Abstract
Background:
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) syndrome are highly prevalent respiratory conditions. Their coexistence is referred to as the overlap syndrome. They are both related to pulmonary hypertension (PH) development. This study investigated the effects of OSA on PH in patients with COPD and the associated factors.
Methods:
Consecutive patients with stable COPD were recruited for an observational cross-sectional study from September 2016 to May 2018 at Peking University Third Hospital. In total, 106 patients with COPD were enrolled and performed home portable monitoring and echocardiography. OSA was defined by an apnea hypopnea index (AHI) ≥10 events/h. Based on OSA absence or presence, patients were divided into the COPD with OSA and COPD without OSA groups. Factors affecting pulmonary artery pressure (PAP) and PH were identified using univariate analysis and logistic regression models.
Results:
In the 106 patients with COPD, the mean age was 69.52 years, 91.5% were men, and the mean forced expiratory volume in 1 s (FEV1) percentage of predicted was 56.15%. Fifty-six (52.8%) patients with COPD were diagnosed with OSA, and 24 (22.6%) patients with COPD were diagnosed as PH. Compared with COPD without OSA group, the median PAP in COPD with severe OSA group increased by 5 mmHg (36.00 [26.00–50.00] mmHg vs. 31.00 [24.00–34.00] mmHg, P = 0.036). COPD with percent of night-time spent with oxygen saturation below 90% (T90) > 10% group had higher PAP than COPD with T90 ≤ 1% group (36.00 [29.00–50.00)] mmHg vs. 29.00 [25.50–34.00] mmHg, F = 7.889, P = 0.007). Univariate analysis revealed age, FEV1% predicted, T90, and Charlson index had statistically significant effects on PH. Multiple regression analysis showed a significant and independent effect of both FEV1% predicted (odds ratio [OR] = 3.46; 95% confidence interval [CI]: 1.15–10.46; P = 0.028) and AHI (OR = 3.20; 95% CI: 1.09–19.35; P = 0.034) on PH.
Conclusions:
Patients with COPD with OSA are more susceptible to PH, which is associated with declining lung function and increased severity of OSA. Thus, nocturnal hypoxemia and OSA in elderly patients with COPD should be identified and treated.
Keywords: Chronic obstructive pulmonary disease, Echocardiography, Obstructive sleep apnea, Pulmonary hypertension
Introduction
Chronic obstructive pulmonary disease (COPD) occurs in 13.7% of adults in people aged 40 years or older in China.[1,2] Owing to the development of an aging population worldwide, the prevalence and mortality rate of COPD are increasing year by year. Obstructive sleep apnea (OSA) accounts for approximately 4.1% of the Chinese population.[3,4] OSA could cause daytime sleepiness, affect the quality of life and increase mortality of the individuals. The coexistence of both disorders is often referred to as the overlap syndrome, which occurs in approximately 1% of adults.[5]
Pulmonary hypertension (PH) is defined as an increase in mean pulmonary arterial pressure (PAP) >25 mmHg at rest as assessed by right heart catheterization.[6] Right heart failure is fatal in patients with COPD, and PH secondary to COPD is the primary cause of right heart failure. The prevalence of PH and cor pulmonale in COPD cases varies from 20.0% to 62.4%. PH is mainly due to chronic hypoxic pulmonary vasoconstriction of the small pulmonary arteries, eventually resulting in vascular remodeling.[7] In the recent 20 years, limited epidemiologic data have suggested that the prevalence of PH in OSA ranges from 17% to 53%.[8–12] Intermittent hypoxia, negative pleural pressure, and endothelial dysfunction have been shown to play an important role in the pathogenesis of PH in OSA.[13]
OSA can cause severe hypoxemia and hypercapnia at night. Hence, it is speculated that patients with OSA have a higher prevalence of PH, but few studies have investigated this relationship. Thus, the purpose of the current study was to investigate the effects of OSA on PH in patients with COPD and the associated factors by comparing the PAP and proportion of PH in patients with COPD with OSA and without OSA.
Methods
Ethical approval
The study was approved by the Ethics Committee of Third Hospital of Peking University (No. IRB00006761-M2016164). All patients gave their informed consent.
Patient recruitment
This cross-sectional study was performed from September 2016 to May 2018 at Peking University Third Hospital. Patients with stable COPD were invited to participate in study. Participants were allocated into either the group with OSA or into the group without OSA based on whether home portable monitoring evaluation revealed an apnea hypopnea index (AHI) of ≥10 events/h or <10 events/h, respectively. Inclusion criteria were as follows: (1) patients age >40 years; (2) outpatients diagnosed with COPD according to the criteria recommended by China Guidelines of COPD (revised version 2013)[14]; and (3) patients with stable COPD with no acute exacerbation of symptoms and upper respiratory tract infection in the 6 weeks preceding the study. Exclusion criteria were as follows: (1) patients with an active medical, neurologic or psychiatric disorder that could impact the results of the questionnaire; (2) patients with comorbidities that affected PH (such as acute heart failure, congenital cardiovascular disease, pulmonary embolism, obesity hypoventilation syndrome, central sleep apnea, neuromuscular disease, pulmonary arteritis caused by autoimmune diseases, and other chronic pulmonary diseases such as fibrosis); and (3) patients with near-terminal illness. During a clinical examination, height, weight, and neck circumference were measured. Exposure history of noxious particles, exacerbations in the past 12 months, therapy information, and comorbidities were recorded during a physician-led interview. Participants completed questionnaires related to the assessments of COPD and OSA. Pulmonary function data of patients in the last 6 months were recorded. Patients were asked to perform echocardiography and home portable monitoring.
Pulmonary function tests
A diagnosis of COPD was confirmed by reviewing the medical records for a clinical diagnosis of COPD[14] and spirometric data meeting the diagnostic criteria of global initiative for obstructive lung disease (GOLD) 2017.[15] Pulmonary function tests were performed with a spirometer (Medgraphics, Elite Series DL, St. Paul, MN, USA).
Home portable monitoring
All participants underwent nocturnal sleep monitoring using the ApneaLink™ (ResMed, MAP Medicine Technology, Martinsried, Germany) device at home. The device recorded the patient's nasal respiratory pressure signal, thoraco-abdominal movement, and oxygen saturation during sleep.[16] All physiologically important respiratory events were identified using the 2017 American Academy of Sleep Medicine (AASM) definition.[17] Apnea was diagnosed when the peak signal excursion dropped by ≥90% of pre-event baseline levels as determined using a nasal pressure sensor for ≥10 s. Hypopnea was diagnosed when the peak signal excursion dropped by ≥30% of pre-event baseline levels as determined using a nasal pressure sensor for ≥10 s in association with ≥4% arterial oxygen desaturation. The OSA severity was categorized based on the AHI as normal (<5), mild (≥5 to 15), moderate (≥15 to 30), and severe (≥30). Considering that OSA was more common in the elderly, we categorized OSA using an AHI of ≥10 events/h.
Echocardiography
All patients underwent transthoracic echocardiography examinations at rest with a two-dimensional, color-flow Doppler apparatus (Vivid E9, GE Vingmed Ultrasound A/S, Strandpromenaden 45, N-3191 Horten, Norway). Subjects were placed in the left lateral position by an experienced ultrasound physician for examination and image acquisition. The right side of the heart (right atrium, tricuspid valve, right ventricle) was investigated to evaluate the right heart performance and to calculate the PAP. When tricuspid regurgitation was recorded using the color-flow Doppler, the maximum velocity (V) of tricuspid incompetence was calculated with a continuous Doppler study of at least four consecutive beats. Right ventricular pressure (RVP) was derived using the equation RVP = 4V2 + right atrial pressure (RAP). For standardization, a RAP of 5 mmHg was assumed for all patients unless clear features were present such as an inferior vena cava diameter of >2.1 cm with <50% collapsibility. The estimated RVP is considered to represent the PAP, if there is no evidence of pulmonary valvular dysfunction. Tricuspid regurgitation velocity (TRV) >2.8 m/s and systolic PAP >36 mmHg on the echocardiographic examination were regarded as PH.[18]
Questionnaires
Daytime sleepiness was assessed using the Epworth sleepiness scale (ESS). The patient was diagnosed with daytime sleepiness when the ESS score was ≥9 points.[19] Dyspnea was quantified using the modified Medical Research Council Dyspnea scale (mMRC).[20] Symptoms were also quantified by use of COPD assessment test (CAT).[21] Hospital anxiety depression scale (HADS) was a self-reported, 14-item depression, and anxiety screening instrument assessing the severity of symptoms in medically ill patients.[22] Quality of life in patients with COPD was evaluated using St. George respiratory questionnaire (SGRQ).[23] Significant comorbidities were recorded and quantified according to the well-established Charlson index.[24,25]
Exacerbation frequency
An acute exacerbation (AE) of COPD was defined as an acute worsening of respiratory symptoms that resulted in additional therapy. The definition of severe exacerbation was that patient required hospitalization or visits the emergency room. Patients were asked about the number of exacerbations in the most recent 12 months.[15]
Primary outcomes and secondary outcomes
Primary outcomes were the PAP and proportion of PH. Secondary outcomes were exacerbation frequency, depression, anxiety, quality of life, and comorbidities.
Statistical analysis
Descriptive data with normal distribution were expressed as mean ± standard deviation (SD). Descriptive data without normal distribution were expressed as medians (interquartile range [IQR]) and frequencies were expressed as percentages. Comparisons were made between the COPD without OSA group and COPD with OSA group using Chi-squared tests for categorical variables, and t tests or Wilcoxon rank-sum tests for continuous variables depending on distribution. To compare patient characteristics among different groups, statistical analyses were performed using Chi-squared tests for categorical variables, and one-way analysis of variance for continuous variables. The relationship between two continuous variables was determined by measuring the Pearson correlation coefficient. Univariate analysis and a logistic regression model were used to obtain determinants of PAP and PH. A value of P < 0.05 was considered significant for all analyses. All statistical analyses were performed with SPSS version 17.0 (SPSS, Chicago, IL, USA).
Results
Of the 159 screened individuals with COPD, 106 participated in the study and completed home portable monitoring and echocardiography. The mean age was 69.52 years, 91.5% were men, and the median body mass index (BMI) was 23.77 kg/m2 [Table 1]. In total, 56 individuals were allocated to the COPD with OSA group and 50 were allocated to COPD without OSA group [Figure 1].
Table 1.
Baseline characteristics of COPD without OSA group and COPD with OSA group.
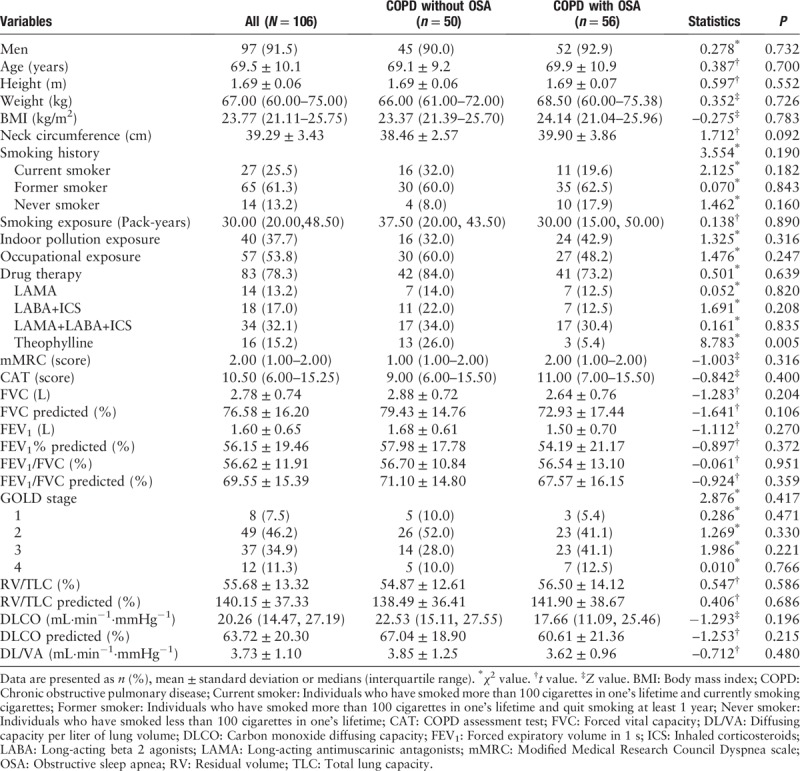
Figure 1.
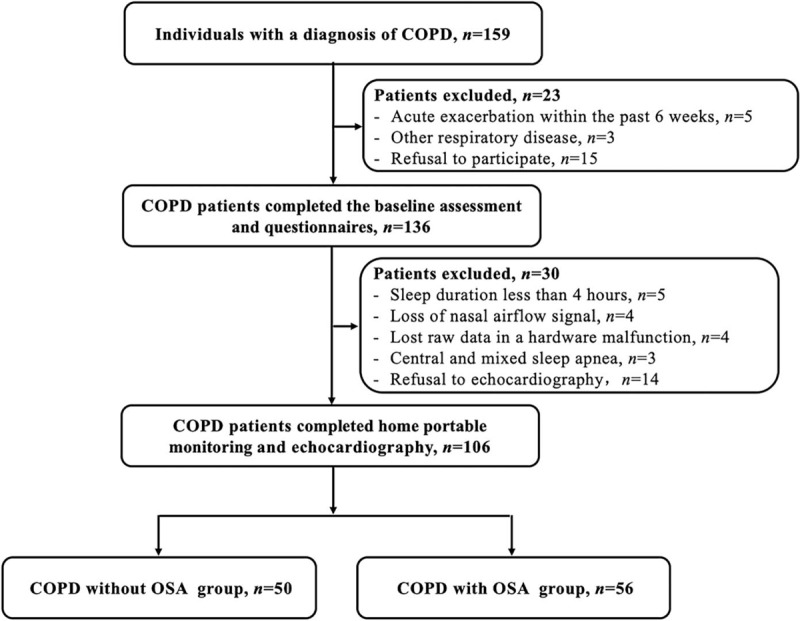
Study workflow for individuals enrolled in the study. COPD: Chronic obstructive pulmonary disease; OSA: Obstructive sleep apnea (apnea hypopnea index ≥10 events/h).
Baseline characteristics of COPD without OSA group and COPD with OSA group
In 106 patients with COPD, 56 patients (52.8%) had an AHI ≥10 events/h and were considered to have COPD with OSA. Individuals in the COPD without OSA and COPD with OSA groups did not differ by sex, age, BMI, neck circumference, or exposure history of noxious particles. The proportion of patients undergoing drug therapy for stable COPD in the COPD without OSA and COPD with OSA groups was 84.0% and 73.2%, respectively (χ2 = 0.501, P = 0.639). However, the use of theophylline in the COPD without OSA group was 20.4% higher than that in COPD with OSA group (5.4% vs. 26.0%, χ2 = 8.783, P = 0.005). Spirometry demonstrated on an average, moderate and severe COPD. The post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio and FEV1% predicted of the two groups were similar (56.54% vs. 56.70%, t = −0.061, P = 0.951; 54.19% vs. 57.98%, t = −0.897, P = 0.372, respectively) [Table 1].
Sleep measures in COPD without OSA group and COPD with OSA group
Eighty-four participants (79.2%) with COPD snored and 37 participants (34.9%) with COPD were daytime sleepiness. The prevalence of snoring in the COPD without OSA group was similar to that in the COPD with OSA group (82.1% vs. 76.0%, χ2 = 0.606, P = 0.479). The prevalence of sleepiness between the COPD with and without OSA groups did not show statistical differences (42.9% vs. 26.0%, χ2 = 1.231, P = 0.102). Compared with COPD individuals without OSA, individuals with OSA had elevated AHI (20.00 [13.13–34.50] events/h vs. 5.05 [3.00–6.83] events/h, Z = −0.861, P < 0.001), more severe minimal oxygen desaturation (minimal SpO2) (83.50 [76.00–87.00]% vs. 88.50 [86.00–91.00] %, Z = −5.429, P < 0.001), and greater percentage of night-time spent with oxygen saturation below 90% (T90) (3.10 [0.50–26.25] % vs. 0 [0–1.00] %, Z = −5.054, P < 0.001) [Table 2].
Table 2.
Sleep measures in the COPD without OSA group and COPD with OSA group.

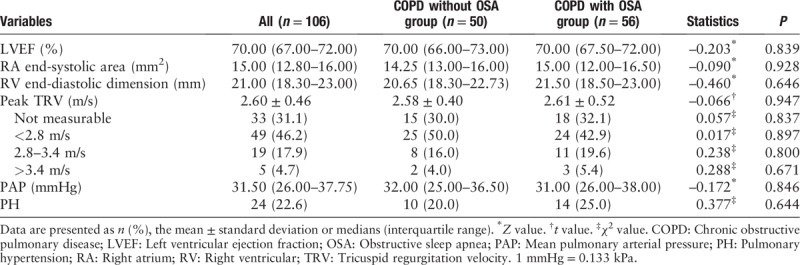
Echocardiography in the COPD without OSA group and COPD with OSA group
The left ventricular ejection fraction (LVEF) did not show significant difference between the two study groups (70.00 [67.50–72.00] % vs. 70.00 [66.00–73.00] %, Z = −0.203, P = 0.839). The mean peak TRV (2.61 ± 0.52 m/s vs. 2.58 ± 0.40 m/s, t = −0.066, P = 0.947) and median PAP (31.00 [26.00–38.00] mmHg vs. 32.00 [25.00–36.50] mmHg, Z = −0.172, P = 0.846) were similar in the COPD with OSA group and the COPD without OSA group. In total, 10 of 50 (20.0%) COPD without OSA participants and 14 of 56 (25.0%) COPD with OSA participants had PH (χ2 = 0.377, P = 0.644) [Table 3].
Table 3.
Echocardiography in the COPD without OSA group and COPD with OSA group.
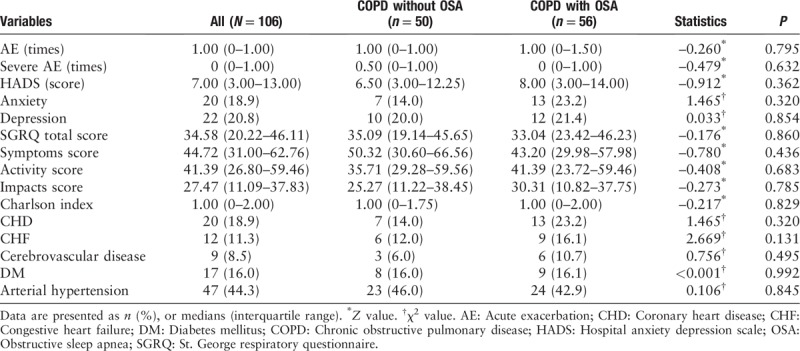
Exacerbation frequency, HADS, SGRQ, and Charlson Index in COPD without OSA group and COPD with OSA group
There were no differences in exacerbation frequency (1.00 [0–1.50] vs. 1.00 [0–1.00], Z = −0.260, P = 0.795), HADS score (8.00 [3.00–14.00] vs. 6.50 [3.00–12.25], Z = −0.912, P < 0.362), total SGRQ score (33.04 [23.42–46.23] vs. 35.09 [19.14–45.65], Z = −0.176, P = 0.860) and Charlson index (1.00 [0–2.00] vs. 1.00 [0–1.75], Z = −0.217, P = 0.829) between groups. The prevalence of coronary heart disease (23.2% vs. 14.0%, χ2 = 1.465, P = 0.320), congestive heart failure (16.1% vs. 12.0%, χ2 = 2.669, P = 0.131), cerebrovascular disease (10.7% vs. 6.0%, χ2 = 0.756, P = 0.495), diabetes mellitus (16.1% vs. 16.0%, χ2 < 0.001, P = 0.992), and arterial hypertension (42.9% vs. 46.0%, χ2 = 0.106, P = 0.845) was not significantly increased in the COPD with OSA group compared with the COPD without OSA group [Table 4].
Table 4.
Exacerbation frequency, HADS, SGRQ, and Charlson index in the COPD without OSA group and COPD with OSA group.
Echocardiography and secondary outcomes grouped according to AHI, minimal SpO2, and T90
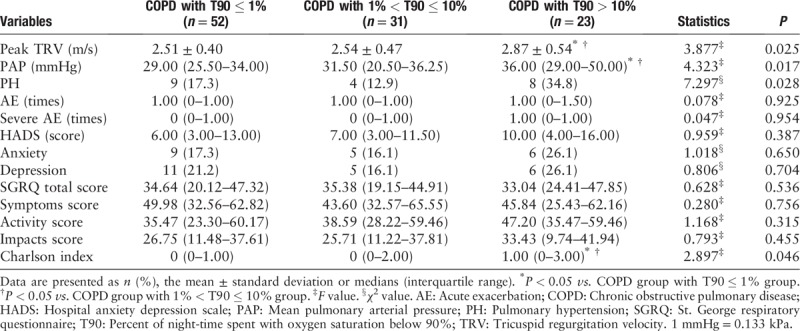
Patients with COPD were divided into four categories based on their AHI: COPD without OSA group (AHI < 5), COPD with mild OSA group (5 ≤ AHI < 15), COPD with moderate OSA group (15 ≤ AHI < 30), and COPD with severe OSA group (AHI ≥ 30). Compared with COPD without OSA group, the median PAP in COPD with severe OSA group increased by 5 mmHg (36.00 [26.00–50.00] mmHg vs. 31.00 [24.00–34.00] mmHg, P = 0.036). The mean peak TRV (2.88 ± 0.63 m/s vs. 2.53 ± 0.39 m/s, P = 0.047), median PAP (36.00 [26.00–50.00] mmHg vs. 30.50 [26.00–35.75] mmHg, P = 0.024), and Charlson index (1.00 [0.25–2.75] vs. 0 (0–1.75), P = 0.022) in the COPD with severe OSA group were higher than those in the COPD with mild OSA group [Table 5].
Table 5.
Echocardiography and secondary outcomes grouped according to AHI.
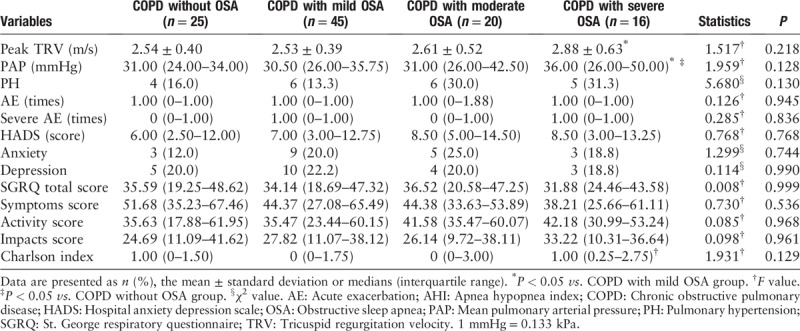
According to the degree of nocturnal hypoxemia, patients with COPD were divided into COPD without hypoxemia group (minimal SpO2 ≥ 90%), COPD with mild hypoxemia group (85% ≤ minimal SpO2 < 90%), COPD with moderate hypoxemia group (80% ≤ minimal SpO2 < 85%), and COPD with severe hypoxemia group (minimal SpO2 < 80%). There were no statistical differences in the median PAP, proportion of PH, and secondary outcomes between the four groups [Table 6].
Table 6.
Echocardiography and secondary outcomes grouped according to minimal SpO2.
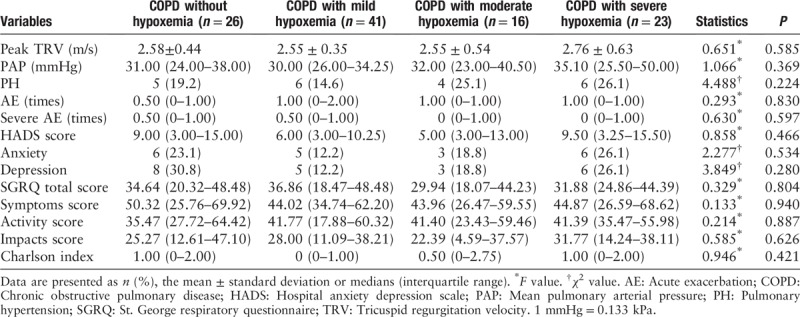
Based on T90, the patients were also divided into three groups, including COPD with T90 ≤ 1%, 1% < T90 ≤ 10% and T90 > 10%. We found that the mean peak TRV (2.87 ± 0.54 m/s vs. 2.51 ± 0.40 m/s, P = 0.008), median PAP (36.00 [29.00–50.00] mmHg vs. 29.00 [25.50–34.00] mmHg, P = 0.007), and Charlson index (1.00 [0–3.00] vs. 0 [0–1.00], P = 0.019) in the COPD with T90 > 10% group were significantly higher than those in COPD with T90 ≤ 1% group [Table 7].
Table 7.
Echocardiography and secondary outcomes grouped according to T90.
The Pearson correlations between the three factors (AHI, minimal SpO2, and T90) and PAP are shown in Figure 2. AHI and minimal SpO2 were not linearly correlated with PAP (r = 0.180, P = 0.121; r = −0.189, P = 0.103); only T90 was positively correlated with PAP (r = 0.252, P = 0.028).
Figure 2.
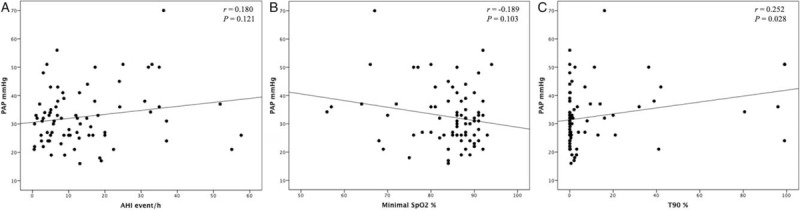
Correlation between (A) Apnea hypopnea index (AHI), (B) minimal oxygen desaturation (minimal SpO2), and (C) percent of night-time spent with oxygen saturation below 90% (T90) and pulmonary artery pressure (PAP) in COPD subjects. COPD: Chronic obstructive pulmonary disease.
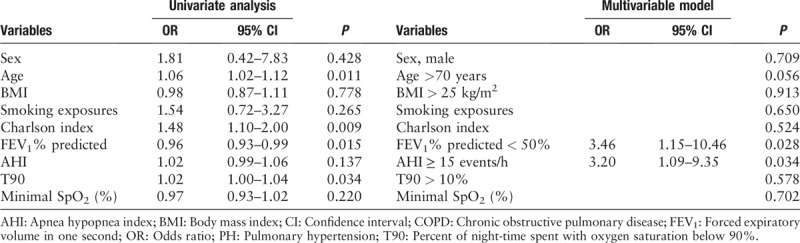
Univariate and logistic regression analysis for the variables associated with PH in subjects with COPD
Univariate analysis performed on data in patients with COPD revealed significant effects of age, FEV1% predicted, T90, and Charlson index on PH. According to the regression model, FEV1% predicted <50% increased the risk of PH by 3.46 times (odds ratio [OR] = 3.46; 95% confidence interval [CI]: 1.15–10.46; P = 0.028) and AHI ≥15 events/h increased the risk of PH by 3.20 times (OR = 3.20; 95% CI: 1.09–19.35; P = 0.034). Moderate to severe OSA and GOLD stage 2 or higher were independent factors contributing to PH in subjects with COPD [Table 8].
Table 8.
Univariate and logistic regression analysis for the variables associated with PH in subjects with COPD.
Discussion
This study demonstrated that patients with COPD with OSA were more susceptible to PH, which might be associated with declining lung function and increased OSA severity. The main findings of this study are: (1) 56 patients (52.8%) with COPD were diagnosed as OSA, and 24 patients (22.6%) with COPD had PH; (2) COPD with severe OSA group and COPD with T90 >10% group had higher PAP; (3) multiple regression analysis revealed significant and independent effects of both FEV1% predicted and AHI on PH. These findings were consistent with our hypothesis that OSA is an aggravating factor of PAP and PH in patients with COPD.
In our study, we showed a high prevalence of OSA in patients with COPD, which was similar to the high prevalence reported in other studies.[26–28] This might be related to the fact that most of the subjects were elderly and had moderate to severe lung injury. Previous studies have found that the prevalence of OSA increased with age.[29,30] Considering the average age was 70 years in our study, we used AHI ≥10 events/h as a diagnostic criterion. Moreover, we found that the use of theophylline in COPD with OSA group was higher than that in COPD without OSA group. Previous studies have shown that theophylline could improve AHI, nocturnal hypoxia and sleep-related gas exchange in OSA.[31,32] Theophylline may have a stimulant effect on central respiratory drive and the upper airway muscles. It is unclear if theophylline is a more suitable drug for patients with COPD with OSA. In addition, 79.2% of patients with COPD snored and 34.9% of patients with COPD were daytime sleepiness. However, snoring and daytime sleepiness were not significant clinical features for identifying OSA in patients with COPD. At present, there is no recognized tool for screening OSA in patients with COPD. For patients with COPD, we need to further explore simpler identifiable features and screening methods for OSA. Sleep monitoring is needed to diagnose OSA for patients with COPD.
The present study revealed the prevalence of PH in 22.6% patients with COPD. Previous studies showed 38.7% to 62.4% cases of PH in patients with COPD.[33–35] In patients with COPD, increased PAP is an independent predictor of future exacerbations and life expectancy reduction. Decrease in the pulmonary vascular bed and chronic hypoxia are two main mechanisms of increased pulmonary vascular resistance and subsequent PH COPD.[7,36] In the present study, with increasing duration of hypoxemia, a significant increase in PAP was observed. However, we did not directly observe a significant increase in PAP with increasing severity of OSA and hypoxemia. Compared with AHI, the duration of hypoxemia may be more relevant to PH. Therefore, further studies involving larger sample size are needed to understand better clinical and biochemical profile of patients with OSA.
The prevalence of OSA-related PH varies from 17% to 53% in studies using right heart catheterization.[10,13,37] In general, older age, high BMI, worse nocturnal desaturations, and poor lung function are closely related to PH in OSA.[10,38] The occurrence of PH was mainly related to BMI and nocturnal hypoxia, and AHI was not an independent risk factor for PH. However, those studies were not limited to patients with OSA alone and subjects might have chronic cardiopulmonary disease, such as COPD. It is difficult to determine whether PH is due to intermittent hypoxemia caused by sleep apnea or persistent hypoxemia associated with chronic cardiopulmonary disease. Some studies have attempted to control the effect of cardiopulmonary disease as a confounding factor. Small sample studies have shown that the prevalence of PH in OSA without lung or heart disease was 20.7% to 41.0%.[39] Most studies have found that OSA-induced PH was mild to moderate, and some studies have challenged the effect of AHI on PH.[11,12,37,40] Few studies have focused on PH in patients with COPD with OSA. The coexistence of OSA may have a synergistic adverse effect on pulmonary hemodynamics leading to right ventricular dysfunction in patients with COPD. Chaouat et al[41] have reported that the prevalence of PH in patients with overlap syndrome is 29% higher than that in patients with OSA alone. Hawrylkiewicz et al[42] have suggested that PH was very common (14/17, 82.4%) in patients with OS, but did not correlate with the severity of nocturnal desaturation in OS patients. Consistent with other research reports, we also found that patients with COPD with OSA developed more severe hypoxemia at night. In a study by Kendzerska et al,[43] the degree of hypoxemia had a better ability to predict PH than did AHI in individuals with COPD and OSA. In addition, they demonstrated that co-occurrence of COPD and severe OSA has a synergistic effect on cardiovascular events and mortality.
Although our results suggested that apnea-hypopnea was an independent risk factor for PH in patients with COPD, this observation is still controversial. In this present study, we found that AHI and oxygen desaturation index (ODI) did not differ between the groups. This might be explained by the fact that patients with COPD were more likely to experience hypoxemia at night. An increase in upper respiratory resistance during night sleep in patients with COPD is almost always accompanied by hypoxemia. Therefore, hypoxemia occurs with apnea-hypopnea in COPD. Previous studies found the primary determinant of oxygen desaturation during repetitive airway obstruction was the duration of obstruction rather than the number of obstructions, and that hypoxemia was a main factor in elevating PAP.[44] However, repetitive airway obstruction can cause repeated negative changes in intrathoracic pressure, which can lead to increased intrathoracic venous reflux, resulting in right ventricular hypertrophy and PH. A systematic review and meta-analysis showed that patients with OSA exhibited right ventricular dilatation, increased wall thickening, and altered RV function. [45] Repetitive airway obstruction can also cause microarousal and changes in sleep structure. The average PAP during rapid eye movement (REM) sleep is higher than that during non-REM sleep.[46,47] The increase in sympathetic nerve excitation and catecholamine secretion caused by apnea-hypopnea, as well as inflammation, oxidative stress, and endothelial dysfunction caused by intermittent hypoxia have been suggested to play a role in the pathogenesis of PH in OSA.
Our study has a few limitations. This study was cross-sectional, and we did not observe the compliance and efficacy of positive pressure ventilation therapy in patients with COPD with OSA. We failed to diagnose OSA using polysomnography, and could not assess the quality and stage of sleep in patients with COPD. Compared with polysomnography, the ApneaLink device is a simple, easy-to-use and reliable device with high sensitivity and specificity in calculating AHI. Apnea Link has been shown to underestimate and overestimate the AHI of OSA patients;[16,48] however, we used AHI ≥10 events/h as a criterion for diagnosing OSA to reduce errors. In recent years, echocardiography has been recommended as a first non-invasive screening and diagnostic technique for PH.[18] The accuracy of Doppler echocardiography in evaluating PAP has been verified using right heart catheterization. Patients with TRV-estimated elevated PAP have an intermediate or high risk of PH. This study can help in diagnosis and treatment of these patients with COPD in a timely manner.
In conclusion, we observe that patients with COPD have a high prevalence of OSA. COPD with OSA patients are more susceptible to PH, which is associated with declining lung function and increased OSA severity. The severity of airflow obstruction, apnea-hypopnea and nocturnal hypoxia play important roles in the pathogenesis of PH in patients with COPD. Our observations can help understand the clinical and physiologic characteristics of individuals with COPD, with and without OSA and to identify suspected PH in COPD. Moreover, OSA and nocturnal hypoxemia deserve attention in elderly patients with COPD. The effect of the interaction between COPD and OSA on PH needs further confirmation. Furthermore, whether sleep apnea can promote PAP, or whether this interaction is bidirectional needs further study.
Funding
This work was supported by the grants from the Chronic Non-Communicable Diseases Prevention and Control Research of National Key Research and Development Program of China (No. 2016YFC1304301), Precision Medical Project of National Key Research and Development Program (No. 2016YFC0903601 and 2016YFC0901102), and the Beijing New-star Plan of Science and Technology program (No. Z171100001117124).
Conflicts of interest
None.
Footnotes
How to cite this article: Sun WL, Wang JL, Jia GH, Mi WJ, Liao YX, Huang YW, Hu Z, Zhang LQ, Chen YH. Impact of obstructive sleep apnea on pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chin Med J 2019;00:00–00. doi: 10.1097/CM9.0000000000000247
References
- 1.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018; 391:1706–1717. doi: 10.1016/s0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 2016; 387:251–272. doi: 10.1016/s0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 3.Ip SM, Lam B, Lauder IJ, Tsang KW, Chung K-F, Mok Y-W, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest 2001; 119:62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Wei C, Huang L, Wang W, Liang D, Lei Z, et al. Prevalence of signs and symptoms suggestive of obstructive sleep apnea syndrome in Guangxi, China. Sleep Breath 2014; 18:375–382. doi: 10.1007/s11325-013-0896-2. [DOI] [PubMed] [Google Scholar]
- 5.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration 2005; 72:142–149. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 6.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62:42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Sakao S, Voelkel NF, Tatsumi K. The vascular bed in COPD: pulmonary hypertension and pulmonary vascular alterations. Eur Respir Rev 2014; 23:350–355. doi: 10.1183/09059180.00007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bady E, Achkar A, Pascal S, Orvoen-Frija E, Laaban JP. Pulmonary arterial hypertension in patients with sleep apnoea syndrome. Thorax 2000; 55:934–939. doi: 10.1136/thorax.55.11.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong HT, Chee KH, Chong AW. Pulmonary hypertension and echocardiogram parameters in obstructive sleep apnea. Eur Arch Otorhinolaryngol 2017; 274:2601–2606. doi: 10.1007/s00405-017-4491-1. [DOI] [PubMed] [Google Scholar]
- 10.Minai OA, Ricaurte B, Kaw R, Hammel J, Mansour M, McCarthy K, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol 2009; 104:1300–1306. doi: 10.1016/j.amjcard.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 11.Sanner BM, Doberauer C, Konermann M, Sturm A, Zidek W. Pulmonary hypertension in patients with obstructive sleep apnea syndrome. Arch Intern Med 1997; 157:2483–2487. doi: 10.1001/archinte.1997.00440420115011. [PubMed] [Google Scholar]
- 12.Alchanatis M, Tourkohoriti G, Kakouros S, Kosmas E, Podaras S, Jordanoglou JB. Daytime pulmonary hypertension in patients with obstructive sleep apnea: the effect of continuous positive airway pressure on pulmonary hemodynamics. Respiration 2001; 68:566–572. doi: 10.1159/000050574. [DOI] [PubMed] [Google Scholar]
- 13.Wong HS, Williams AJ, Mok Y. The relationship between pulmonary hypertension and obstructive sleep apnea. Curr Opin Pulm Med 2017; 23:517–521. doi: 10.1097/MCP.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 14.Chronic Obstructive Pulmonary Disease Group, Chinese Medical Association. Guideline for diagnosis and treatment of chronic obstructive pulmonary disease [in Chinese]. Chin J Tuberc Respir Dis 2013; 36:255–264. doi: 10.3760/cma.j.issn.1001-0939.2013.04.007. [Google Scholar]
- 15.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2017 report. Available at: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd Accessed December 16, 2016. [Google Scholar]
- 16.Nigro CA, Dibur E, Malnis S, Grandval S, Nogueira F. Validation of ApneaLink Ox( for the diagnosis of obstructive sleep apnea. Sleep Breath 2013; 17:259–266. doi: 10.1007/s11325-012-0684-4. [DOI] [PubMed] [Google Scholar]
- 17.Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med 2017; 13:665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 19.Kendzerska TB, Smith PM, Brignardello-Petersen R, Leung RS, Tomlinson GA. Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med Rev 2014; 18:321–331. doi: 10.1016/j.smrv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J 2009; 34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 22.Dowson C, Laing R, Barraclough R, Town I, Mulder R, Norris K, et al. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002; 52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Mitsuma S, Kobayashi A, Yanagida M, Nakayasu K, Hasegawa Y, et al. COPD and disease-specific health status in a working population. Respir Res 2013; 14:61.doi: 10.1186/1465-9921-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roffman CE, Buchanan J, Allison GT. Charlson comorbidities index. J Physiother 2016; 62:171.doi: 10.1016/j.jphys.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Degroot V, Beckerman H, Lankhorst G, Bouter L. How to measure comorbiditya critical review of available methods. J Clin Epidemiol 2003; 56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 26.Basoglu OK, Gunduz C, Tasbakan MS. Prevalence of overlap syndrome in chronic obstructive pulmonary disease patients without sleep apnea symptoms. Clin Respir J 2016; 8:236–242. doi: 10.1111/crj.12493. [DOI] [PubMed] [Google Scholar]
- 27.Soler X, Gaio E, Powell FL, Ramsdell JW, Loredo JS, Malhotra A, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015; 12:1219–1225. doi: 10.1513/AnnalsATS.201407-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shawon MS, Perret JL, Senaratna CV, Lodge C, Hamilton GS, Dharmage SC. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev 2017; 32:58–68. doi: 10.1016/j.smrv.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Gamaldo AA, Beydoun MA, Beydoun HA, Liang H, Salas RE, Zonderman AB, et al. Sleep disturbances among older adults in the United States, 2002–2012: nationwide inpatient rates, predictors, and outcomes. Front Aging Neurosci 2016; 8:266.doi: 10.3389/fnagi.2016.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leppanen T, Toyras J, Mervaala E, Penzel T, Kulkas A. Severity of individual obstruction events increases with age in patients with obstructive sleep apnea. Sleep Med 2017; 37:32–37. doi: 10.1016/j.sleep.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Mulloy E, McNicholas WT. Theophylline in obstructive sleep apnea a double-blind evaluation. Chest 1992; 101:753–757. doi: 10.1378/chest.101.3.753. [DOI] [PubMed] [Google Scholar]
- 32.Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1993; 148:1030–1036. doi: 10.1164/ajrccm/148.4_Pt_1.1030. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber A, Cemmi F, Ambrosino N, Ceriana P, Lastoria C, Carlucci A. Prevalence and predictors of obstructive sleep apnea in patients with chronic obstructive pulmonary disease undergoing inpatient pulmonary rehabilitation. COPD 2018; 1–6. doi: 10.1080/15412555.2018.1500533. [DOI] [PubMed] [Google Scholar]
- 34.Gupta KK, Roy B, Chaudhary S, Mishra A, Patel ML, Singh J, et al. Prevalence of pulmonary artery hypertension in patients of chronic obstructive pulmonary disease and its correlation with stages of chronic obstructive pulmonary disease, exercising capacity, and quality of life. J Family Med Prim Care 2018; 7:53–57. doi: 10.4103/jfmpc.jfmpc_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soler X, Liao SY, Marin JM, Lorenzi-Filho G, Jen R, DeYoung P, et al. Age, gender, neck circumference, and Epworth sleepiness scale do not predict obstructive sleep apnea (OSA) in moderate to severe chronic obstructive pulmonary disease (COPD): the challenge to predict OSA in advanced COPD. PLoS One 2017; 12:e0177289.doi: 10.1371/journal.pone.0177289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32:1371–1385. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 37.Sajkov D, Wang T, Saunders NA, Bune AJ, Neill AM, Douglas Mcevoy R. Daytime pulmonary hemodynamics in patients with obstructive sleep apnea without lung disease. Am J Respir Crit Care Med 1999; 159:1518–1526. doi: 10.1164/ajrccm.159.5.9805086. [DOI] [PubMed] [Google Scholar]
- 38.Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest 1996; 109:380–386. doi: 10.1378/chest.109.2.380. [DOI] [PubMed] [Google Scholar]
- 39.Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51:363–370. doi: 10.1016/j.pcad.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Nagaoka M, Goda A, Takeuchi K, Kikuchi H, Finger M, Inami T, et al. Nocturnal hypoxemia, but not sleep apnea, is associated with a poor prognosis in patients with pulmonary arterial hypertension. Circ J 2018; 82:3076–3081. doi: 10.1253/circj.CJ-18-0636. [DOI] [PubMed] [Google Scholar]
- 41.Chaouat A, Weitzenblum E, Warrior J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995; 151:82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 42.Hawryłkiewicz I, Sliwiński P, Górecka D, Plywaczewski R, Zieliński JZ. Pulmonary haemodynamics in patients with OSAS or an overlap syndrome. Monaldi Arch Chest Dis 2004; 61:148–152. doi: 10.4081/monaldi.2004.693. [DOI] [PubMed] [Google Scholar]
- 43.Kendzerska T, Leung RS, Aaron SD, Ayas N, Sandoz JS, Gershon AS. Cardiovascular outcomes and all-cause mortality in patients with obstructive sleep apnea and chronic obstructive pulmonary disease (overlap syndrome). Ann Am ThoracSoc 2019; 16:71–81. doi: 10.1513/AnnalsATS.201802-136OC. [DOI] [PubMed] [Google Scholar]
- 44.Iwase N, Kikuchi Y, Hida W, Miki H, Taguchi O, Satoh M, et al. Effects of repetitive airway obstruction on O2 saturation and systemic and pulmonary arterial pressure in anesthetized dogs. Am Rev Respir Dis 1992; 146:1402–1410. doi: 10.1164/ajrccm/146.6.1402. [DOI] [PubMed] [Google Scholar]
- 45.Maripov A, Mamazhakypov A, Sartmyrzaeva M, Akunov A, MurataliUulu K, Duishobaev M, et al. Right ventricular remodeling and dysfunction in obstructive sleep apnea: a systematic review of the literature and meta-analysis. Can Respir J 2017; 2017:1587865.doi: 10.1155/2017/1587865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niijima M, Kimura H, Edo H, Shinozaki T, Kang J, Masuyama S, et al. Manifestation of pulmonary hypertension during REM sleep in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 1999; 159:1766–1772. doi: 10.1164/ajrccm.159.6.9808064. [DOI] [PubMed] [Google Scholar]
- 47.Choi E, Park DH, Yu JH, Ryu SH, Ha JH. The severity of sleep disordered breathing induces different decrease in the oxygen saturation during rapid eye movement and non-rapid eye movement sleep. Psychiatry Investig 2016; 13:652–658. doi: 10.4306/pi.2016.13.6.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ragette R, Wang Y, Weinreich G, Teschler H. Diagnostic performance of single airflow channel recording (ApneaLink) in home diagnosis of sleep apnea. Sleep Breath 2010; 14:109–114. doi: 10.1007/s11325-009-0290-2. [DOI] [PubMed] [Google Scholar]


