Supplemental Digital Content is available in the text
Keywords: Receptor interacting protein kinase-3, Coronary artery disease, Necroptosis, Atherosclerosis, Acute coronary syndrome, Biomarker
Abstract
Background:
Necroptosis plays an important role in human atherosclerosis and atheroma development. Since receptor interacting protein kinase-3 (RIP3) acts as a key mediator of necroptosis, this study aimed to explore its relationship between plasma RIP3 levels and coronary artery disease (CAD) and discover a potential new biomarker for screening CAD subtypes and severity.
Methods:
A total of 318 patients with CAD who had coronary angiography and 166 controls in Peking Union Medical College Hospital from September 2017 to January 2018 were enrolled in this study. Patients with CAD were divided into three subgroups: patients with stable coronary artery disease (SCAD), patients with unstable angina (UA), and patients with myocardial infarction (MI). The severity of atherosclerosis was determined by Gensini score (GSS). Logistic regression was used to determine the relationship between plasma RIP3 levels and CAD. The correlation between plasma RIP3 and GSS was calculated using multiple linear regression models.
Results:
Overall, plasma RIP3 levels were significantly higher than serum RIP3 levels. Plasma RIP3 levels in patients with CAD were significantly higher than those in controls. Plasma RIP3 levels were strongly associated with CAD (odds ratio: 6.00, 95% confidence interval 3.04–11.81; P < 0.001). Plasma RIP3 levels increased linearly from controls to patients with SCAD, then patients with UA, and finally to patients with MI. We found a significantly positive correlation between proportion of cases of acute coronary syndrome in subjects and their plasma RIP3 level quartile. Plasma RIP3 levels were also associated with GSS (B 0.027; standard error 0.012; P < 0.05).
Conclusions:
Plasma RIP3 levels were independently associated with CAD. Plasma RIP3 levels could potentially supplement clinical assessment to screen CAD and determine CAD severity.
Introduction
Coronary artery disease (CAD) is common in developed countries and remains the leading cause of death.[1,2] A study has focused on the pathogenesis of atherosclerosis and the development of CAD.[3] However, several questions remain unanswered. First, it would be better if new biomarkers could be found to assist in screening CAD subtypes based on current definitions. Second, we need more convenient and non-invasive methods to evaluate the coronary artery atheromatous severity instead of coronary computed tomography angiography and coronary arteriography. Moreover, it is hard but important to select patients who might be suffering with acute coronary syndrome (ACS) from others so that appropriate and timely prevention and treatment can be given.
Emerging pieces of evidence have suggested that necroptosis might play an important role in several inflammatory conditions and diseases, including human atherosclerosis and atheroma development.[4] Receptor interacting protein kinase-3 (RIP3) is currently regarded as a critical regulator of programmed necrosis/necroptosis.[5] As far as we know, circulating RIP3 levels have been considered important markers in diagnosing and predicting some diseases, including sepsis,[6] acute kidney injury,[7] and ST-segment elevation myocardial infarction (MI).[8] It was reported that the levels of serum RIP3 were not altered significantly in patients with ST-segment elevation MI compared to controls.[8] However, the association between RIP3 levels and CAD required further confirmation in a large-scale study. And the differences between plasma RIP3 levels and serum RIP3 levels have not been discussed.
The aim of this study was to confirm the relationship between plasma RIP3 levels and CAD. Furthermore, we tried to explore the relationship between plasma RIP3 levels and various CAD subtypes and severity.
Methods
Ethical approval
This study was approved by the Ethics Committee of Peking Union Medical College Hospital and conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Study population
From September 2017 to January 2018, we consecutively recruited Chinese patients with CAD aged between 30 and 100 years who were hospitalized for coronary angiography in the Peking Union Medical College Hospital. Patients with the presence of ≥50% stenosis in ≥1 main coronary artery were diagnosed as having CAD. Patients with CAD were further divided into three subgroups as follows: (1) patients with stable coronary artery disease (SCAD); (2) patients with unstable angina (UA); and (3) patients with MI. They were also categorized according to the number of pathological branches involved. Chinese volunteers free from a known history of CAD (as controls) were recruited from the Peking Union Medical College Hospital at the same time. Subjects with acute renal injury, sepsis, or shock were excluded from the study.
UA was defined as a normal measurement of cardiac troponin and with at least one of the following criteria: prolonged (>20 min) angina pain at rest, new onset angina (Class II or III according to the Classification of the Canadian Cardiovascular Society), recent destabilization of previously stable angina with at least Canadian Cardiovascular Society Class III angina characteristics (crescendo angina), or post-MI angina.[9] SCAD was defined as angina that did not fulfill the above UA criteria.[10] MI was defined as a rise and/or fall of cardiac troponin with at least one value above the 99th percentile upper reference limit and with at least one of the following: (1) ischemia symptoms; (2) new or presumed new significant ST-segment-T wave changes or new left bundle branch block; (3) development of pathological Q waves in the electrocardiogram; (4) imaging evidence of new viable myocardium loss or new regional wall motion abnormality; and (5) identification of an intra-coronary thrombus by angiography or autopsy.[11]
Coronary angiography and image interpretation
Coronary angiography was performed with a conventional angiography unit (Integris H; Philips Medical Systems, Amsterdam, the Netherlands). Coronary artery stenosis was imaged in the center of the field from multiple projections. Using the quantitative coronary analysis system, the coronary atherosclerotic lesion severity was evaluated from at least two projections by two interventional cardiologists with at least 5 years of experience who were blinded to the patients’ clinical information. Coronary angiographies were performed to determine the severity of atherosclerosis by the Gensini score (GSS).[12]
Plasma/serum collection and plasma/serum RIP3 level quantification
For the patients with MI, peripheral blood was whether drawn from the radial or femoral artery before coronary angiography or from the vein in the morning on the day after admission according to their disease subtypes and treatment guidelines. For the patients with SCAD and UA, peripheral fasting blood was drawn from the vein in the morning the day after admission. For the controls, peripheral fasting blood was drawn from the vein in the morning. Plasma blood was centrifuged at 1500 r/min for a period of 10 min at 20°C, and the supernatant was collected. Serum blood was centrifuged at 1000 r/min for a period of 10 min at 20°C, and the supernatant was collected. They were collected into sodium heparin tubes (BD) and stored at −80°C within 4 h until RIP3 levels were tested. Plasma/serum RIP3 levels were detected with ELISA kits (Cusabio, Wuhan, China) according to the manufacturer's instructions.
Other parameters
Subjects’ body mass index was calculated as weight (kg) divided by height squared (m2). Blood pressure was measured with a standard mercury sphygmomanometer by specially trained nurses with the patient in a seated position after ≥5 min of rest. Hypertension and diabetes were diagnosed using the current guidelines.[13,14] Peripheral venous blood was taken from the antecubital vein early in the morning while the patient was in a fasting state and before any medications were administered. The levels of each lipid profile component (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides), creatinine, and high-sensitivity C reactive protein were measured.
Statistical analysis
The Shapiro-Wilk test was used to determine normality of the data distribution for sample n < 50. The Kolmogorov-Smirnov test was used to determine normality of the data distribution for sample n ≥ 50. Normally distributed data were expressed as the mean ± standard deviation, and data with non-normal distributions were expressed as the median (Q1, Q3). Continuous, normally distributed variables between two groups were analyzed by Student t-test. The Mann-Whitney U test was applied for data of this type that were not normally distributed. Continuous, normally distributed variables among four groups were analyzed by a one-way analysis of variance. The Kruskal-Wallis H test was applied for data of this type that were not normally distributed. Categorical variables were summarized as count and percentage and were compared by the Chi-square test. Logistic regression was used to determine the relationship between plasma RIP3 levels and CAD after adjusting for confounding factors. The Youden's index in the receiver operating characteristic (ROC) curves was used to determine the optimal cut-off of plasma RIP3 levels for detecting CAD. Plasma RIP3 levels were compared among controls and patients in different subtypes of CAD using the analysis of variance type of trend analysis. To explore the relationship between plasma RIP3 levels and proportion of cases of ACS in subjects, all subjects were divided into four categories according to the quartiles of plasma RIP3 levels. The correlation between plasma RIP3 and GSS was calculated using multiple linear regression models and adjusted for confounding factors. A P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS Statistics software, version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Participant characteristics
A total of 484 subjects were enrolled in this study, including 166 controls and 318 patients with CAD (patients with SCAD, n = 93; patients with UA, n = 153; and patients with MI, n = 72). The mean ages were 60.1 ± 9.4 years for controls and 64.4 ± 10.8 years for patients with CAD. Traditional cardiovascular risk factors and New York Heart Association classes in the study population are summarized in Table 1. Most patients with CAD were male and more likely to have a history of hypertension and diabetes. CAD groups exhibited relatively higher high-sensitivity C-reactive protein levels, compared with the controls.
Table 1.
Baseline characteristics of study participants.
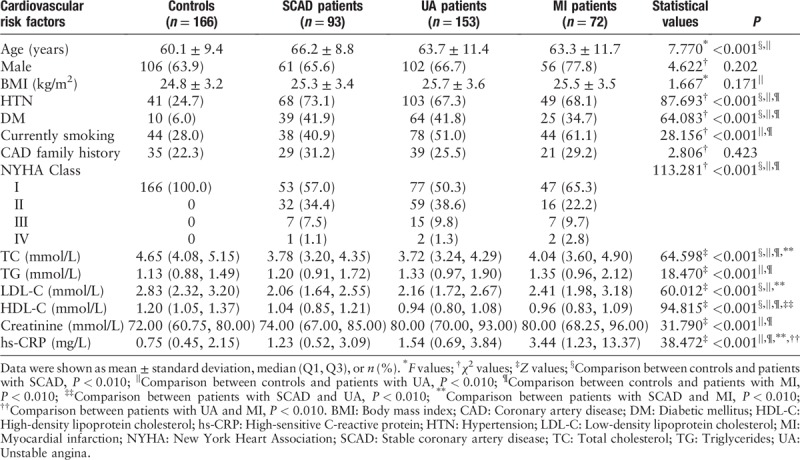
Differences between plasma and serum RIP3 levels
Both plasma and serum levels of RIP3 were tested in 23 controls, 20 patients with SCAD, 20 patients with UA, and 20 patients with MI. The median levels of plasma RIP3 were significantly higher than corresponding serum levels in total (433.75 [296.28, 516.11] pg/mL vs. 39.20 [20.43, 65.33] pg/mL, Z = 257.000, P < 0.001) and in each group (controls: 244.28 [171.34, 287.03] pg/mL vs. 24.37 [16.26, 39.44] pg/mL, Z = 0.000, P < 0.001; patients with SCAD: 426.98 [395.70, 493.35] pg/mL vs. 34.67 [22.00, 51.31] pg/mL, Z = 0.000, P < 0.001; patients with UA: 464.98 [455.20, 512.51] pg/mL vs. 30.21 [16.08, 61.10] pg/mL, Z = 0.000, P < 0.001; patients with MI: 551.34 [473.64, 656.40] pg/mL vs. 105.23 [59.68, 178.03] pg/mL, Z = 0.000, P < 0.001) [Figure 1].
Figure 1.
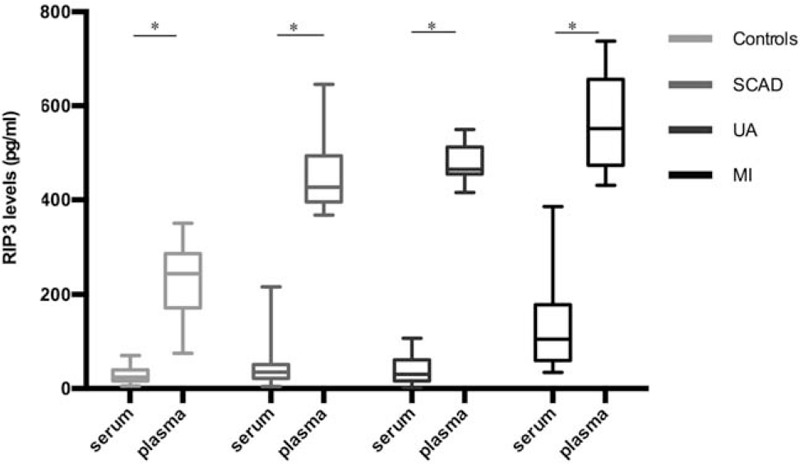
Plasma and serum levels of RIP3 in controls, patients with SCAD, patients with UA, and patients with MI. Short horizontal lines denote minimum value and maximum value; long horizontal lines denote 25th, 50th, and 75th percentiles. ∗P < 0.001, plasma levels vs. serum levels (Mann-Whitney U test was used). MI: Myocardial infarction; RIP3: Receptor interacting protein kinase-3; SCAD: Stable coronary artery disease; UA: Unstable angina.
Biomarker for CAD and plasma RIP3 cut-off level
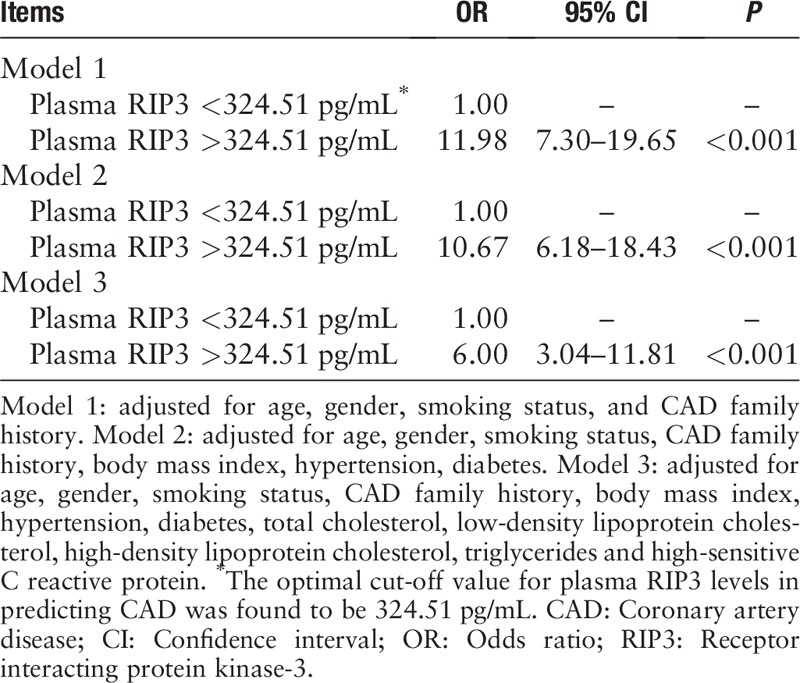
We tried to determine if plasma levels of RIP3 could potentially serve as a marker for CAD. We expanded the sample size of RIP3 plasma level testing to include all 318 patients with CAD and 166 controls. The median levels of plasma RIP3 in patients with CAD were significantly higher than that of controls (406.87 [311.51, 516.59] pg/mL vs. 241.61 [175.83, 318.13] pg/mL, Z = 9565.000, P < 0.001). To diminish the confounding effects of traditional CAD risk factors, we conducted a logistic regression analysis. The Youden's index in the ROC curve was used to determine the optimal cut-off of plasma RIP3 levels for detecting CAD. According to the ROC curve analysis, the optimal cut-off value for plasma RIP3 levels in predicting CAD was found to be 324.51 pg/mL, with a specificity of 80.0% and sensitivity of 73.0% (area under curve = 0.819, 95% confidence interval = 0.779–0.859) [Figure 2]. After adjusting for confounding factors, plasma RIP3 levels still remained strongly associated with CAD (P < 0.001) [Table 2].
Figure 2.
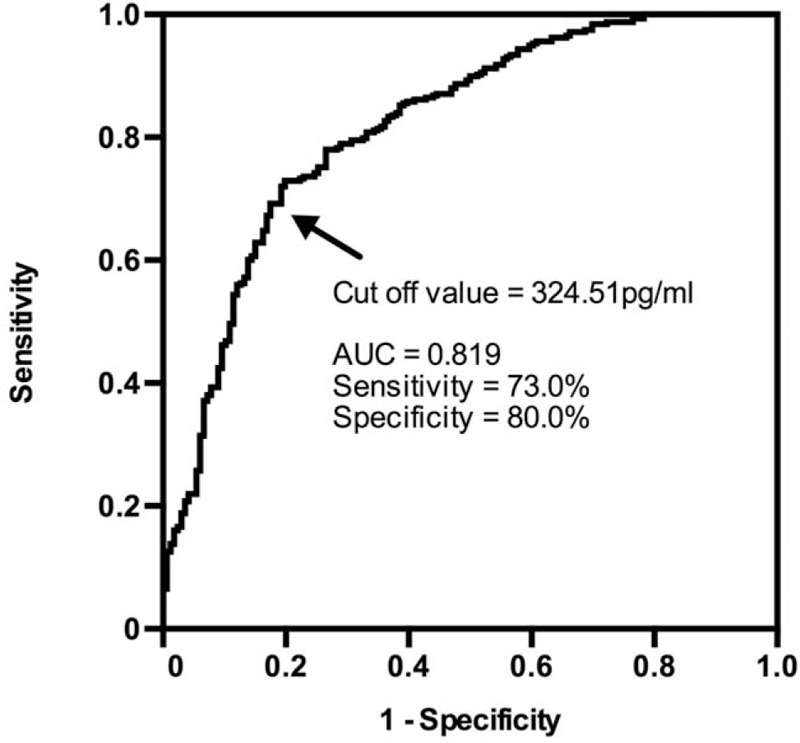
Receiver operating characteristic curve to detect CAD. The optimal cut-off value for plasma RIP3 levels in predicting CAD was found to be 324.51 pg/mL. CAD: Coronary artery disease; RIP3: Receptor interacting protein kinase-3.
Table 2.
Logistic regression analyses of plasma RIP3 levels in diagnosing the occurrence of CAD.
Association between plasma RIP3 levels and CAD severity
Plasma RIP3 levels increased linearly from controls (241.61 [175.83, 318.13] pg/mL), to patients with SCAD (388.39 [328.8, 501.66] pg/mL), then patients with UA (386.91 [303.14, 478.67] pg/mL), and finally to patients with MI (455.04 [343.36, 607.44] pg/mL; P for linear trend < 0.001) [Figure 3]. We did not find a similar linear relationship in plasma RIP3 levels when comparing patients with normal coronary arteries (245.24 [177.47, 319.58] pg/mL), single vessel disease (403.53 [311.99, 505.46] pg/mL), double vessel disease (416.56 [297.74, 500.04] pg/mL), and triple vessel disease (401.26 [311.09, 521.91] pg/mL) [Supplemental Figure 1]. However, there were significant differences in plasma RIP3 levels between the subjects with normal coronary arteries and patients with different pathological branches involved.
Figure 3.
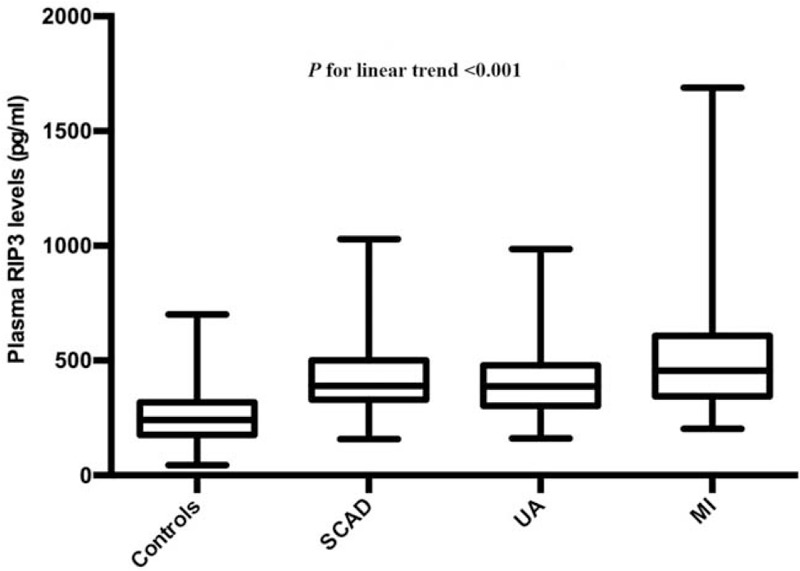
Plasma RIP3 levels in controls and different subtypes of CAD. Short horizontal lines denote minimum value and maximum value; long horizontal lines denote 25th, 50th, and 75th percentiles. The analysis of variance type of trend analysis was used. CAD: Coronary artery disease; MI: Myocardial infarction; RIP3: Receptor interacting protein kinase-3; SCAD: Stable coronary artery disease; UA: Unstable angina.
To explore the relationship between plasma RIP3 levels and proportion of cases of ACS in subjects, all subjects were divided into four categories according to which quartile their plasma RIP3 level fell into. Clinical characteristics are listed in Supplementary Table 1. Subjects with higher plasma RIP3 levels were older and were more likely to suffer from comorbidities. Moreover, they had higher levels of high-sensitivity C-reactive protein. Notably, we found a significant positive correlation between proportion of cases of ACS in subjects and the plasma RIP3 level quartile [Supplementary Figure 2].
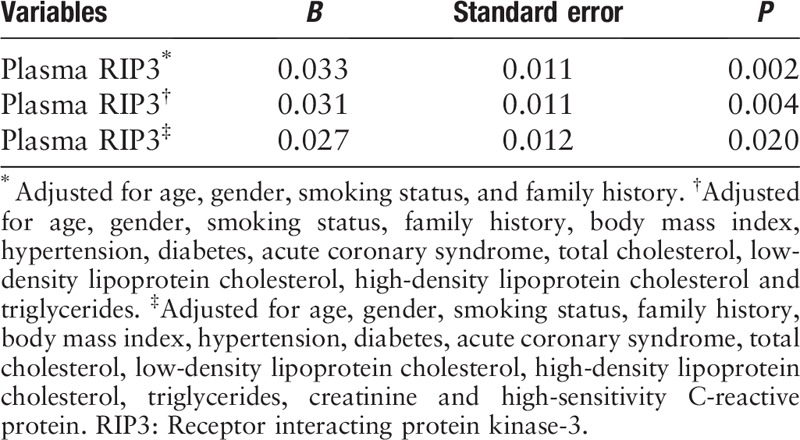
We adopted multiple linear regression models to determine the correlation between plasma RIP3 levels and GSS. Plasma RIP3 levels were associated with GSS after adjustments for traditional cardiovascular risk factors (P < 0.05) [Table 3].
Table 3.
Regression analysis of factors associated with Gensini score.
Discussion
This study confirmed that in patients with CAD plasma RIP3 levels were significantly higher than controls and they were strongly associated with CAD, even after adjusting for confounding factors. We believed that plasma RIP3 is an independent CAD risk factor and might be regarded as a surrogate marker for CAD screening. Plasma RIP3 levels were also found to be positively correlated with coronary arterial atherosclerosis severity and its clinical manifestations. This indicated that plasma RIP3 could be used to predict disease severity.
The human RIP3 gene is located on chromosome 14,[15] and its messenger RNA encodes a polypeptide of 518 amino acids. Several studies have pointed out that RIP3 is essential for the execution of tumor necrosis factor-induced necroptosis downstream of RIP1.[16–18] In addition, through the analysis of RIP3 deficient mice, researchers have implicated the role of necroptosis in atherosclerosis,[19] alcoholic liver disease,[20] and retinal degeneration.[21,22] The critical pro-necroptotic factor RIP3 is found to be present and activated in human atherosclerotic plaque.[23] RIP3 deficient, atherosclerosis-prone low-density lipoprotein receptor-deficient, or apolipoprotein E-deficient mice had a significant reduction in advanced atherosclerotic lesions. In addition, in vitro cellular studies showed that RIP3 deletion prevented primary necrosis of macrophages.[19] These findings indicated a driver function of RIP3 in atherosclerotic necrosis. In the present study, plasma RIP3 levels were associated with GSS. However, the extent to which necroptosis and its mediator RIP3 play a role in atherosclerosis is unknown.
It is notable that we demonstrated a significant association between plasma RIP3 level quartiles and prevalence of ACS cases, which might shed light in predicting adverse events in patients with CAD. In 1989, Muller et al[24] proposed the concept of “vulnerable plaque.” Predominantly derived from pathological observations at autopsies, the “plaque-rupture” hypothesis and new views of vulnerable atherosclerotic coronary plaque features were established.[25] Acute coronary thrombosis and subsequent acute MI are now regarded as the results of sudden plaque rupture or erosion.[26,27] And efforts have been made to identify high-risk patients using advanced imaging methods to find “vulnerable” coronary sites. However, these methods have many disadvantages, such as price, inconvenience, and poor efficacy.[28] Karunakaran et al[23] found that necroptotic cell death in humans was activated in advanced atherosclerotic plaques and expression of RIP3 and mixed lineage kinase domain-like protein was increased with unstable carotid atherosclerosis. This indicated that RIP3 might correlate with lesion vulnerability. The elevated plasma RIP3 levels we found in patients with MI might be due to the rupture of atherosclerotic plaques with their subsequent release into circulating plasma. This finding might also be related to thrombus formations in the coronary artery. Zhang et al[29] demonstrated a role for RIP3 in promoting in vivo thrombosis and hemostasis. RIP3 inhibitors dose-dependently inhibited platelet aggregation in vitro and prevented arterial thrombus formation in vivo.[29]
In this study, we found that plasma levels of RIP3 were significantly higher than serum levels of RIP3 in human patients. Circulating RIP3 levels have been considered important markers in diagnosing and predicting diseases. Kashlov et al[8] figured out that levels of serum RIP3 were not altered significantly in patients with ST-segment elevation MI, compared to controls. However, in our study, we pointed out that plasma levels of RIP3 were significantly higher than the corresponding serum levels. Zhang et al[29] confirmed that RIP3 was expressed in platelets, and the differences between serum and plasma values might be influenced by the platelets at the time of blood coagulation.
Some limitations of our study should also be considered. This study was a cross-sectional study and it was impossible to assess variables’ contributions over time. Therefore, further studies, utilizing a prospective cohort, are needed to confirm the association between plasma RIP3 levels and CAD. The underlying mechanism behind our findings remains uncertain, since the source of RIP3 detected in plasma is still unclear. Additionally, participants in our study came from China; thus, generalizability to other ethnic or racial populations may not be valid. Similar research is necessary in other regions to assess the general application of our findings.
In conclusion, the results of this study confirmed that compared to serum, plasma RIP3 levels were detected in significantly higher concentrations. Plasma RIP3 could be used as a surrogate marker for CAD screening or a predictor for the severity of CAD progression. Furthermore, plasma RIP3 levels were positively associated with CAD subtypes and proportion of cases of ACS in subjects. Accordingly, elevated plasma RIP3 levels may indicate the need for a careful evaluation for the presence and progression of CAD.
Funding
This research was supported by grants from the National Natural Science Foundation of China (No. 81670329 and No. 81601431) and Chinese Academy of Medical Sciences Initiative for Innovative Medicine (No. 2017-I2M-2-002 and No. 2016-I2M-3-011).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Hu XM, Chen X, Pang HY, Liu HH, Chen PP, Shi JL, Tang S, Wu ZH, Zhang SY. Plasma levels of receptor interacting protein kinase-3 correlated with coronary artery disease. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000225
Xiao-Min Hu and Xi Chen contributed equally to this study.
References
- 1.Keys A. Coronary heart disease – the global picture. Atherosclerosis 1975; 22:149–192. doi: 10.1016/0021-9150(75)90001-5. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Roger VL, Dunlay SM, Go AS, Rosamond WD. Challenges of ascertaining national trends in the incidence of coronary heart disease in the United States. J Am Heart Assoc 2014; 3:e001097.doi: 10.1161/jaha.114.001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ndrepepa G, Tada T, Fusaro M, Cassese S, King L, Hadamitzky M, et al. Association of coronary atherosclerotic burden with clinical presentation and prognosis in patients with stable and unstable coronary artery disease. Clin Res Cardiol 2012; 101:1003–1011. doi: 10.1007/s00392-012-0490-9. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med 1999; 340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature 2015; 517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Li J, Gao HM, Xing YH, Lin Z, Li HJ, et al. Necroptosis regulated proteins expression is an early prognostic biomarker in patient with sepsis: a prospective observational study. Oncotarget 2017; 8:84066–84073. doi: 10.18632/oncotarget.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shashaty MG, Reilly JP, Sims CA, Holena DN, Qing D, Forker CM, et al. Plasma levels of receptor interacting protein kinase-3 (RIP3), an essential mediator of necroptosis, are associated with acute kidney injury in critically Ill trauma patients. Shock 2016; 46:139–143. doi: 10.1097/SHK.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashlov JK, Donev IS, Doneva JG, Valkov VD, Kirkorova AD, Ghenev PI, et al. Serum levels of RIPK3 and troponin I as potential biomarkers for predicting impaired left ventricular function in patients with myocardial infarction with ST segment elevation and normal troponin I levels prior percutaneous coronary intervention. Biosci Trends 2016; 10:294–299. doi: 10.5582/bst.2016.01077. [DOI] [PubMed] [Google Scholar]
- 9.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 10.Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013; 34:2949–3003. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Glob Heart 2012; 7:275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983; 51:606.doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018; 41:S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2018; 71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappa B nuclear translocation and localizes to mitochondria. FEBS Lett 2000; 473:285–291. doi: 10.1016/s0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- 16.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009; 137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He S, Wang L, Miao L, Wang T, Du F, Zhao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009; 137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009; 325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 19.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 2013; 3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 2013; 57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami Y, Matsumoto H, Roh M, Giani A, Kataoka K, Morizane Y, et al. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ 2014; 21:270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A 2010; 107:21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karunakaran D, Geoffrion M, Wei L, Gan W, Richards L, Shangari P, et al. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv 2016; 2:e1600224.doi: 10.1126/sciadv.1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 1989; 79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 25.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014; 114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 26.Wang TS, Li XM, Zhuang SW, Tian B, Jin C, Wang S, et al. Comparison of coronary plaque composition between patients with acute coronary syndrome and stable coronary artery disease (in Chinese). Chin J Cardiol 2008; 36:994–998. doi: 10.3321/j.issn:0253-3758.2008.11.009. [PubMed] [Google Scholar]
- 27.Libby P. Mechanisms of acute coronary syndromes. N Engl J Med 2013; 369:883–884. doi: 10.1056/NEJMc1307806. [DOI] [PubMed] [Google Scholar]
- 28.Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, et al. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging 2012; 5:941–955. doi: 10.1016/j.jcmg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhang J, Yan R, Tian J, Zhang Y, Zhang J, et al. Receptor-interacting protein kinase 3 promotes platelet activation and thrombosis. Proc Natl Acad Sci U S A 2017; 114:2964–2969. doi: 10.1073/pnas.1610963114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


