Abstract
Background:
Acute kidney injury (AKI) is a serious complication in critically ill patients with septic shock treated in the intensive care unit. Renal replacement therapy (RRT) is a treatment for severe AKI; however, the time of initiation of RRT and factors that affect the recovery of kidney function remains unclear. This study was to explore whether early initiation of RRT treatment for fluid management to reduce central venous pressure (CVP) can help to improve patients’ kidney function recovery.
Methods:
A retrospective analysis of septic patients who had received RRT treatment was conducted. Patients received RRT either within 12 h after they met the diagnostic criteria of renal failure (early initiation) or after a delay of 48 h if renal recovery had not occurred (delayed initiation). Parameters such as patients’ renal function recovery at discharge, fluid balance, and levels of CVP were assessed.
Results:
A total of 141 patients were eligible for enrolment: 40.4% of the patients were in the early initiation group (57 of 141 patients), and 59.6% were in the delayed initiation group (84 of 141 patients). There were no significant differences in the characteristics at baseline between the two groups, and there were no differences in 28-day mortality between the two groups (χ2 = 2.142, P = 0.143); however, there was a significant difference in the recovery rate of renal function between the two groups at discharge (χ2 = 4.730, P < 0.001). More importantly, early initiation of RRT treatment and dehydration to reduce CVP are more conducive to the recovery of renal function in patients with AKI.
Conclusion:
Compared with those who received delayed initiation RRT, patients who received early-initiation RRT for dehydration to reduce CVP have enhanced kidney function recovery.
Keywords: Sepsis, Acute kidney injury, Renal replacement therapy, Central venous pressure
Introduction
Acute kidney injury (AKI) is a serious complication in critically ill patients treated in the intensive care unit (ICU) because of high mortality and economic conditions.[1–3] Sepsis is the leading cause of AKI in the ICU, and approximately 45% to 70% of all AKI is associated with sepsis.[4–6] Compared with patients with AKI without septic shock, patients with AKI and septic shock may have a different response to renal replacement therapy (RRT).[7]
The most common treatment of septic AKI is RRT when supportive therapy and the level of endogenous renal function is not sufficient to meet the patients’ metabolic demands.[8,9] Many studies have focused on methods of RRT.[10,11] However, in the absence of complications, such as hyperkalemia, the appropriate time of initiation of RRT, and the key indicators affecting the recovery of renal function remain unclear. Recently, several conflicting results have been reported in studies comparing an early strategy with a delayed strategy for the initiation of RRT.[12] Therefore, whether early initiation of RRT can promote renal function recovery and improve survival rate and whether there are risks of early RRT associated with renal function recovery in patients with septic AKI are still unknown.
Several studies determine “recovery” at hospital discharge.[13,14] The recent Acute Disease Quality Initiative conference suggests differentiating AKI from acute kidney disease (AKD) and chronic kidney disease (CKD), which may provide a framework for defining recovery in terms of time after the sentinel event.[15] AKD was initially proposed to encompass any acute condition that impacts on long-term kidney function.[16]
Fluid overload at the initial time of RRT is a well-known predictor of patient survival.[17] Some studies emphasized the association between the speed of fluid accumulation and ICU mortality.[18] However, fluid overload has been mostly quantified as an arithmetical calculation, and it does not accurately reflect the actual liquid state of the body, especially the interstitial edema pressure and reflux resistance. Other studies have demonstrated that an increased central venous pressure (CVP) was associated with the development of AKI because a higher CVP is accompanied by microcirculation impairment and increased organ reflux resistance.[19,20] It is not clear whether early initiation of RRT for dehydration alone or to reduce CVP is conducive to the recovery of renal function.
Thus, this retrospective study examined the records of patients with septic AKI admitted to the department of critical care medicine of our hospital from May 2016 to February 2018. Baseline and 5-day clinical data for all enrolled patients were retrieved, and we investigated factors that may affect renal function recovery and the effect of different RRT treatment initiation schedules on patient mortality.
Methods
Ethics approval
This study was approved by the Peking Union Medical College Hospital Ethics Committee (No. SK-411), and the need for informed consent was waived because the study was non-interventional and retrospective.
Patient selection
Patients admitted to the department of critical care medicine of our hospital from May 2016 to February 2018 who had been diagnosed with septic AKI and who received RRT were consecutively selected for the study. Patients who were younger than 18 years old, who were pregnant women, or who had pre-existing CKD were excluded from the study. For the assessment of the effect of RRT on the recovery of renal function after acute renal injury, patients treated with RRT before ICU admission or who had been treated with RRT for non-renal indications or for end-stage renal failure on long-term dialysis were excluded. AKI caused by permanent occlusion or surgical lesion of the renal artery, glomerulonephritis, interstitial nephritis, vasculitis, post-renal obstruction, or hemolytic uremic syndrome or thrombotic thrombocytopenic purpura were excluded. We also excluded patients for prior kidney transplantation and excluded those who had undergone RRT for <24 h or who had an ICU stay of <72 h.
Definition of early and delayed initiation of RRT
AKI was diagnosed based on changes in the serum creatinine (Cr), urine output, or both. Cr measurements were performed at least once per day. Every patient had a urinary catheter, and urine output was measured every hour. The early initiation of RRT was started within 12 h after the onset of AKI that was determined to be at the failure stage of the risk, injury, failure, loss, and end-stage kidney disease (RIFLE) classification. With the delayed initiation, RRT was initiated after a delay of 48 h if renal function did not spontaneously recover and if no condition meeting the criteria for emergency RRT developed.[21] The failure stage of the RIFLE classification is characterized as follows: oliguria (urine output <0.3 mL per kilogram of body weight per hour for ≥24 h), anuria for 12 h or more, or a serum Cr level three times the baseline level (or ≥4 mg per decilitre [≥350 μmol per litre] accompanied by a rapid increase of ≥0.5 mg per decilitre [≥44 μmol per litre]).[22]
RRT procedure
RRT was performed with the Baxter system in the ICU. Bicarbonate-buffered solution was used with a replacement fluid amounting to 30 to 40 mL/kg per hour, with a blood flow rate of 150 to 200 mL/min. Heparin or low-molecular-weight heparin was used as an anticoagulant in the treatment modalities. The dosage of heparin or low-molecular-weight heparin was regulated according to the patient's blood coagulation state. Changes in blood and replacement fluid flow rates and dialysate composition, as well as type of anticoagulant, were dictated by the patients’ clinical condition. According to the standard requirements of our department, the CVP level of patients in RRT is maintained at the lowest possible level within the allowable range, and RRT was discontinued if renal recovery defined by urine output (>400 mL/24 h without and 1000 mL/24 h with diuretic treatment) and Cr clearance (>20 mL/min) occurred.
Data collection
Baseline data and biochemical parameters measured over 5 days after ICU admission were collected, including age, gender, infection site, complications potentially related to AKI (such as hypertension, diabetes, coronary heart disease), use and/or change in doses of vasopressor drugs, duration of mechanical ventilation, duration of RRT, Cr, daily urine output, fluid balance in the first 5 days after initiation of RRT, ICU length of stay, hospital length of stay, and mortality at 90 days. In addition, clinical parameters and severity scores, such as CVP, mean arterial pressure (MAP), diastolic blood pressure (DAP), mean perfusion pressure, diastolic perfusion pressure (DPP), Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, and sequential organ failure assessment (SOFA) scores, were also collected.
Outcome measures
Despite many limitations, the most obvious definition of complete recovery from AKI is the absence of AKI criteria. Partial recovery can then be defined as a decrease in AKI stage.[23] Kidney function is defined as non-recovery when patients need intermittent hemodialysis. Therefore, the recovery of renal function was divided into three categories: complete recovery, partial recovery, and non-recovery, and we evaluated renal function recovery at discharge.
Statistical analysis
The unreasonable values, such as abnormal outliers, were considered missing values. The results are expressed as the mean standard deviation for normal data or median (interquartile range) for non-normal data. The χ2 test was used for qualitative data, one-way analysis of variance was used for normal quantitative data, and the Mann-Whitney U test or Kruskal-Wallis H test was used for non-normal quantitative data to determine differences between groups. Ordinal polytomous logistic regression was used to analyze the relationship between factors and outcomes. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
General characteristics
A total of 141 patients with septic AKI were enrolled: 57 patients were in the early initiation group, and 84 were in the delayed initiation group. A flow chart is shown in Figure 1. In general, the main infection sites were the lungs (20.9%), blood (20.6%), abdominal cavity (9.9%), soft tissue (4.8%), and other sites (29.9%, such as in the urinary system, bone marrow, and intracranial tissue). Gram-negative bacteria (48.7%) were the most common organisms. There was no statistically significant difference in infection site between the two groups (P = 0.563). Parameters such as age, gender, body mass index, basic disease (such as hypertension, coronary heart disease, and diabetes), APACHE II score, SOFA score, and the application of vasoactive drugs or extracorporeal membrane oxygenation were not different between the two groups (P > 0.05). The patients’ demographic and clinical characteristics are shown in Table 1.
Figure 1.
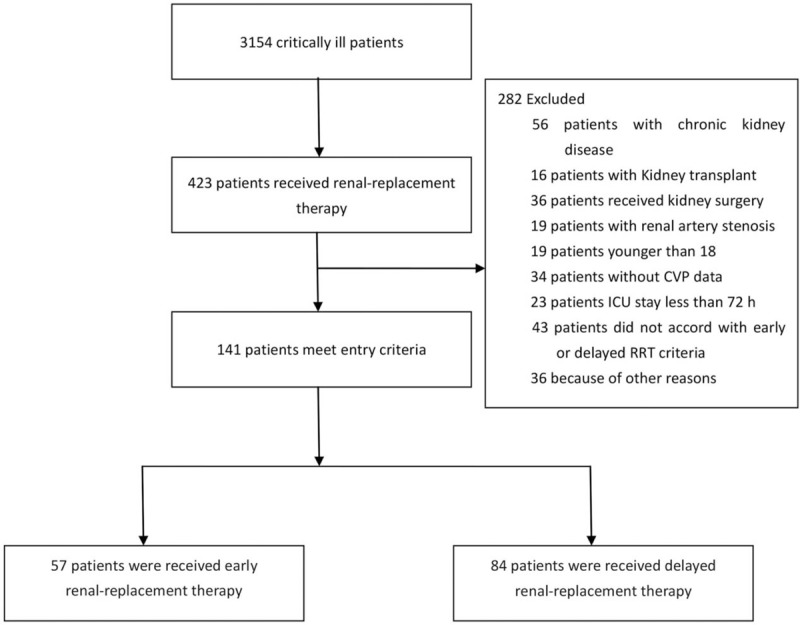
The flowchart of this study on patients with septic acute kidney injury. CVP: Central venous pressure; RRT: Renal replacement therapy.
Table 1.
Baseline clinical and biological data of patients who received RRT.
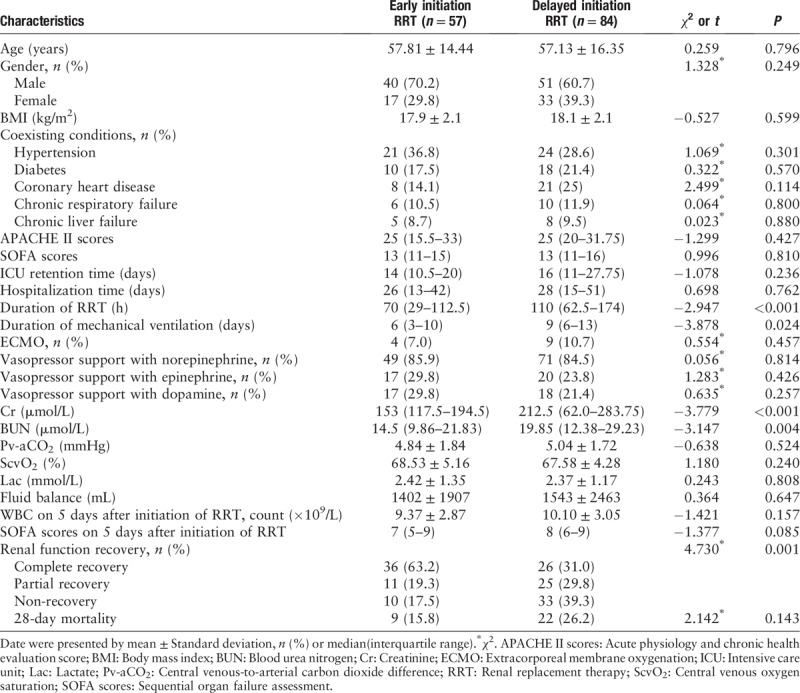
There were differences in the duration of mechanical ventilation among the two groups (6 vs. 9 days, P = 0.024) [Table 1], and the total RRT time was significantly different between the two groups (P < 0.001). However, the ICU retention time and hospitalization time were not different between the two groups (14 vs. 16 days, P = 0.236; 26 vs. 28 days, P = 0.762, respectively). Cr and blood urea nitrogen (BUN) levels were different at the start of RRT between the two groups. There were no differences between the two groups in mortality at discharge. However, renal function recovery between the two groups showed significant differences.
Patients were divided into three groups according to the recovery of renal function, and we analyzed the factors that may affect renal function recovery. Our study found that there was no significant differences in Cr, BUN, MAP, or DAP at the initiation of RRT (P > 0.05). However, the early or delayed strategy of RRT was different between different groups, and the level of CVP, DPP, and fluid balance was different between those groups. At the same time, we did not find any difference in the venous-to-arterial carbon dioxide (Pv-aCO2) or ScvO2 of different groups, which means that the cardiac function of those patients was not a factor affecting renal function in any of the three groups [Table 2].
Table 2.
Baseline clinical and biological data of patients with different renal function recovery.
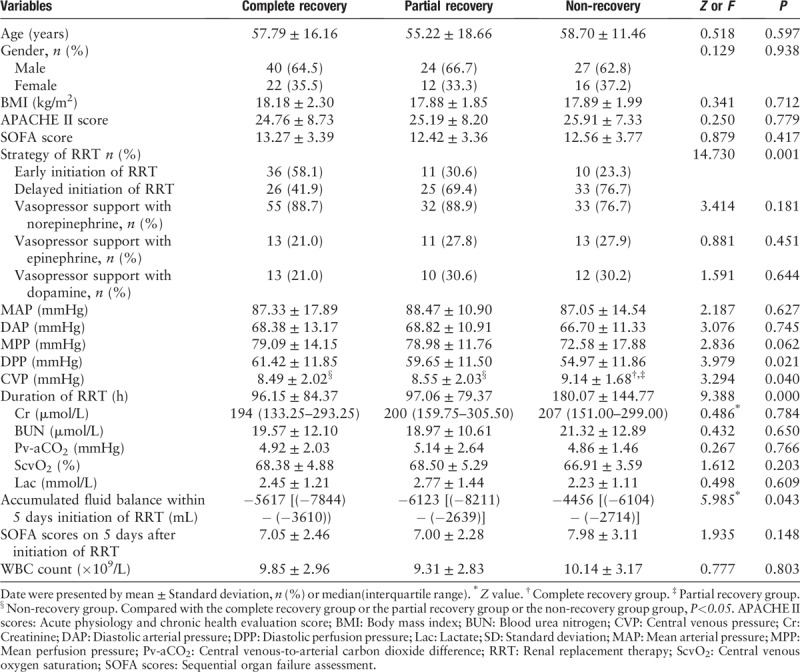
When we evaluated the CVP levels in different renal function recovery conditions, we found that in those patients who received early initiation RRT, CVP decreased significantly and correlated with rapid renal function recovery at discharge (P < 0.05) [Figure 2].
Figure 2.
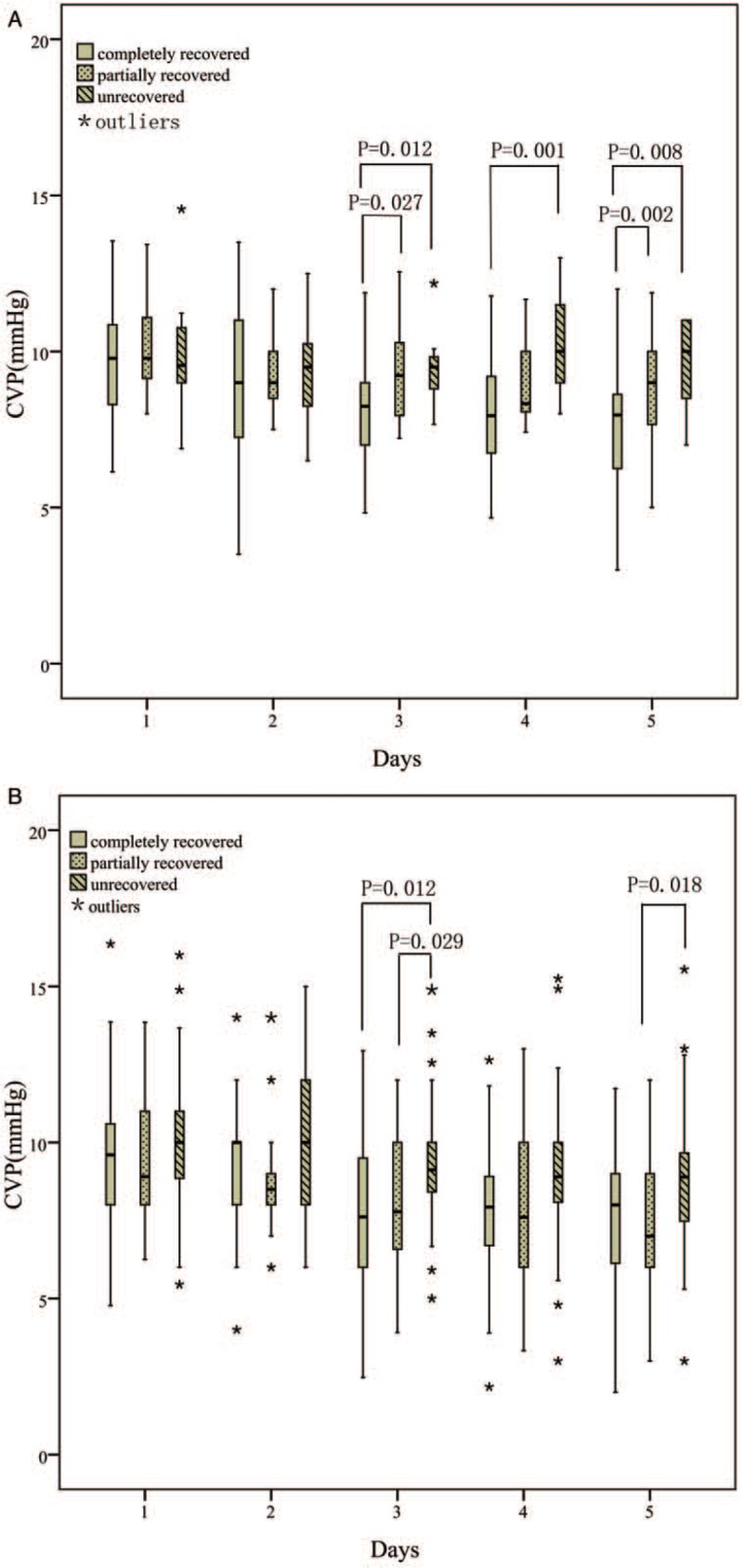
Relationship between renal function recovery and CVP. (A) Relationship between renal function recovery and CVP in patients who received early initiation of RRT; (B) Relationship between renal function recovery and CVP in patients who received delayed initiation RRT. CVP: Central venous pressure; RRT: Renal replacement therapy.
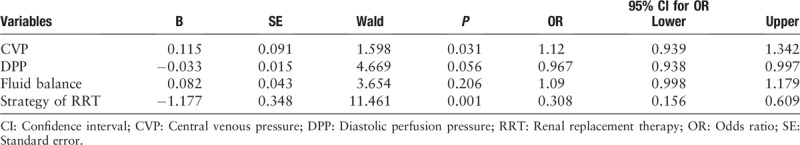
We conducted an ordinal polytomous logistic regression analysis of the factors that may affect the recovery of renal function, and we found that early strategy of RRT and a decline of CVP can promote the recovery of renal function (P = 0.031, P = 0.001; respectively) [Table 3].
Table 3.
Ordinal ploytomous logistic regression analysis for possible risk factors for renal function recovery.
Discussion
This study indicated that in patients with septic AKI, early initiation RRT for fluid management improved the recovery of kidney function. In addition, the recovery of renal function was correlated with the decrease of CVP level, this effect was more prominent in patients who received early initiation RRT.
The timing of initiation of RRT depends on numerous factors and is, therefore, a complex process.[24] Some previous studies suggested that a survival advantage is associated with early RRT.[25,26] Recently, two randomized, controlled studies explored this question, but they came to the opposite conclusion.[12,27] Our trial found no significant difference in mortality at 28 days between the early initiation renal replacement group and the delayed strategy group; this result is consistent with previous reports.[27] Although there was no difference in mortality between the two groups, we found some notable differences in clinical outcomes that merit further discussion. First, we found that delayed initiation of RRT was generally associated with a longer duration of RRT support and mechanical ventilation time. Second, we found that delayed initiation of RRT was associated with inferior renal recovery and a higher rate of dialysis dependence at hospital discharge.
The cause of kidney injury is not only the toxic effect of Cr and BUN but also the damaging effect of a high level of CVP and fluid overload on the kidney with a long duration.[28] The pathophysiology of congestive renal failure includes reduced transglomerular pressure, elevated renal interstitial pressure, and enhanced proinflammatory pathways.[29] Earlier initiation of dialysis in AKI might improve the control of acid-base status and the removal of toxins; these changes can reduce the burden on the kidneys, thereby preventing further deterioration of renal function.[30] The effect of fluid management and CVP on the recovery of renal function in RRT treatment is still unclear.
In this study, we compared patients with different levels of recovery of renal function and found that the recovery of renal function was correlated with the initiation of RRT, CVP levels, liquid equilibrium state, and DPP levels. Further regression analysis of these factors showed that the main factors affecting the recovery of renal function were the initiation of RRT and the levels of CVP. The liquid equilibrium state was statistically insignificant in the regression analysis; a possible explanation for this phenomenon is that the liquid overload state in patients is not positively correlated with CVP and that the backward resistance of the systemic circulation may be more important than the overall liquid balance state in patients with AKI.[31]
Previous studies have found that the baseline CVP of patients with AKI is higher than that of patients without AKI.[19] Damman et al[32] confirmed that CVP affected kidney function not only in patients with heart failure but also in patients with normal heart function. Our study found that the CVP levels of patients with rapid renal function recovery were lower than those whose kidney function recovered more gradually; this phenomenon was more obvious in patients who received early-initiation RRT. More importantly, when different renal function recovery situations were compared, there were no differences in other factors such as the Pv-aCO2 and ScvO2, suggesting that there is no significant difference in heart function between patients with different kidney function recovery. These results fully demonstrated that early application of RRT to reduce CVP was crucial for renal function recovery.
Maintaining optimal blood pressure is an important aspect of preventing AKI, especially for vasopressor-dependent patients.[33] MAP is widely used as an index for the optimal blood pressure.[34] However, a recent observational study revealed that lower DAP and higher CVP were associated with septic AKI, while MAP was not.[35] Another observational study reported that decreased MAP was not associated with AKI, suggesting that only DPP and CVP may be associated with septic AKI.[36] Our study confirmed that high CVP and low DPP were important factors affecting renal function recovery. At the same time, it is more likely that different levels of CVP lead to different DPP. Therefore, high CVP is the most important factor affecting renal perfusion pressure.
There were several limitations to this study. First, this was a retrospective study, and the number of patients was small, which was likely to introduce bias. Second, various factors, such as positive end-expiratory pressure was not investigated. Third, the lack of long-term renal function indicators and the speed of renal function recovery may have an impact on long-term renal function and quality of life. Additional studies are required to investigate the effect of different treatment strategies on long-term renal function.
In conclusion, our study confirmes that early initiation RRT for fluid management to reduce CVP levels is conducive to the recovery of renal function in patients with sepsis kidney injury compared with the delayed-initiation RRT strategy.
Conflicts of interest
None.
Footnotes
How to cite this article: Xing ZQ, Liu DW, Wang XT, Long Y, Zhang HM, Pan P, Su LX. Early initiation renal replacement therapy for fluid management to reduce central venous pressure is more conducive to renal function recovery in patients with acute kidney injury. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000240
References
- 1.Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, et al. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med 2011; 39:2659–2664. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste EA, Kellum JA, Katz NM, Rosner MH, Haase M, Ronco C. Epidemiology of acute kidney injury. Contrib NephrolV 165 2010; 1–8. doi: 10.1159/000313737. [DOI] [PubMed] [Google Scholar]
- 3.Kim SY, Kim YN, Shin HS, Jung Y, Rim H. The influence of hypophosphatemia on outcomes of low- and high-intensity continuous renal replacement therapy in critically ill patients with acute kidney injury. Kidney Res Clin Pract 2017; 36:240–249. doi: 10.23876/j.krcp.2017.36.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care (London, England) 2012; 16:313.doi: 10.1186/cc11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quenot JP, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, et al. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care (London, England) 2013; 17:R65.doi: 10.1186/cc12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, et al. Acute kidney injury in sepsis. Intensiv Care Med 2017; 43:816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 8.Ronco C, Ricci Z, De Backer D, Kellum JA, Taccone FS, Joannidis M, et al. Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care (London, England) 2015; 19:146.doi: 10.1186/s13054-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neri M, Villa G, Garzotto F, Bagshaw S, Bellomo R, Cerda J, et al. Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Crit Care (London, England) 2016; 20:318.doi: 10.1186/s13054-016-1489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonventre JV. Daily hemodialysis – will treatment each day improve the outcome in patients with acute renal failure? N Engl J Med 2002; 346:362–364. doi: 10.1056/nejm200201313460512. [DOI] [PubMed] [Google Scholar]
- 11.Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet (London, England) 2006; 368:379–385. doi: 10.1016/s0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 12.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstadt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN Randomized Clinical Trial. JAMA 2016; 315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 13.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after Acute Kidney Injury. Am J Respir Crit Care Med 2017; 195:784–791. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heung M, Steffick DE, Zivin K, Gillespie BW, Banerjee T, Hsu CY, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis 2016; 67:742–752. doi: 10.1053/j.ajkd.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 16.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 17.Neyra JA, Li X, Canepa-Escaro F, Adams-Huet B, Toto RD, Yee J, et al. Cumulative fluid balance and mortality in septic patients with or without acute kidney injury and chronic kidney disease. Crit Care Med 2016; 44:1891–1900. doi: 10.1097/ccm.0000000000001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garzotto F, Ostermann M, Martin-Langerwerf D, Sanchez-Sanchez M, Teng J, Robert R, et al. The dose response multicentre investigation on fluid assessment (DoReMIFA) in critically ill patients. Crit Care (London, England) 2016; 20:196.doi: 10.1186/s13054-016-1355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vellinga NA, Ince C, Boerma EC. Elevated central venous pressure is associated with impairment of microcirculatory blood flow in sepsis: a hypothesis generating post hoc analysis. BMC Anesthesiol 2013; 13:17.doi: 10.1186/1471-2253-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyere R, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018; 379:1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 22.Lin CY, Chen YC. Acute kidney injury classification: AKIN and RIFLE criteria in critical patients. World J Crit Care Med 2012; 1:40–45. doi: 10.5492/wjccm.v1.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettila V, Prowle JR, et al. Renal recovery after acute kidney injury. Intensiv Care Med 2017; 43:855–866. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou YH, Huang TM, Wu VC, Wang CY, Shiao CC, Lai CF, et al. Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care (London, England) 2011; 15:R134.doi: 10.1186/cc10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care (London, England) 2011; 15:R72.doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis 2008; 52:272–284. doi: 10.1053/j.ajkd.2008.02.371. [DOI] [PubMed] [Google Scholar]
- 27.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. New Engl J Med 2016; 375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 28.Gibney N, Hoste E, Burdmann EA, Bunchman T, Kher V, Viswanathan R, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol 2008; 3:876–880. doi: 10.2215/cjn.04871107. [DOI] [PubMed] [Google Scholar]
- 29.Ross EA. Congestive renal failure: the pathophysiology and treatment of renal venous hypertension. J Card Fail 2012; 18:930–938. doi: 10.1016/j.cardfail.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Wald R, Bagshaw SM. The timing of renal replacement therapy initiation in acute kidney injury: is earlier truly better?∗. Crit Care Med 2014; 42:1933–1934. doi: 10.1097/ccm.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 31.Chen KP, Cavender S, Lee J, Feng M, Mark RG, Celi LA, et al. Peripheral edema, central venous pressure, and risk of AKI in critical illness. Clin J Am Soc Nephrol 2016; 11:602–608. doi: 10.2215/cjn.08080715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009; 53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 33.Pilcher DV, Scheinkestel CD, Snell GI, Davey-Quinn A, Bailey MJ, Williams TJ. High central venous pressure is associated with prolonged mechanical ventilation and increased mortality after lung transplantation. J Thorac Cardiovasc Surg 2005; 129:912–918. doi: 10.1016/j.jtcvs.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 34.Sato R, Luthe SK, Nasu M. Blood pressure and acute kidney injury. Crit Care (London, England) 2017; 21:28.doi: 10.1186/s13054-017-1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care (London, England) 2013; 17:R278.doi: 10.1186/cc13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong BT, Chan MJ, Glassford NJ, Martensson J, Bion V, Chai SY, et al. Mean arterial pressure and mean perfusion pressure deficit in septic acute kidney injury. J Crit Care 2015; 30:975–981. doi: 10.1016/j.jcrc.2015.05.003. [DOI] [PubMed] [Google Scholar]


