Abstract
Background:
Human-immunodeficiency virus (HIV) infection is increasing worldwide and nontuberculous mycobacteria (NTM) is an established microbiologic cause of pulmonary disease, lymphadenitis, and disseminated disease in cases of advanced immune suppression. Data on patients coinfected with HIV and NTM are limited. Thus, this study aimed to analyze the clinical characteristics, drug resistance, and pathogen spectrum of patients coinfected with both HIV and NTM in the Chengdu area of China.
Methods:
Data of 59 patients coinfected with both HIV and NTM collected from the Public Health Clinical Center of Chengdu, between January 2014 and December 2018, were analyzed. NTM drug sensitivity testing was performed using the microporous plate ratio method. Data were analyzed using SPSS 19.0, and the change in drug resistance rate was analyzed using the chi-square (χ2) test.
Results:
Seven species/complex of NTM were identified from patients coinfected with HIV and NTM in this study, with Mycobacterium avium–intracellulare complex (52.5%) and M. kansasii (27.1%) as the predominant species. Male patients were more affected 50/59 (84.7%); the mean age of the 59 cases was 45 years. The clinical characteristics mainly included anemia (86.4%), cough and expectoration (79.7%). The baseline CD4 count was <50 cells/μL (84.7%). Patients were mainly in advanced acquired immunodeficiency syndrome (AIDS) stage. Chest imaging mainly showed patchy shadows (42.4%) and nodules (32.2%), with various degrees of AIDS-defining diseases. The drug resistance of NTM was severe, and the rate of isoniazid resistance (100.0%) was the highest, followed by rifampicin (94.9%), streptomycin (94.9%), ofloxacin (93.2%), and others. Ethambutol (52.5%) and clarithromycin (33.9%) were relatively low. No significant difference was found in the drug resistance rate of NTM strain against nine antituberculosis drugs in 5 years (P > 0.05).
Conclusions:
The immune level of patients coinfected with HIV and NTM is low in advanced AIDS stage; more male are affected in patients who are mainly infected with MAC and M. kansasii and with serious drug resistance. The drug resistance rate of ethambutol and clarithromycin is relatively low.
Keywords: Clinical features, Drug resistance, HIV, Mycobacterium avium-intracellulare infection, Mycobacterium kansasii, Nontuberculous mycobacteria
Introduction
Mycobacteria other than Mycobacterium tuberculosis complex and M. leprae are known as nontuberculous mycobacteria (NTM). It is a broad classification term applied to a group of approximately 186 currently recognized unique mycobacterium species, and new species are still emerging.[1] NTM infections are present in hypoimmunity and immunocompromised humans, more often in human-immunodeficiency virus (HIV)-infected patients all over the world.[2–4] It is also an important cause of pulmonary and disseminated disease infection and death, especially in the acquired immunodeficiency syndrome (AIDS) stage.[5,6] HIV is one of the major life-threatening viruses spreading in China. Chengdu of Sichuan Province, Southwest China, has the highest incidence of HIV-infected patients.[7–9] This study aimed to analyze the clinical characteristics, drug resistance, and distribution of the pathogen spectrum of patients coinfected with HIV and NTM in the Chengdu area to provide a scientific basis for the prevention, control, diagnosis, and treatment of the disease.
Methods
Ethical approval
The study was approved by the Ethical Review Committee of the Public Health Clinical Center of Chengdu ([No. 2017Y] 025). As this was a retrospective study and all patient information used in this study had been routinely collected through the mandatory notification system, the requirement for informed consent was waived by the ethics committee.
Study design and population
To avoid selection bias, this retrospective study enrolled patients with confirmed consecutive culture-positive NTM with HIV, who were treated in the Public Health Clinical Center of Chengdu from January 2014 to December 2018. A total of 59 patients were randomly selected for this study. HIV was diagnosed based on the Chinese HIV and HIV Infection Diagnostic Criteria (WS293–2008). HIV clinical staging was based on the 1993 Centers for Disease Control diagnostic criteria.[9] The same NTM strain cultured several times in the same patient was not repeatedly counted. The medical records, including their epidemiology, clinical features, imaging, and laboratory information, of all selected participants were recorded.
Culture and identification of bacterial strains
The BACTEC MGIT960 system (Becton Dickinson & Co., Franklin Lakes, NJ, USA) was used for Mycobacterium culture. Pulmonary samples were collected by expectoration, gastric aspiration, and sputum induction. Extrapulmonary samples (pleural fluid, spinal fluid, and lymph nodes) were collected by lumbar puncture, pleural tap, fine needle aspiration, lymph node biopsy, and other techniques. The participants were defined as HIV-infected patients with positive NTM culture results. Their NTM isolates were further grown on the Löwenstein-Jensen (L-J) medium for up to 4 weeks. The p-nitrobenzoic acid and 2-thiophenecarboxylic acid hydrazide were used for NTM identification at first. All collected NTM strains were frozen in the strain bank of the hospital for backup, and the control strain H37Rv was monitored.
Identification of NTM and drug sensitivity
Tuberculous polymerase chain reaction (TB-PCR) was used for further identification of the species/complex level. The TB-PCR was performed according to the instructions of the manufacturer CapitalBio Corporation (Chengdu, China). NTM identification was performed using gene chip (CapitalBioCorporation, Chengdu, China) or matrix-assisted laser desorption/ionization time-of-flight mass spectrometry systems (Microflex LT; Bruker Daltonics, Bremen, Germany). The NTM drug sensitivity testing (DST) kit was purchased from AUTOBIO diagnostics Co., Ltd. (Zhengzhou, China) and used according to the manufacturer's standard procedure. The critical concentration was 0.25 μg/mL for isoniazid, 2 μg/mL for rifampicin, 2 μg/mL for streptomycin, 5 μg/mL for ethambutol, 2 μg/mL for ofloxacin, 2.5 μg/mL for capreomycin, 2.5 μg/mL for amikacin, 10 μg/mL for kanamycin, and 5 μg/mL for clarithromycin. H37Rv was used as a reference strain.
Laboratory quality control
External quality assessment was conducted at the National Tuberculosis Reference Laboratory of the Chinese Center for Disease Control and Prevention. This assessment included smear, culture, and DST. Blinded retesting of a random selection about 10% of isolates from the study laboratory was conducted in a superior laboratory.
Diagnosis and treatment
The NTM-infected patients were categorized and diagnosed according to the Chinese Pulmonary Tuberculosis Diagnostic Criteria (WS288–2017), 2012 Nontuberculous Mycosis Diagnosis and Treatment Expert Consensus,[10] the Chinese TB Volume of Clinical Diagnosis and Treatment guidelines (Chinese Medical Association, 2005), and the updated World Health Organization guidelines. HIV was diagnosed based on the Chinese HIV and HIV Infection Diagnostic Criteria. HIV clinical staging was based on the 1993 Centers for Disease Control diagnostic criteria.[11]
Statistical analysis
Data were analyzed using SPSS Statistics Client 19.0 (SPSS Inc., Chicago, IL, USA). The measurement data of normal distribution were expressed as mean ± standard deviation (SD) and data of non-normal distribution were expressed as median (inter-quartile range [IQR]). The categorical variables were expressed as the number and percentage. The chi-square (χ2) analysis was used to analyze the drug resistance rate of nine antituberculosis drug (ATD) strains of NTM within 5 years; the level of statistical significance was set at P < 0.05.
Results
Patient characteristics
From January 2014 to December 2018, 59 patients coinfected with HIV and NTM were randomly selected. Fifty-six (94.9%) patients had respiratory distress, two (3.4%) had cerebrospinal fluid-related complications, and one (1.7%) had ascites. Fifty patients were male. The mean age was 45 years (range, 22–83 years) [Table 1].
Table 1.
Clinical features of nontuberculous mycobacteria in HIV-infected patients during 2014–2018 in Sichuan, China (n = 59).
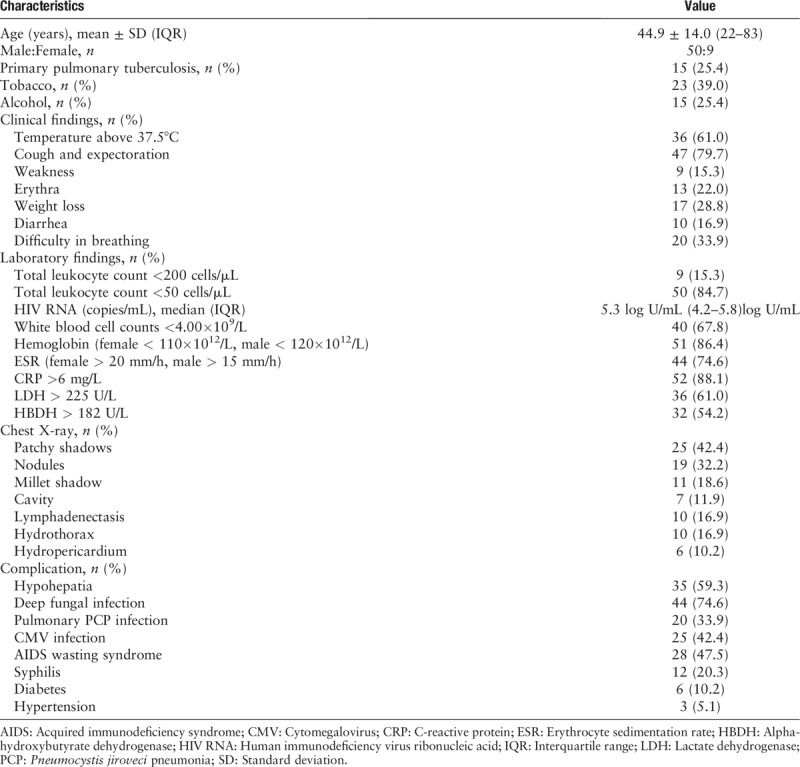
Thirty-four (57.6%) patients acquired HIV through heterosexual contact, eight (13.6%) through homosexual contact, five (8.5%) were injection drug users, one (1.7%) had a history of blood transfusion, one (1.7%) had a history of heterosexual contact and blood transfusion, two (3.4%) had a history of heterosexual contact and were injection drug users, and eight (13.6%) had unknown etiology. Fifteen (25.4%) patients had primary pulmonary tuberculosis. The most common clinical symptoms were cough and expectoration (79.7%), followed by fever (61.0%), difficulty in breathing (33.9%), weight loss (28.8%), diarrhea (16.9%), and weakness (15.3%) [Table 1].
Fifty-one (86.4%) patients had at least one AIDS-defining disease other than NTM infection: cytomegalovirus infection was found in 25/59 (42.4%), pneumocystis pneumonia in 20/59 (33.9%), deep fungal infection in 44/59 (74.6%), and syphilis in 12/59 (20.3%). Fifty-eight (98.3%) patients had at least one underlying condition: hypohepatia was found in 35/59 (59.3%), anemia in 51/59 (86.4%), AIDS wasting syndrome in 28/59 (47.5%), hepatitis B virus infection in 2/59 (3.4%), diabetes mellitus in 6/59 (10.2%), and hypertension in 3/59 (5.1%).
Laboratory and imaging findings
The median CD4 count was 26 cells/μL (range, 1–179 cells/μL), with 50 (84.7%) patients having a CD4 count <50 cells/μL and nine (15.3%) having <200 cells/μL. The median HIV RNA count was 5.3 log U/mL (4.2-5.8)log U/mL. The most common laboratory finding was reduction in white blood cell counts in 40/59 (67.8%) and increase in erythrocyte sedimentation rate in 44/59 (74.6%), C-reactive protein in 52/59 (88.1%), lactate dehydrogenase in 36/59 (61.0%), and alpha-hydroxybutyrate dehydrogenase in 32/59 (54.2%). The main imaging changes shown in chest computed tomography included patchy shadows in 25/59 (42.4%), nodules in 19/59 (32.2%), millet shadow in 11/59 (18.6%), cavity in 7/59 (11.9%), lymphadenectasis in 10/59 (16.9%), hydrothorax in 10/59 (16.9%), and hydropericardium in 6/59 (10.2%) [Table 1].
NTM pathogenic spectrum and drug resistance situation
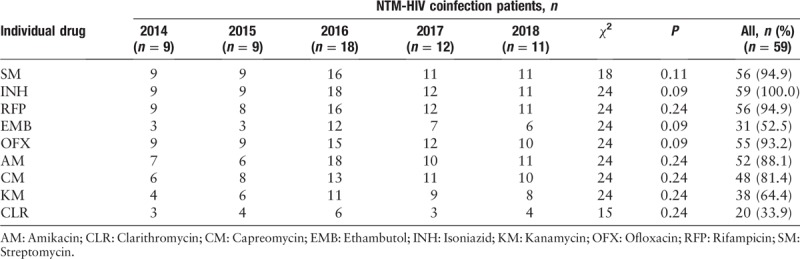
From the 59 patients coinfected with HIV and NTM, seven kinds of NTM species/complex were identified. The most dominant NTM was M. avium–intracellulare complex (MAC), which accounted for 52.5%. The M. kansasii was the next most prevalent species (27.1%), followed by M. chelonae/M. abscessus (13.6%), M. scrofulaceum (3.4%), M. fortuitum (1.7%), and M. xenopi (1.7%). DST of 59 NTM strains showed the resistance rate of the drugs, listed here in the descending order as follows: isoniazid (100.0%), rifampicin (94.9%), streptomycin (94.9%), ofloxacin (93.2%), amikacin (88.1%), capreomycin (81.4%), kanamycin (64.4%), ethambutol (52.5%), and clarithromycin (33.9%). No significant change was observed in the drug resistance rate of NTM strain against nine ATDs in 5 years: isoniazid (χ2 = 24, P = 0.11), rifampicin (χ2 = 24, P = 0.24), streptomycin (χ2 = 18, P = 0.12), ofloxacin (χ2 = 24, P = 0.09), amikacin (χ2 = 24, P = 0.24), capreomycin (χ2 = 24, P = 0.24), kanamycin (χ2 = 24, P = 0.24), ethambutol (χ2 = 24, P = 0.09), and clarithromycin (χ2 = 15, P = 0.24) [Table 2].
Table 2.
Drug resistance of nontuberculous mycobacteria in HIV-infected patients during 2014–2018 in Chengdu, China.
Discussion
NTM exists ubiquitously in the environment. In recent years, reports of NTM infections have increased worldwide.[12–14] NTM causes many kinds of diseases in mostly aggressive people with low immunity. Basic pulmonary disease and HIV infection cause lesions in multiple organs and tissues.[2] A previous study found that in 58 clinical isolations of NTM-caused diseases, 8/58 (17%) patients with NTM isolated from the Sichuan Chengdu area of China had associated HIV infection. The pathogen spectrum of the isolated strains was predominantly MAC and M. kansasii.[12] In this study, a total of 59 patients coinfected with HIV and NTM strains were isolated, mainly including 31 (52.5%) patients with MAC, 17 (27.1%) with M. kansasii, and 8 (13.6%) with M. chelonae/abscessus complex, which was basically consistent with the previous studies and reports in recent years.[8,12,15] The infection rate and distribution of NTM were greatly different from those in the economically developed levels and regions. It has been reported that in Canada and southern England, M. xenopi was second only to MAC in clinically isolated NTM strains.[16] Many reports of human pathogenicity caused by M. scrofulaceum, M. fortuitum, and M. xenopi have been published.[17–19] However, such strains of NTM in HIV-infected patients were rare. In this study, two strains of M. scrofulaceum, one of M. fortuitum, and one of M. xenopi causing lung infections were isolated from the 59 patients. This also means that with the continuous economic development in China, the prevention and treatment of this rare NTM should be emphasized besides the prevention of common NTM pathogenic bacteria, such as MAC and M. kansasii, in patients with AIDS.
HIV-infected patients, especially in the AIDS stage are infected not only with NTM but also with some other pathogenic bacteria because of their weakened immunity. In this study, the 59 patients coinfected with HIV and NTM were associated with different degrees of infection, including deep fungal infection, pneumocystis pneumonia, cytomegalovirus, and syphilis. Fifteen patients (25.4%) had a history of primary tuberculosis. The most common clinical symptoms were fever, cough, expectoration, weight loss, and erythra. Patients coinfected with HIV and NTM also had different degrees of hypohepatia and anemia, and a few with diabetes and hypertension. The patients acquired HIV mainly through homosexual/heterosexual contact, drug abuse, and blood transfusion. This study showed that the CD4 count of the patients were all low, with 84.7% having less than 50 cells/μL CD4 count and 15.3% having less than 200 cells/μL CD4 count. This means that lower CD4 count, especially less than 50 cells/μL, is a risk factor for the NTM infection in HIV-infected patients. At the same time, the majority of patients coinfected with HIV and NTM showed a reduction in the white blood cell counts and increase in ESR, C-reactive protein, lactate dehydrogenase, and alpha-hydroxybutyrate dehydrogenase. The main imaging changes in the chest computed tomography included patchy shadows, nodules, millet shadow, cavity, lymphadenectasis, hydrothorax, and hydropericardium.
Most NTM are naturally drug-resistant to many ATDs. This is also one of the reasons why treatment for HIV-infected NTM infection is difficult and unsuccessful. In China, the drug resistance rate of NTM to first-line ATDs is 96%, and the multidrug resistance rate is up to 84%, according to the fourth national tuberculosis epidemiology sample survey.[20] This study demonstrated that the patients coinfected with HIV and NTM exhibited different degrees of drug tolerance to ATDs. The rate of isoniazid resistance (100.0%) was the highest, followed by rifampicin (94.9%), streptomycin (94.9%), ofloxacin (93.2%), amikacin (88.1%), capreomycin (81.4%), kanamycin (64.4%), ethambutol (52.5%), and clarithromycin (33.9%). For patients coinfected with HIV and NTM disease, timely information on the drug resistance of NTM strains can better guide clinical medication.
In conclusion, the most dominant NTM in HIV-infected patients are MAC and M. kansasii in the Chengdu region of China. All patients have a lower CD4 count. This further explains that lower CD4 count, especially less than 50 cells/μL, is a risk factor for the development of NTM infection in HIV-infected patients. The drug resistance of NTM strains to HIV-infected patients is severe, with relatively low drug resistance to kanamycin, ethambutol, and clarithromycin. These drugs can be given priority in clinic when NTM drug sensitivity results are not obtained.
Funding
This study was supported by grants from the Youth Innovation in Medical Research Subject in Sichuan Province of China (No. Q17020), the Chengdu Science and Technology Bureau (No. 2018JY0383), and the Health Planning Committee of Sichuan Province (No. 18PJ015).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang MD, Liao Y, Li QF, Zhu M, Wu GH, Xu YH, Zhong J, Luo J, Li YJ. Drug resistance and pathogenic spectrum of patients coinfected with nontuberculous mycobacteria and human-immunodeficiency virus in Chengdu, China. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000235
Dong-Mei Wang and Yi Liao contributed equally to this work.
References
- 1.Maura J, Donohue Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect Dis 2018; 10:163.doi: 10.1186/s12879-018-3043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian SN, Zhang LF, Zhang YQ, Yang QW, Wang P, Xu YC, et al. Clinical and laboratory characteristics of patients with nontuberculous mycobacterium bloodstream infection in a tertiary referral hospital in Beijing, China [in Chinese]. Chin Med J 2016; 129:2220–2225. doi: 10.4103/0366-6999.189920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varley CD, Ku JH, Henkle E, Schafer SD, Winthrop KL. Disseminated nontuberculous mycobacteria in HIV-infected patients, Oregon, USA, 2007-2012. Emerg Infect Dis 2017; 23:533–535. doi: 10.3201/eid2303.161708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi J, Nikoomanesh K, Uppal J, Wang S. Progressive disseminated histoplasmosis with concomitant disseminated nontuberculous mycobacterial infection in a patient with AIDS from a nonendemic region (California). BMC Pulm Med 2019; 19:46.doi: 10.1186/s12890-019-0808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn SM, Shi HJ, Wang SH, Lee SK, Park SY, Lee JS, et al. Mycobacterium avium complex infection-related immune reconstitution inflammatory syndrome mimicking lymphoma in an human immunodeficiency virus-infected patient. Infect Chemother 2018; 50:350–356. doi: 10.3947/ic.2018.50.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marino A, Caltabiano E, Zagami A, Onorante A, Zappalà C, Locatelli ME, et al. Rapid emergence of cryptococcal fungemia, Mycobacterium chelonae vertebral osteomyelitis and gastro intestinal stromal tumor in a young HIV late presenter: a case report. BMC Infect Dis 2018; 18:693.doi: 10.1186/s12879-018-3573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang S, Chiu APY, Lin Q, Zeng Z, Li Y, Zhang Y, et al. HIV epidemics in Shenzhen and Chongqing, China. PLoS One 2018; 13:e192849.doi: 10.1371/journal.pone.0192849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Chow EP, Su S, Yiu WL, Zhang X, Iu KI, et al. A systematic review and meta-analysis of the prevalence, trends, and geographical distribution of HIV among Chinese female sex workers (2000-2011): implications for preventing sexually transmitted HIV. Int J Infect Dis 2015; 39:76–86. doi: 10.1016/j.ijid.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Yang Y, Shi X, Mao S, Shi N, Hui X. The spatial distribution pattern of human immunodeficiency virus/acquired immune deficiency syndrome in China. Geospat Health 2016; 11:414.doi: 10.4081/gh.2016.414. [DOI] [PubMed] [Google Scholar]
- 10.Editorial Board of CSTB/Chin J Tuberc Respir Dis. Nontuberculous mycosis diagnosis and treatment expert consensus [in Chinese]. Chin J Tuberc Respir Dis 2016; 39:438–443. doi: 10.3760/cma.j.issn.1001-0939.2012.08.006. [Google Scholar]
- 11.From the Centers for Disease Control and prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA 1993; 269:460. [PubMed] [Google Scholar]
- 12.Wang DM, Zhu M, Xu YH, Luo J, Li YJ, Liu XD, et al. Clinical relevance and drug resistance of nontuberculous mycobacteria, Sichuan area in Chinese [in Chinese]. Chin J Infect Dis 2016; 34:520–523. doi: 10.3760/cma.j.issn.1000-6680.2016.09.002. [Google Scholar]
- 13.Shao Y, Chen C, Song HH, Li GL, Liu Q, Li Y, et al. The epidemiology and geographic distribution of nontuberculous mycobacteria clinical isolates from sputum samples in the Eastern Region of China. PLoS Negl Trop Dis 2015; 9:e0003623.doi: 10.1371/journal.pntd.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CR, Russell C, Soll B, Chow D, Bamrah S, Brostrom R, et al. Bankowski increasing prevalence of nontuberculous mycobacteria in respiratory specimens from US-Affiliated Pacific Island Jurisdictions. Emerg Infect Dis 2018; 24:485–491. doi: 10.3201/eid2403.171301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Deng XZ, Hu FY, Chen WS, Chen XJ, Cai WP, et al. Pathogenic spectrum, clinical features and drug resistance of pneumonia caused by nontuberculous mycobacteria in acquired immunodeficiency syndrome patients in Chinese [in Chinese]. Chin J Infect Dis 2017; 35:3.doi: 10.3760/cma.j.issn.1000-6680.2017.03.004. [Google Scholar]
- 16.Li ZX. Nontuberculous mycobacteria and clinical infections. Beijing Sci Press 2015; 1:151. [Google Scholar]
- 17.Takemoto Y, Tokuyasu H, Ikeuchi T, Nakazaki H, Nakamatsu S, Kakite S, et al. Disseminated Mycobacterium scrofulaceum infection in an immunocompetent host. Intern Med 2017; 15:1931–1935. doi: 10.2169/internalmedicine.56.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamori S, Asakura T, Nishimura T, Tamizu E, Ishii M, Yoshida M, et al. Natural history of Mycobacterium fortuitum pulmonary infection presenting with migratory infiltrates: a case report with microbiological analysis. BMC Infect Dis 2018; 18:1.doi: 10.1186/s12879-017-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluth MH, Vera R, Razeq J, Kramer M, Abu-Lawi KI. Mycobacterium xenopi: evidence for increased rate of clinical isolation. Int J Biomed Sci 2009; 5:96–100. [PMC free article] [PubMed] [Google Scholar]
- 20.Duanmu H. National Technical Steering Group of the Epidemiological Sampling Survey for Tuberculosis. Report on fourth national epidemiological sampling survey of tuberculosis [in Chinese]. Chin J Tubere Respir Dis 2002; 25:3–7. doi: 10.3760/j.issn:1001-0939.2002.01.002. [PubMed] [Google Scholar]


