To the Editor: Kaposiform hemangioendothelioma (KHE) is a rare, locally invasive, vascular tumor of infants and adolescents. It has an annual incidence of approximately 0.071 per 100,000 children.[1,2] KHE has a roughly equal gender ratio.[3] It generally affects the soft tissues presenting as mass lesions with associated cutaneous abnormities and Kasabach-Merritt phenomenon (KMP).[4–6] KHE locating in non-cutaneous sites (such as viscera and retroperitoneum) and those with KMP are reported to be associated with poor prognosis due to a more aggressive disease phenotype.[7] To date, spinal involvement with KHE is rarely encountered in the clinics. A comprehensive review of the literature only identified two cases of spinal KHE occurring in two girls.[4,5] Here, we documented a rare case of KHE of the cervical spine that was successfully treated by surgical resection and thalidomide therapy.
A 40-year-old woman presented with chronic, atraumatic pain in the left neck and shoulder region, which did not worsen over the preceding 4 years. Physical examination showed no neurological deficits and there were no cutaneous lesions present. Laboratory workup included blood routine examination, comprehensive metabolic panel, C-reactive protein, erythrocyte sedimentation rate, and serology tests for tuberculosis (including the MycoDot and T-SPOT tests). All results were within the normal range. Unenhanced computed tomography (CT) scan [Figure 1A] showed lytic lesions involving the vertebral bodies of C4 and C5, as well as the left pedicle and transverse process of the C4 to C5 levels, which extended into the corresponding intervertebral and transverse foramina. Magnetic resonance imaging examination [Figure 1B] showed no associated cord compression but revealed hypo-intense signal changes in C4 and C5 bodies on the sagittal T1-weighted image, which showed marked enhancement after infusion of gadolinium. Malignancy was suspected. For further evaluation of the patient, 18 F-fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT [Figure 1C–1F] was performed, which showed mild FDG-avid disease involving C4 and the intervertebral and transverse foramina of the C4 to C5 levels with a maximum standardized uptake value of 4.6. A CT-guided biopsy of the C4 body was undertaken to establish a diagnosis and histopathological analysis [Figure 1G] was consistent with KHE. Surgery consisting of anterior C4, C5 corpectomy, and autogenous iliac crest grafting with titanium plate fixation was performed after consulting with the patient and her family. Considering its close proximity to vital vascular structures, as well as multiple vertebral involvements and the low-grade nature of KHE in our case, the tumors of the intervertebral and transverse processes were not completely removed during the operation. Subsequent histopathological examinations of the intraoperative specimen confirmed the diagnosis of KHE. Postoperatively, the patient continued treatment with oral thalidomide for 8 months as previously suggested.[4] At the 34-month follow-up, the patient reported no discomfort and was alive with no evidence of disease progression [Figure 1H–1J].
Figure 1.
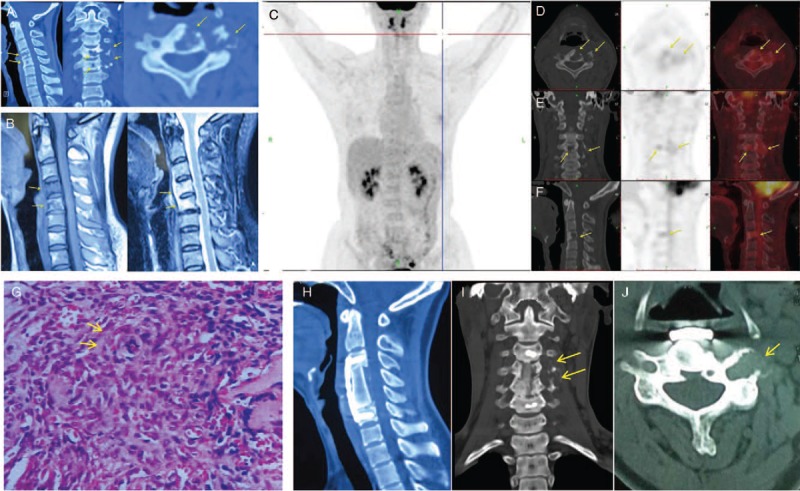
Radiological and histopathological findings of the patient. (A) Unenhanced CT scan showed lytic lesions involving the vertebral bodies of C4 and C5, as well as the left pedicle and transverse process of the C4 to C5 levels, which extended into the corresponding intervertebral and transverse foramina (yellow arrows). (B) Magnetic resonance imaging examination showed no associated cord compression, but revealed hypo-intense signal changes on sagittal T1-weighted image of C4 and C5, which showed marked enhancement after infusion of gadolinium (yellow arrows). (C–F) FDG-PET/CT was performed for further evaluation of the patient. Maximum intensity projection PET (C), transverse (D), coronal (E), and sagittal (F) CT, corresponding PET and fused images (the right side of the D-F images) revealed mild FDG-avid disease involving C4 and the intervertebral and transverse foramina of the C4 to C5 levels (yellow arrows), with a maximum standardized uptake value of 4.6. (G) Histopathological analysis showed glomeruloid-like areas (yellow arrows) characterized by small, thin-walled vascular channels merging with epithelioid cells (hematoxylin and eosin staining; original magnification, ×400), which was consistent with the diagnosis of Kaposiform hemangioendothelioma. The sagittal (H) coronal (I) and transverse (J) CT scans showed that the tumor was largely removed with good bony fusion and no displacement of the titanium plate. The residual tumor (yellow arrows) in the intervertebral and transverse process showed no evidence of progression. CT: Computed tomography; FDG: 18 F-fluorodeoxyglucose; PET: Positron emission tomography.
As patients with KHE can have heterogeneous clinical presentations, it is usually challenging for physicians to define an accurate diagnosis for this disease in a timely fashion.[7] Clinically, the most common manifestations of KHE include immovable enlarging masses with ill-defined margins.[8] Radiologically, it has been reported that heterogeneous hyper-intensity or slight hyper-intensity with speckled hypo-intense signals on the T2-weighted image are significant characteristics of KHE.[8] However, all these findings above are not specific enough to form a diagnosis.[8] Although a definite diagnosis can be reached for patients with typical clinical features related to KHE and KMP,[9] the diagnosis can still remain difficult for solitary KHE, especially in rare sites, such as the bones or testicles.[8] In this situation, a histopathological examination is necessary to confirm the diagnosis,[7,8] which was well exemplified in our case. The patient showed no clinical manifestations consistent with KHE and KMP, a preoperative pathological result helped make the diagnosis and then guided subsequent treatment decisions. Currently, information from 18F-FDG-PET/CT studies in KHE is largely lacking.[10] The present report provided a detailed record of 18F-FDG-PET/CT characteristics in KHE. In this case, although a slightly increased FDG uptake was observed, the diagnosis of KHE remains challenging as the patients had no cutaneous skin changes, laboratory changes (specifical thrombocytopenia), and no superficial or deep soft tissue masses. However, 18F-FDG PET/CT is valuable for evaluating the metabolic activity of the lesion, detecting infectious pathologies, identifying metastatic or primary malignant diseases as well as revealing other occult lesions.
Due to the rare nature of KHE, there is currently no standardized management strategy for this tumor entity. Researchers have advocated complete resection of these tumors whenever possible to maximize patient benefit.[3] However, due to an infiltrative feature of the tumor, a high risk of bleeding as well as the anatomic site of the lesion, complete resection is often demanding in clinical practice.[3,7] Supporting this, non-total tumor removal has been described in the literature,[4] similar to our case. In this situation, adjuvant chemotherapy has been recommended to achieve local control of the disease.[4,8] Although no optimal therapeutic agent or combination of agents has been determined for KHE at present,[3] published data have shown that treatment with corticosteroids or vincristine can be successful in 10% to 27% and 60% to 70% of cases, respectively.[11,12] Furthermore, several recent studies have shown that sirolimus can display a higher efficacy rate probably by inhibiting angiogenesis and lymphangiogenesis of KHE, with more than 90% of patients experiencing improvement in symptoms and/or complications.[3,13] As complete regression of KHE is rare, researchers have recommended discontinuing subsequent chemotherapy when the high-risk symptom has improved and the lesions are stable on serial imaging.[3] The patient received thalidomide therapy because this drug has also been demonstrated to have the ability to inhibit angiogenesis in human cancers[14] and showed efficacy in the treatment of spinal KHE.[2] This choice of treatment may be further justified by the subsequent satisfactory outcome during the follow-up period.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and efforts will be made to conceal the identity of the patient, although anonymity cannot be guaranteed.
Conflicts of interest
None.
Footnotes
How to cite this article: Liu FS, Zou MX, Zheng BW, Wang XB, Lyu GH, Li J. A report on Kaposiform hemangioendothelioma in the cervical spine. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000217
References
- 1.Beaubien ER, Ball NJ, Storwick GS. Kaposiform hemangioendothelioma: a locally aggressive vascular tumor. J Am Acad Dermatol 1998; 38:799–802. doi: 10.1016/S0190-9622(98)70461-X. [DOI] [PubMed] [Google Scholar]
- 2.Croteau SE, Liang MG, Kozakewich HP, Alomari AI, Fishman SJ, Mulliken JB, et al. Kaposiform hemangioendothelioma: atypical features and risks of Kasabach-Merritt phenomenon in 107 referrals. J Pediatr 2013; 162:142–147. doi: 10.1016/j.jpeds.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji Y, Chen S, Xiang B, Li K, Xu Z, Yao W, et al. Sirolimus for the treatment of progressive kaposiform hemangioendothelioma: a multicenter retrospective study. Int J Cancer 2017; 141:848–855. doi: 10.1002/ijc.30775. [DOI] [PubMed] [Google Scholar]
- 4.Lisle JW, Bradeen HA, Kalof AN. Kaposiform hemangioendothelioma in multiple spinal levels without skin changes. Clin Orthop Relat Res 2009; 467:2464–2471. doi: 10.1007/s11999-009-0838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y, Qiu G, Zhao H, Liang J, Shi X. Kaposiform hemangioendothelioma with adolescent thoracic scoliosis: a case report and review of literature. Eur Spine J 2011; 20 Suppl 2:S309–S313. doi: 10.1007/s00586-011-1731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar M, Mulliken JB, Kozakewich HP, Robertson RL, Burrows PE. Thrombocytopenic coagulopathy (Kasabach-Merritt phenomenon) is associated with Kaposiform hemangioendothelioma and not with common infantile hemangioma. Plast Reconstr Surg 1997; 100:1377–1386. doi: 10.1097/00006534-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Ji Y, Chen S, Li L, Yang K, Xia C, Li L, et al. Kaposiform hemangioendothelioma without cutaneous involvement. J Cancer Res Clin Oncol 2018; 144:2475–2484. doi: 10.1007/s00432-018-2759-5. [DOI] [PubMed] [Google Scholar]
- 8.Hu PA, Zhou ZR. Clinical and imaging features of Kaposiform hemangioendothelioma. Br J Radiol 2018; 91:20170798.doi: 10.1259/bjr.20170798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drolet BA, Trenor CC, 3rd, Brandão LR, Chiu YE, Chun RH, Dasgupta R, et al. Consensus-derived practice standards plan for complicated Kaposiform hemangioendothelioma. J Pediatr 2013; 163:285–291. doi: 10.1016/j.jpeds.2013.03.080. [DOI] [PubMed] [Google Scholar]
- 10.Dong A, Zhang L, Wang Y, He T, Zuo C. Abdominal Kaposiform hemangioendothelioma associated with lymphangiomatosis involving mesentery and ileum: a case report of MRI, CT, and 18F-FDG PET/CT findings. Medicine (Baltimore) 2016; 95:e2806.doi: 10.1097/MD.0000000000002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Li J, Qu X, Yan W, Zhang L, Zhang S, et al. Clinical outcomes for systemic corticosteroids versus vincristine in treating Kaposiform Hemangioendothelioma and tufted angioma. Medicine (Baltimore) 2016; 95:e3431.doi: 10.1097/MD.0000000000003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu XH, Li JY, Qu XH, Yan WL, Zhang L, Yang C, et al. Treatment of Kaposiform hemangioendothelioma and tufted angioma. Int J Cancer 2016; 139:1658–1666. doi: 10.1002/ijc.30216. [DOI] [PubMed] [Google Scholar]
- 13.Ji Y, Yang K, Chen S, Peng S, Lu G, Liu X. Musculoskeletal complication in Kaposiform hemangioendothelioma without Kasabach-Merritt phenomenon: clinical characteristics and management. Cancer Manag Res 2018; 10:3325–3331. doi: 10.2147/CMAR.S171223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherbet GV. Therapeutic potential of thalidomide and its analogues in the treatment of cancer. Anticancer Res 2015; 35:5767–5772. [PubMed] [Google Scholar]


