Abstract
Introduction
According to the literature, early cholecystectomy is necessary to avoid complications related to gallstones after an initial episode of acute biliary pancreatitis (ABP). A randomised, controlled multicentre trial (the PONCHO trial) revealed that in the case of gallstone-induced pancreatitis, early cholecystectomy was safe in patients with mild gallstone pancreatitis and reduced the risk of recurrent gallstone-related complications, as compared with interval cholecystectomy. We hypothesise that carrying out a sphincterotomy (ES) allows us to delay cholecystectomy, thus making it logistically easier to perform and potentially increasing the efficacy and safety of the procedure.
Methods/Design
EMILY is a prospective, randomised, controlled multicentre trial. All patients with mild ABP, who underwent ES during the index admission or in the medical history will be informed to take part in EMILY study. The patients will be randomised into two groups: (1) early cholecystectomy (within 6 days after discharge) and (2) patients with delayed (interval) cholecystectomy (between 45 and 60 days after discharge). During a 12-month period, 93 patients will be enrolled from participating clinics. The primary endpoint is a composite endpoint of mortality and recurrent acute biliary events (that is, recurrent ABP, acute cholecystitis, uncomplicated biliary colic and cholangitis). The secondary endpoints are organ failure, biliary leakage, technical difficulty of the cholecystectomy, surgical and other complications.
Ethics and dissemination
The trial has been registered internationally ISRCTN10667869, and approved by the relevant organisation, the Scientific and Research Ethics Committee of the Hungarian Medical Research Council (EKU/2018/12176–5).
Trial registration number
ISCRTN10667869; Pre-results.
Keywords: acute biliary pancreatitis, cholecystectomy, endoscopic sphincterotomy
Strengths and limitations of this study.
The study is designed as a prospective, randomised-controlled trial to achieve conclusion on the highest evidence level to provide the first evidence concerning the possible benefits of sphincterotomy (ES) on timing cholecystectomy, it is (i) multinational, (ii) multicentric, (iii) internationally registered and (iv) the prestudy protocol is published.
Only high volume, expert centres can join to the study. They have to provide (i) laparoscopically trained surgeons with >100 laparoscopic procedures performed and (ii) if endoscopic retrograde cholangiopancreatography (ERCP)/ES is provided during the index admission, trained gastroenterologist with >50 ES completed within a year must be on duty.
The study enjoys continuous support from (i) an International Translational Advisory Board including top, well-established experts from different area of research field (ii) an Independent Data Management Board.
The final conclusion can be achieved with low number of patients within a relatively short period of time.
The study will provide evidence in a selected population (mild acute biliary pancreatitis (ABP) who underwent ERCP+ES) and no evidence concerning the usefulness of ES in moderate and severe ABP.
Introduction
Acute pancreatitis is one of the leading gastrointestinal causes of acute hospital admissions.1 2 In most cases, it is caused by gallstones or oedema.3 Gallstone-induced pancreatitis involves a pathophysiological factor, namely a distal common channel of the biliary and pancreatic ducts, which can be found in 80% of acute biliary pancreatitis (ABP).4 ABP is a clinical entity with high rates of morbidity (15%–50%) and mortality (2%-5%).5 After ABP, several complications may occur; recurrent acute pancreatitis, cholestasis and fistula affecting the hepatobiliary system or other biliary events, such as acute cholecystitis, obstruction of the common biliary duct, cholangitis or biliary colic.6 7 Interval cholecystectomy after mild ABP is associated with a high risk of readmission for recurrent biliary events, especially after recurrent ABP.8 The international practice guidelines recommend that in case of cholangitis or choledocholthiasis, an ERCP should be performed to clear the bile duct with endoscopic sphincterotomy (ES). In addition, cholecystectomy should also be performed to avoid complications related to recurrent biliary events.9 10 In patients with clinically severe pancreatitis, with local complications, such as pancreatic necrosis or organ failure, the intervention, namely the laparoscopic cholecystectomy (LC), is delayed 6 months until complications are resolved.11 In cases of mild ABP, cholecystectomy is recommended between days 7 and 21.4 The latest studies show that after discharge of patients with ABP, cholecystectomy could reduce the risk of a recurrent ABP and other gallstone-induced complications.12 In this setting, surgeons still prefer delayed cholecystectomy for efficacy and safety and for logistical reasons.13 Some publications draw attention to ERCP/ES, which could reduce mortality and the formation of severe biliary complications.3 14 The aim of the EMILY trial is to combine a surgical treatment and a gastroenterological procedure to investigate if ES with delayed cholecystectomy (within 45–60 days after discharge) compared with ES with early cholecystectomy (within 6 days after discharge) could reduce recurrent biliary events.
Methods
Design
EMILY is a prospective, randomised-controlled, multicentre trial. The patients are randomised into two groups: (1) Patients who undergo early cholecystectomy (within 6 days after discharge) and (2) patients who undergo delayed (interval) cholecystectomy (between 45 and 60 days after discharge). During a 12-month period, 93 patients will be enrolled from participating clinics. The primary endpoint is a composite endpoint of mortality and recurrent acute biliary events (which are recurrent ABP, acute cholecystitis, uncomplicated biliary colic and cholangitis). The secondary endpoints are: organ failure, biliary leakage, technical difficulty of cholecystectomy and surgical and other complications.
This study was structured following the SPIRIT 201315 guideline defining standard protocol items for clinical trials and got the relevant ethical approval EKU/2018/12176–5 (Scientific and Research Ethical Committee, Medical Research Council, Hungary).
Trial organisation, committees and boards
The coordinator and designer of the EMILY study is the Centre for Translational Medicine at the University of Pécs Medical School (coordinating institution and sponsor, http://www.tm-centre.org) and the Hungarian Pancreatic Study Group (HPSG-coordinating society, http://www.pancreas.hu). The HPSG was established in 2011 to stimulate research in pancreatic diseases.
Until now, it has launched three international observational clinical studies in 201416–18 (EASY, APPLE and PINEAPPLE) and two interventional studies (PREPAST19—2014 and GOULASH20—2017) and has published the relevant guidelines for pancreatic diseases to improve patient care in pancreatology.21–24
The following committees and boards will be involved: Steering Committee (SC): The committee will be led by PH (corresponding investigator, gastroenterologist and internal medicine specialist).
The members in Szeged (HU) will be: LC (gastroenterologist), GL (surgeon); Debrecen (HU): MP (gastroenterologist), KP (gastroenterologist), ZS (surgeon); Pécs (HU): ÁV (gastroenterologist), DK (surgeon), AV (surgeon), LB (anaesthesiologist), DK (surgeon), PH (gastroenterologist); Székesfehérvár (HU): FI (gastroenterologist), ÁA (surgeon); Targu Mures (RO): IT (gastroenterologist), LPK (surgeon), MD (surgeon), Cluj Napoca (RO): BS (surgeon), TM (gastroenterologist). KM, ZN and JA are trial management specialists, IN (statistician), whereas AS leads the multidisciplinary core facility which will assist the scientists to run the study successfully. The SC will make decisions concerning all relevant questions including drop outs during the study.
International Translational Advisory Board (ITAB): The board will consist of a gastroenterologist (MML), a surgeon and two basic scientists (JN, MST, OHP). The ITAB will continuously monitor the progress of the study and will advise the SC.
The study was designed by the SC and ITAB. It was funded by the University of Pécs, Medical School. The sponsor was not involved in the design of the study and will have no access to database or the randomisation code.
The study also contains an independent physician and safety manager as required by the ethical regulation.
Study population
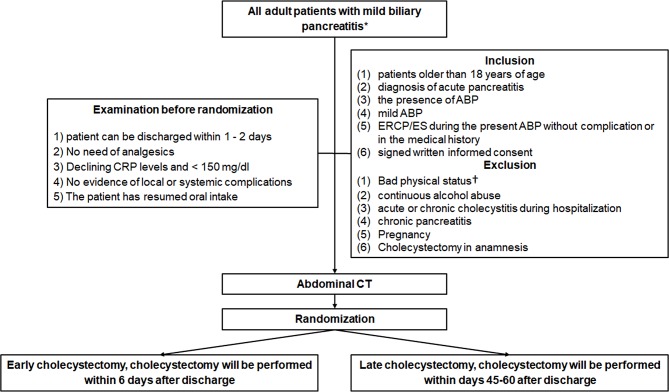
All patients with mild ABP (according to the revised Atlanta classification25) will be informed of the possibility to take part in the EMILY trial. After the consent form is signed, participants will be randomised into two groups if they meet all the inclusion and no exclusion criteria (figure 1).
Figure 1.
The flowchart of participants according to SPIRIT 2013 guideline.15 *No pancreatic necrosis, no transient or persistent organ failure (>48 hours) is present with any of the following three definitions: (1) diagnosis of gallstones on imaging, (2) alanine aminotransferase level >2 times higher than normal values with ALT >AST. †ASA IV or V patients and ASA III>75 years old. ABP, acute biliary pancreatitis; ASA, American Society of Anesthesiologists; CRP, C reactive protein; ES, endoscopic sphincterotomy.
Inclusion criteria
The criteria for inclusion in the study are: (1) patients older than 18 years of age; (2) diagnosis of acute pancreatitis (at least two of the following three symptoms: upper abdominal pain, serum lipase or amylase is three times higher than the upper limit of normal and characteristic findings for acute pancreatitis on imaging); (3) the presence of ABP (any of the following three definitions): diagnosis of gallstones on imaging and alanine aminotransferase level >2 times higher than normal values with ALT>AST; (4) mild ABP (meaning no pancreatic necrosis, no transient or persistent organ failure (>48 hours) is present; (5) ERCP/ES either during the index admission or in the medical history without complication; (6) signed written informed consent (all included patient will sign the consent which contains the information about the trial and procedures) (figure 1).
Exclusion criteria
A patient’s bad physical status can be an exclusion criterion. American Society of Anesthesiologists (ASA) III patients>75 years old; ASA IV or V patients, will be excluded. Patients with continuous alcohol abuse, acute or chronic cholecystitis during hospitalisation, chronic pancreatitis, pregnancy, previous cholecystectomy will also be excluded (figure 1).
Time of randomisation
Five criteria are described by the PONCHO trial.26 If these five criteria are met, the informed consent will be signed by the patient and a control abdominal CT will be carried out before discharge. These criteria are the following: (1) anticipation on the part of the treating physician that the patient can be discharged; (2) the patient has no abdominal pain and there is no need for analgesics; (3) declining C reactive protein levels and <150 mg/L; (4) no evidence of local or systemic complications (eg, no fever); (5) oral feeding is tolerated for 24 hours. The patient must be randomised on the day of the discharge.
Randomisation
The method of randomisation is the following: The patient can be randomised by the study coordinator using a randomisation module with sealed envelope. Patient data will be uploaded with the help of the administrator to the database, which will be followed by the randomisation. This randomisation module will allocate the participants to the two different groups. This method makes it impossible for researchers to predict the allocation of the patients involved in the study. It is impossible to conceal the distribution of the patients in this study because the patients need to be scheduled for either an early cholecystectomy or a delayed cholecystectomy (figure 1).
Allocation will be carried out based on predefined randomisation lists created separately for each recruiting centre. The allocation sequence will be prepared with a variable block size by an allocation ratio 1:1 by the Independent Data Management Board (IDMB).
Blinding
In prevention of patient’s selection to group A and B trial participants, care providers and outcome assessors will be blinded until the allocation, as no access to randomisation sequence. From assignment to intervention blinding cannot be provided considering the study characteristics (exact date of cholecystectomy). The allocation sequence is unblinded only to data analysts who are completely independent form medical team (decision making) and data collection.
Endpoints
Primary endpoint
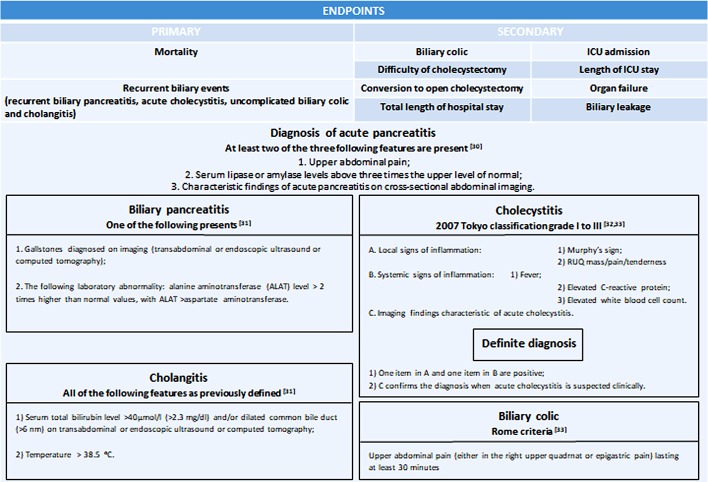
The primary endpoint is a composite endpoint, which is based on mortality and on recurrent biliary events (which are recurrent ABP, acute cholecystitis, uncomplicated biliary colic and cholangitis). The observation period is 3 months. We decide based on criteria in figure 2 if a complication is present or not.
Figure 2.
The evaluation of primary and secondary endpoints.30–33
Secondary endpoints
We hypothesise that cholecystectomy for ABP between days 45–60 after discharge in patients with ES is as effective and safe as early cholecystectomy (within 6 day after discharge). In order to evaluate this, we will observe the following parameters: the number of biliary colic registered for the patient, difficulty of cholecystectomy (on a scale of 0–10, 0=easy, 5=moderately difficult, 10=hard, rate of conversion to open cholecystectomy, total length of hospital stay, need for intensive care unit (ICU) admission and total length of ICU stay, organ failure and biliary leakage (figure 2).
Treatment protocol
Randomisation:
Group A. Early cholecystectomy
Group B. Delayed cholecystectomy
We randomise patients into two groups after discharge (figure 3):
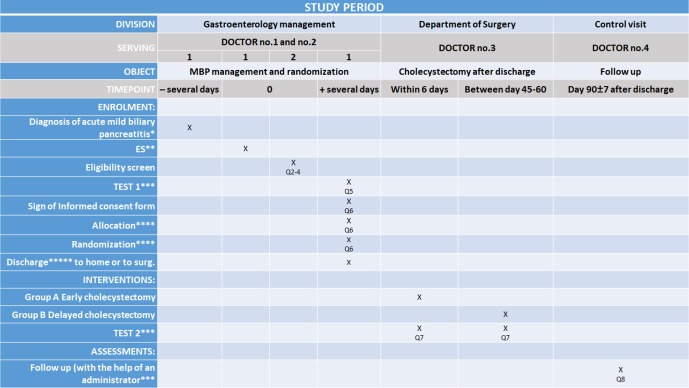
Figure 3.
Schedule of enrolment, interventions and assessments according to the SPIRIT 2013 statement.15 *Diagnosis of acute biliary pancreatitis (any of the following three definitions): diagnosis of gallstones on imaging, and alanine aminotransferase level >2 times higher than normal values with ALT >AST. In the first 24 hours of admission, all patients will undergo either an ultrasonography or a contrast-enhanced CT to detect if the gallbladder contains gallstones and to determinate the diameter of the common bile duct. ABP is mild, when there is no pancreatic necrosis or no transient or persistent organ failure (>48 hours). **If it is necessary to perform endoscopic sphincterotomy during the current admission or ES in the medical history also acceptable. ***Data will be collected in a personalised database and follow-up will consist of questionnaires. The patient will be asked to note every biliary event during the follow-up period and will be contacted in person within the 90 days after discharge to collect information. After data collection, we can draw conclusions about the treatment strategy. Improperly completed datasheets and incorrect data upload will be avoided and controlled by the administrator. (Q5, Q7, Q8, Q=question) **** The patient can be randomised by using a randomisation module with sealed envelope. Patient data will be uploaded to the data base, which will be followed by the randomisation. This randomisation module will allocate the participants to the two different groups. This method makes it impossible for researchers to predict the allocation of the patients involved in the study. It is impossible to conceal the distribution of the patients in this study because the patients need to be scheduled for either an early cholecystectomy or a delayed cholecystectomy. Allocation will be carried out based on predefined randomisation lists created separately for each recruiting centre. The allocation sequence will be prepared with a variable block size and with an allocation ratio 1:1 by the IDMB. *****The criteria are the following: (1) anticipation on the part of the treating physician that the patient can be discharged within 1 or 2 days; (2) no need for analgesics; (3) declining C reactive protein levels and <150 mg/L; (4) no evidence of local or systemic complications (eg, no fever); (5) oral feeding is tolerated for 24 hours and (6) ERCP/ES either during the index admission or in the medical history without complication. Before discharge or transfer to surgery department. ES, endoscopic sphincterotomy; IDMB, Independent Data Management Board.
Group A: The patient is randomised to the early cholecystectomy group, and cholecystectomy will be performed within 6 days after discharge.
Group B: The patient is randomised to the delayed cholecystectomy group, and the cholecystectomy will be carried out between 45 and 60 days after discharge.
Discontinuing or the modification of the allocated interventions for a trial participant is based on surgical causes like contraindicated opus, need for conversion to open cholecystectomy or when the patient does not present to the hospital for cholecystectomy. Switching over the two interventions is not possible considering the trial characteristics; however, in case of acute cholecystitis, acute cholecystectomia can be performed independently from this trial. The case must be presented to SC.
Surgical details and quality control
If it will be the first ERCP/ES performed in the patient’s medical history, it will be performed according to the European Society of Gastrointestinal Endoscopy (ESGE) guidelines.27 The LC will follow the European Association Guidelines for Endoscopic Surgery.28 The patients will be operated on by laparoscopically trained surgeons with >100 laparoscopic procedures performed and by a trained gastroenterologist with >50 ES completed within a year must be on duty if ERCP/ES is provided during the index admission. Centres which intend to randomise at least 15 patients and are able to perform an early cholecystectomy and ERCP/ES are eligible to participate in the study. In those centres, ES data will then be collected on the incidence of choledocholithiasis, percentage bile duct injury, duration and perceived difficulty (on a scale of 0–10).
Diagnosing and treating ABP
In the first 24 hours of admission, all patients will undergo either an ultrasonography or a contrast-enhanced CT to detect if the gallbladder contains gallstones and to determinate the diameter of the common bile duct. ERCP should be performed only in the case of cholangitis or choledocholthiasis, to clear the bile duct with ES as described in the IAP/APA guideline. When only the laboratory parameters suggest common bile duct obstruction or choledocholthiasis, MRCP/EUS should be carried out.10
Data collection and follow-up
Data will be collected in a personalised database, and follow-up will consist of questionnaires (online supplementary file). The patient will be asked to note every biliary event during the follow-up period and will be contacted in person within 90 days after discharge to collect information. After data collection, we can draw conclusions about the treatment strategy. Improperly completed datasheets and incorrect data upload will be avoided and controlled by an administrator.
bmjopen-2018-025551supp001.pdf (558.2KB, pdf)
The personal information about enrolled participants will only be shared with IDMB as uploaded data for randomisation, after data analysis only randomisation code will be used for identification to protect confidentiality during, and after the trial. Only the principal investigator and the IDMB will have access to the final trial dataset. However, only identification code is used, and we can keep aside duplicated patient’s data as cholecystectomy cannot be performed twice.
Sample size estimation method
Primary endpoint
A composite of gallstone-related complications or mortality occurring within 6 months after discharge.
Hypothesis
With regard to our hypothesis, based on a non-inferiority design, there is no difference between the two groups (5%) in mortality or readmission for gallstone-related complications within 3 months after discharge.
Starting point
Sample size estimation was based on the results obtained by the PONCHO trial carried out on 264 patients, where a non-significant difference of 14% was obtained between the two study groups (3% in the same-admission cholecystectomy group compared with 17% in the interval admission group). Thus, using the hypothesised 5% for the occurrence of the primary endpoint in the same-admission cholecystectomy group and a max difference of 14% given by the results of the PONCHO trial a total sample size of 93 was obtained using a 10% drop-out rate. The sample size estimation results are listed in figure 4.
Figure 4.
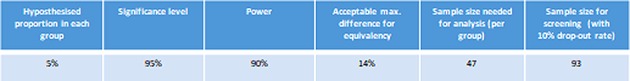
The listed parameters were used to estimate results for the current sample size.
Data management and statistical analyses
Data will be handled by an independent Clinical Research Organiser. Electronic case report form (eCRF) will be used. The Investigator will ensure that the data in the eCRF are accurate, complete and legible. Detailed data flow will be described in a Data Management Plan. Data from completed eCRFs will be validated under the direction of the Data Manager according to a Data Cleaning Plan (DCP). Adverse events will be coded using MedDRA according to GCP, GLP, FDA 21CFR PART11 and other relevant regulatory requirements.
Safety Analysis Set (all patients enrolled in the study), Per Protocol Set (all enrolled patients who finished the study conforming to the requirements of the study protocol) and Intention to Treat (all randomised participants who start on a treatment, excluding consent withdrawals) will be performed.
Baseline patient and disease characteristics will be analysed using descriptive analysis. Demographic and baseline characteristics will be summarised for the overall study population. Descriptive statistics for both the primary and secondary parameters will be analysed similarly.
Subgroup analyses will be performed concerning the imaging alterations: (1) no gallstones on imaging, (2) gallstone. Since we cannot exclude the possibility of fibrosis after earlier ES, we will perform a subgroup analysis during the interim analysis as well. If the results obtained from the interim analysis indicate that there could be significant difference between index admission and earlier ES, we will modify the trial protocol from the single-population (the same-admission endoscopic sphincterotomy or ES in the medical history) two-arm (two groups: (1) early cholecystectomy; (2) delayed cholecystectomy) set up to a two-population two-arm set up (four groups: (1) early or (2) delayed cholecystectomy with index admission ES, (3) early or (4) delayed cholecystectomy in patients having earlier ES). The required patients’ number will be adjusted in both populations accordingly. In case of important protocol modifications, IDMB will report to the SC. SC will discuss and if the adverse effect is confirmed, it will be reported to the relevant institutional and national ethical committee http://www.ett.hu/tukeb.htm
Premature termination of the study
In the interests of patient safety, an interim analysis will be conducted after 15 patients and after half of the presumed number of patients (45) have completed the study. IDMB will perform an independent assessment of the trial related documents and activities, with the aim of ensuring the respect of subjects’ right, safety and well-being and to guarantee the plausibility of clinical data. Similarity of groups at baseline will be also checked. The study will also be stopped if the two groups’ results differ significantly (p<0.001). The study will be discontinued if the difference between the planned number of patients and the actual number is higher than 60% within 1 year. IDMB will report to SC.
Centres
The trial will be launched in four Hungarian (Szeged, Debrecen, Pécs and Székesfehérvár) and two Romanian centres (Targu Mures and Cluj Napoca), after which the study will be open to other centres. In all cases, the IDMB will conduct an audit of the centre and will report to the SC. The SC maintains the right to decide whether a centre meets the required quality to join the study.
The full protocol will be available for public in an open access journal.
Publication policy
We would like to publish the results in one of the internationally highly recognised journals. Centres providing more than 25 patients can provide 4 authors to the authorship list: 2 surgeons and 2 gastroenterologists.
Patient and public involvement
This prestudy protocol contains no results and data, therefore patients and or public were not involved.
Discussion
In the case of early LC, while dissection and logistics are more difficult6 7 compared with delayed (interval) cholecystectomy, it is still more effective. Delayed cholecystectomy in a mild form of ABP is preferred by many surgeons, but a number of complications can occur: recurrent ABP, acute cholecystitis, obstruction of ductus choledochus and uncomplicated biliary colic.6 7 After ERCP/ES is performed, the common bile duct is cleared, the complications caused by gallstones are significantly reduced.29 The EMILY study is designed to determine if ERCP/ES for mild ABP aids in delaying the cholecystectomy to day 45–60 after discharge among patients with ABP.
If an ES aids in delaying a cholecystectomy, then we can reduce early cholecystectomy-related complications and the surgeons can proceed with a safer, easier cholecystectomy using this method of treatment.
Supplementary Material
Footnotes
DK and PH contributed equally.
Contributors: LPK, KM, DK, ÁV, LC, MP, FI, ÁA, MT and PH designed the study. As a member of the ITAB MML, JN, MS-T and OHP gave advice and will continuously monitor the progress of the study. LPK, KM, PH, ZS and KP drafted the manuscript. GL, SB, AV, LB, MD, NZ, JA and AS edited the manuscript. IN carried out the sample size calculation. ZS, KP, IT, NZ, JA and AS edited the figures and tables. All authors read and approved the final manuscript. During the study IT, ÁV, LC, MP and MT are going to manage the endoscopic treatments. DK, GL, ZS, MD and SB are responsible for cholecystectomies. ITAB and SC members are listed ahead.
Funding: Center costs (IT, biostatistics, trial organisation and so on) are covered by the University of Pécs Medical School, Momentum Grant of the Hungarian Academy of Sciences (LP2014-10/2014) and Economic Development and Innovation Operative Programme Grant and Highly Cited Publication Grant of the National Research, Development and Innovation Office (GINOP-2.3.2- 15-2016-00048 Stay Alive, KH-125678 and EFOP 3.6.2-16-2017-00006 Live Longer) and Translational Medicine Foundation. Since no additional treatment is necessary for the study, the general healthcare costs are covered by the National Healthcare System (University of Pécs-Medical School). This study was designed with help of the Centre for Translational Medicine at the University of Pécs. This centre is committed to improve patient’s life with research activities like registries, observational and interventional trial organisations (https://tm-centre.org).
Competing interests: None declared.
Ethics approval: Scientific and Research Ethics Committee of the Hungarian Medical Research Council (EKU/2018/12176-5).
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: Additional information and future plan: Blood samples (serum and plasma) will be stored from all patients in order to study laboratory parameters later if required (eg, the laboratory could not measure it), and in order to build up a biobank for later clinical studies to which all participants will give informed consent. The samples will be stored at –80°C. The post-trial care will follow the routine treatment protocols. In case if a patient suffers a harm during hospitalisation, all of the responsibility is taken by the hospital where the patient is treated.
Patient consent for publication: Not required.
References
- 1. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87. 10.1053/j.gastro.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252–61. 10.1053/j.gastro.2013.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schepers NJ, Bakker OJ, Besselink MG, et al. Early biliary decompression versus conservative treatment in acute biliary pancreatitis (APEC trial): study protocol for a randomized controlled trial. Trials 2016;17:5 10.1186/s13063-015-1132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Uhl W, Müller CA, Krähenbühl L, et al. Acute gallstone pancreatitis: timing of laparoscopic cholecystectomy in mild and severe disease. Surg Endosc 1999;13:1070–6. [DOI] [PubMed] [Google Scholar]
- 5. Halász A, Pécsi D, Farkas N, et al. Hungarian Pancreatic Study Group. Outcomes and timing of endoscopic retrograde cholangiopancreatography for acute biliary pancreatitis. Dig Liver Dis 2019. 10.1016/j.dld.2019.03.018 [DOI] [PubMed] [Google Scholar]
- 6. Working Party of the British Society of Gastroenterology Association of Surgeons of Great Britain and Ireland Pancreatic Society of Great Britain and Ireland Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut 2005;54 Suppl 3:iii1–iii9. 10.1136/gut.2004.057026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forsmark CE, Baillie J. AGA Institute Clinical Practice and Economics Committee AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology 2007;132:2022–44. 10.1053/j.gastro.2007.03.065 [DOI] [PubMed] [Google Scholar]
- 8. van Baal MC, Besselink MG, Bakker OJ, et al. for the Dutch Pancreatitis Study Group: Interval cholecystectomy after mild biliary pancreatitis is associated with a high risk of readmission for recurrent biliary events, especially recurrent biliary pancreatitis. Annals of Surgery 2012;255:860–6. [DOI] [PubMed] [Google Scholar]
- 9. Banks PA, Freeman ML. Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101:2379–400. 10.1111/j.1572-0241.2006.00856.x [DOI] [PubMed] [Google Scholar]
- 10. Uhl W, Warshaw A, Imrie C, et al. IAP Guidelines for the Surgical Management of Acute Pancreatitis. Pancreatology 2002;2:565–73. 10.1159/000067684 [DOI] [PubMed] [Google Scholar]
- 11. Nealon WH, Bawduniak J, Walser EM. Appropriate timing of cholecystectomy in patients who present with moderate to severe gallstone-associated acute pancreatitis with peripancreatic fluid collections. Ann Surg 2004;239:741–51. 10.1097/01.sla.0000128688.97556.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. da Costa DW, Bouwense SA, Schepers NJ, et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): a multicentre randomised controlled trial. Lancet 2015;386:1261–8. 10.1016/S0140-6736(15)00274-3 [DOI] [PubMed] [Google Scholar]
- 13. Lankisch PG, Weber-Dany B, Lerch MM. Clinical perspectives in pancreatology: compliance with acute pancreatitis guidelines in Germany. Pancreatology 2005;5:591–3. 10.1159/000087501 [DOI] [PubMed] [Google Scholar]
- 14. Uomo G, Manes G, Laccetti M, et al. Endoscopic sphincterotomy and recurrence of acute pancreatitis in gallstone patients considered unfit for surgery. Pancreas 1997;14:28–31. 10.1097/00006676-199701000-00005 [DOI] [PubMed] [Google Scholar]
- 15. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hritz I, Hegyi P. Early Achievable Severity (EASY) index for simple and accurate expedite risk stratification in acute pancreatitis. J Gastrointestin Liver Dis 2015;24:177–82. 10.15403/jgld.2014.1121.242.easy [DOI] [PubMed] [Google Scholar]
- 17. Párniczky A, Mosztbacher D, Zsoldos F, et al. Analysis of Pediatric Pancreatitis (APPLE Trial): pre-study protocol of a multinational prospective clinical trial. Digestion 2016;93:105–10. 10.1159/000441353 [DOI] [PubMed] [Google Scholar]
- 18. Zsoldos F, Párniczky A, Mosztbacher D, et al. Pain in the early phase of pediatric pancreatitis (pineapple trial): Pre-study protocol of a multinational prospective clinical trial. Digestion 2016;93:121–6. 10.1159/000441352 [DOI] [PubMed] [Google Scholar]
- 19. Dubravcsik Z, Madácsy L, Gyökeres T, et al. Preventive pancreatic stents in the management of acute biliary pancreatitis (PREPAST trial): pre-study protocol for a multicenter, prospective, randomized, interventional, controlled trial. Pancreatology 2015;15:115–23. 10.1016/j.pan.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 20. Márta K, Szabó AN, Pécsi D, et al. High versus low energy administration in the early phase of acute pancreatitis (GOULASH trial): protocol of a multicentre randomised double-blind clinical trial. BMJ Open 2017;7:e015874 10.1136/bmjopen-2017-015874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubravcsik Z, Farkas G, Hegyi P, et al. [Autoimmune pancreatitis. Evidence based management guidelines of the Hungarian Pancreatic Study Group]. Orv Hetil 2015;156:292–307. 10.1556/OH.2015.30061 [DOI] [PubMed] [Google Scholar]
- 22. Hritz I, Czakó L, Dubravcsik Z, et al. [Acute pancreatitis. Evidence-based practice guidelines, prepared by the Hungarian Pancreatic Study Group]. Orv Hetil 2015;156:244–61. 10.1556/OH.2015.30059 [DOI] [PubMed] [Google Scholar]
- 23. Párniczky A, Czakó L, Dubravcsik Z, et al. [Pediatric pancreatitis. Evidence based management guidelines of the Hungarian Pancreatic Study Group]. Orv Hetil 2015;156:308–25. 10.1556/OH.2015.30062 [DOI] [PubMed] [Google Scholar]
- 24. Takács T, Czakó L, Dubravcsik Z, et al. [Chronic pancreatitis. Evidence based management guidelines of the Hungarian Pancreatic Study Group]. Orv Hetil 2015;156:262–88. 10.1556/OH.2015.30060 [DOI] [PubMed] [Google Scholar]
- 25. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 26. Bouwense SA, Besselink MG, van Brunschot S, et al. Pancreatitis of biliary origin, optimal timing of cholecystectomy (PONCHO trial): study protocol for a randomized controlled trial. Trials 2012;13:225 10.1186/1745-6215-13-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2016;48:657–83. 10.1055/s-0042-108641 [DOI] [PubMed] [Google Scholar]
- 28. Edmund AMN, Hans T, Kum CK, et al. EAES Clinical Practice Guidelines on Laparoscopic Cholecystectomy, Appendectomy, and Hernia Repair (1994) In: Neugebauer E, Sauerland S, Fingerhut AB, Millat B, Buess GF, eds EAES guidelines for endoscopic surgery: Twelve years evidence-based surgery in EuropeSpringer Berlin Heildelberg, 2006:265–89. [Google Scholar]
- 29. Testoni PA. Acute recurrent pancreatitis: Etiopathogenesis, diagnosis and treatment. World J Gastroenterol 2014;20:16891 10.3748/wjg.v20.i45.16891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Santvoort HC, Besselink MG, de Vries AC, et al. Early endoscopic retrograde cholangiopancreatography in predicted severe acute biliary pancreatitis: a prospective multicenter study. Ann Surg 2009;250:68–75. 10.1097/SLA.0b013e3181a77bb4 [DOI] [PubMed] [Google Scholar]
- 31. Keus F, de Jong JA, Gooszen HG, et al. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev 2006;4:CD006231 10.1002/14651858.cd006231 [DOI] [PubMed] [Google Scholar]
- 32. Mayumi T, Takada T, Kawarada Y, et al. Results of the Tokyo Consensus Meeting Tokyo Guidelines. J Hepatobiliary Pancreat Surg 2007;14:114–21. 10.1007/s00534-006-1163-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil 2017;23:151–63. 10.5056/jnm16214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-025551supp001.pdf (558.2KB, pdf)