Abstract
Hemobilia refers to bleeding from and/or into the biliary tract and is an uncommon but important cause of gastrointestinal hemorrhage. Reports of hemobilia date back to the 1600s, but due to its relative rarity and challenges in diagnosis, only in recent decades has hemobilia been more critically studied. The majority of cases of hemobilia are iatrogenic and caused by invasive procedures involving the liver, pancreas, bile ducts and/or the hepatopancreatobiliary vasculature, with trauma and malignancy representing the two other leading causes. A classic triad of right upper quadrant pain, jaundice, and overt upper gastrointestinal bleeding has been described (i.e. Quincke’s triad), but this is present in only 25%–30% of patients with hemobilia. Therefore, prompt diagnosis depends critically on having a high index of suspicion, which may be based on a patient’s clinical presentation and having recently undergone (peri-) biliary instrumentation or other predisposing factors. The treatment of hemobilia depends on its severity and suspected source and ranges from supportive care to advanced endoscopic, interventional radiologic, or surgical intervention. Here we provide a clinical overview and update regarding the etiology, diagnosis, and treatment of hemobilia geared for specialists and subspecialists alike.
Keywords: Hemobilia, Upper gastrointestinal hemorrhage, Etiology, Diagnosis, Imaging, Hepatopancreatobiliary interventions
1. Introduction
1.1. Overview
Hemobilia, in its most elemental sense, refers to the occurrence of extravasated gross blood in the biliary tract. The most common causes of hemobilia are iatrogenic, traumatogenic, and neoplastic. Though hemobilia remains an uncommon cause of digestive tract bleeding, its incidence has gradually increased as the arsenal of minimally-invasive hepatopancreatobiliary procedures has expanded. More hepatopancreatobiliary procedures has come the advent of new approaches, including advanced endoscopic and interventional radiologic techniques, to diagnose and treat hemobilia.1 Nevertheless, the diagnosis of hemobilia can be clinically challenging, and the ideal treatment approach may not be readily apparent or available.
In this narrative review, we provide a comprehensive yet concise overview of the etiology, diagnosis and treatment of hemobilia based on the published biomedical literature. We present both historical perspectives as well as clinical updates and current practices which are relevant for specialists as well as subspecialists.
1.2. Historic background
The first known report of hemobilia was from Francis Glisson, in 1654, who described the clinical presentation of a nobleman whom in the midst of a sword fight suffered a fatal blow to the right upper quadrant, leading to massive (upper) gastrointestinal bleeding and death. Postmortem, the source of bleeding was found to be from a liver laceration, which in turn led to the landmark description of hemobilia. Antonie Portal was the first to publish a case of hemobilia identified antemortem, reporting suspected hemobilia that was later confirmed on autopsy in 1777.2 Portal drew important attention to the difficulty in identifying the pinpoint source of bleeding. A problem still faced today in many cases.3 One hundred years later, Quincke4 identified the clinical triad of right upper quadrant pain, jaundice, and upper gastrointestinal tract bleeding, known as “Quincke’s triad”. By the 1900s, there were many scattered case reports of biliary tract hemorrhage, though the term hemobilia was not actually coined in the published literature until 1948.5
2. Epidemiology
Though uncommon, hemobilia is an important cause of upper gastrointestinal hemorrhage. Published data on the topic are mainly in the form of case reports and three large case series. In 1973, Sandblom5 reported a series reviewing 355 cases, including 59 iatrogenic cases (16.6%) and 137 (38.6%) traumatogenic cases. In 1987, Yoshida et al.6 published a series of 103 patients with hemobilia, of whom 41% were iatrogenic and 19% were traumatogenic, thus reversing the relative contributions (compared to prior reports) in favor of iatrogenic causes, citing increasing hepatobiliary interventional procedures as the primary contributing factor. This finding was subsequently validated in 2001 by Green et al.7 in a series of 222 patients, among whom 65% had an iatrogenic cause and only 6% had a traumatogenic cause (Fig. 1). This is consistent with more recent reports indicating iatrogenic injury as the leading cause of hemobilia, accounting for over 50% of all cases.
Fig. 1. Evolving etiologies of hemobilia over time.
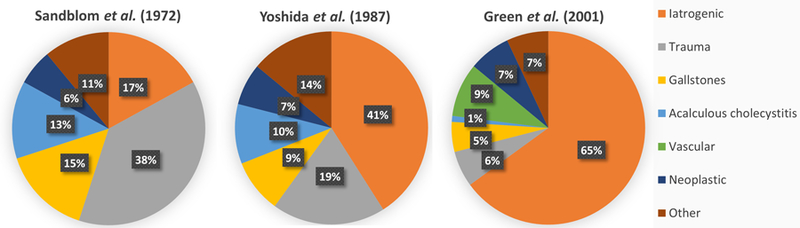
A transition can be seen from traumatogenic to iatrogenic cases of hemobilia over time.5–7
3. Clinical presentation
The classic presentation of hemobilia is formally known as Quincke’s triad: jaundice, right upper quadrant abdominal pain, and upper gastrointestinal hemorrhage, but the presentation of all three together only occur in 22%—35% of cases.7,8 Typically, hemobilia can present as hematemesis, melena, or hematochezia, with or without choluria, depending on the rate of bleeding and anatomical factors (e.g. post-bilioenteric surgical anatomy). However, the clinical presentation of hemobilia often depends on the etiology. For example, patients with a percutaneous transhepatic biliary drain (PTBD) may present with bloody output from the biliary drain.
The timing of presentation can also vary and possibly aid in diagnosis. Endoscopic retrograde cholangiopancreatography (ERCP)-related hemobilia tends to present immediately or within a few days after the inciting biliary duct injury (e.g. sphincterotomy or biliary stricturoplasty).9,10 Hemobilia can emanate from venous or arterial sources; the former tends to be lower volume or self-limited compared to the latter (except in cases of portal hypertension, in which case it may be larger volume and/or persistent). Regardless of the source, the difference in density and biochemical properties between blood and bile causes phase separation of the two within the biliary tree, and as bleeding stops, the blood that entered the biliary tract begins to clot, serving as a physical impediment to biliary outflow.
Blood clots in the biliary tree can cause mechanical biliary obstruction, symptomatic jaundice, and right upper quadrant or epigastric pain. In addition, clots also cause biliary stasis and predispose to development of acute cholangitis.9,11 Because of their similar echogenicity, it is important to recognize that clots can masquerade as biliary stones on imaging studies, thus requiring a high index of suspicion congruent with the clinical presentation. Biochemically, hemobilia may present with anemia and/or abnormal liver enzymes. The pattern of liver enzyme elevation depends on the etiology of hemobilia, as well as the severity and time point in the course of hemobilia. Classically, elevations are seen in serum bilirubin and alkaline phosphatase, with a degree of elevation depending on the severity of the obstruction caused by clots and blood. However, isolated elevated alkaline phosphatase or a mixed cholestatic-hepatocellular pattern has also been reported.12,13 In one case series of 37 patients with hemobilia, mean total bilirubin was reported to be 10.5 mg/dL, alkaline phosphatase 834 IU/L, aspartate aminotransferase 353 IU/L, and mean alanine aminotransferase 243 IU/L.14
4. Causes of hemobilia
There are many possible causes of hemobilia, including iatrogenic, traumatogenic, neoplastic, inflammatory, infectious, and vascular etiologies. More recently, iatrogenic causes of hemobilia (though still relatively rare) have superseded other causes in most series/populations and can be generally categorized by procedural type.
4.1. Iatrogenic causes
4.1.1. Percutaneous interventions
Common causes of iatrogenic hemobilia include percutaneous liver biopsy, diagnostic percutaneous transhepatic cholangiography (PTC), and PTBD placement.15 With regard to the risk of hemobilia due to percutaneous liver biopsy, the published literature shows some discrepancy. For example, a recent study by Zhou et al.17 found that hemobilia accounts for 3% of all major complications of percutaneous liver biopsy, whereas a larger retrospective study reported only a 0.005% risk (4 cases of hemobilia out of 68,276 liver biopsies). The discrepancy between studies may ostensibly be due to an increasing number of higher risk biopsies being performed (e.g. due to detection and subsequent sampling of smaller or more central hepatic lesions) or to improved diagnosis and better reporting of hemobilia over time.16,17
Percutaneous interventions can also cause hemobilia by inadvertently “nicking” a vascular structure, with the risk being higher in cases such as a non-dilated biliary tree (i.e. a smaller target) or portal vein thrombosis. Rivera et al.18 compared hemobilia caused by PTBD vs. PTC and found that the risk of hemobilia is higher with PTBD (2.2%) than with PTC alone (0.7%). This three-fold increase in hemobilia with PTBD compared to PTC alone may be due to the greater size of the aperture made in the bile duct wall with PTBD and the presence of a foreign material remaining in the duct which can serve as a cause of inflammation or erosion.9 A retrospective cohort study had similar results, citing that the risk of hepatic artery injury was 2.6% with PTBD and 0.7% with PTC.19
Other interventional procedures which may result in hemobilia include ultrasound-guided radiofrequency ablation, transarterial chemoembolization, and transjugular intrahepatic portosystemic shunt placement.20–22 Though potentially high risk, these interventions collectively comprise a much smaller proportion of cases due to their relative rarity.
4.1.2. Endoscopic hepatopancreatobiliary interventions
The main endoscopic procedure associated with hemobilia is ERCP, and in particular, endoscopic sphincterotomy. Sphincterotomy-associated bleeding typically occurs at the level of cut papillary sphincter, but blood can occasionally reflux from the duodenum into the biliary tree.5 In general, the risk of hemobilia depends on the invasiveness of the maneuvers performed during ERCP (e.g. stricturoplasty, extraction of large stones) as well as patient-level variables such as coagulopathy and presence of diseased tissue (e.g. friable, hypervascular tumor). Additional risk factors for ERCP-related hemobilia include the presence of variant anatomy (particularly if not recognized a priori), aggressive biliary balloon dilation or intraductal biopsy acquisition, and vascular anomalies (e.g. hereditary hemorrhagic telangiectasia).
Other endoscopic interventions which merit mention include endoscopic ultrasound (EUS)-guided fine needle aspiration or biopsy of pancreatobiliary lesions and trans-biliary ductal drainage procedures (e.g. EUS-guided choledochoduodenostomy and hepaticogastrostomy). These procedures are being increasingly performed by advanced endoscopists and can result in hemobilia given their complexity and frequent underlying presence of patient-level comorbidities.9,23–26
4.1.3. Surgical interventions
Complications of both laparoscopic and laparotomic surgeries performed near the cystic and right hepatic artery can involve lacerations or other injuries of nearby structures in a manner which can lead to hemobilia. Cholecystectomy, liver transplantation, and pancreaticoduodenectomy are examples of surgeries which have been reported to cause hemobilia, often through the formation of hepatic artery pseudoaneurysms.27–30 Bile is known to cause endothelial damage to blood vessels, and iatrogenic injury that occurs to both the arterial and biliary structures can lead to delayed healing and the formation of pseudoaneurysms.31 The majority of pseudoaneurysms occur in the right hepatic artery, though they can occur in any artery near the hepatobiliary system.32,33
4.2. Non-iatrogenic causes
4.2.1. Malignancy
Arguably the most common spontaneous cause of hemobilia is primary or metastatic hepatobiliary malignancy.2 Hepatobiliary malignancies, including cholangiocarcinoma, pancreatic cancer, gallbladder cancer, liver metastasis, and hepatocellular carcinoma (HCC) have all been associated with hemobilia.34 All-cause malignancies account for 10% of total hemobilia cases (Green et al.,7 2001). This is thought to be due to more friable tissue and vasculature, leading to an increased propensity for spontaneous hemorrhage.17 Likewise, metastasis of other malignancies to the hepatobiliary tract can cause hemobilia that may be difficult to detect on convention imaging and can present initially in other forms such as biliary obstruction.35
Manolakis et al.36 reported a case of hemobilia as the initial manifestation of cholangiocarcinoma in a patient with the classic triad of abdominal pain, gastrointestinal bleeding, and jaundice but in whom imaging studies, including ultrasound, magnetic resonance imaging (MRI) /magnetic resonance cholangiopancreatography (MRCP) and magnetic resonance angiography (MRA) and initial ERCP failed to identify a mass. It was on repeat ERCP that the biliary ductal system was noted to have been infiltrated with tumor and lined by friable tissue with blood clots evident in the papilla of Vater, consistent with hemobilia. This case highlights the rare but important consideration of hemobilia and underlying malignancy in a patient presenting with classic symptoms despite a lack of previous trauma or instrumentation.36
Like cholangiocarcinoma, HCC is a highly vascular tumor and can cause hemobilia with biliary ductal invasion.37 Additionally, there are numerous case reports of spontaneous rupturing of HCC leading to hemorrhagic shock and end organ dysfunction. While rates of spontaneous rupture are extremely rare (<3% reported), it remains a life-threatening complication with high mortality rates (>50%).38 Metastasis of other malignancies to the hepatobiliary tract can also cause hemobilia that may be difficult to detect on convention imaging and can present initially in other forms such as biliary obstruction.35 Most cases of hemobilia caused by malignancy can be treated with procedures such as radiofrequency ablation or transcatheter arterial embolization (TACE). Additionally, emergent hepatic resection, when technically feasible, has been shown to be a safe and appropriate treatment option for patients with tumor bleeding from HCC.
4.2.2. Treatment of malignancy
While endovascular modalities are often used to treat hemobilia, these procedures themselves can also inadvertently lead to hemobilia.27 Radiofrequency ablation (RFA) used for early-stage HCC has been shown to cause hemobilia, possibly by causing fistulas to form between the punctured blood vessels and the biliary duct.39 The frequency of hemobilia after ultrasound-guided percutaneous liver RFA range from 0% to 0.5%.40–42 For computed tomography (CT) -guided RFA, a single center study of 195 patients found the rate of post-RFA hemobilia to be 8.2%.39 TACE using drug-eluting beads has also been associated with hemobilia.43 There are also reports of certain drugs such as sorafenib, which has been shown to increase the risk of bleeding events in general, leading to hemobilia in patients with HCC invading into the biliary tract.44
4.2.3. Portal biliopathy
Hemobilia can rarely occur due to portal biliopathy, with or without preceding biliary tract intervention. Portal biliopathy occurs as a result of hypertension of the peri-biliary (e.g. choledochal) venous plexus, often in patients with portal vein thrombosis and ensuing portal cavernomas, and manifests radiographically and cholangiographically with multifocal biliary stenosis from enlarged, tortuous venous structures encircling the bile duct. Hemobilia in this context often requires interventional radiologic or other advanced intervention as the bleeding is not from an abnormality of the biliary epithelium.
4.2.4. Chronic ductal obstruction
Chronic obstruction of the pancreatobiliary tract can potentially lead to inflammation, erosion, and fistulization with adjacent vascular structures and resultant hemobilia.45 As mentioned earlier, intrabiliary clots which form due to hemobilia can be mistaken as stones on various imaging studies. Even when gallstones are present, however, there can still be concurrent hemobilia, especially in cases wherein the stone erodes through the cystic artery or other vascular, analogous to how a stone can erode and fistulize into the duodenum and cause duodenal outlet obstruction in Bouveret’s syndrome.27 It is worth mentioning here that, although not classified as hemobilia, hemosuccus pancreaticus (also referred to as Wirsungorrhagia) can occur via pathophysiologically similar mechanisms, e.g. pancreatitis eroding into the splenic artery and causing bleeding into the main pancreatic duct.46,47
4.2.5. Intraductal infection
The most clinically significant cause of infectious hemobilia is “tropical hemobilia”, a result of parasitic infestation of the biliary tract. Commonly implicated organisms include roundworms (e.g. Ascaris lumbricoides), the Chinese liver fluke (Clonorchis sinensis), and the sheep liver fluke (Fasciola hepatica). Echinococcal infections can also cause hemobilia indirectly, as hydatid cysts may cause inflammation of perivascular tissue, weakening of vessel walls, and/or pseudoaneurysm formation with resultant bleeding into the biliary tract. China, Korea, and Vietnam carry the highest incidence of ascariasis and subsequently higher rates of hemobilia secondary to this infection.27
5. Diagnosis
Hemobilia should be suspected in any patient with an unclear source of gastro-intestinal (GI) bleed, recent blunt force or penetrating trauma to the upper abdomen, or biliary instrumentation or manipulation, particularly in the context of contemporaneous signs or symptoms of biliary obstruction. The diagnosis of hemobilia can be challenging because it is often simply not suspected or raised in the differential diagnosis due to its uncommon occurrence, particularly in cases where there is no history of recent biliary tract instrumentation or trauma. Imaging can be highly helpful for making the initial diagnosis and guiding therapeutic options, though findings may be nonspecific, again prompting a need for clinical suspicion and careful correlation. Upper endoscopy with direct visualization of blood or clot emerging from the biliary tract essentially confirms the diagnosis (Fig. 2), as can angiography. These and other modalities are discussed further below.
Fig. 2. Proposed algorithm for the diagnosis and management of hemobilia.
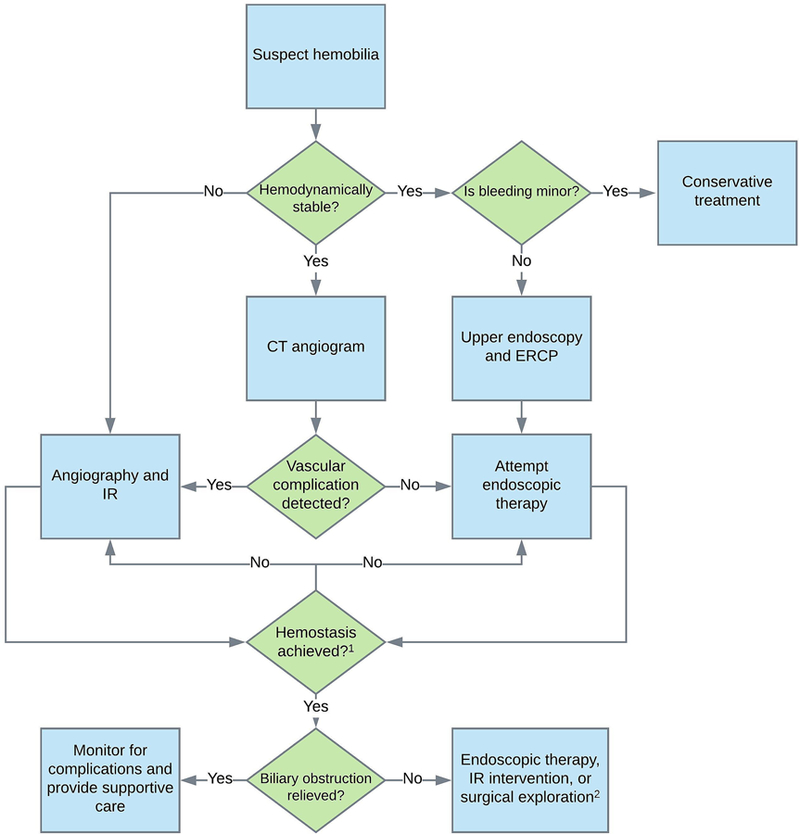
(i) Vascular complications include hepatic artery aneurysms, pseudoaneurysms, and cholangio-venous or arterio-ductal fistulae. (ii) If endoscopic therapy is not successful in achieving hemostasis, IR techniques should be attempted. The converse may also apply, depending on the clinical scenario. (iii) Surgical exploration is indicated for biliary obstruction (or hemostasis) when endoscopic and IR techniques fail or are not applicable. Abbreviations: CT, computed tomography; IR, interventional radiology; ERCP, endoscopic retrograde cholangiopancreatography.
5.1. Computed tomography
CT of the abdomen with angiography protocol has become a first choice diagnostic test for hemobilia. The advantages of contrast-enhanced CT include its non-invasive nature and low radiation exposure compared to conventional angiography, rapid results, and excellent diagnostic performance characteristics.46,48,49
5.2. Upper endoscopy and ERCP
Up to 60% of hemobilia cases can be diagnosed by upper endoscopy.49 Depending on anatomical and other factors, a duodenoscope (i.e. side-viewing scope) may be needed to visualize the major papilla and assess for clots or other evidence of hemobilia (Figs. 3A and B). ERCP can be used to further visualize the biliary tree and may offer therapeutic options in patients with hemobilia and associated biliary obstruction. Characteristic ERCP findings that suggest the presence of blood clots include amorphous, tubular, or cast-like filling defects with otherwise unexplained common bile duct or peri-hilar ductal dilation.50
Fig. 3. Hemobilia emanating from a recently placed fully-covered self-expanding metallic stent.
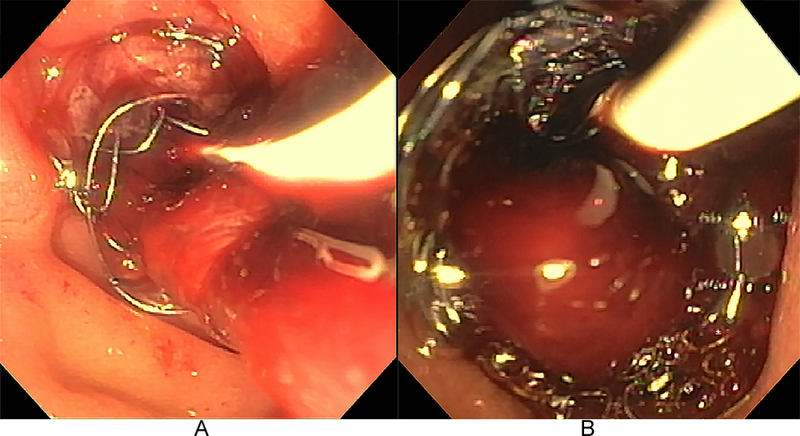
Esophagogastrojejunoscopy demonstrated numerous red-maroon clots occluding the metallic stent, requiring extensive intraductal irrigation and clot extraction as well as concomitant correction of coagulopathy.
If EUS is available, it may be used as an adjunctive non-invasive method to evaluate for vascular aneurysms and blood clots within the biliary tree when ERCP findings are equivocal.50–52 EUS can also be used to detect portal biliopathy-related bleeding (e.g. in the context of portal hypertension with intra- or para-choledochal varices).46
5.3. Angiography
Although formal angiography is no longer used as a first-line study, it remains the gold-standard for both diagnosis and treatment of hemobilia in most settings. If the bleeding vessel has not already been identified on non-invasive imaging, the first angiographic study should be a celiac arteriogram with delayed phase imaging to visualize both the hepatic arteries as well as the portal vein. It is necessary to ensure that the portal vein is patent prior to hepatic artery embolization because the liver is supplied by both the hepatic artery and the portal vein; performing hepatic artery embolization when the portal vein is thrombosed or otherwise obstructed could potentially cause significant hepatic ischemia. This is especially important in patients who are liver transplant recipients as the transplanted liver does not receive as much blood from the portal vein as a native liver, thus making it more dependent on the hepatic artery for its blood supply. Patients with cirrhosis and hereditary hemorrhagic telangiectasia involving the liver are also at risk for hepatic ischemia for the same reason.53
Angiographic evaluation typically progresses in a stepwise fashion; if celiac arteriography does not reveal a clear source of bleeding, then the catheter should be advanced and arteriographies of both the left and right hepatic arteries should be performed. If this does not reveal a source, then the superior mesenteric artery should be selectively interrogated. Contrast extravasation into the biliary tree, peripheral arterial truncation, arterial transection, pseudoaneurysms, and arterioportal fistula are all suggestive of arterial injury.54
5.4. Other diagnostic modalities
Lesser used methods to aid in the initial diagnosis include MRCP, abdominal ultrasonography (US), and surgical exploration. MRCP is a non-invasive alternative to ERCP but lacks the therapeutic options that ERCP offers and also requires more time for image acquisition. Abdominal US has been used to evaluate for the presence of blood within the gallbladder, but its diagnostic effectiveness is limited due to its limited ability to visualize the biliary ducts, particular the distal common bile duct and in patients with truncal obesity. Surgical exploration is usually reserved as a final option in which other modalities are unable to identify or resolve the hemobilia.46
6. Management
Management of hemobilia consists of two main objectives: achieving hemostasis and maintaining bile flow. The latter is important because the formation of blood clots within the biliary tract can cause complications such as obstructive jaundice, acute cholangitis, acute cholecystitis, and pancreatitis.9
The approach to management depends on several factors, including the suspected source of bleeding (arterial vs. venous bleeding), degree of hemodynamic instability, and etiology/cause (Fig. 4). All patients should have a type and screen performed and be closely monitored for hemodynamic instability. Patients who present with minor hemobilia can potentially be addressed with conservative treatment, including intravenous fluids and correction of coagulopathy. Major hemobilia that causes significant hemoglobin drop or persistent bleeding typically requires endoscopic, radiologic, or rarely, surgical intervention. Hemodynamically unstable patients should go directly to interventional radiology for hepatic angiogram and embolization or to surgery. If there are signs or symptoms of cholangitis with or without concomitant sepsis or septic shock, broad-spectrum intravenous antibiotics should be administered promptly. Vasopressors may be necessary in cases of major hemobilia as part of resuscitative measures and as a bridge to therapeutic intervention.
Fig. 4. Common endoscopic accessories relevant to management of hemobilia.
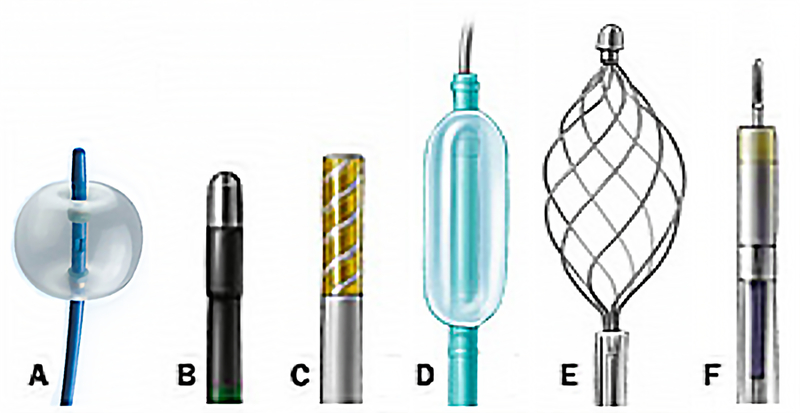
(A) extraction balloon; (B) heater probe; (C) bipolar probe; (D) dilation balloon; (E) retrieval basket; (F) injection needle.
6.1. Conservative treatment
Minor hemobilia, which typically presents as blood-tinged output from a biliary drainage catheter, is often due to injury related to PTBD catheters and can often be treated conservatively. Exchanging a PTBD catheter with a larger sized one and adjusting its position such that the side-holes of the tube are not in the same location as potential portal vein transgression sites can also help tamponade blood by increasing pressure on the walls of the bile ducts. Minor hemobilia will often resolve with maturation of the surgically created tract. A tractogram or “tubogram”, an imaging study where contrast is injected into the tract to visualize its course and patency, can be performed if bleeding persists or if there is impaired drainage (e.g. due to obstruction from clot material). If hemobilia persists, options such as embolization of the existing percutaneous tract and creation of a new tract can be considered.53
6.2. Advanced endoscopic techniques
For hemodynamically stable hemobilia without clear arterial sources of bleeding or significant vascular abnormalities on non-invasive imaging, upper endoscopy (with a duodenoscope or a clear endcap-outfitted gastroscope) and ERCP are typically the initial therapeutic procedure of choice because of their utility in concurrently managing both bleeding and biliary obstruction.55
There are a wide variety of endoscopic techniques to achieve hemostasis, the choice of which to implement will depend on the cause (e.g. trauma), location (e.g. common hepatic duct), and source (e.g. para-choledochal vein) of hemobilia. For instance, post-sphincterotomy hemobilia, which typically is a result of injury to the posterior branch of the superior pancreaticoduodenal artery (itself a branch of the gastroduodenal artery) during sphincterotomy, can be treated by spraying diluted epinephrine (1:10,000) over the area of hemorrhage, injection of epinephrine into the adjacent tissue, monopolar or bipolar coagulation, fibrin sealant injection, hemoclipping, balloon tamponading, and stent placement.56–62 These methods are most useful when the site of bleeding is distal, e.g. located at the level of the papilla or ampulla. When hemobilia is from a more proximal (e.g. peri-hilar) bleeding source, other accessories and methods to treat the hemobilia tend to be needed, including devices to extract intraductal clots, e.g. extraction balloon catheters and retrieval baskets (Fig. 4), followed by stent placement, among other options (Fig. 5). One case report has also described the use of endobiliary radiofrequency ablation for hemorrhage secondary to malignant hemobilia.63
Fig. 5. Cholangiographic images of hemobilia.
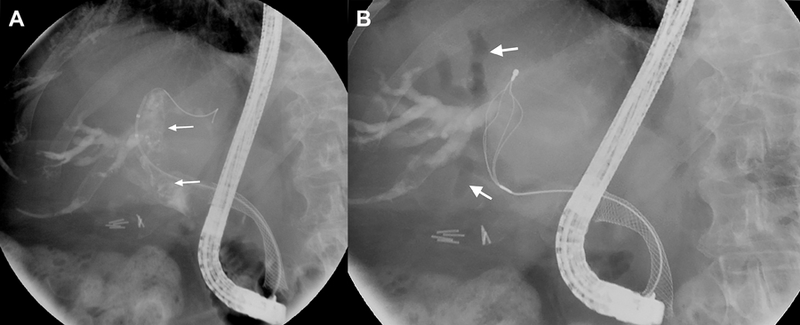
(A) Intraductal lucencies (arrows) suggestive of occlusive clot formation are seen fluoroscopically. A self-expanding metallic stent is also noted in the biliary tree. (B) A flower basket is used to extract clot from the biliary tree. Resultant air cholangiograms are seen, with arrows denoting pneumobilia.
The use of biliary stents merits additional discussion. Stents have been shown to achieve immediate hemostasis in certain cases and work by creating a tamponade effect on the bile wall while also maintaining luminal patency and thus bile flow. It can act as salvage therapy when other methods fail and as a bridge to more permanent therapy through interventional radiology or surgery.64 Both metal and plastic stents has been used successfully for hemobilia resulting from sphincterotomy, ductal dilation for biliary stenosis, fine needle aspiration (e.g. pancreatic), bile duct biopsy, and malignancy, among other causes. Fully covered self-expanding metallic stents (FCSEMs) appear to have better tamponading effects and patency and have thus largely supplanted plastic stents.64–67
Stenting can be performed in conjunction with other methods and techniques, for example, balloon tamponade can be performed by inserting a dilation balloon catheter into the common bile duct as a temporizing measure until blood flow has slowed adequately enough to permit visualization and stent placement as a more durable treatment.59 In addition, endoscopic nasobiliary drainage can help treat hemobilia and offers some unique advantages, but it is not commonly performed (primarily due to the associated discomfort).68
6.3. Transcatheter arterial embolization
As the cause of hemobilia has shifted from traumatogenic to iatrogenic over the years, radiologic intervention has become the gold standard for both diagnosis and management of persistent or hemodynamically unstable hemobilia. Angiography with transcatheter arterial embolization (TAE) should be considered as the initial therapy of choice if non-invasive imaging shows significant arterial extravasation, the presence of large arterial aneurysms or pseudoaneurysms, presence of arterio-biliary fistulae, and/or intrahepatic or extrahepatic vascular lesions. The success rate of TAE has been reported to be as high as 80% to 100%.69,70 TAE should be avoided, however, in patients with liver allografts, cirrhosis with concurrent shock, and portal vein thrombosis given these patients have compromised collateral blood flow from the portal vein, as a result of which TAE can lead to ischemic liver injury.46 Such patients may benefit from arterial stenting (as a tamponading measure) instead.
Once the bleeding site has been identified angiographically, superselection of the injured artery via threading of a microcatheter to the target area is performed, followed by TAE using coils. Coiling should be performed in a distal-to-proximal fashion to avoid back bleeding via intrahepatic arterial collaterals.53 Pseudoaneurysms should be embolized with coils from the two ends to reduce the risk of enlarging the aneurysm. Alternatives to coils include Gelfoam, polyvinyl alcohol (PVA) particles, and liquid embolic agents such as n-butyl-2-cyanoacrylate (NBCA), onyx, or thrombin. There have also been case reports of percutaneous injection of thrombin into pseudoaneurysms under ultrasound guidance.71 The method of TAE depends on the anatomy of the hepatic arteries, presence of vasospasms, tortuosity of vessels, and operator/center experience. For instance, liquid embolic agents may be helpful in patients with tortuous vessels or when there are several smaller feed into an aneurysm but require experienced radiologists due to the risk spilling the agent and causing embolization of non-target arteries or the biliary ducts.72
If selective embolization of the bleeding artery cannot be performed, non-selective embolization of the left or right hepatic artery may be performed. In patients who are hemodynamically unstable, embolization of the main hepatic artery can be performed if the patient is a poor surgical candidate, though recognizing the increased risk of liver necrosis.53 It is currently not recommended to empirically embolize any hepatic arteries if no bleeding source is detected due to this very risk, even with patent portal veins. Furthermore, because the bile ducts are supplied primarily by the hepatic arteries rather than the portal vein, there is a risk of biliary ischemia and resultant multifocal strictures.8
Complications of TAE include hepatic abscesses, postembolization syndrome, hyperaminotransaminasemia, hepatic ischemia, and hepatic infarction or rarely failure.49 A study of 72 patients who underwent TAE showed that 55 experienced hepatic ischemia evidenced by transiently elevated serum liver enzymes, while 3 experienced focal hepatic infarcts in the areas corresponding to the embolized arterial branches.70
6.4. Vascular stenting
An alternative to embolization, as alluded to earlier, is the placement of a covered stent across the site of vascular injury. Stenting has the advantage of preserving flow through the artery, which may be beneficial, if not crucial, in patients with liver transplants or compromised portal vein flow. The diameter of most hepatic vessels is similar to the size of coronary vessels, making coronary stents ideal for this application. Stent diameter should be slightly oversized by about 10%—20% of the diameter of the target vessel and extend approximately 10mm to either side/end of the site of injury to ensure proper tamponade.73,74
6.5. Surgery
Surgical intervention is rarely necessary and usually reserved for failed endoscopic, endovascular, and/or percutaneous therapies. However, it is first-line if pseudoaneurysms are infected or if they are compressing other vascular structures. Surgery may also be indicated if cholecystitis is present, among other uncommon scenarios. Options for surgery include hepatic artery ligation, pseudoaneurysm excision, or hepatic segmentectomy/lobectomy with the potential for concurrent cholecystectomy if cholecystitis is present or the gallbladder neck is involved. Although surgery has a high success rate of above 90%, it is also associated with a high mortality of up to 10%.8
6.6. Managing complications of hemobilia and of treatment thereof
Complications that arise from hemobilia should be managed as they would be in any other scenario. For example, cholecystitis should be treated with early cholecystectomy, as it carries a high mortality rate with rates of gallbladder perforation between 2%—15%.75 Acute pancreatitis is another complication that can occur due to obstruction of the ampulla or more proximal main pancreatic duct by blood clots and reverse flow of blood into the pancreatic ducts and should be managed medically, and in some instances by ERCP. Biliary strictures can form following hepatic artery embolization because the vascular supply for the biliary tree comes mostly from the hepatic artery, and these will generally require treatment with endoscopic or percutaneous balloon dilation.8
7. Conclusions
Hemobilia is an unusual but important cause of GI bleeding and most commonly due to hepatopancreatobiliary tract procedures, regional trauma, and malignancy. The diagnosis can be challenging due to its uncommon occurrence, particularly in cases where there is no history of recent biliary tract instrumentation or trauma. CT angiography and endoscopy/ERCP have become common initial diagnostic testing modalities due to their versatility in excluding other causes of bleeding, low contrast requirement, and relative safety. Most cases of minor hemobilia can be treated conservatively or with minimally-invasive endoscopic management. Major hemobilia, characterized as refractory to conservative measures or leading to hemodynamic instability, should be managed by interventional radiology in conjunction with endoscopy/ERCP. While TAE is a mainstay, vascular stenting has gained traction as an alternative to embolization due to the preservation of hepatic arterial blood supply. Surgery is typically reserved as a last resort due to its high mortality rate and invasive nature relative to alternative approaches. Although the gold standard for management remains angiography, new technologies and techniques such as advanced endoscopic and radiologic procedures have become an attractive alternative for both the diagnosis and treatment of hemobilia.
Acknowledgements
This work was supported by the USA National Institutes of Health grant NIDDK DK057993 (to NFL).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Parekh J, Corvera CU. Hemorrhagic cholecystitis. Arch Surg 2010; 145: 202–204. [DOI] [PubMed] [Google Scholar]
- 2.Bates D English manuscripts of Francis glisson (1): From anatomia hepatis (the anatomy of the liver), 1654. Andrew Cunningham. Isis 1996; 87: 357–358. [Google Scholar]
- 3.Merrell SW, Schneider PD. Hemobilia--evolution of current diagnosis and treatment. West J Med 1991; 155: 621–625. [PMC free article] [PubMed] [Google Scholar]
- 4.Quincke H Ein fall von aneurysma der leberarterie. Berl Klin Wochenschr 1871; 30: 349–352. [Google Scholar]
- 5.Sandblom P Hemobilia. Surg Clin North Am 1973; 53: 1191–1201. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida J, Donahue PE, Nyhus LM. Hemobilia: Review of recent experience with a worldwide problem. Am J Gastroenterol 1987; 82: 448–453. [PubMed] [Google Scholar]
- 7.Green MH, Duell RM, Johnson CD, Jamieson NV. Haemobilia. Br J Surg 2001; 88: 773–786. [DOI] [PubMed] [Google Scholar]
- 8.Murugesan SD, Sathyanesan J, Lakshmanan A, et al. Massive hemobilia: A diagnostic and therapeutic challenge. World J Surg 2014; 38: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 9.Navuluri R Hemobilia. Semin Intervent Radiol 2016; 33: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goffette PP, Laterre P-F. Traumatic injuries: Imaging and intervention in post-traumatic complications (delayed intervention). Eur Radiol 2002; 12: 994–1021. [DOI] [PubMed] [Google Scholar]
- 11.Bismuth H Hemobilia. N Engl J Med 1973; 288: 617–619. [DOI] [PubMed] [Google Scholar]
- 12.Barriga J, Mathews C, Marino C, Tombazzi C. 71 Hemobilia after laparoscopic cholecystectomy: Case report. J Investig Med 2006; 54: S268.4–S268. [Google Scholar]
- 13.Senadhi V, Arora D, Arora M, Dutta S. Hemobilia caused by a ruptured hepatic cyst: A case report. J Med Case Rep 2011; 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KH, Kim TN. Etiology, clinical features, and endoscopic management of hemobilia: A retrospective analysis of 37 cases. Korean J Gastroenterol 2012; 59: 296–302. [DOI] [PubMed] [Google Scholar]
- 15.Gurakuqi GC, Stadlbauer V, Portugaller HR, Högenauer C, Trauner M, Stauber RE. Fatal hemobilia resulting from an iatrogenic arteriobiliary fistula as a rare complication of transjugular liver biopsy. Eur J Gastroenterol Hepatol 2008; 20: 83–86. [DOI] [PubMed] [Google Scholar]
- 16.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol 1986; 2: 165–173. [DOI] [PubMed] [Google Scholar]
- 17.Zhou HB. Hemobilia and other complications caused by percutaneous ultrasound-guided liver biopsy. World J Gastroenterol 2014; 20: 3712–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera-Sanfeliz GM, Assar OSA, LaBerge JM, et al. Incidence of important hemobilia following transhepatic biliary drainage: Left-sided versus right-sided approaches. Cardiovasc Intervent Radiol 2004; 27: 137–139. [DOI] [PubMed] [Google Scholar]
- 19.Fidelman N, Bloom AI, Kerlan RK Jr, et al. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: Experience with 930 patients. Radiology 2008; 247: 880–886. [DOI] [PubMed] [Google Scholar]
- 20.González-Abraldes J, Moitinho E, García-Pagán JC, et al. Selective arterial embolization for life threatening hemobilia after transjugular intrahepatic portosystemic shunt placement. J Hepatol 2001; 34: 174–176. [DOI] [PubMed] [Google Scholar]
- 21.Rössle M, Siegerstetter V, Huber M, Ochs A. The first decade of the transjugular intrahepatic portosystemic shunt (TIPS): State of the art. Liver 1998; 18: 73–89. [DOI] [PubMed] [Google Scholar]
- 22.Kaswala D, Gandhi D, Moroianu A, et al. Hemobilia secondary to transjugular intrahepatic portosystemic shunt procedure: A case report. J Clin Med Res 2012; 1: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa Macedo T, Maldonado R, Valente A, et al. Hemobilia in hereditary hemorrhagic telangiectasia: An unusual complication of endoscopic retrograde cholangiopancreatography. Endoscopy 2003; 35: 531–533. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Zhu J, Xing L, Wang Y, Jin Z, Li Z. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc 2016; 83: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 25.Khan MA, Akbar A, Baron TH, et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. Dig Dis Sci 2016; 61: 684–703. [DOI] [PubMed] [Google Scholar]
- 26.Tsou YK, Liu NJ, Jan YY. Biliary obstruction caused by hemobilia after endoscopic sphincterotomy in a patient with an anomalous location of papilla of vater. Gastrointest Endosc 2008; 68: 1232–1234. [DOI] [PubMed] [Google Scholar]
- 27.Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep 2010; 12: 121–129. [DOI] [PubMed] [Google Scholar]
- 28.Balsara KP, Dubash C, Shah CR. Pseudoaneurysm of the hepatic artery along with common bile duct injury following laparoscopic cholecystectomy. Surg Endosc 1998; 12: 276–277. [DOI] [PubMed] [Google Scholar]
- 29.Gachabayov M, Kubachev K, Mityushin S, Zarkua N. Recurrent hemobilia due to right hepatic artery pseudoaneurysm. Clin Med Res 2017; 15: 96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari A, Hammad T, Sharma H, et al. Unusual clinical presentation of hemobilia with recurrent vasovagal episodes. Case Rep Gastroenterol 2017; 11: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vachhani PG, Copelan A, Remer EM, Kapoor B. Iatrogenic hepatopancreaticobiliary injuries: A review. Semin Intervent Radiol 2015; 32: 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tessier DJ, Fowl RJ, Stone WM, et al. Iatrogenic hepatic artery pseudoaneurysms: An uncommon complication after hepatic, biliary, and pancreatic procedures. Ann Vasc Surg 2003; 17: 663–669. [DOI] [PubMed] [Google Scholar]
- 33.Badillo R, Darcy MD, Kushnir VM. Hemobilia due to cystic artery pseudoaneurysm: A rare late complication of laparoscopic cholecystectomy. ACG Case Rep J 2017; 4: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogura T, Okuda A, Higuchi K. Hemobilia due to hepatocellular carcinoma: Cholangioscopic findings and novel endoscopic hemostasis. Hepatobiliary Pancreat Dis Int 2018; 17: 275–277. [DOI] [PubMed] [Google Scholar]
- 35.Abdelfatah MM, Mudireddy PR. Hemobilia: A rare cause of intermittent biliary obstruction. VideoGIE 2018; 3: 236–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manolakis AC, Kapsoritakis AN, Tsikouras AD, Tsiopoulos FD, Psychos AK, Potamianos SP. Hemobilia as the initial manifestation of cholangiocarcinoma in a hemophilia B patient. World J Gastroenterol 2008; 14: 4241–4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawaguchi Y, Ogawa M, Maruno A, Ito H, Mine T. A case of successful placement of a fully covered metallic stent for hemobilia secondary to hepatocellular carcinoma with bile duct invasion. Case Rep Oncol 2012; 5: 682–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battula N, Madanur M, Priest O, et al. Spontaneous rupture of hepatocellular carcinoma: A Western experience. Am J Surg 2009; 197: 164–167. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh M-F, Chen C-B, Chen Y-L, Chou C-T. Hemobilia after CT-guided radiofrequency ablation of liver tumors: Frequency, risk factors, and clinical significance. Abdom Radiol (NY) 2018. [DOI] [PubMed]
- 40.Kim SH, Lim HK, Choi D, et al. Changes in bile ducts after radiofrequency ablation of hepatocellular carcinoma: Frequency and clinical significance. AJR Am J Roentgenol 2004; 183: 1611–1617. [DOI] [PubMed] [Google Scholar]
- 41.Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg 2004; 239: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005; 234: 961–967. [DOI] [PubMed] [Google Scholar]
- 43.Nishi M, Saeki I, Yamasaki T, et al. Hemobilia immediately after transcatheter arterial chemoembolization using drug-eluting beads for hepatocellular carcinoma with intrahepatic bile duct invasion. Hepatol Res 2018; 48: 329–332. [DOI] [PubMed] [Google Scholar]
- 44.Verset G, Maréchal R, Bali MA, Devière J, Van Laethem JL. Fatal hemobilia in advanced hepatocellular carcinoma invading biliary tract after treatment with sorafenib and biliary stenting. Ann Oncol 2010; 21: 1381–1382. [DOI] [PubMed] [Google Scholar]
- 45.Luu MB, Deziel DJ. Unusual complications of gallstones. Surg Clin North Am 2014; 94: 377–394. [DOI] [PubMed] [Google Scholar]
- 46.Cathcart S, Birk JW, Tadros M, Schuster M. Hemobilia: An uncommon but notable cause of upper gastrointestinal bleeding. J Clin Gastroenterol 2017; 51: 796–804. [DOI] [PubMed] [Google Scholar]
- 47.Tabibian JH, Tabibian N, Aguet JC. Choledochal cyst complications presenting as duodenal obstruction in an 82-year-old patient with gallbladder agenesis. Dig Dis Sci 2009; 54: 184–187. [DOI] [PubMed] [Google Scholar]
- 48.Wen F, Dong Y, Lu ZM, Liu ZY, Li W, Guo QY. Hemobilia after laparoscopic cholecystectomy: Imaging features and management of an unusual complication. Surg Laparosc Endosc Percutan Tech 2016; 26: e18–e24. [DOI] [PubMed] [Google Scholar]
- 49.Feng W, Yue D, ZaiMing L, et al. Iatrogenic hemobilia: Imaging features and management with transcatheter arterial embolization in 30 patients. Diagn Interv Radiol 2016; 22: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KH, Kim TN. Etiology, clinical features, and endoscopic management of hemobilia: A retrospective analysis of 37 cases. Korean J Gastroenterol 2012; 59: 296–302. [DOI] [PubMed] [Google Scholar]
- 51.Konerman MA, Zhang Z, Piraka C. Endoscopic ultrasound as a diagnostic tool in a case of obscure hemobilia. ACG Case Rep J 2016; 3: e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trakarnsanga A, Sriprayoon T, Akaraviputh T, Tongdee T. Massive hemobilia from a ruptured hepatic artery aneurysm detected by endoscopic ultrasound (EUS) and successfully treated. Endoscopy 2010; 42 Suppl 2: E340–E341. [DOI] [PubMed] [Google Scholar]
- 53.Saad WEA, Davies MG, Darcy MD. Management of bleeding after percutaneous transhepatic cholangiography or transhepatic biliary drain placement. Tech Vasc Interv Radiol 2008; 11: 60–71. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava DN, Sharma S, Pal S, et al. Transcatheter arterial embolization in the management of hemobilia. Abdom Imaging 2006; 31: 439–448. [DOI] [PubMed] [Google Scholar]
- 55.Abdelhafez M, Phillip V, Hapfelmeier A, et al. Cap assisted upper endoscopy for examination of the major duodenal papilla: A randomized, blinded, controlled crossover study (CAPPA Study). Am J Gastroenterol 2017; 112: 725–733. [DOI] [PubMed] [Google Scholar]
- 56.Sniderman KW, Morse SS, Rapoport S, Ross GR. Hemobilia following transhepatic biliary drainage: Occlusion of an hepatoportal fistula by balloon tamponade. Radiology 1985; 154: 827. [DOI] [PubMed] [Google Scholar]
- 57.Komaki Y, Kanmura S, Funakawa K, et al. A case of hereditary hemorrhagic telangiectasia with repeated hemobilia arrested by argon plasma coagulation under direct peroral cholangioscopy. Gastrointest Endosc 2014; 80: 528–529. [DOI] [PubMed] [Google Scholar]
- 58.Moparty RK, Brown RD, Layden TJ, Chirravuri V, Wiley T, Venu RP. Dissolution of blood clots in the biliary ducts with a thrombolytic agent infused through nasobiliary catheter. Gastrointest Endosc 2002; 56: 436–438. [DOI] [PubMed] [Google Scholar]
- 59.Bagla P, Erim T, Berzin TM, Chuttani R. Massive hemobilia during endoscopic retrograde cholangiopancreatography in a patient with cholangiocarcinoma: A case report. Endoscopy 2012; 44 Suppl 2 UCTN: E1. [DOI] [PubMed] [Google Scholar]
- 60.Katsinelos P, Paroutoglou G, Beltsis A, et al. Endoscopic hemoclip placement for postsphincterotomy bleeding refractory to injection therapy: Report of two cases. Surg Laparosc Endosc Percutan Tech 2005; 15: 238–240. [DOI] [PubMed] [Google Scholar]
- 61.Katsinelos P, Kountouras J, Chatzimavroudis G, et al. Endoscopic hemostasis using monopolar coagulation for postendoscopic sphincterotomy bleeding refractory to injection treatment. Surg Laparosc Endosc Percutan Tech 2010; 20: 84–88. [DOI] [PubMed] [Google Scholar]
- 62.Leung JW, Chan FK, Sung JJ, Chung SC. Endoscopic sphincterotomy-induced hemorrhage: A study of risk factors and the role of epinephrine injection. Gastrointest Endosc 1995; 42: 550–554. [DOI] [PubMed] [Google Scholar]
- 63.Linz CM, Modi RM, Krishna SG. A dual-modality approach of endobiliary radiofrequency ablation and self-expandable metal stent placement to control malignant hemobilia. Endoscopy 2017; 49: E21–E22. [DOI] [PubMed] [Google Scholar]
- 64.Goenka MK, Harwani Y, Rai V, Goenka U. Fully covered self-expandable metal biliary stent for hemobilia caused by portal biliopathy. Gastrointest Endosc 2014; 80: 1175. [DOI] [PubMed] [Google Scholar]
- 65.Shinjo K, Matsubayashi H, Matsui T, et al. Biliary hemostasis using an endoscopic plastic stent placement for uncontrolled hemobilia caused by transpapillary forceps biopsy (with video). Clin J Gastroenterol 2016; 9: 86–88. [DOI] [PubMed] [Google Scholar]
- 66.Song J-Y, Moon JH, Choi HJ, et al. Massive hemobilia following transpapillary bile duct biopsy treated by using a covered self-expandable metal stent. Endoscopy 2014; 46 Suppl 1 UCTN: E161–E162. [DOI] [PubMed] [Google Scholar]
- 67.Barresi L, Tarantino I, Ligresti D, Curcio G, Granata A, Traina M. Fully covered self-expandable metal stent treatment of spurting bleeding into the biliary tract after endoscopic ultrasound-guided fine-needle aspiration of a solid lesion of the pancreatic head. Endoscopy 2015; 47 Suppl 1 UCTN: E87–E88. [DOI] [PubMed] [Google Scholar]
- 68.Noro T, Kawasaki N, Ohdaira H, Takizawa R, Suzuki N, Suzuki Y. A new endoscopic method: Percutaneous endoscopic trans-gastric biliary drainage as an option for biliary drainage 2013; 37: 937–940. [Google Scholar]
- 69.Marynissen T, Maleux G, Heye S, et al. Transcatheter arterial embolization for iatrogenic hemobilia is a safe and effective procedure: Case series and review of the literature. Eur J Gastroenterol Hepatol 2012; 24: 905–909. [DOI] [PubMed] [Google Scholar]
- 70.Choi SH, Gwon DI, Ko G-Y, et al. Hepatic arterial injuries in 3110 patients following percutaneous transhepatic biliary drainage. Radiology 2011; 261: 969–975. [DOI] [PubMed] [Google Scholar]
- 71.Kumar A, Sheikh A, Partyka L, Contractor S. Cystic artery pseudoaneurysm presenting as a complication of laparoscopic cholecystectomy treated with percutaneous thrombin injection. Clin Imaging 2014; 38: 522–525. [DOI] [PubMed] [Google Scholar]
- 72.Cagli B, Tuncel SA, Sengul E, et al. Hemobilia and occult cystic artery stump bleeding after a laparoscopic cholecystectomy: Endovascular treatment with N-butyl cyanoacrylate. Prague Med Rep 2011; 112: 132–136. [PubMed] [Google Scholar]
- 73.Hardman RL, Taussky P, Kim R, O’Hara RG. Post-transplant hepatic artery pseudoaneurysm treated with the pipeline flow-diverting stent. Cardiovasc Intervent Radiol 2015; 38: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 74.Krokidis ME, Hatzidakis AA. Acute hemobilia after bilioplasty due to hepatic artery pseudoaneurysm: Treatment with an ePTFE-covered stent. Cardiovasc Intervent Radiol 2009; 32: 605–607. [DOI] [PubMed] [Google Scholar]
- 75.Suchniak-Mussari KA, Foreman BA, Sharma A, Shah T, Dye CE. Transjugular liver biopsy and the bloody mess that follows: A rare case of hemobilia and hemocholecystitis. ACG Case Rep J 2016; 3: e108. [DOI] [PMC free article] [PubMed] [Google Scholar]


