Abstract
Objective
The 3-N-butylphthalide (NBP) comprises one of the chemical constituents of celery oil. It has a series of pharmacologic mechanisms including reconstructing microcirculation, protecting mitochondrial function, inhibiting oxidative stress, inhibiting neuronal apoptosis, etc. Based on the complex multi-targets of pharmacologic mechanisms of NBP, the clinical application of NBP is increasing and more clinical researches and animal experiments are also focused on NBP. The aim of this review was to comprehensively and systematically summarize the application of NBP on neurologic diseases and briefly summarize its application to non-neurologic diseases. Moreover, recent progress in experimental models of NBP on animals was summarized.
Data sources
Literature was collected from PubMed and Wangfang database until November 2018, using the search terms including “3-N-butylphthalide,” “microcirculation,” “mitochondria,” “ischemic stroke,” “Alzheimer disease,” “vascular dementia,” “Parkinson disease,” “brain edema,” “CO poisoning,” “traumatic central nervous system injury,” “autoimmune disease,” “amyotrophic lateral sclerosis,” “seizures,” “diabetes,” “diabetic cataract,” and “atherosclerosis.”
Study selection
Literature was mainly derived from English articles or articles that could be obtained with English abstracts and partly derived from Chinese articles. Article type was not limited. References were also identified from the bibliographies of identified articles and the authors’ files.
Results
NBP has become an important adjunct for ischemic stroke. In vascular dementia, the clinical application of NBP to treat severe cognitive dysfunction syndrome caused by the hypoperfusion of brain tissue during cerebrovascular disease is also increasing. Evidence also suggests that NBP has a therapeutic effect for neurodegenerative diseases. Many animal experiments have found that it can also improve symptoms in other neurologic diseases such as epilepsy, cerebral edema, and decreased cognitive function caused by severe acute carbon monoxide poisoning. Moreover, NBP has therapeutic effects for diabetes, diabetes-induced cataracts, and non-neurologic diseases such as atherosclerosis. Mechanistically, NBP mainly improves microcirculation and protects mitochondria. Its broad pharmacologic effects also include inhibiting oxidative stress, nerve cell apoptosis, inflammatory responses, and anti-platelet and anti-thrombotic effects.
Conclusions
The varied pharmacologic mechanisms of NBP involve many complex molecular mechanisms; however, there many unknown pharmacologic effects await further study.
Keywords: 3-N-butylphthalide, Pharmacological mechanisms, Microcirculation, Mitochondria, Ischemic stroke
Introduction
3-N-butylphthalide (NBP) comprises a family of optical isomers that includes l-3-N-butylphthalide (l-NBP), d-3-N-butylphthalide (d-NBP), and dl-3-N-butylphthalide (dl-NBP).[1] L-NBP is one of the chemical constituents in celery oil, whereas dl-NBP is synthetic and an important neuroprotective drug for the treatment of neurologic diseases. Its chemical formula is C12H14O2 and molar mass is 190.24 g/mol. Dl-NBP is a fat-soluble substance that can freely pass across the blood-brain barrier. Further, it is absorbed rapidly, with a peak blood concentration seen at 1.25 h. Moreover, it has a long-lasting pharmacologic effect, as the elimination half-life of dl-NBP is 11.84 h. Its main target organs are brain and adipose tissue, and dl-NBP undergoes extensive metabolism in the human body; the major metabolites of this compound in human plasma were found to be 3-OH-NBP, 10-OH-NBP, 11-COOH-NBP, and 10-CO-NBP.
The main pharmacologic effects of NBP include reconstructing microcirculation, protecting mitochondrial function, inhibiting oxidative stress, inhibiting inflammatory responses, and inhibiting neuronal apoptosis. It has also been found to have effects including anti-platelet aggregation, anti-thrombosis, and anti-atherosclerosis. The role of NBP in reconstructing microcirculation and protecting mitochondrial function can improve the pathologic process of ischemic stroke with multiple targets. NBP is now more and more clinically used in the treatment of ischemic stroke. Secondly, the study found that NBP has a corresponding effect on the treatment of some neurodegenerative diseases such as Alzheimer disease (AD), dementia, amyotrophic lateral sclerosis, and dyskinesia diseases such as Parkinson disease, as well as other neurologic diseases such as carbon monoxide (CO) poisoning, traumatic central nervous system injury, autoimmune disease, and seizures, but there is less clinical application of NBP in the above diseases. As a result, there is still much room for its development in clinical application. Even in non-neurologic diseases, studies have found that NBP has certain effects on diabetes, diabetic cataract, and atherosclerosis. Due to the diverse targets of NBP, more and more researchers are focusing on its animal experiments and clinical experiments.
Butylphthalide and its effect on reconstructing microcirculation
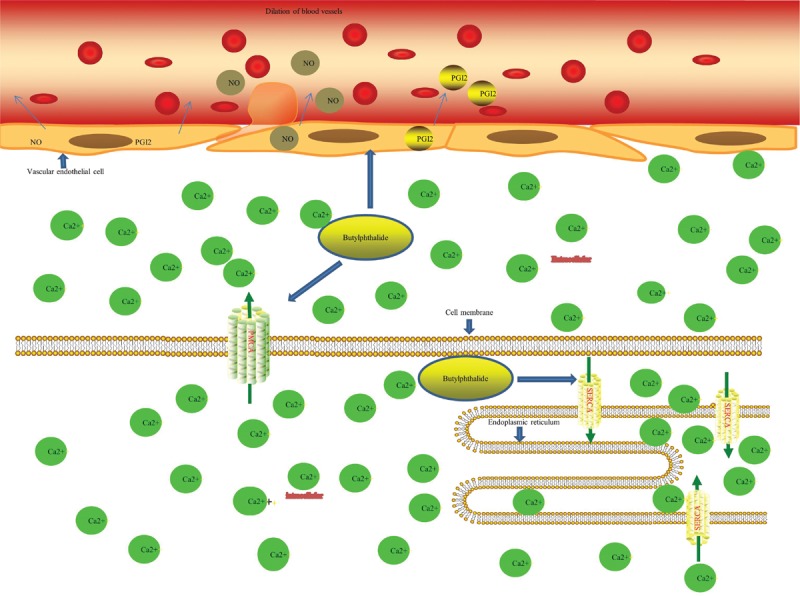
Numerous studies have shown that dl-NBP facilitates reconstruction of the microcirculation, during which it can reduce arachidonic acid (AA) content. Leukotriene is a metabolite of AA, of which leukotriene B4 is an important mediator of various inflammatory reactions and is involved in the development of atherosclerosis.[2] Studies have found that aggregation of isolated platelets induced by AA, adenosine diphosphate (ADP), and collagen can be inhibited by l-NBP.[1] Study also found that NBP inhibits human platelet phosphodiesterase and elevated 3,5-cyclic adenosine monophosphate levels in platelets.[3] In addition to exerting anti-platelet and anti-atherosclerosis effects in stroke, it expands blood vessels and improves cerebral blood flow. It can improve nitric oxide (NO) and PGI2 contents in cerebral vascular endothelial cells, and NO and PGI2 are important vasodilators. In an experimental study on ischemia-reperfusion (I/R) in rats, NBP was found to increase the PGI2/thromboxane A2 (TXA2) ratio, and consequently PGI2 exerted functional effects on blood vessel dilation to improve perfusion during ischemic brain tissue.[4] TXA2 is a thromboxane commonly used as a vasoconstrictor to activate platelets and aggregate them. In another study, NBP was also found to reduce thrombin or collagen-induced TXA2 synthesis by inhibiting cytosolic phospholipases A2 (cPLA2) phosphorylation, which was accompanied by a significant decrease in intracellular calcium mobilization.[3] This mechanism would play a role in anti-platelet aggregation and the improvement microcirculation.[3] NBP also inhibits the release of free radicals and improves the activity of anti-oxidant enzymes during ischemic cerebral injury.[5] Such pharmacologic actions would ultimately produce a series of effects including protecting the integrity of vascular structure and restoring vessel diameter, improving local microcirculation, and increasing blood flow and the number of capillaries during cerebral ischemia [Figure 1].
Figure 1.
NBP improves NO and PGI2 contents in cerebral vascular endothelial cells and reduces intracellular calcium concentrations by improving the activities of PMCA and SERCA. These effects protect the integrity of the vascular structure and restore vessel diameter, improving local microcirculation. NBP: 3-N-butylphthalide; NO: Nitric oxide; PGI2: Prostaglandin I 2; PMCA: Plasma membrane Ca2+-ATPase; SERCA: Sarco/endoplasmic reticulum Ca2+-ATPase; ADP: Adenosine diphosphate; ATP: Adenosine triphosphate.
Butylphthalide and its effect on protecting mitochondrial function
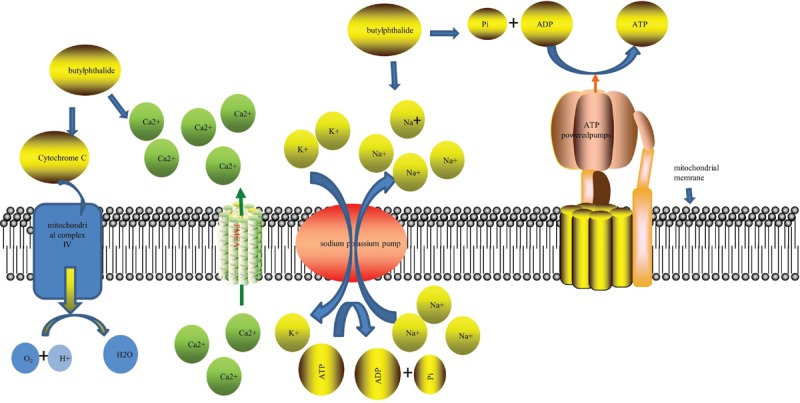
Regarding the protection of mitochondrial function, early animal studies showed that dl-NBP improves the activities of Na/K-ATPase and Ca-ATPase in the mitochondrion. As is known, Na/K-ATPase and Ca-ATPase are important enzymes that maintain cell membrane potential and participate in material transportation to regulate cell volume [Figure 2]. NBP can maintain mitochondrial membrane fluidity and the stability of the mitochondrial membrane, preventing mitochondrial swelling.[6] Previous studies have also shown that NBP might act directly on mitochondrial complex IV to increase its activity.[7] Studies have also found that NBP suppresses the release of cytochrome c in cells after ischemia in mice.[8] Another study found that NBP can increase cytochrome c oxidase levels.[9] It is known that cytochrome c is present in mitochondria. On the mitochondrial inner membrane, this protein represents a link in the electron transfer of redox reactions, and NBP exerts its effects by modulating mitochondrial redox.[7,8] (S)-ZJM-289 is a novel NO release derivative of NBP, which has stronger anti-platelet and anti-thrombotic effects than NBP. In oxygen-glucose-deprivation/reperfusion (OGD/R), also known as the in vitro ischemia model, the carbon dioxide and oxygen (O2) concentrations in the incubator are precisely controlled by direct introduction of nitrogen (N2) and the neuronal growth medium is deprived of sugar at the same time, thereby causing the environment of cultured neurons to be hypoxic and sugar deficient, simulating the body's condition of cerebral ischemia.[10] (S)-ZJM-289 attenuates OGD/R-induced neuronal damage and prevents mitochondrial membrane depolarization by reducing intracellular calcium overload and reactive oxygen species (ROS) accumulation.[10] Moreover, it retains the activity of the respiratory chain complex.[9]
Figure 2.
Dl-NBP improves the activities of PMCA and Na/K-ATPase in the mitochondrion. NBP might act directly on mitochondrial complex IV to increase its activity. dl-NBP: dl-3-N-butylphthalide; NBP: 3-N-butylphthalide; PMCA: Plasma membrane Ca2+-ATPase; PGI2: prostaglandin I2; ADP: Adenosine diphosphate; ATP: Adenosine triphosphate.
Butylphthalide and Diseases
Application of NBP in clinical practice is becoming increasingly extensive for the treatment of nervous system diseases. In addition, it might be beneficial for some non-neurologic diseases. NBP was originally a drug used to treat ischemic stroke, which is the leading cause of death and long-term disability in China,[11] seriously affecting daily life and health. Since 2002, NBP has been approved by the Chinese Drug Administration for the treatment of cerebral ischemia in clinical trials. Second, NBP also has beneficial effects on AD, an incurable disease for which there are no treatment options capable of stopping or reversing its progression.[12] NBP, due to its neuroprotective effects, represents a multi-targeted approach to treat the pathology of AD, as demonstrated by several studies. In addition to its effect on several common neurologic diseases, NBP also has beneficial effects on vascular dementia (VaD), Parkinson disease, and brain damage after CO poisoning; studies have even found that NBP can suppress pathogenesis and play a neuroprotective role in cerebral edema, epilepsy, nervous system trauma, and autoimmune diseases. Moreover, in recent years, there has been an increase in research on the effects of NBP on non-neurologic diseases. This review focuses on the specific mechanism of NBP in neurologic diseases, and also mentions the possible mechanism of NBP in non-neurologic diseases.
Butylphthalide and Stroke
Cerebral infarction is caused by the reduction or cessation of blood flow in the local blood supply artery of the brain tissue. This reduction of blood flow results in ischemia and hypoxia of the corresponding brain tissue, leading to necrosis and softening of the tissue. The main pathophysiologic mechanisms of cerebral infarction include vascular stenosis, occlusion, and brain microcirculation ischemia, and hypoxia caused by thrombosis, causing energy metabolism disorder and apoptosis. NBP can play a series of roles, such as improving local microcirculation, protecting mitochondrial function, improving energy metabolism, and inhibiting neuronal apoptosis to treat ischemic stroke.
Butylphthalide and local microcirculation
Over the past several decades, multiple studies have demonstrated that NBP can block multiple pathologic processes associated with ischemic cerebral injury caused by stroke and significantly improve local microcirculation, increasing blood flow and the number of capillaries in the ischemic area.[8,13–16] NBP can reduce the content of AA and increase NO and prostaglandin I2 (PGI2) levels in cerebral vascular endothelial cells. NO and PGI2 are molecules that can expand blood vessels, and expanding blood vessels in the area of cerebral infarction can improve local blood perfusion. Moreover, NBP can up-regulate the expression of vascular endothelial growth factor, promote the formation of new blood vessels, and increase the number of new capillaries in the ischemic area to improve microcirculation. NBP and NBP derivatives specifically inhibit AA-induced platelet aggregation.[17] Specifically, the compound NOSH-NBP-5 is a derivative of NBP and inhibits AA- and ADP-induced platelet aggregation in vitro. In addition, it can mediate the release of moderate levels of NO and hydrogen sulfide, which helps to improve cardiovascular and cerebral circulation. Further, NBP inhibits thrombus formation in a dose-dependent manner, and l-NBP was found to be the most effective of all NBP isomers.[18] This compound significantly protects mice from collagen- and adrenaline-induced thrombosis.[1] When 100 mg/kg of l-NBP was orally administered to rats, the bleeding time was increased 2.1-fold compared to that in the control group.[1] At the same dose, ex vivo platelet aggregation induced by ADP, collagen, and AA was inhibited and an anti-thrombotic effect was also observed.[1] Regarding the protection of microvessels, studies have found that NBP attenuates oxygen glucose deprivation (OGD)-induced mitochondrial superoxide, peroxynitrite formation and decreases superoxide dismutase (SOD) activity, mitochondrial rupture, and loss of mitochondrial membrane potential.[1] In a study model of human umbilical vein endothelial cells, it was found that NBP may increase the expression of hypoxia-inducible factor-1α (HIF-1α) induced by OGD. Generally, when cells are exposed to OGD, the expression of HIF-1α is increased, but the level of HIF-1α is further increased after treatment with NBP, which is a transcriptionally active nuclear protein whose activity involves angiogenesis. Therefore, its enhanced expression promotes blood vessel growth and increases local circulating blood volume.[19] Similarly, in another study, the expression of vascular endothelial growth factor (VEGF) and HIF-1α was up-regulated after NBP treatment within 24 h of ischemic stroke, and angiogenesis was enhanced to rescue brain tissue.[15]
Butylphthalide and mitochondrial function
Experiments have shown that NBP can increase the activity of Na+-K+ ATPase and Ca2+-ATPase in the mitochondrial membrane. These enzymes maintain electrolyte stability inside and outside the mitochondrial membrane, maintaining membrane stability, participating in material transport, and also maintaining cell volume. BNP significantly improves the decrease in neuronal mitochondrial complex IV activity induced by low glucose and hypoxia, increasing the activity of SOD and glutathione peroxidase (GSH-Px) in mitochondria, and increasing the content of malondialdehyde (MDA) in mitochondria. All of these effects play an important role in maintaining mitochondrial function and improving energy metabolism.[8,12–15] Studies have also shown that BNP can reduce the release of cytochrome c in mouse cells and increase the activity of cytochrome c oxidase and suppress caspase-dependent apoptosis.[8,20] In one study, NBP was found to inhibit the production of ROS by inhibiting nicotinamide adenine dinucleotide (NADH)-pan-quinone oxidase. Since NADH-pan-quinone oxidase is located in the mitochondrial layer and is highly enriched with glial cells, it has also been found that the anti-ROS effect of NBP depends on glial cells rather than neurons.[7] The study concluded that NBP can modulate the function of NADH-ubiquinone oxidoreductase by competitively embedding itself into the complex, further affecting mitochondrial respiration during cerebrovascular disease.[7] NBP can also increase the rate of adenosine triphosphate (ATP) metabolism in permanent middle cerebral artery occlusion (pMCAO) rats, and it was found that the content of glutamic acid and glutamine in the brain tissue of these animals was increased after treatment with NBP.[21] In addition, the level of aspartic acid in the brain tissue of these rats was also increased. It is further speculated that NBP might increase the rate of malate passage in pMCAO rats.[21,22]
Butylphthalide and other effects on ischemic stroke
NBP has an important effect in decreasing brain edema, preserving the blood-brain barrier (BBB), and increasing the level of circulating endothelial progenitor cells.[8,12–15] It can also reduce oxidative damage and neuronal apoptosis and inhibit inflammatory responses.[8,12–15] The inflammatory response plays an important role in ischemic brain injury and NBP can up-regulate hepatocyte growth factor, down-regulate Toll-like receptor 4 (TRL4), and inhibit activation of the TRL4/nuclear factor kβ (NF-kβ) pathway to exert its anti-inflammatory effect, which plays a role in brain protection.[23] One study found that cerebral I/R injury induces the phosphorylation of extracellular-regulated protein kinases (ERKs); phosphorylated ERK (pERK) further induces the phosphorylation of Golgi reassembly stacking protein 65 (GRASP65), and this molecule promotes the inflammatory response and oxidative stress. It was also found in this study that NBP can reduce levels of pERK, thereby reducing inflammation and oxidative stress to exert neuroprotective effects.[24] In a rat model of I/R, comparing a BNP group to a sham-operated group, we found that NBP could significantly reduce infarct size, apoptosis, BBB damage, and water content. In addition, we found that it could reduce ROS and MDA levels in the brains of rats with cerebral I/R injury and increase SOD activity in brain tissue. Results showed that NBP could also inhibit p38 and c-Jun N-terminal kinase (JNK) expression and protect the brain from I/R damage.[24] Moreover, NBP can up-regulate the expression of heat shock protein 70 (HSP70) to prevent neuronal I/R injury by inhibiting neuronal apoptosis.[25] Studies have also shown that this compound can inhibit apoptosis by modulating caspase-3, caspase-9, and B-cell lymphoma-2 (Bcl-2) levels and inhibit the mitogen-activated protein kinase family and phosphatidylinositol 3-kinase (PI3K)/protein kinase B signaling, which has an anti-apoptotic role.[26] In addition, NBP can increase the mRNA and protein expression of VEGF and transforming growth factor-β1 in the infarct region of a rat cerebral infarction model, suggesting a possible mechanism through which this molecule can protect ischemic brain tissue.[27] As stated, in 2002, NBP was approved by the China Food and Drug Administration for clinical trials for the treatment of cerebral ischemia.[28] According to the results of a recent clinical trial, the efficiency of NBP for the treatment of ischemic cerebrovascular disease is as high as 74.7%, with a low incidence of adverse reactions.[29] Further, a series of studies has shown that NBP is safe for clinical treatment. Although the pharmacologic mechanism of NBP in ischemic stroke has not been fully elucidated, it is expected that the use of this drug will increase for the treatment of stoke because of its effects on multiple pathologic processes associated with ischemic cerebral injury, in addition to its efficacy.
Butylphthalide and Alzheimer Disease
Synapse impairment in the AD brain is an early event leading to cognitive dysfunction.[30] Most oxidative stress localizes to the synapse, and synapse loss is the basic cause of cognitive decline during this disease.[30] In one study, dl-NBP was found to reduce oxidative stress and ameliorate synaptic plasticity.[31] It was also found to diminish levels of soluble amyloid beta and amyloid beta oligomers in the Sprague-Dawley (SD) rat brain.[31] However, in the AβPPswe/PS1dE9 (AβPP/PS1) mouse model, Peng et al[32] found that l-NBP had no effect on Aβ plaque deposition in the brain and Aβ levels in brain homogenates, but they did note a reduction in tau hyperphosphorylation at Ser199, Thr205, Ser396, and Ser404, as well as down-regulation of tau-related kinase phosphorylation. Some studies have also found that the number of synapses and spines in hippocampus regions increases following treatment with l-NBP and that this change can be attributed to anti-oxidative and anti-inflammatory mechanisms.[32] In previous experiments, NBP was found to inhibit activation of the domain-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome, and enhance the interaction between thioredoxin-interacting protein (TXNIP) and NLRP3 by up-regulating nuclear factor erythroid 2-related factor 2 (Nrf2).[33] It is also speculated that disruption of the Nrf2-TXNIP-TXNIP-thioredoxin (TrX) system might be involved in the imbalance of cellular redox homeostasis in the AD brain and the pathologic process of inflammatory injury.[33] Moreover, it was found that NBP can partially reverse the increased expression of caspase-3, caspase-9, and cytochrome c induced by Aβ25-35, and down-regulate the anti-apoptotic protein Bcl-2, significantly suppressing Aβ25-35-induced mitochondrial dysfunction.[34] NBP also attenuates Aβ-induced astrocyte activation and neuroinflammation by inhibiting the NF-kB signaling pathway.[35] In the study of hydrogen peroxide (H2O2)-induced neuronal injury, the protective effect of l-NBP on neural stem cells (NSCs) induced by H2O2-induced neurologic injury and its possible mechanism were evaluated.[36] The results indicate that l-NBP increases the proliferation of NSCs by up-regulating cyclin D1, and PI3K/Akt may be a possible target for this process.[36] Immunofluorescence staining in this experiment showed that l-NBP can promote the differentiation of NSCs into neurons. L-NBP protects H2O2-induced damage in NSCs by promoting proliferation, migration, and neural differentiation of NSCs, suggesting that l-NBP may provide a new entry point for the treatment of AD.[36] Synaptophysin (SYN) and post-synaptic density 95 (PSD-95) are markers of pre-synaptic and post-synaptic terminals, respectively, which represent the structural basis of learning and memory plasticity. SYN not only directly affects synaptic structure, but also may affect synaptic plasticity by regulating neurotransmitters[37] PSD may regulate the structure of synaptic connections and transmit membrane receptor signals by interacting with various proteins.[38,39]Accelerated aging mice constitute an accelerated aging animal model based on aging rather than genetic mutations. The senescence-accelerated mouse (SAM) consists of a SAM-producing strain (SAMP) and an accelerated aging mouse-resistant strain.[40]After 3 months of administration of dl-NBP, the levels of SYN and PSD-95 in SAMP 8 mice were significantly elevated.[41] In addition, the brain-derived neurotrophic factor (BNDF)/tropomyosin-related kinase B (TrkB) signaling pathway forms and consolidates memory by regulating synapse formation and plasticity.[41] BNDF and TrkB levels are significantly reduced in SAMP 8 mice, but dl-NBP treatment significantly increases both of the above in SAMP 8 mice.[41] Long-term dl-NBP therapy may reduce cognitive decline in SAMP 8 mice by enhancing BDNF/TrkB signaling pathway through the regulation of structural synaptic plasticity.[41] It has also speculated that the distribution and expression of synaptic proteins SYN and PSD-95 may be regulated by the BDNF/TrkB signaling pathway.[42,43] Similarly, in a study by Lei et al,[44] l-NBP significantly increased the expression of BDNF and nerve growth factor in the hippocampal of APP/PS1 mice, and increased tyrosine and Akt phosphorylation and activation of Akt signaling pathway. At the same time, the proliferation, migration, and differentiation of hippocampal NSCs were also enhanced.[44] This study suggests that l-NBP may enhance the proliferation and activation of hippocampal stem cells by mediating the activation of BDNF/TrkB/cyclic adenosine monophosphate-response element binding protein/Akt signaling pathway. It is therefore beneficial for treating AD.[44] However, NBP treatment for AD is still in the trial stage and the therapeutic effects and pharmacologic mechanisms of this drug in AD remain to be elucidated.[45] Regardless, NBP could represent an etiologic treatment for AD.
Butylphthalide and Vascular Dementia
Several studies have also shown that NBP might be effective for the management of dementias such as VaD. Recent studies have also shown that NBP is also effective for vascular cognitive dysfunction without dementia.[46,47] The main pathologic mechanism of VaD is related to the post-cleavage release of amyloid precursor protein amyloid beta peptide (AB1–40) and AB1–42.[48] Increasing evidence suggests that VaD is associated with a group of diverse pathologies affecting cerebrovascular circulation.[49] The restoration of blood flow after cerebral ischemia is the most important way to prevent irreversible tissue damage, but it can also lead to tissue damage.[50] VaD can be caused by excitotoxicity, oxidative stress, and inflammatory reactions after I/R. Previous studies had shown that l-NBP can improve cognitive function and decrease neuronal damage. In addition, a recent study also showed that l-NBP can increase the phosphorylation of Akt in the hippocampus.[50] The Akt signaling pathway is important for the pathogenesis of degenerative diseases.[51] For example, it was found that activating this pathway might have neuroprotective effect through the suppression of neuronal death after stroke.[51] Studies have also found that l-NBP can increase the expression of brain neurotrophic-derived factor, TrkB, PI3K, and Akt in the hippocampus of APP/PS1 transgenic mice and improve memory impairment in mice.[52] In chick embryo chorioallantoic membrane tests, the expression of growth factors, VEGF, VEGF receptor, and basic fibroblast growth factor (BFGF) are increased after treatment with NBP, suggesting that NBP might improve VaD by promoting angiogenesis.[53] Further, the effect of NBP treatment on VaD might be related to enhanced levels of energy metabolism in brain cells, thus promoting anti-oxidant effects and reducing the damage to neurons mediated by oxygen-free radicals.[54] Experiments have shown that NBP can increase anti-oxidant enzyme activity, such as that of SOD,[54] the most important anti-oxygen-free radical enzyme in the body, which can eliminate free radicals generated during human metabolism. SOD is also an indicator of free radical scavenging ability. NBP can also decrease the level of MDA, a marker of lipid peroxidation metabolism, as well as toxicity of the final products. Its content directly reflects the in vivo rate of lipid peroxidation and can indirectly reflect the severity of oxygen-free radical damage to tissues and cells.
Butylphthalide and Parkinson Disease
The pathogenesis of Parkinson disease comprises the loss of dopaminergic neurons in the substantia nigra pars compacta,[55,56] and includes oxidative stress, mitochondrial dysfunction, calcium overload, and excitotoxic amino acid toxicity. Some targets of NBP coincide with the major pathologic pathways of Parkinson disease. In one study, SD rats were treated with rotenone to simulate Parkinson disease, and those rats were randomly divided into an NBP treatment group and a control group.[57] The level of GSHand the activity of SOD in the brain tissue of the treatment group were found to be significantly increased; however, the level of MDA was decreased and the number of tyrosine hydroxylase (TH)-positive cells in the substantia nigra was significantly increased.[57] The number of TH-positive cells is the most important pathologic indicator of whether a drug has dopaminergic activity and neuroprotective effects.[57] Thus, this study provides evidence that NBP could be used for Parkinson disease treatment.[57] In the Parkinson disease model induced by 1-methyl-4-phenylpyridinium ion (MPP+), NBP was found to enhance the colocalization of α-synuclein with microtubule-associated protein light chain 3 (LC3) and up-regulate protein levels of LC3-II, suggesting that NBP can protect PC12 cells against MPP+-induced neurotoxicity by activating the degradation of α protein via autophagy.[58,59]
Butylphthalide and Brain Edema
There is less literature regarding the effect of NBP on the treatment cerebral edema secondary to cerebral infarction. The permeability of the BBB plays an extremely important role in brain edema. Brain damage usually causes damage to the BBB, especially related to ischemic stroke.[60,61] Pathologic factors disrupt the integrity of the BBB by disrupting the normal function of endothelial cells and reducing the production of tight junction proteins.[62] VEGF or BFGF can enhance the function of endothelial cell membrane after injury, thereby restoring the function of BBB.[63] The recovery of BBB function can significantly reduce brain edema formation and cell damage.[63] In a rat model of cerebral edema after concussion brain injury, it is speculated that NBP can also repair the function of BBB by increasing the content of VEGF or BFGF in brain edema tissue, thereby reducing brain edema.[64] Matrix metalloproteinase 9 (MMP-9) is an enzyme that can destroy the BBB by degrading the extracellular matrix, whereas tissue inhibitor of metalloproteinasis 1 (TIMP-1) is a specific inhibitor of MMP-9. In a study of cerebral edema secondary to ischemic cerebral infarction using SD rats, NBP was found to significantly decrease the expression of MMP-9 and increase the expression of TIMP-1.[21] As a result, the permeability of the BBB was reduced, and brain edema was suppressed.[21] It was also found that NBP can protect the BBB by alleviating damage due to oxygen-free radicals and improving brain blood circulation.[6] NBP is also effective for brain edema secondary to CO poisoning, and it might alleviate brain edema by inhibiting the down-regulation of ZO-1 and claudin-5 proteins, which preserve the barrier function.[65]
Butylphthalide and Cognitive Dysfunction in Rats after Acute Severe CO Poisoning
In recent years, an increasing number of studies have determined the effects of NBP on cognitive dysfunction in rats after acute severe CO poisoning. As is known, CO poisoning can cause serious cognitive impairment. The expression of calpain 1 and calmodulin-dependent protein kinase II (CAMKII) protein increased after CO exposure and these molecules can lead to cognitive impairment. Some researchers have found that calpain 1 and CAMKII levels are decreased in CO-poisoned rat brain tissue after NBP treatment.[66] Thus, they speculated that NBP can improve cognitive impairment caused by CO poisoning.[66] NBP also maintains the integrity of the hippocampal ultrastructure[67] and can play a neuroprotective role with respect to brain damage after acute CO poisoning. This is achieved by improving mitochondrial function and balancing the expression of anti-apoptotic and pro-apoptotic genes, which might be related to the fact that NBP treatment can up-regulate Bcl-2 and down-regulate Bax mRNA expression.[68] Further, one study showed that this drug might inhibit neuronal apoptosis and down-regulate the expression of Nogo and NgR1, consequently protecting brain tissue upon exposure to CO poisoning.[69] Compared to those with hyperbaric oxygenation therapy, the combination of NBP and hyperbaric oxygen can significantly increase the mini-mental state examination (MMSE) score and reduce the National Institute of Health stroke scale (NIHSS) score in patients with CO poisoning; MMSE and NIHSS scores are the most commonly used rating scales for stroke patients.[70]
Butylphthalide and Traumatic Central Nervous System Injury
In one study, NBP was found to alleviate secondary spinal cord injury by inhibiting endoplasmic reticulum stress-induced apoptosis, thereby promoting neurologic and locomotor functional recovery. This was associated with the up-regulation of endoplasmic reticulum stress-related proteins such as the 78,000 glucose-regulated protein, activating transcription factor 6 (ATF-6), ATF-4, protein disulfide isomerase (PDI), x-box-binding protein-1 (XBP-1), and CCAAT enhancer-binding protein homologous protein (CHOP).[71] In a guinea pig model of traumatic spinal cord injury, the permeability of the blood-spinal cord barrier increased, leading to rapid entry of blood cell and plasma components into the spinal cord.[72] Researchers found that NBP can prevent the degradation of adhesion and tight junction proteins, which would diminish the permeability of the blood-spinal cord barrier.[72] NBP might also inhibit inflammation during traumatic brain injury by suppressing the up-regulation of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 1β (IL-1β).[72] In traumatic brain injury, NBP also improves the level of BDNF, VEGF, endothelial-derived NO synthase, and matrix metalloprotein (MMP-9).[73]
Butylphthalide and Autoimmune Disease
In a model of autoimmune myositis in guinea pigs, NBP was observed to improve the calcium pump activity of the muscle mitochondrial membrane and the sarcolemmal membrane, increasing the expression of Foxp3 mRNA and reducing the expression of inflammatory factors, indicating that NBP might be an effective drug to treat autoimmunity in muscle.[74] In addition, in a similar model, studies also found that NBP can act as an anti-oxidant to protect muscle mitochondria and muscle cells from oxidative damage, by enhancing SOD and catalase activity, decreasing MDA activity, and enhancing ATPase activity on the mitochondrial membrane and muscle fiber membrane.[75] Further, in a mouse model of autoimmune myelitis, NBP was found to attenuate the progression of this disease by inhibiting phosphoglycerate mutase family member 5-induced necrosis and inflammation in microglia.[76]
Butylphthalide and Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a type of motor neuron disease. NBP can obviously reduce the decrease in motor unit number estimation, delaying the amplitude of gastrocnemius compound muscle action potential, slowing down the process of gastrocnemius atrophy.[77] NBP might thus play a major role in reducing the loss of nerve cells, inhibiting the expression of TNF-α, NF-kB, and P65, and improving levels of Nrf2 and heme oxygenase-1.[78] However, the exact mechanisms of ALS require further study, and the pharmacologic mechanisms associated with the effect of NBP on ALS also remain unclear.
Butylphthalide and Epilepsy
An increasing number of studies has found that epilepsy might be closely related to the function of mitochondria. Seizures can damage the function of these organelles, and mitochondrial dysfunction leads to epilepsy attacks through a series of mechanisms. Enzyme IV activity in the mitochondria of epilepsy patients is decreased significantly, and NBP can improve the activity of this protein by inhibiting calcium overload, as well as inhibiting a series of mechanisms that destroy the mitochondrial membrane structure. It has been observed that NBP can decrease neuronal hyperexcitability through post-synaptic phospho-α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor 2 (GluA2)-lacking calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors (CP-AMPARs) in a rat model of epilepsy.[79] AMPAR mediates the post-synaptic effects of glutamate in synapses. Further, NBP was found to have an anti-convulsant effect in a pentylenetetrazol-induced seizure model in vivo.[79] In the 4-aminopyridine epilepsy model, epileptic activity in the cornu ammonis 1 (CA1) region of hippocampal slices is reduced and the AMPA current is selectively regulated by post-synaptic CP-AMPARs.[79] In addition, studies have found that NBP can maintain the balance of excitation and suppression systems by reducing the levels of glucosuria (Glu) and Glu/gamma-aminobutyric acid (GABA) and relieving seizures.[80] One study showed that NBP can improve learning and memory dysfunction by mitigating neuronal loss caused by chronic epilepsy in hippocampal CA1, CA3, and dentate gyrus areas. Glutamate decarboxylase (GAD65/67) is a GABA synthase that determines GABA levels during post-natal synaptic maturation.[81] The results of our study also suggested that the anti-epileptic effect of NBP might be due to the up-regulation of GAD65/67 and the reversal of neuron loss.[81] In this study, we found that NBP might have a therapeutic effect on anxiety and depression by increasing BDNF and Klotho mRNA levels in mice with epilepsy induced by pilocarpine.[81] BDNF is a neurotrophic factor that promotes the development of immature neurons, increases the survival and synaptic plasticity of adult neurons, and protects against depression and anxiety.[81] Using a mouse model of chronic epilepsy induced by pilocarpine, we found that NBP not only reduced the severity of epileptic seizures and abnormal electroencephalogram, but also saved cognitive and mood disorders in these epileptic mice. Possible underlying mechanisms may be related to the protective role of NBP in reducing neuronal loss and restoring the expression of synaptic proteins such as PSD-95 and GAD65/67.[82] PSD-95 is a scaffold protein associated with synaptic maturation and synaptic stability, strength, and plasticity. GAD65/67 is an important enzyme in the synthesis of GABA and a key enzyme in synaptic function.[83–85] In addition, NBP also increases transcription of the neuroprotective factors BDNF and Klotho. BDNF and Klotho are known neuroprotective factors that improve cognitive and/or mental behavior.[82,86] These findings suggest that NBP may be a beneficial drug for treatment of epilepsy.
Butylphthalide and Non-neurologic Diseases
NBP not only has important effects on neurologic diseases, but also can function in non-neurologic diseases such as diabetes, diabetic cataract, and atherosclerosis. In Tian study, NBP was found to significantly increase SOD levels, thereby reducing TNF-α, IL-1β, and IL-6 levels in the hippocampus of NBP-treated animals, which suggests that NBP could be effective against diabetes.[87] NBP might also have a protective effect on diabetic brain damage by enhancing VEGF expression and inhibiting the key apoptosis-associated enzyme caspase-3.[88] Numerous studies have demonstrated that reactive oxygen species (ROS) have a central role in the pathogenesis of diabetic cataract. These molecules can directly disturb the physiologic function of cellular macromolecules and eventually lead to lens opacification. As is known, NBP can significantly reduce oxidative damage and up-regulate the expression of anti-oxidant enzymes.[89] In one study, NBP was found to be beneficial for the treatment of diabetic cataract by decreasing the production of ROS and blood glucose levels.[90] Some research has also found that NBP can reduce the expression of vascular cell adhesion molecule-1 in the aorta and delay the occurrence of atherosclerosis by lowering blood lipid levels and inhibiting inflammation.[90] However, the exact mechanisms through which this agent decreases blood lipid levels and blood glucose are not yet clear. The protective role of NBP in acute myocardial infarction has been recognized.[91,92] Recent studies have also found that NBP can improve cardiac function and ventricular remodeling and inhibit ventricular arrhythmias and inhibit atrial fibrosis in a rat model of myocardial infarction. PI3k/Akt/Nrf2/anti-oxidant response element signaling pathway may contribute to its anti-ventricular remodeling.[93,94]
Conclusion
NBP is a compound that can be extracted from celery seeds. In recent years, researchers have become interested in studying the pharmacologic effects of NBP, since its initial application for ischemic stroke has shown great promise. The most widely known pharmacologic properties of NBP include microcirculation protection, mitochondrial protection, inhibition of inflammatory responses, and the inhibition of apoptosis, as well as anti-platelet, anti-thrombotic, and endothelial progenitor cell mobilizing effects. These mechanisms essentially cover the pathogenesis of most neurologic diseases. In recent years, it has been reported that NBP has therapeutic effects on neurologic diseases and those effects involve many different molecular mechanisms including various signaling pathways. Understanding the mechanisms associated with the effect of NBP on many diseases is still in the experimental stage (using rats) and the research of different groups could elicit contradictory molecular mechanisms. These points indicate that our understanding of the pharmacologic effects of NBP is still not sufficient and also demonstrate the complexity of the molecular and pharmacologic effects of this drug. The complex and diverse molecular mechanisms associated with NBP make it a hot topic for research in addition to revealing the prospects for its future use in medicine.
Conflicts of interest
None.
Footnotes
How to cite this article: Chen XQ, Qiu K, Liu H, He Q, Bai JH, Lu W. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000289
References
- 1.Peng Y, Zeng X, Feng Y, Wang X. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J Cardiovasc Pharmacol 2004; 43:876–881. doi: 10.1097/00005344-200406000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Zhang J, Wang J, Sun T, Xiao H, Zhang JS. Association between genetic variants of the leukotriene biosynthesis pathway and the risk of stroke: a case-control study in the Chinese Han population. Chin Med J 2013; 126:254–259. doi: 10.3760/cma.j.issn.0366-6999.20121113. [PubMed] [Google Scholar]
- 3.Ye J, Zhai L, Zhang S, Zhang Y, Chen L, Hu L, et al. DL-3-n-butylphthalide inhibits platelet activation via inhibition of cPLA2-mediated TXA2 synthesis and phosphodiesterase. Platelets 2015; 26:736–744. doi: 10.3109/09537104.2014.989826. [DOI] [PubMed] [Google Scholar]
- 4.Chong ZZ, Feng YP. Effects of dl-3-n-butylphthalide on production of TXB2 and 6-keto-PGF1 alpha in rat brain during focal cerebral ischemia and reperfusion. Acta Pharmacol Sin 1997; 18:505–508. doi: 10.1016/S0014-2999(97)81950-4. [PubMed] [Google Scholar]
- 5.Zhao W, Luo C, Wang J, Gong J, Li B, Gong Y, et al. 3-N-butylphthalide improves neuronal morphology after chronic cerebral ischemia. Neural Regen Res 2014; 9:719–726. doi: 10.4103/1673-5374.131576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong J, Feng YP. The protective effect of butylphthalide against mitochondrial injury during cerebral ischemia [in Chinese]. Acta Pharmaceutica Sinica 2000; 35:408–412. doi: 10.3321/j.issn:0513-4870.2000.06.003. [Google Scholar]
- 7.Wang Y, Qi W, Zhang L, Ying Z, Sha O, Li C, et al. The novel targets of DL-3-n-butylphthalide predicted by similarity ensemble approach in combination with molecular docking study. Quant Imaging Med Surg 2017; 7:532–536. doi: 10.21037/qims.2017.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, Zhou W, Hu Z. Chinese herbal extract dl-3n-butylphthalide: a commonly used drug for the treatment of ischemic stroke as a novel therapeutic approach to treat neurodegenerative diseases. Neural Regenerat Res 2011; 6:2773–2778. doi: 10.3969/j.issn.1673-5374.2011.35.009. [Google Scholar]
- 9.Chang Q, Wang XL. Effects of chiral 3-n-butylphthalide on apoptosis induced by transient focal cerebral ischemia in rats. Acta Pharmacol Sin 2003; 24:796–804. doi: 10.1021/ar020267y. [PubMed] [Google Scholar]
- 10.Zhao Q, Zhang C, Wang X, Chen L, Ji H, Zhang Y. (S)-ZJM-289, a nitric oxide-releasing derivative of 3-n-butylphthalide, protects against ischemic neuronal injury by attenuating mitochondrial dysfunction and associated cell death. Neurochem Int 2012; 60:134–144. doi: 10.1016/j.neuint.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Wang K. The fate of medications evaluated for ischemic stroke pharmacotherapy over the period 1995-2015. Acta Pharm Sin B 2016; 6:522–530. doi: 10.1016/j.apsb.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pchitskaya EI, Zhemkov VA, Bezprozvanny IB. Dynamic microtubules in Alzheimer's disease: association with dendritic spine pathology. Biochemistry (Mosc) 2018; 83:1068–1074. doi: 10.1134/s0006297918090080. [DOI] [PubMed] [Google Scholar]
- 13.Liu CL, Liao SJ, Zeng JS, Lin JW, Li CX, Xie LC, et al. dl-3n-butylphthalide prevents stroke via improvement of cerebral microvessels in RHRSP. J Neurol Sci 2007; 260:106–113. doi: 10.1016/j.jns.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Xu J, Wang Y, Li N, Xu L, Yang H, Yang Z. L-3-n-butylphthalide improves cognitive deficits in rats with chronic cerebral ischemia. Neuropharmacology 2012; 62:2424–2429. doi: 10.1016/j.neuropharm.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Liao SJ, Lin JW, Pei Z, Liu CL, Zeng JS, Huang RX. Enhanced angiogenesis with dl-3n-butylphthalide treatment after focal cerebral ischemia in RHRSP. Brain Res 2009; 1289:69–78. doi: 10.1016/j.brainres.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Yun W, Zhang Q, Cai X, Li X, Hui G, et al. Mobilization of circulating endothelial progenitor cells by dl-3-n-butylphthalide in acute ischemic stroke patients. J Stroke Cerebrovasc Dis 2016; 25:752–760. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Liu Q, Tan M, Wen S, Pi R, Lin D. Synthesis and biological evaluation of n-butylphthalide derivatives as anti-platelet aggregation agents. Nat Prod Res 2016; 1–4. doi: 10.1080/14786419.2015.1136907. [DOI] [PubMed] [Google Scholar]
- 18.Wang XL, Wang ZY, Ling JJ, Zhang YH, Yin J. Synthesis and biological evaluation of nitric oxide (NO)-hydrogen sulfide (H2S) releasing derivatives of (S)-3-n-butylphthalide as potential antiplatelet agents [in Chinese]. Chin J Nat Med 2016; 14:946–953. doi: 10.1016/s1875-5364(17)30021-3. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Zhang B, Tao Y, Wang Y, Wei H, Zhao J, et al. DL-3-n-butylphthalide protects endothelial cells against oxidative/nitrosative stress, mitochondrial damage and subsequent cell death after oxygen glucose deprivation in vitro. Brain Res 2009; 1290:91–101. doi: 10.1016/j.brainres.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Li Y, Ogle M, Zhou X, Song M, Yu SP, et al. DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res 2010; 1359:216–226. doi: 10.1016/j.brainres.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong ZZ, Feng YP. dl-3-n-butylphthalide attenuates reperfusion-induced blood-brain barrier damage after focal cerebral ischemia in rats. Acta Pharmacol Sin 1999; 20:696–700. [PubMed] [Google Scholar]
- 22.Liu RZ, Fan CX, Zhang ZL, Zhao X, Sun Y, Liu HH, et al. Effects of Dl-3-n-butylphthalide on cerebral ischemia infarction in rat model by mass spectrometry imaging. Int J Mol Sci 2017; 18: doi: 10.3390/ijms18112451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Guo ZF, Xu YM, Li YS, Song JG. N-Butylphthalide (NBP) ameliorated cerebral ischemia reperfusion-induced brain injury via HGF-regulated TLR4/NF-kappaB signaling pathway. Biomed Pharmacother 2016; 83:658–666. doi: 10.1016/j.biopha.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 24.Zhu BL, Xie CL, Hu NN, Zhu XB, Liu CF. Inhibiting of GRASP65 phosphorylation by DL-3-N-butylphthalide protects against cerebral ischemia-reperfusion injury via ERK signaling. Behav Neurol 2018; 2018:5701719.doi: 10.1155/2018/5701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Niu LJ, Qi FM, Guo L. Effect of 3-n-butylphthalide pretreatment on expression of the HSP70 after brain ischemia/reperfusion [in Chinese]. Chin J Appl Physiol 2015; 31:136–140. doi: 10.13459/j.cnki.cjap.2015.02.011. [PubMed] [Google Scholar]
- 26.Sun B, Feng M, Tian X, Lu X, Zhang Y, Ke X, et al. DL-3-n-Butylphthalide protects rat bone marrow stem cells against hydrogen peroxide-induced cell death through antioxidation and activation of PI3K-Akt pathway. Neurosci Lett 2012; 516:247–252. doi: 10.1016/j.neulet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Sun L, Xuan X, Wang J. Impacts of N-butylphthalide on expression of growth factors in rats with focal cerebral ischemia. Bosn J Basic Med Sci 2016; 16:102–107. doi: 10.17305/bjbms.2016.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Zhang QY, Hua WY, Huang M, Zhou WJ, Lou K, et al. Pharmacokinetics, safety and tolerability of L-3-n-butylphthalide tablet after single and multiple oral administrations in healthy Chinese volunteers. Bras Pharm Sci 2015; doi: 10.1590/s1984-82502015000300004. [Google Scholar]
- 29.Cui LY, Zhu YC, Gao S, Wang JM, Peng B, Ni J, et al. Ninety-day administration of dl-3-n-butylphthalide for acute ischemic stroke: a randomized, double-blind trial. Chin Med J 2013; 126:3405–3410. doi: 10.3760/cma.j.issn.0366-6999.20123240. [PubMed] [Google Scholar]
- 30.Zhang Y, Huang LJ, Shi S, Xu SF, Wang XL, Peng Y. L-3-n-butylphthalide rescues hippocampal synaptic failure and attenuates neuropathology in aged APP/PS1 mouse model of Alzheimer's disease. CNS Neurosci Ther 2016; 22:979–987. doi: 10.1111/cns.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CY, Wang ZY, Xie JW, Wang T, Wang X, Xu Y, et al. Dl-3-n-butylphthalide-induced upregulation of antioxidant defense is involved in the enhancement of cross talk between CREB and Nrf2 in an Alzheimer's disease mouse model. Neurobiol Aging 2016; 38:32–46. doi: 10.1016/j.neurobiolaging.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Peng Y, Hu Y, Xu S, Li P, Li J, Lu L, et al. L-3-n-butylphthalide reduces tau phosphorylation and improves cognitive deficits in AbetaPP/PS1-Alzheimer's transgenic mice. J Alzheimers Dis 2012; 29:379–391. doi: 10.3233/jad-2011-111577. [DOI] [PubMed] [Google Scholar]
- 33.Wang CY, Xu Y, Wang X, Guo C, Wang T, Wang ZY. Dl-3-n-butylphthalide inhibits NLRP3 inflammasome and mitigates Alzheimer's-like pathology via Nrf2-TXNIP-TrX axis. Antioxid Redox Signal 2019; 30:1411–1431. doi: 10.1089/ars.2017.7440. [DOI] [PubMed] [Google Scholar]
- 34.Lei H, Zhao CY, Liu DM, Zhang Y, Li L, Wang XL, et al. l-3-n-Butylphthalide attenuates beta-amyloid-induced toxicity in neuroblastoma SH-SY5Y cells through regulating mitochondrion-mediated apoptosis and MAPK signaling. J Asian Nat Prod Res 2014; 16:854–864. doi: 10.1080/10286020.2014.939586. [DOI] [PubMed] [Google Scholar]
- 35.Wang HM, Zhang T, Huang JK, Sun XJ. 3-N-butylphthalide (NBP) attenuates the amyloid-beta-induced inflammatory responses in cultured astrocytes via the nuclear factor-kappaB signaling pathway. Cell Physiol Biochem 2013; 32:235–242. doi: 10.1159/000350139. [DOI] [PubMed] [Google Scholar]
- 36.Wang S, Huang L, Zhang Y, Peng Y, Wang X, Peng Y. Protective effects of L-3-n-butylphthalide against H2O2-induced injury in neural stem cells by activation of PI3K/Akt and Mash1 pathway. Neuroscience 2018; 393:164–174. doi: 10.1016/j.neuroscience.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science 2009; 323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, et al. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature 1998; 396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 39.Lin H, Jacobi AA, Anderson SA, Lynch DR. D-serine and serine racemase are associated with PSD-95 and glutamatergic synapse stability. Front Cell Neurosci 2016; 10:34.doi: 10.3389/fncel.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gainotti G, Quaranta D, Vita MG, Marra C. Neuropsychological predictors of conversion from mild cognitive impairment to Alzheimer's disease. J Alzheimers Dis 2014; 38:481–495. doi: 10.3233/jad-130881. [DOI] [PubMed] [Google Scholar]
- 41.Lv C, Ma Q, Han B, Li J, Geng Y, Zhang X, et al. Long-term DL-3-n-butylphthalide treatment alleviates cognitive impairment correlate with improving synaptic plasticity in SAMP8 mice. Front Aging Neurosci 2018; 10:200.doi: 10.3389/fnagi.2018.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci 2000; 3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- 43.Chen X, Wang X, Tang L, Wang J, Shen C, Liu J, et al. Nhe5 deficiency enhances learning and memory via upregulating Bdnf/TrkB signaling in mice. Am J Med Genet B Neuropsychiatr Genet 2017; 174:828–838. doi: 10.1002/ajmg.b.32600. [DOI] [PubMed] [Google Scholar]
- 44.Lei H, Zhang Y, Huang L, Xu S, Li J, Yang L, et al. L-3-n-butylphthalide regulates proliferation, migration, and differentiation of neural stem cell in vitro and promotes neurogenesis in APP/PS1 mouse model by regulating BDNF/TrkB/CREB/Akt pathway. Neurotox Res 2018; 34:477–488. doi: 10.1007/s12640-018-9905-3. [DOI] [PubMed] [Google Scholar]
- 45.Song FX, Wang L, Liu H, Wang YL, Zou Y. Brain cell apoptosis inhibition by butylphthalide in Alzheimer's disease model in rats. Exp Ther Med 2017; 13:2771–2774. doi: 10.3892/etm.2017.4322. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Jia J, Wei C, Liang J, Zhou A, Zuo X, Song H, et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement 2016; 12:89–99. doi: 10.1016/j.jalz.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Ni J, Han F, Yuan J, Wang H, Shen DC, Xu Y, et al. The discrepancy of neurological diseases between china and western countries in recent two decades. Chin Med J 2018; 131:886–891. doi: 10.4103/0366-6999.229905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaundal M, Zameer S, Najmi AK, Parvez S, Akhtar M. Betulinic acid, a natural PDE inhibitor restores hippocampal cAMP/cGMP and BDNF, improve cerebral blood flow and recover memory deficits in permanent BCCAO induced vascular dementia in rats. Eur J Pharmacol 2018; 832:56–66. doi: 10.1016/j.ejphar.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Lu W, Lei Q, Tao X, You H, Xie P. Salvianolate increases heat shock protein expression in a cerebral ischemia-reperfusion injury model. Neural Regen Res 2013; 8:2327–2335. doi: 10.3969/j.issn.1673-5374.2013.25.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huai Y, Dong Y, Xu J, Meng N, Song C, Li W, et al. L-3-n-butylphthalide protects against vascular dementia via activation of the Akt kinase pathway. Neural Regen Res 2013; 8:1733–1742. doi: 10.3969/j.issn.1673-5374.2013.19.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene 2003; 22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 52.Xiang J, Pan J, Chen F, Zheng L, Chen Y, Zhang S, et al. L-3-n-butylphthalide improves cognitive impairment of APP/PS1 mice by BDNF/TrkB/PI3K/AKT pathway. Int J Clin Exp Med 2014; 7:1706–1713. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Lu L, Chan WM, Huang Y, Wai MS, Yew DT. Effects of DL-3-n-butylphthalide on vascular dementia and angiogenesis. Neurochem Res 2012; 37:911–919. doi: 10.1007/s11064-011-0663-3. [DOI] [PubMed] [Google Scholar]
- 54.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci 2005; 25:9794–9806. doi: 10.1523/jneurosci.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen C, Zheng H, Hu Z. Association between Parkinson's disease and risk of prostate cancer in different populations: an updated meta-analysis. Sci Rep 2017; 7:13449.doi: 10.1038/s41598-017-13834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014; 311:1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 57.Zhao ZW, Xiong N, Zhang ZT, Jia M, Liang ZH, Cao XB, et al. Therapeutic effect of butylphthalide on the rotenone-induced hemiparkinsonian model of rats [in Chinese]. Chin J Neuroimmunol Neurol 2010; 17:38–41. doi: 10.3969/j.issn.1006-2963.2010.01.013. [Google Scholar]
- 58.Huang JZ, Chen YZ, Su M, Zheng HF, Yang YP, Chen J, et al. dl-3-n-Butylphthalide prevents oxidative damage and reduces mitochondrial dysfunction in an MPP(+)-induced cellular model of Parkinson's disease. Neurosci Lett 2010; 475:89–94. doi: 10.1016/j.neulet.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 59.Liu K, Huang J, Chen R, Zhang T, Shen L, Yang J, et al. Protection against neurotoxicity by an autophagic mechanism. Braz J Med Biol Res 2012; 45:401–407. doi: 10.1590/s0100-879x2012007500039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao H, Deng M, Yang B, Hu Z, Tang J. Pretreatment with 17beta-estradiol attenuates cerebral ischemia-induced blood-brain barrier disruption in aged rats: involvement of antioxidant signaling. Neuroendocrinology 2018; 106:20–29. doi: 10.1159/000455866. [DOI] [PubMed] [Google Scholar]
- 61.Deng S, Liu H, Qiu K, You H, Lei Q, Lu W. Role of the Golgi apparatus in the blood-brain barrier: Golgi protection may be a targeted therapy for neurological diseases. Mol Neurobiol 2018; 55:4788–4801. doi: 10.1007/s12035-017-0691-3. [DOI] [PubMed] [Google Scholar]
- 62.Ji HR, Kong LW, Kong W, Zhao SM, Kong XY, Chen M, et al. Effect of pretreatment with NBP on brain edema and blood brain barrier in rats during cerebral ischemia reperfusion injury [in Chinese]. J Apoplexy Nerv Dis 2014; 31:698–700. [Google Scholar]
- 63.Li QF, Kong SY, Deji QZ, He L, Zhou D. Effects of dl-3-n-butylphthalide on expression of VEGF and bFGF in rat brain with permanent focal cerebral ischemia [in Chinese]. J Sichuan Univ 2008; 39:84–88. [PubMed] [Google Scholar]
- 64.Feng L, Sharma A, Niu F, Huang Y, Lafuente JV, Muresanu DF, et al. TiO2-nanowired delivery of DL-3-n-butylphthalide (DL-NBP) attenuates blood-brain barrier disruption, brain edema formation, and neuronal damages following concussive head injury. Mol Neurobiol 2018; 55:350–358. doi: 10.1007/s12035-017-0746-5. [DOI] [PubMed] [Google Scholar]
- 65.Bi M, Zhang M, Guo D, Bi W, Liu B, Zou Y, et al. N-Butylphthalide alleviates blood-brain barrier impairment in rats exposed to carbon monoxide. Front Pharmacol 2016; 7:394.doi: 10.3389/fphar.2016.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bi MJ, Sun XN, Zou Y, Ding XY, Liu B, Zhang YH, et al. N-Butylphthalide improves cognitive function in rats after carbon monoxide poisoning. Front Pharmacol 2017; 8:64.doi: 10.3389/fphar.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Q, Ding X, Bi W, Wang J, Zou Y. Effects of N-butylphthalide on the expressions of calpain 1 and CaMK II in hippocampus in rats with acute severe carbon monoxide poisoning [in Chinese]. Chin Crit Care Med 2017; 29:1127–1132. doi: 10.3760/cma.j.issn.2095-4352.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Li Q, Cheng Y, Bi M, Lin H, Chen Y, Zou Y, et al. Effects of N-butylphthalide on the activation of Keap1/Nrf-2 signal pathway in rats after carbon monoxide poisoning. Environ Toxicol Pharmacol 2015; 40:22–29. doi: 10.1016/j.etap.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Cheng Y, Bi MJ, Kang H, Qu Y, Lin H, et al. Effects of N-butylphthalide on the expressions of Nogo/NgR in rat brain tissue after carbon monoxide poisoning. Environ Toxicol Pharmacol 2015; 39:953–961. doi: 10.1016/j.etap.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Xiang W, Xue H, Wang B, Li Y, Zhang J, Jiang C, et al. Efficacy of N-butylphthalide and hyperbaric oxygen therapy on cognitive dysfunction in patients with delayed encephalopathy after acute carbon monoxide poisoning. Med Sci Monit 2017; 23:1501–1506. doi: 10.12659/msm.899499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Z, Zhou Y, Huang Y, Wang Q, Zheng B, Zhang H, et al. Dl-3-n-butylphthalide improves functional recovery in rats with spinal cord injury by inhibiting endoplasmic reticulum stress-induced apoptosis. Am J Transl Res 2017; 9:1075–1087. [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng B, Zhou Y, Zhang H, Yang G, Hong Z, Han D, et al. Dl-3-n-butylphthalide prevents the disruption of blood-spinal cord barrier via inhibiting endoplasmic reticulum stress following spinal cord injury. Int J Biol Sci 2017; 13:1520–1531. doi: 10.7150/ijbs.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y, Lee JH, Chen D, Gu X, Caslin A, Li J, et al. DL-3-n-butylphthalide induced neuroprotection, regenerative repair, functional recovery and psychological benefits following traumatic brain injury in mice. Neurochem Int 2017; 111:82–92. doi: 10.1016/j.neuint.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen J, Wang J, Zhang J, Pu C. Effect of butylphthalide intervention on experimental autoimmune myositis in guinea pigs. Exp Ther Med 2018; 15:152–158. doi: 10.3892/etm.2017.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen J, Wang J, Zhang J, Pu C. 3-n-Butylphthalide reduces the oxidative damage of muscles in an experimental autoimmune myositis animal model. Exp Ther Med 2017; 14:2085–2093. doi: 10.3892/etm.2017.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Bi Y, Xia Z, Shi W, Li B, Li B, et al. Butylphthalide ameliorates experimental autoimmune encephalomyelitis by suppressing PGAM5-induced necroptosis and inflammation in microglia. Biochem Biophys Res Commun 2018; 497:80–86. doi: 10.1016/j.bbrc.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 77.Feng XH, Yuan W, Peng Y, Liu MS, Cui LY. Therapeutic effects of dl-3-n-butylphthalide in a transgenic mouse model of amyotrophic lateral sclerosis. Chin Med J 2012; 125:1760–1766. doi: 10.3760/cma.j.issn.0366-6999.2012.10.014. [PubMed] [Google Scholar]
- 78.Feng X, Peng Y, Liu M, Cui L. DL-3-n-butylphthalide extends survival by attenuating glial activation in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology 2012; 62:1004–1010. doi: 10.1016/j.neuropharm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 79.Yang Q, Hu YD, Wang XF, Zheng FS. Dl-3n-butylphthalide reduces epileptiform activity through GluA2-lacking calcium-permeable AMPARs in epilepsy models. Oncotarget 2017; 8:98242–98257. doi: 10.18632/oncotarget.21529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han L, Wang Q, Liu X. The effect of butylphthalide on amino acid in the brain of epileptic mice. Minerva Pediatr 2016. [DOI] [PubMed] [Google Scholar]
- 81.Liao ET, Lin YW, Huang CP, Tang NY, Hsieh CL. Electric stimulation of ear reduces the effect of Toll-like receptor 4 signaling pathway on kainic acid-induced epileptic seizures in rats. Biomed Res Int 2018; 2018:5407256.doi: 10.1155/2018/5407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye X, Rong Z, Li Y, Wang X, Cheng B, Cheng Y, et al. Protective role of L-3-n-butylphthalide in cognitive function and dysthymic disorders in mouse with chronic epilepsy. Front Pharmacol 2018; 9:734.doi: 10.3389/fphar.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science 2000; 290:1364–1368. doi: 10.1126/science.290.5495.1364. [PubMed] [Google Scholar]
- 84.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron 2006; 52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez E, Collins MO, Frank RAW, Zhu F, Kopanitsa MV, Nithianantharajah J, et al. Arc requires PSD95 for assembly into postsynaptic complexes involved with neural dysfunction and intelligence. Cell Rep 2017; 21:679–691. doi: 10.1016/j.celrep.2017.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 1995; 267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 87.Tian Z, Wang J, Wang Y, Zhang M, Zhou Y. Effects of butylphthalide on cognitive decline in diabetic rats. Mol Med Rep 2017; 16:9131–9136. doi: 10.3892/mmr.2017.7700. [DOI] [PubMed] [Google Scholar]
- 88.Zhang T, Jia W, Sun X. 3-n-Butylphthalide (NBP) reduces apoptosis and enhances vascular endothelial growth factor (VEGF) up-regulation in diabetic rats. Neurol Res 2010; 32:390–396. doi: 10.1179/016164110x12670144526264. [DOI] [PubMed] [Google Scholar]
- 89.Wang F, Ma J, Han F, Guo X, Meng L, Sun Y, et al. DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci Rep 2016; 6:19396.doi: 10.1038/srep19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao N, Yang QY, Liu YM. Effects of butylphthalide on atherosclerosis lesion and VCAM-1 expression in aortic wall of ApoE-/- mice [in Chinese]. Chin J Pathophysiol 2016; 32:1037–1042. doi: 10.3969/j.issn.1000-4718.2016.06.013. [Google Scholar]
- 91.Wang YG, Li Y, Wang CY, Ai JW, Dong XY, Huang HY, et al. L-3-n-Butylphthalide protects rats’ cardiomyocytes from ischaemia/reperfusion-induced apoptosis by affecting the mitochondrial apoptosis pathway. Acta Physiol (Oxf) 2014; 210:524–533. doi: 10.1111/apha.12186. [DOI] [PubMed] [Google Scholar]
- 92.Tian X, He W, Yang R, Liu Y. Dl-3-n-butylphthalide protects the heart against ischemic injury and H9c2 cardiomyoblasts against oxidative stress: involvement of mitochondrial function and biogenesis. J Biomed Sci 2017; 24:38.doi: 10.1186/s12929-017-0345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qiu H, Ma J, Wu H, Ding C. DL-3-n-butylphthalide improves ventricular function, and prevents ventricular remodeling and arrhythmias in post-MI rats. Naunyn Schmiedebergs Arch Pharmacol 2018; 391:627–637. doi: 10.1007/s00210-018-1490-8. [DOI] [PubMed] [Google Scholar]
- 94.Qiu H, Wu H, Ma J, Cao H, Huang L, Qiu W, et al. DL-3-n-Butylphthalide reduces atrial fibrillation susceptibility by inhibiting atrial structural remodeling in rats with heart failure. Naunyn Schmiedebergs Arch Pharmacol 2018; 391:323–334. doi: 10.1007/s00210-017-1457-1. [DOI] [PubMed] [Google Scholar]


