyperlipidemia, diabetes, current or former smoking, and alcohol use. In addition, depression was assessed with the Patient Health Questionnaire 9-item (PHQ-9), and anxiety was assessed with Generalized Anxiety Disorder 7-item (GAD-7). Furthermore, we utilized the Gensini scoring system to value the stenosis of each coronary artery, as it had been described previously.[21]
Blood collection
Beta-blockers and caffeine-containing products were withheld for 24 h prior to stress testing. Patient blood samples for biomarkers analysis were obtained at resting period in the morning after an 8-h fast and 5 min after mental arithmetic. The blood was collected into plastic tubes with ethylenediamine tetraacetic acid (final concentration of 0.5–1%). The tubes were then centrifuged and the supernatant collected and stored at −80°C until analysis. Biomarkers included high sensitivity cardiac troponin I (hs-cTnI), N-terminal prohormone of brain natriuretic peptide (NT-proBNP), creatine kinase-muscle/brain (CK-MB), myoglobin, CRP, D-dimer. These biomarkers concentrations were measured by AFIAS-6 (AFIAS; automated fluorescent immunoassay system; Chimax Boditech Co. Ltd., Shanghai, China).
MSIMI diagnosis
Mental arithmetic was used as a mental stress task. Patients were asked to perform a series of subtraction with encouragement (subtracted 7 sequentially from a four-digit number) while being urged to calculate faster and more accurately in 5 min.
Left ventricular wall motion images were acquired before and during the stressor (mental arithmetic). Heart rate and blood pressure were recorded at rest, peri-stressor and post-stressor. Diagnostic criteria of MSIMI[22] included the following: compared to rest, any development or worsening of wall motion abnormality; or reduction of left ventricular ejection fraction (LVEF) ≥5%. The echocardiography was performed by a professional and experienced echocardiographic sonographer, calculating LVEF from 3 to 5 beats, and assessing wall motion from 30 to 40 frames of systole from one cardiac cycle according to the latest American Society of Echocardiography's 17-segment model.[10,23] Each segment was graded and scored as normal (1 = normal or hyperdynamic) or abnormal (2 = hypokinetic, 3 = akinetic, 4 = dyskinetic, or 5 = aneurysmal) wall motion. A wall motion score index (WMSI) was calculated as the sum of the segmental wall motion scores divided by the total number of the scored segments. According to the echocardiography images, patients were assigned to MSIMI positive group and MSIMI negative group.
Statistical analysis
Statistical comparisons of the two groups were examined on baseline demographic and clinical characteristics, the value of biomarkers, blood pressure, heart rate, and LVEF. Continuous variables of normal distribution were displayed as mean ± standard deviation, and independent t test or paired t test was used for group comparison. Continuous variables of skewed distribution were displayed as median (P25- P75), and non-parametric test of two independent samples or two related samples was used for group comparison. Categorical variables were displayed as absolute values and percentage, and Chi-square was used for group comparison. Linear regression was used to assess the relationship between the value of biomarkers at rest and post-stressor.
To establish hs-cTnI and NT-proBNP cut-off values with appropriate sensitivity and specificity, ROC curves were plotted, and the area under the curve (AUC) was estimated. The optimal hs-cTnI and NT-proBNP cut-off value was determined as the value that maximized the Youden index, calculated as (sensitivity + specificity) − 1. Logistic regression was used to test the predictive value of the hs-cTnI and NT-proBNP cut-off and the other selected parameters. The P value <0.05 (two-tailed) was considered for statistical significance. IBM SPSS 24.0 (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis.
Results
Study characteristics
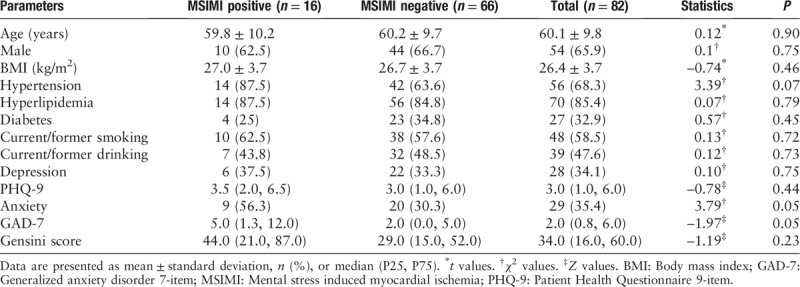
We enrolled a total of 82 patients with stable CAD in this study. The mean age was 60.1 ± 9.8 years, 65.9% were males, 32.9% had diabetes mellitus, 68.3% had hypertension, 85.4% had hyperlipidemia, 34.1% had depression, and 35.4% had anxiety. The incidence of MSIMI was 19.5%. As many as 16 patients were assigned to MSIMI positive group and 66 patients were assigned to MSIMI negative group. No significant differences in demographic features were observed between the two groups. And there was no difference of coronary artery stenosis between MSIMI positive and negative groups (44.0 [21.0, 87.0] vs. 29.0 [15.0, 52.0], Z = −1.19, P = 0.23) [Table 1].
Table 1.
Study characteristics in patients with and without MSIMI.
Biomarkers comparison in different groups
Comparisons between MSIMI positive group and MSIMI negative group
Compared with those without MSIMI, the median resting hs-cTnI level was slightly higher in patients with MSIMI (0.015 [0.009–0.040] ng/mL vs. 0.009 [0.009–0.010] ng/mL; Z = −1.95, P = 0.05), the median post-stressor hs-cTnI was significantly higher in patients with MSIMI (0.020 [0.009–0.100] ng/mL vs. 0.009 [0.009–0.010] ng/mL; Z = −2.45, P = 0.01) [Tables 2 and 3].
Table 2.
Biomarkers comparisons between patients with and without MSIMI.
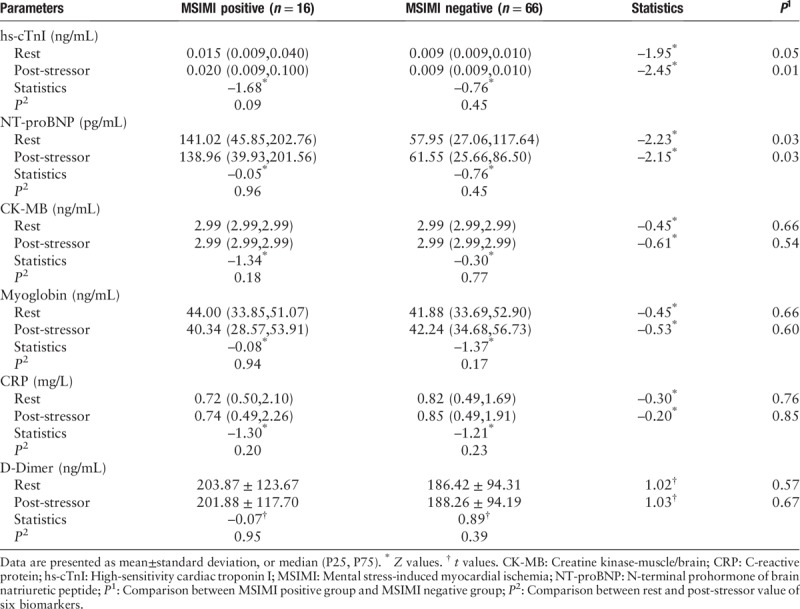
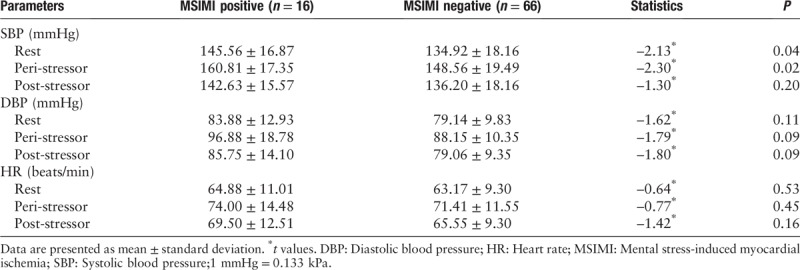
Table 3.
BP, HR comparisons between patients with and without MSIMI.
Compared with those without MSIMI, the median resting NT-proBNP level was significantly higher in patients with MSIMI (141.02 [45.85–202.76] pg/mL vs. 57.95 [27.06–117.64] pg/mL; Z = −2.23, P = 0.03), the median post-stressor NT-proBNP was significantly higher in patients with MSIMI (138.96 [39.93–201.56] pg/mL vs. 61.55 [25.66–86.50] pg/mL; Z = −2.15, P = 0.03) [Table 2].
There was no significance in the median resting CK-MB comparison between MSIMI positive and MSIMI negative groups (2.99 [2.99–2.99] ng/mL vs. 2.99 [2.99–2.99] ng/mL; Z = −0.45, P = 0.66). There was no significance in the median post-stressor CK-MB comparison between MSIMI positive and MSIMI negative groups (2.99 [2.99–2.99] ng/mL vs. 2.99 [2.99–2.99] ng/mL; Z = −0.61, P = 0.54) [Table 2].
There was no significance in the median resting myoglobin comparison between MSIMI positive and MSIMI negative groups (44.00 [33.85–51.07] ng/mL vs. 41.88 [33.69–52.90] ng/mL; Z = −0.45, P = 0.66). There was no significance in the median post-stressor myoglobin comparison between MSIMI positive and MSIMI negative groups (40.34 [28.57–53.91] ng/mL vs. 42.24 [34.68–56.73] ng/mL; Z = −0.53, P = 0.60) [Table 2].
There was no significance in the median resting CRP comparison between MSIMI positive and MSIMI negative groups (0.72 [0.50–2.10] mg/L vs. 0.82 [0.49–1.69] mg/L; Z = −0.30, P = 0.76). There was no significance in the median post-stressor CRP comparison between MSIMI positive and MSIMI negative groups (0.74 [0.49–2.26] mg/L vs. 0.85 [0.49–1.91] mg/L; Z = −0.20, P = 0.85) [Table 2].
There was no significance in the resting D-dimer comparison between MSIMI positive and MSIMI negative groups (203.87 ± 123.67 vs. 186.42 ± 94.31 ng/mL; t = 1.02, P = 0.57). There was no significance in the post-stressor D-dimer comparison between MSIMI positive and MSIMI negative groups (201.88 ± 117.70 vs. 188.26 ± 94.19 ng/mL; t = 1.03, P = 0.67) [Table 2].
Comparison between rest and post-stressor
In MSIMI positive and negative groups, there were no significance in the comparison between rest and post-stressor biomarkers [Table 2].
hs-cTnI
Regarding the unitary linear association between rest hs-cTnI and post-stressor hs-cTnI, we had built the linear equation as: Y = 0.008 + 1.08X (Y: hs-cTnI post-stressor, X: rest hs-cTnI). The absolute value of standardized coefficients beta demonstrated the interaction of rest hs-cTnI and post-stressor hs-cTnI [Figure 2].
Figure 2.
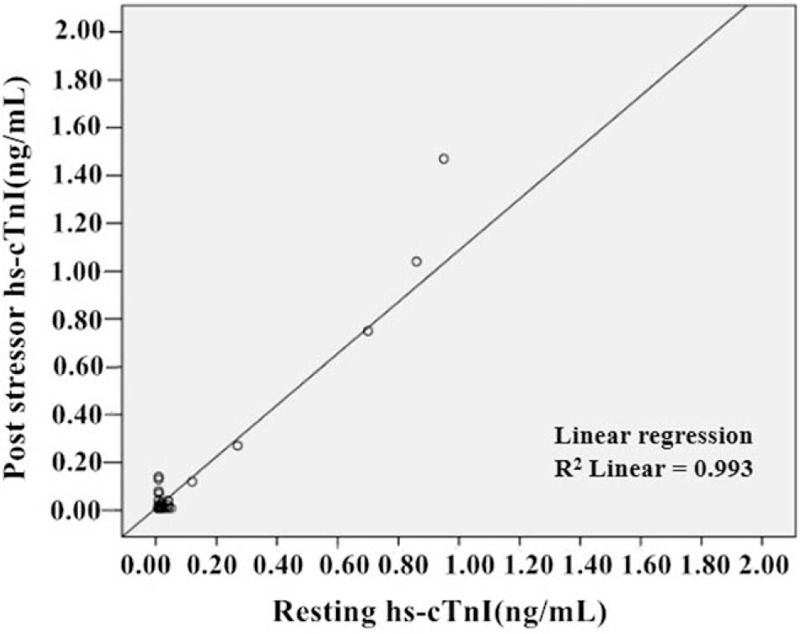
Unitary linear regression of resting and post-stressor hs-cTnI. hs-cTnI: High-sensitivity cardiac troponin I.
Post-stressor hs-cTnI
For asymptotic significance is 0.094, we could not describe ROC curve for rest hs-cTnI and MSIMI.
The ROC curve, in assessing the utility of post-stressor hs-cTnI as a predictor of MSIMI, yielded a cut-off value of 0.015 ng/mL, with a sensitivity of 56.3% and a specificity of 79.7% (AUC: 0.67, 95% confidence interval [CI]: 0.51–0.83; P = 0.037); a positive predictive value (PPV) of 40.9% and a negative predictive value (NPV) of 87.9%, a positive likelihood ratio of 2.77 and a negative likelihood ratio of 0.55. As a matter of fact, 56.3% of patients who developed MSIMI had post-stressor hs-cTnI ≥ 0.015 ng/mL [Figure 3].
Figure 3.
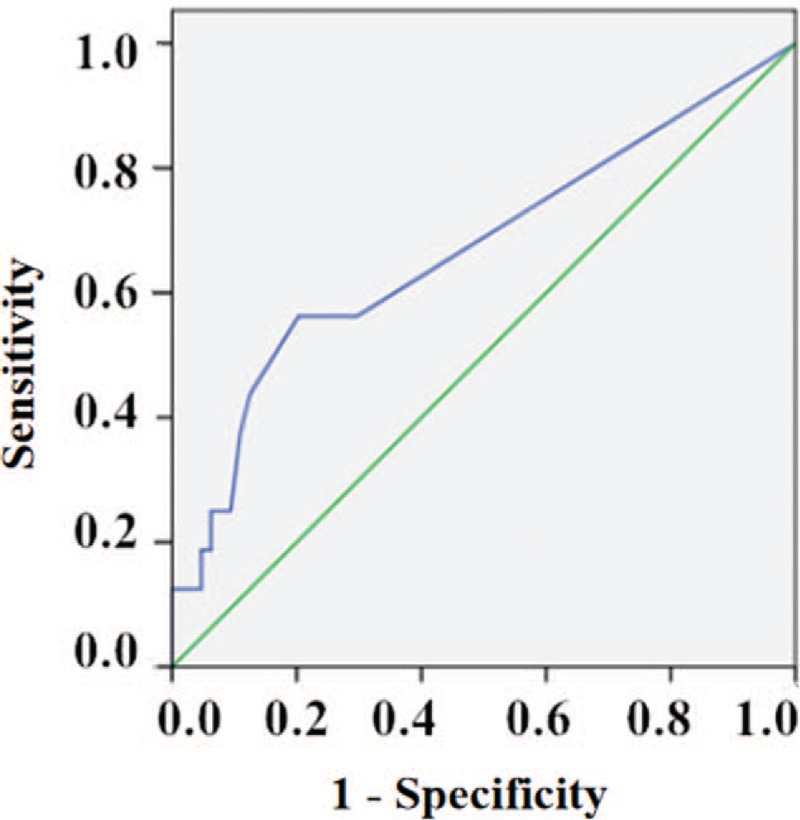
Receiver operating characteristic curve in assessing the utility of post-stressor hs-cTnI as a predictor of MSIMI, yielded a cut-off value of 0.015 ng/mL, with a sensitivity of 56.3% and a specificity of 79.7% (AUC: 0.67, 95% CI: 0.51–0.83; P = 0.037). AUC: Area under the curve; CI: Confidence interval; hs-cTnI: High-sensitivity cardiac troponin I; MSIMI: Mental stress-induced myocardial ischemia.
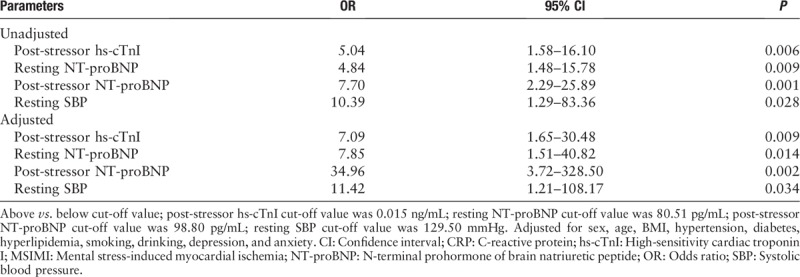
Before adjustment, the logistic regression analysis showed that patients presenting a post-stressor hs-cTnI ≥ 0.015 ng/mL had a five-fold increase in the risk of developing MSIMI (odds ratio [OR]: 5.04; 95% CI: 1.58–16.10; P = 0.006). After adjustment for sex, age, body mass index (BMI), hypertension, diabetes, hyperlipidemia, smoking, drinking, depression, and anxiety, the multiple logistic regression analysis showed that patients presenting a post-stressor hs-cTnI ≥ 0.015 ng/mL had seven-fold increase in the risk of developing MSIMI (OR: 7.09; 95% CI: 1.65–30.48; P = 0.009) [Table 4].
Table 4.
Factors associated with MSIMI in patients with CAD.
NT-proBNP
Regarding the unitary linear association between rest NT-proBNP and post-stressor NT-proBNP, we had built the linear equation as: Y = 23.20 + 0.7X (Y: NT-proBNP post-stressor; X: rest NT-proBNP). The absolute value of standardized coefficients beta demonstrated the interaction of rest NT-proBNP and post-stressor NT-proBNP [Figure 4].
Figure 4.
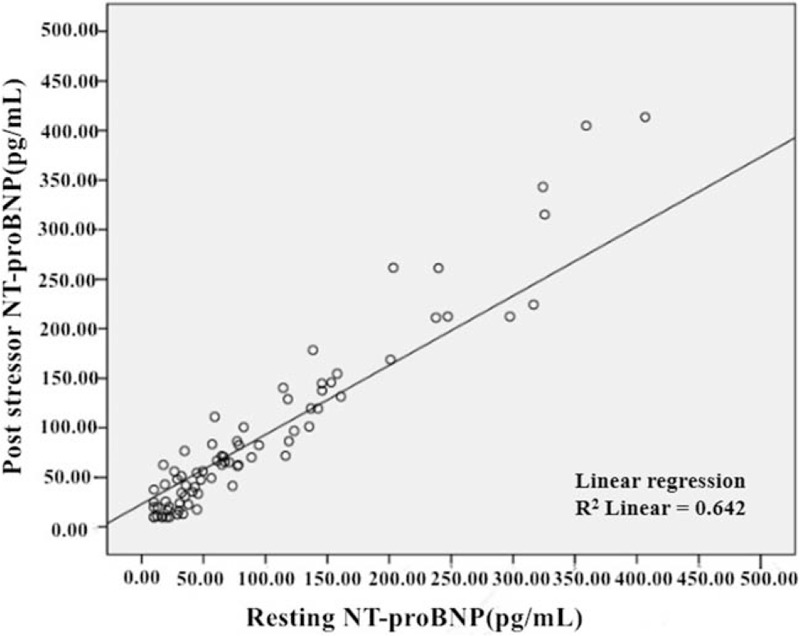
Unitary linear regression of resting and post-stressor NT-proBNP. NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Resting NT-proBNP
The ROC curve, in assessing the utility of rest NT-proBNP as a predictor of MSIMI, yielded a cut-off value of 80.51 pg/mL, with a sensitivity of 68.8% and a specificity of 68.7% (AUC: 0.68, 95% CI: 0.54–0.83; P = 0.026); a PPV of 35.5% and an NPV of 89.8%, a positive likelihood ratio of 2.19 and a negative likelihood ratio of 0.45. As a matter of fact, 68.8% of patients who developed MSIMI had rest NT-proBNP ≥ 80.51 pg/mL [Figure 5].
Figure 5.
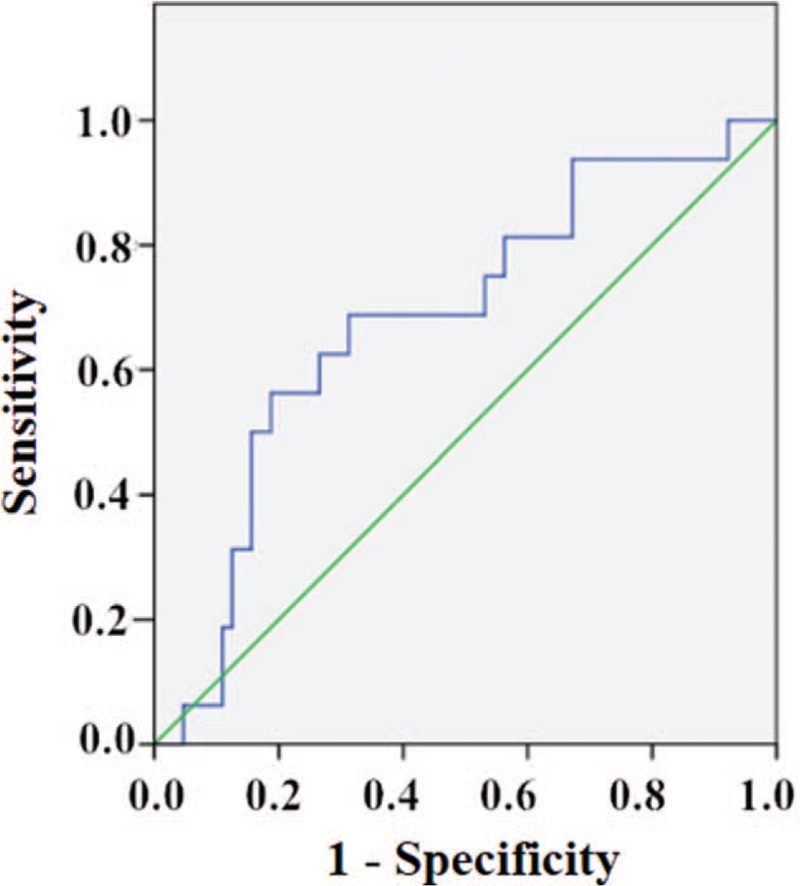
Receiver operating characteristic curve, in assessing the utility of rest NT-proBNP as a predictor of MSIMI, yielded a cut-off value of 80.51 pg/mL, with a sensitivity of 68.8% and a specificity of 68.7% (AUC: 0.68, 95% CI: 0.54–0.83; P = 0.026). AUC: Area under the curve; CI: Confidence interval; MSIMI: Mental stress-induced myocardial ischemia; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Before adjustment, the logistic regression analysis showed patients presenting a rest NT-proBNP ≥ 80.51 pg/mL had a nearly five-fold increase in the risk of developing MSIMI (OR: 4.84; 95% CI: 1.48–15.78; P = 0.009). After adjustment for sex, age, BMI, hypertension, diabetes, hyperlipidemia, smoking, drinking, depression and anxiety, the multiple logistic regression analysis showed that patients presenting a rest NT-proBNP ≥ 80.51 pg/mL had nearly eight-fold increase in the risk of developing MSIMI (OR: 7.85; 95% CI: 1.51–40.82; P = 0.014) [Table 4].
Post-stressor NT-proBNP
In assessing the utility of post-stressor NT-proBNP as a predictor of MSIMI, the ROC curve yielded a cut-off value of 98.80 pg/mL, with a sensitivity of 68.8% and a specificity of 77.8% (AUC: 0.68, 95% CI: 0.51–0.84; P = 0.032); a PPV of 44% and an NPV of 90.7%, a positive likelihood ratio of 3.10 and a negative likelihood ratio of 0.40. As a matter of fact, 68.8% of patients who developed MSIMI had post-stressor NT-proBNP ≥ 98.80 pg/mL [Figure 6].
Figure 6.
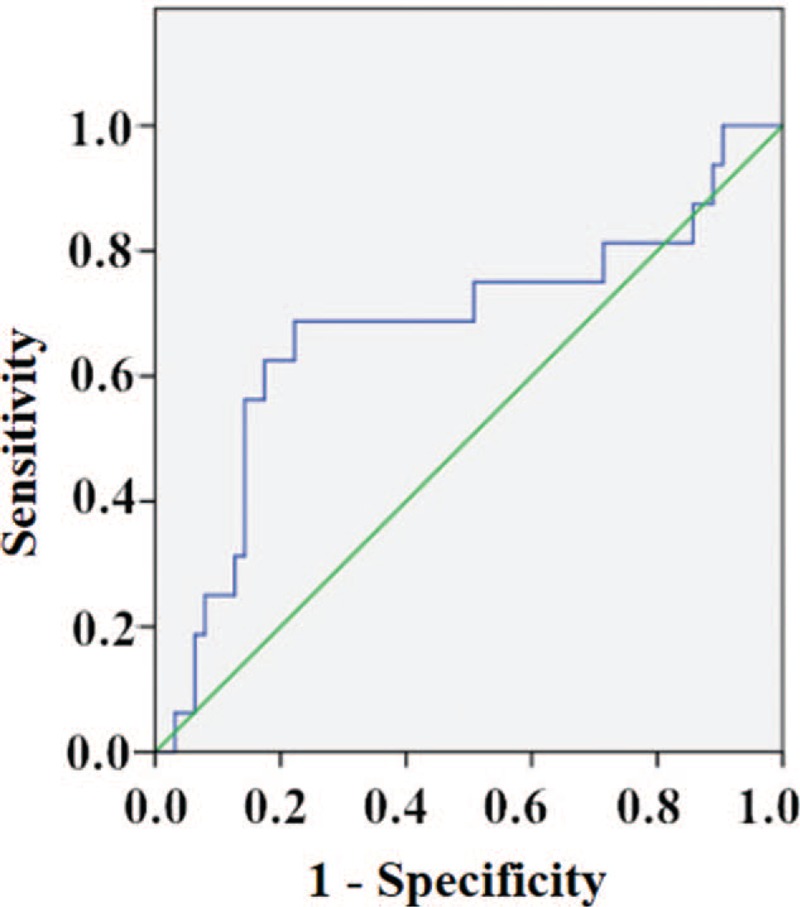
Receiver operating characteristic curve of post-stressor NT-proBNP and MSIMI. In assessing the utility of post-stressor NT-proBNP as a predictor of MSIMI, the ROC curve yielded a cut-off value of 98.80 pg/mL, with a sensitivity of 68.8% and a specificity of 77.8% (AUC: 0.68, 95% CI: 0.51–0.84; P = 0.032). AUC: Area under the curve; CI: Confidence interval; MSIMI: Mental stress-induced myocardial ischemia; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Before adjustment, the logistic regression analysis showed patients presenting a post-stressor NT-proBNP ≥ 98.80 pg/mL had a nearly eight-fold increase in the risk of developing MSIMI (OR: 7.70; 95% CI: 2.29–25.89; P = 0.001). After adjustment for sex, age, BMI, hypertension, diabetes, hyperlipidemia, smoking, drinking, depression, and anxiety, the multiple logistic regression analysis showed that patients presenting a post-stressor NT-proBNP ≥ 98.80 pg/mL had a 35-fold increase in the risk of developing MSIMI (OR: 34.96; 95% CI: 3.72–328.50; P = 0.002) [Table 4].
Blood pressure
comparisons between MSIMI positive group and MSIMI negative group
Compared to patients in MSIMI negative group, resting systolic blood pressure (SBP) value (145.56 ± 16.87 mmHg vs. 134.92 ± 18.16 mmHg, Z = −2.13, P = 0.04) and peri-stressor SBP value (160.81 ± 17.35 mmHg vs. 148.56 ± 19.49 mmHg, Z = −2.30, P = 0.02) were significantly higher in patients with MSIMI, but post-stressor SBP value was slightly higher without significance (142.63 ± 15.57 mmHg vs. 136.20 ± 18.16 mmHg, Z = −1.30, P = 0.20).
No significance was found in the comparison of resting DBP (83.88 ± 12.93 mmHg vs. 79.14 ± 9.83 mmHg, Z = −1.62, P = 0.11), peri-stressor DBP (96.88 ± 18.78 mmHg vs. 88.15 ± 10.35 mmHg, Z = −1.79, P = 0.09), post-stressor DBP (85.75 ± 14.10 mmHg vs. 79.06 ± 9.35 mmHg, Z = −1.80, P = 0.09), resting HR (64.88 ± 11.01 beats/min vs. 63.17 ± 9.30 beats/min, Z = −0.64, P = 0.53), peri-stressor HR (74.00 ± 14.48 vs. 71.41 ± 11.55 beats/min, Z = −0.77, P = 0.45), post-stressor HR (69.50 ± 12.51 beats/min vs. 65.55 ± 9.30 beats/min, Z = −1.42, P = 0.16) between MSIMI positive group and MSIMI negative group.
Rest SBP
The ROC curve, in assessing the utility of rest SBP as a predictor of MSIMI, yielded a cut-off value of 129.50 mmHg, with a sensitivity of 93.8% and a specificity of 40.9% (AUC: 0.66, 95% CI: 0.54–0.79; P = 0.045); a PPV of 27.8% and an NPV of 96.4%, a positive likelihood ratio of 1.59 and a negative likelihood ratio of 0.15. Interestingly, 93.8% of patients who developed MSIMI had rest SBP ≥ 129.50 mmHg [Figure 7].
Figure 7.
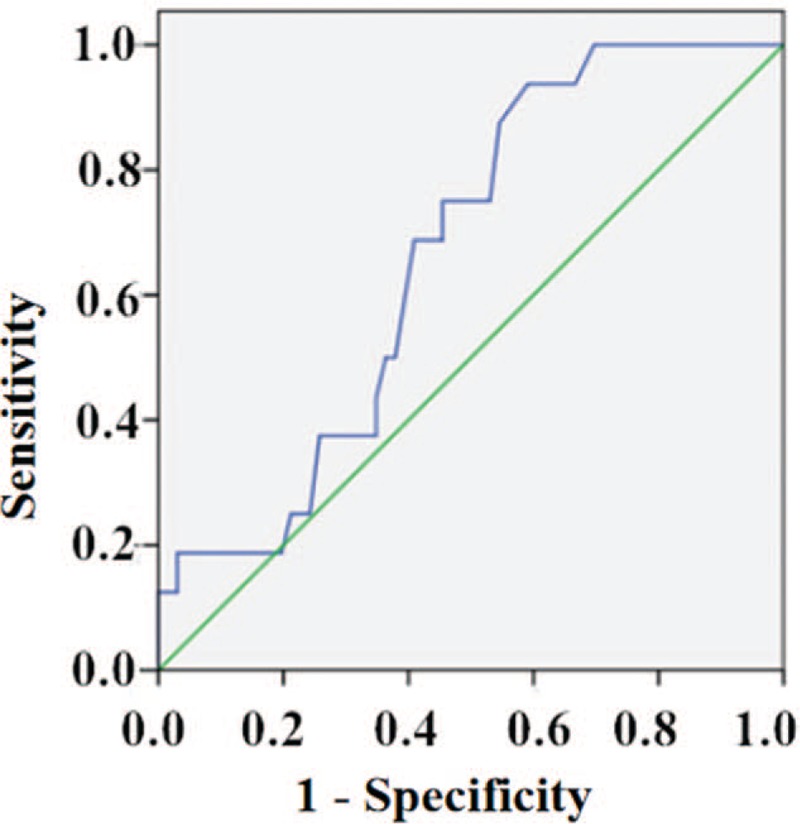
Receiver operating characteristic curve of rest SBP and MSIMI. In assessing the utility of rest SBP as a predictor of MSIMI, yielded a cut-off value of 129.50 mmHg, with a sensitivity of 93.8% and a specificity of 40.9% (AUC: 0.66, 95% CI: 0.54–0.79; P = 0.045). AUC: Area under the curve; CI: Confidence interval; MSIMI: Mental stress-induced myocardial ischemia; SBP: Systolic blood pressure.
Before adjustment, the logistic regression analysis showed patients presenting a rest SBP ≥ 129.50 mmHg had a nearly ten-fold increase in the risk of developing MSIMI (OR: 10.39; 95% CI: 1.29–83.36; P = 0.028). After adjustment for sex, age, BMI, hypertension, diabetes, hyperlipidemia, smoking, drinking, depression, and anxiety, the multiple logistic regression analysis showed that patients presenting a rest SBP ≥ 129.50 mmHg had an 11-fold increase in the risk of developing MSIMI (OR: 11.42; 95% CI: 1.21–108.17; P = 0.034) [Table 4].
Cardiographic indicators
No significance was found in the comparison of resting LVEF between MSIMI positive group and MSIMI negative group (66.06 ± 6.61% vs. 62.62 ± 8.04%, Z = −1.80, P = 0.07), while there was significance in the comparison of peri-stressor LVEF between the two groups (62.19 ± 5.98% vs. 64.26 ± 8.43%, Z = −2.15, P = 0.03).
No significance was found in the comparison of resting WMSI between MSIMI positive group and MSIMI negative group (1.00 [1.00, 1.00] vs. 1.00 [1.00, 1.00] score, Z = −0.42, P = 0.67); and there was no significance in the comparison of peri-stressor WMSI between the two groups (1.00 [1.00, 1.00] vs. 1.00 [1.00, 1.00] score, Z = −0.36, P = 0.72) [Table 5].
Table 5.
LVEF, WMSI comparisons between patients with and without MSIMI.
Discussions
The study results show that patients with CAD with MSIMI have slightly higher baseline resting hs-cTnI levels, significantly greater resting NT-proBNP levels, and significantly higher SBP when compared with those without MSIMI. After the 5-min mental stress task, those who develop MSIMI exhibit higher elevation of post-stressor hs-cTnI and NT-proBNP levels, and peri-stressor SBP compared with those without MSIMI. Using the ROC curves, the optimal cut-off points of post-stressor hs-cTnI, resting NT-proBNP, post-stressor NT-proBNP, and resting SBP were identified. After the adjustment for basic characteristics, the multiple logistic regression analysis showed that: (1) patients presenting a post-stressor hs-cTnI levels ≥0.015 ng/mL had a seven-fold increase in the risk of developing MSIMI; (2) a rest NT-proBNP ≥80.51 pg/mL had nearly eight-fold increase; (3) a post-stressor NT-proBNP ≥ 98.80 pg/mL had 35-fold increase; and (4) a rest SBP ≥129.50 mmHg had 11-fold increase.
According to previous literature, MSIMI is associated with a worse prognosis in subjects with stable CAD, as demonstrated in a two-fold increased risk of future cardiac events.[24] Nevertheless, a large body of research demonstrates that MSIMI is a silent phenomenon.[1] Therefore, it is difficult to screen patients susceptible to MSIMI. Williams[6] suggested that post-myocardial infarction patients with higher state and trait anger were more likely to develop myocardial ischemia during a stress challenge. Pimple[25] reported that the higher frequency of angina during the day was associated MSIMI. Technologic advances have introduced ECG, echocardiography, SPECT in detecting myocardial ischemia and standard criteria of MSIMI diagnosis have been used.[1] So far, we could not draw any significant relationship between coronary artery stenosis and MSIMI from our study. Furthermore, due to the important and evolving role of biomarkers, some researchers have focused on the association between biomarkers and MSIMI previously.
Cardiac troponins are currently the most sensitive and specific biochemical markers used for myocardial infarction. There are three types of troponins including, troponin-C, -I, and -T. The clinical measurement of serum hs-cTnI has become an important tool in the diagnosis of acute myocardial infarction. Hs-cTnI level was found to be higher in patients with exercise-induced myocardial ischemia (ESIMI) in Lee and his colleagues’ study.[26] Hammadah et al[27] investigated 587 patients with CAD who underwent both mental stress and conventional stress, assessing myocardial ischemia by SPECT. They concluded that higher resting levels of hs-cTnI were associated with MSIMI or ESIMI. From the results of our study, we happen to hold the same view that patients with CAD with MSIMI have slightly higher hs-cTnI level, and exhibit higher elevations of hs-cTnI after 5-min mental stress task, and at the optimal cut-off points of post-stressor hs-cTnI ≥ 0.015 ng/mL have seven-fold increase in the risk of developing MSIMI. Results observed above may be a reflection of the extensive functions of hs-cTnI in cardiac muscle fibers contraction.
NT-proBNP is produced predominately by the cardiac ventricular myocytes and is released in response to myocardial stress and filling pressures, and is involved in maintaining intravascular volume homeostasis; moreover, it has been proved to be a strong and independent predictor of obstructive CAD causing myocardial ischemia.[28] An elevation of NT-proBNP gene has demonstrated the close link between NT-proBNP and chronic stable angina, which is characterized by myocardial ischemia, while the concentration of NT-proBNP could decrease after percutaneous coronary revascularization.[29] NT-proBNP is now considered to be a useful marker and has a high degree of diagnostic accuracy in clinical practice and cardiovascular research as a diagnostic tool for the occurrence and severity of heart failure and MACEs.[30] In addition, we have found a close association between NT-proBNP and MSIMI in patients with CAD. We infer that NT-proBNP is involved in the pathophysiologic process of mental stress. For example, Feinkohl et al[31] had done a cross-sectional analysis in 1066 diabetes patients, and they suggested that raised plasma NT-proBNP was significantly associated with poorer cognitive function and depression.
Other studies have concluded that there is more obvious elevation of DBP during mental stress when compared to SBP.[32] Krantz's study[33] showed that after controlling for baseline diastolic blood pressure and group status, there was a significantly 2.4-fold higher relative risk of cardiac events for high versus low stress-induced peak diastolic blood pressure responses compared to low. On the contrary, we found that higher levels of SBP were associated with MSIMI and increased risk of MSIMI. We infer the possible reason may be related to the age and disease history and coronary artery calcium of patients recruited in this study. Further research is needed to clarify our point.
To our knowledge, this is a rare study focused on the association between hs-cTnI, NT-proBNP, and blood pressure, and MSIMI in Chinese patients with CAD. However, we have not found any significance of CK-MB, CRP, D-dimer, myoglobin, and MSIMI, which may be related to the small sample size and biomarkers detection time. And we need more researches to prove which of the three predictors is more powerful or whether the combination is better. Furthermore, we plan to conduct a larger multicenter study involving a larger number of patients from around China, selecting more related biomarkers, exploring potential interventions of pharmacologic and behavioral approaches, and we should have a 3- to 5-year follow-up to further study the association of MSIMI and MACEs.
In conclusion, the present study shows that patients with CAD have higher hs-cTnI levels, and/or greater NT-proBNP levels and/or SBP may be at higher risk of suffering from MSIMI when compared with those without MSIMI, indicating that hs-cTnI, NT-proBNP, SBP might be potential predictors of MSIMI. We will expand our study in the future involving patients and healthy volunteers from different study centers, and further develop suitable treatments for MSIMI.
Acknowledgements
Many thanks to the volunteer patients and the nurses who helped to withdraw the blood sample. The authors expressed deep thanks to Pro. Rong and her colleagues who contributed much to analyze the biomarkers.
Funding
The study was supported by grants from the Beijing Municipal Science & Technology Commission (No. Z151100003915085), and National Key Basic Research Program of China (973 program; No. 2015CB554402).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu MY, Yang Y, Zhang LJ, Pu LH, He DF, Liu JY, Hafeez A, Ding YC, Ma H, Geng QS. Potential predictors for mental stress-induced myocardial ischemia in patients with coronary artery disease. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000260
References
- 1.Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur Heart J 2003; 24:690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Samad Z, Boyle S, Becker RC, Williams R, Kuhn C, et al. Prevalence and clinical characteristics of mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Coll Cardiol 2013; 61:714–722. doi: 10.1016/j.jacc.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammadah M, Sullivan S, Pearce B, Al Mheid I, Wilmot K, Ramadan R, et al. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun 2018; 68:90–97. doi: 10.1016/j.bbi.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soufer R, Jain H, Yoon AJ. Heart-brain interactions in mental stress-induced myocardial ischemia. Curr Cardiol Rep 2009; 11:133–140. doi: 10.1007/s11886-009-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 6.Williams RB. Anger and mental stress-induced myocardial ischemia: mechanisms and clinical implications. Am Heart J 2015; 169:4–5. doi: 10.1016/j.ahj.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Jiang W, Babyak MA, Rozanski A, Sherwood A, O’Connor CM, Waugh RA, et al. Depression and increased myocardial ischemic activity in patients with ischemic heart disease. Am Heart J 2003; 146:55–61. doi: 10.1016/S0002-8703(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 8.Tolentino JC, Schmidt JJ, Schmidt GJ, Mesquita CT, Schmidt SL. Mental stress-induced myocardial ischemia related to generalized anxiety disorder in a patient with acute coronary syndrome and normal coronary arteries. Clin Nucl Med 2016; 41:e487–e490. doi: 10.1097/RLU.0000000000001348. [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, et al. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am J Cardiol 2014; 114:187–192. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun JL, Boyle SH, Samad Z, Babyak MA, Wilson JL, Kuhn C, et al. Mental stress-induced left ventricular dysfunction and adverse outcome in ischemic heart disease patients. Eur J Prev Cardiol 2017; 24:591–599. doi: 10.1177/2047487316686435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alenezi F, Brummett BH, Boyle SH, Samad Z, Babyak MA, Alzaeim N, et al. Usefulness of myocardial annular velocity change during mental stress to predict cardiovascular outcome in patients with coronary artery disease (from the responses of mental stress-induced myocardial ischemia to escitalopram treatment trial). Am J Cardiol 2017; 120:1495–1500. doi: 10.1016/j.amjcard.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W, Boyle SH, Ortel TL, Samad Z, Velazquez EJ, Harrison RW, et al. Platelet aggregation and mental stress induced myocardial ischemia: results from the REMIT study. Am Heart J 2015; 169:496–507.e1. doi: 10.1016/j.ahj.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wawrzyniak AJ, Dilsizian V, Krantz DS, Harris KM, Smith MF, Shankovich A, et al. High concordance between mental stress-induced and adenosine-induced myocardial ischemia assessed using SPECT in heart failure patients: hemodynamic and biomarker correlates. J Nucl Med 2015; 56:1527–1533. doi: 10.2967/jnumed.115.157990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan M, York KM, Li H, Li Q, Lucey DG, Fillingim RB, et al. Usefulness of peripheral arterial tonometry in the detection of mental stress-induced myocardial ischemia. Clin Cardiol 2009; 32:1–6. doi: 10.1002/clc.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He DF, Zhang LJ, Yang Y, Pu LH, Xu LY, Liu MY. Mental stress-induced myocardial ischemia in patients with stable coronary heart disease: a relevant clinic study [in Chinese]. Chin J Evid Based Cardiovasc Med 2018; 10:377–381. doi: 10.3969/j.issn.1674-4055.2017. [Google Scholar]
- 16.Jarolim P, Morrow DA. Use of high sensitivity cardiac troponin assays as an adjunct to cardiac stress testing. Clin Biochem 2016; 49:419–420. doi: 10.1016/j.clinbiochem.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Shah R, Burg MM, Vashist A, Collins D, Liu J, Jadbabaie F, et al. C-reactive protein and vulnerability to mental stress-induced myocardial ischemia. Mol Med 2006; 12:269–274. doi: 10.2119/2006-00077.Shah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews KA, Zhu S, Tucker DC, Whooley MA. Blood pressure reactivity to psychological stress and coronary calcification in the Coronary Artery Risk Development in Young Adults Study. Hypertension 2006; 47:391–395. doi: 10.1161/01.HYP.0000200713.44895.38. [DOI] [PubMed] [Google Scholar]
- 19.Obuchowski NA. Computing sample size for receiver operating characteristic studies. Invest Radiol 1994; 29:238–243. doi: 10.1097/00004424-199402000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Obuchowski NA, Mcclish DK. Sample size determination for diagnostic accuracy studies involving binormal ROC curve indices. Stat Med 1997; 16:1529–1542. doi: 10.1002/(sici)1097-0258(19970715)16:13<1529::aid-sim565>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Kahraman S, Dogan A, Ziyrek M, Usta E, Demiroz O, Ciftci C. The association between aspirin resistance and extent and severity of coronary atherosclerosis. North Clin Istanb 2018; 5:323–328. doi: 10.14744/nci.2017.26779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle SH, Matson WR, Velazquez EJ, Samad Z, Williams RB, Jr, Sharma S, et al. Metabolomics analysis reveals insights into biochemical mechanisms of mental stress-induced left ventricular dysfunction. Metabolomics 2015; 11:571–582. doi: 10.1007/s11306-014-0718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang RM, Birrig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for Chamber Quantification: a report from the American Society of Echocardiography's guidelines and Standards Committee and the Chamber Quantification Writing Group, Develop in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation 2002; 105:1780–1784. doi: 10.1161/01.cir.0000014491.90666.06. [DOI] [PubMed] [Google Scholar]
- 25.Pimple P, Shah AJ, Rooks C, Douglas Bremner J, Nye J, Ibeanu I, et al. Angina and mental stress-induced myocardial ischemia. J Psychosom Res 2015; 78:433–437. doi: 10.1016/j.jpsychores.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G, Twerenbold R, Tanglay Y, Reichlin T, Honegger U, Wagener M, et al. Clinical benefit of high-sensitivity cardiac troponin I in the detection of exercise-induced myocardial ischemia. Am Heart J 2016; 173:8–17. doi: 10.1016/j.ahj.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Hammadah M, Al MI, Wilmot K, Ramadan R, Alkhoder A, Obideen M, et al. Association between high-sensitivity cardiac troponin levels and myocardial ischemia during mental stress and conventional stress. JACC Cardiovasc Imaging 2018; 11:603–611. doi: 10.1016/j.jcmg.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caselli C, Prontera C, Liga R, De Graaf MA, Gaemperli O, Lorenzoni V, et al. Effect of coronary atherosclerosis and myocardial ischemia on plasma levels of high-sensitivity troponin T and NT-proBNP in patients with stable angina. Arterioscler Thromb Vasc Biol 2016; 36:757–764. doi: 10.1161/ATVBAHA.115.306818. [DOI] [PubMed] [Google Scholar]
- 29.Gupta A, Beig JR, Tramboo NA, Afroze D, Hafeez I, Rather HA. The effect of percutaneous coronary revascularization on plasma N-terminal pro-B-type natriuretic peptide levels in stable coronary artery disease. Indian Heart J 2018; 70:282–288. doi: 10.1016/j.ihj.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang LJ, Li N, Li Y, Zeng XT, Liu MY. Cardiac biomarkers predicting MACE in patients undergoing noncardiac surgery: a meta-analysis. Front Physiol 2019; 9:1923.doi: 10.3389/fphys.2018.01923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinkohl I, Sattar N, Welsh P, Reynolds RM, Deary IJ, Strachan MW, et al. Association of N-terminal pro-brain natriuretic peptide with cognitive function and depression in elderly people with type 2 diabetes. PLoS One 2012; 7:e44569–e144569. doi: 10.1371/journal.pone.0044569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Psycho-cardiology Group of Chinese General Practitioner Society of Chinese Medical Doctor Association. Chinese expert consensus on the diagnosis and treatment of mental stress-induced myocardial ischemia in patients with stable coronary artery disease [in Chinese]. Chin J Cardiol 2016; 44:12–18. doi: 10.3760/cma.j.issn.0253-3758.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol 1999; 84:1292–1297. doi: 10.1016/s0002-9149(99)00560-3. [DOI] [PubMed] [Google Scholar]


