Abstract
Background:
Respondents in longitudinal health interview surveys may inconsistently report their chronic diseases across interview waves. Racial/ethnic minority adults have an increased burden of chronic diseases and may dispute chronic disease reports more frequently.
Objective:
We evaluated the longitudinal association between race/ethnicity, nativity, and language of interview with disputing previously-reported chronic diseases.
Methods:
We performed secondary data analysis of nationally-representative longitudinal data (Health and Retirement Study, HRS 1998–2010) of adults 51 years and older (n = 23,593). We estimated multilevel mixed-effects logistic models of disputes of previously-reported chronic disease (hypertension, heart disease, lung disease, diabetes, cancer, stroke, arthritis).
Results:
Approximately 22% of HRS respondents disputed prior chronic disease self-reports across the entire study period; 21% of non-Latino white, 20.5% of non-Latino black, and 28% of Latino respondents disputed. In subgroup comparisons of model-predicted odds using post-estimation commands, Latinos interviewed in Spanish have 34% greater odds of disputing compared with non-Latino whites interviewed in English and 35% greater odds of dispute relative to non-Latino blacks interviewed in English.
Conclusions:
The odds of disputing a prior chronic disease report were substantially higher for Latinos who were interviewed in Spanish compared to non-Latino white or black counterparts interviewed in English, even after accounting for other sociodemographic factors, cognitive declines, and time-in-sample considerations. Our findings point toward leveraging of multiple sources of data to triangulate information on chronic disease status as well as investigating potential mechanisms underlying the higher probability of dispute among Spanish-speaking Latino respondents.
Keywords: race/ethnicity, language of interview, chronic disease, self-report, longitudinal analysis
INTRODUCTION
Large, population-based health interview surveys are used to monitor important health outcomes for older adults and to understand population-level disease trends and how these trends may be affected by changing economic and social circumstances1–3. Consequently, it is important for surveys to provide a good accounting of population-level estimates of chronic diseases. This task becomes more difficult and nuanced when collecting chronic disease self-reports in repeated interviews on the same study respondents over time.
An important concern in using self-reported disease indicators is the reliability of responses across interview waves4,5. Health interview surveys ask participants at each wave whether they have been diagnosed by a doctor with certain chronic diseases. In subsequent interviews, individuals may or may not consistently report prior “yes” responses for disease diagnoses. Thus, the longitudinal patterns of chronic disease responses for a respondent may exhibit a clinically-consistent or inconsistent pattern5. Respondents who report never having been diagnosed with a chronic disease, respondents who always report having been diagnosed with the disease, and respondents who initially report not having been diagnosed but then report having been diagnosed at a follow-up interview all display clinically-consistent patterns. In contrast, respondents who report having a chronic disease in an earlier interview, but in subsequent interviews dispute having that disease, are considered to have inconsistent patterns of longitudinal chronic disease reports. Not only do these inconsistent patterns present difficulties for researchers attempting to interpret and analyze these data, they may also indicate uncertainty of the respondents about whether or not they have a given chronic disease. Because chronic diseases are incurable and persist throughout the remaining lifespan, the expected reporting pattern should reflect this chronicity despite effective treatment or reduced symptomatology6.
Decades of research focused on the illness representations model suggests that individuals’ perceptions and acknowledgement of their own diseases hinges strongly on their experiences with the severity and symptoms of their diseases7. Individuals who experience intermittent symptoms or who have well-controlled or treated disease may not consistently report these diseases8. In addition, inconsistency in the self-report of chronic diseases may be more likely among population subgroups. For example, individuals with multiple chronic diseases may experience or develop increasing cognitive deficits over time and so be prone to longitudinal inconsistency in their reporting of their diseases9,10. Similarly, individuals with poor health literacy or those with lower levels of education may have a limited understanding of their chronic illness, which could result in inconsistent reporting11,12. Potential survey-related reasons for inconsistent reporting—such as recall bias during the 2-year intervening period between data collection waves, data collection via in-person or telephone modes of interview, or translation of survey items—may also be compounded among specific population subgroups. There is an extensive literature that attempts to understand differences in chronic disease morbidity related to race, ethnicity, education, socioeconomic status, and access to healthcare resources. The general consensus in this research points toward complex and intertwined mechanisms that underlie these differences13–15. The factors that affect chronic disease morbidity may similarly influence the self-reporting of chronic diseases. Prior work examining patterns of longitudinal inconsistency in chronic disease reports found that cognitive impairment, proxy status, age, Latino ethnicity, and wealth were predictors of clinically-inconsistent patterns5. Examining the relationship between key factors associated with morbidity disparities for minority racial and ethnic population groups is an important step to further understanding the sociodemographic predictors of longitudinally-inconsistent reporting patterns.
Lack of clarity about one’s own chronic diseases might be a particularly worrisome sign for members of vulnerable populations, particularly those from racial and ethnic backgrounds who contend with a disproportionate burden of chronic disease16–18. Inconsistent reporting of chronic diseases—particularly diseases which must be actively managed (e.g., diabetes)—may indicate less-than-optimal levels of watchfulness and control. Lack of acknowledgement of the permanency of a chronic disease diagnosis, not receiving consistent health messaging from health care providers about chronic disease, or not receiving health information may be exacerbating disparities in chronic disease-related health outcomes. It is critical to understand whether disputing chronic disease diagnoses over time occurs more frequently among minority racial/ethnic middle-aged and older adults. A fuller understanding of correlates of chronic disease disputes may provide insight and opportunities to improve programs that target patient-provider communication and health literacy efforts.
The purpose of our study was to investigate the association between race/ethnicity, nativity, and language of interview on the odds of disputing chronic disease reports over time. We hypothesized that respondents of Latino backgrounds, foreign-born respondents, and respondents who were interviewed in Spanish would be more likely to dispute previous self-reported chronic disease status.
METHODS
Data and Study Design
The Health and Retirement Study (HRS) is a biennial, ongoing, prospective health interview survey of adults age 51 years and older that began in 19923. It is based on a multistage area probability sample of households that is nationally representative, enabling results to be generalized to the United States population19. Extensive documentation of the HRS study design and questionnaire can be found on the HRS website (http://hrsonline.isr.umich.edu). Due to comparability of measures we examined data from 1998–2010 for this study. The HRS is sponsored by the National Institute on Aging and performed by the Institute for Social Research at the University of Michigan; it has been approved by the University of Michigan Health Sciences Institutional Review Board.
Study Population
We included community-dwelling age-eligible study participants (51 years or older). When the respondent was unable to be interviewed for a survey wave (e.g., due to health problems), a proxy respondent, most often the spouse or partner and sometimes the adult children of the respondent, answered questions for that respondent according to HRS protocol. We include data from proxy respondents so as not to introduce bias from excluding these responses; however, we conducted sensitivity analyses to compare results with and without proxy data and found no substantive differences in our findings (not presented, available upon request).
A total of 24,156 respondents were interviewed and followed-up during the period 1998–2010. We excluded participants (N=433) who reported “other” as their racial/ethnic category (and do not identify as Latino) because of heterogeneity within this group, had invalid sampling weights (N=67), or had missing data on model covariates across all waves in the observation period (N= 63). As a result, we conducted secondary data analysis of 23,593 individuals.
Outcome measure
Each HRS biennial core survey wave provides self-reported information on seven chronic diseases (hypertension, heart disease, lung disease, diabetes, cancer, stroke, arthritis). The dependent variable in this study was disputing a prior affirmative response to any of seven chronic diseases in any given wave (including respondents who may have disputed more than one chronic disease), which were queried—“(Has a doctor ever told you that you have [X chronic condition]?\ Our records from your last interview [in [PREV WAVE IW MONTH], [PREV WAVE IW YEAR]\in [PREV WAVE IW YEAR] show that you have had [X chronic condition].)” Disputes occur when, at any of the follow-up time points, respondents provide a response category of “3: Disputes previous wave record, but now has condition” or “4: Disputes previous wave record, does not have condition” after interviewers probe disputed responses to clarify whether the respondent has since been told by a doctor that they have the condition. The time-varying outcome variable for dispute was coded as binary (yes = 1) to reflect dispute of one or more chronic disease disputes in any given wave.
Covariates
Main independent variables.
We included several key independent variables to test our hypothesis that respondents from minority racial and ethnic backgrounds, particularly Latinos, and respondents who gave their interviews in the Spanish language would demonstrate greater odds of disputing a prior chronic disease response over time. We included race/ethnicity (mutually-exclusive categories for: non-Latino White, non-Latino Black, and Latino), nativity (foreign born = 1), and Spanish language of interview (Spanish = 1, time-varying covariate) in the analyses. We included a Latino ethnicity by language of interview interaction term to assess whether language of interview moderated the effect of race/ethnicity on disputing a previous response.
Sociodemographic factors.
To control for important socioeconomic and demographic characteristics, we included age (in years, time-varying covariate), gender (female=1), education (number of school years completed), marital status (married/partnered = 1, time-varying covariate), and wealth (net worth, in thousands of dollars, time-varying covariate) in the analyses.
Cognition and proxy interview status.
For self-respondents, the presence of cognitive impairment is determined using a validated performance-based measure, a modified version of the Telephone Interview for Cognitive Status (TICS). We defined cognitive impairment (yes=1, time-varying) as a score of 0–11 on the 27-point cognitive scale20. For respondents unable to complete the interview, we used a score of 3 out of an 11-point scale comprised of the proxy’s assessment of the respondent’s memory, the proxy’s assessment of the respondent’s Instrumental Activities of Daily Living difficulties, and the interviewer’s assessment of the respondent’s cognitive impairment21,22. An indicator variable for proxy interview status identified interviews given by proxy rather than self at each interview wave (proxy =1, time-varying).
Other covariates.
To account for the number of occasions respondents had to dispute a prior response, we computed a categorical count variable indicating number of waves the respondent participated in the study.
Statistical Analysis
We conducted secondary data analyses of longitudinal HRS data from 1998–2010. Analyses were weighted (using HRS wave-by-wave analytic weights) to account for complex sample design and differential non-response and respondent attrition. If a respondent was missing a weight for a particular wave, the adjacent wave weight was used in accordance with HRS recommendations19,23.
We estimated multilevel mixed-effects logistic models with Stata’s svy: melogit routine for longitudinal binary response data to account for nested disputed responses within individuals over time. We assessed predictors of disputed responses to chronic disease questions over time in a series of sequential hierarchical models: (1) Model 1, the unconditional model, included time; (2) Model 2 added the number of waves participated in and mutually-exclusive racial and ethnic indicator variables (with omitted non-Latino whites as the reference group); (3) Model 3 added Spanish language of interview; (4) Model 4 added an interaction term for Latino ethnicity and Spanish language of interview; and (5) Model 5, the fully-adjusted model, incorporated sociodemographic covariates. Continuous covariates were grand-mean centered to facilitate interpretation24. We report adjusted odds ratios (OR) and 95% confidence intervals (CI) for each model (Table 3). To facilitate interpretation of binary interaction terms, we calculated the model-predicted, marginal odds of dispute for each race/ethnic group by language of interview using the Stata post-estimation margins command and calculated odds ratios comparing the likelihood of dispute between groups (e.g. Latino/Spanish interview vs. non-Latino Black/English interview). Delta-method standard errors and 95% confidence intervals for odds ratios comparing race/ethnic groups by interview language were calculated using the Stata post-estimation command nlcom25,26. All analyses were conducted in Stata/SE 15 (StataCorp, Collage Station, TX).
Table 3:
Multilevel Mixed Effects Logistic Models for the Odds of Disputing Prior Chronic Disease Response
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Time | 1.02 (1.00, 1.03) |
1.01 (1.00, 1.03) |
1.02 (1.00, 1.03) |
1.02 (1.00, 1.03) |
1.01 (0.99, 1.02) |
| No. of waves participated | -- | 1.00 (0.98, 1.02) |
1.00 (0.98, 1.02) |
1.00 (0.98, 1.02) |
0.98 (0.96, 1.00) |
| Latino | -- |
1.24 (1.11, 1.37) |
1.02 (0.87, 1.19) |
0.99 (0.84, 1.17) |
1.07 (0.90, 1.28) |
| Black (non-Latino) | -- | 0.99 (0.90, 1.09) |
0.99 (0.90, 1.09) |
0.99 (0.90, 1.09) |
0.99 (0.90, 1.10) |
| Spanish Interview | -- | -- |
1.46 (1.21, 1.78) |
0.27 (0.09, 0.84) |
0.21 (0.07, 0.66) |
| Spanish Interview*Latino | -- | -- | -- |
5.66 (1.80, 17.77) |
5.53 (1.75, 17.46) |
| Female | -- | -- | -- | -- | 1.01 (0.94, 1.08) |
| Age (years) | -- | -- | -- | -- |
1.03 (1.03, 1.03) |
| Married | -- | -- | -- | -- | 0.96 (0.89, 1.03) |
| Foreign born | -- | -- | -- | -- |
1.16 (1.01, 1.33) |
| Impaired cognition | -- | -- | -- | -- |
1.31 (1.16, 1.48) |
| Proxy respondent | -- | -- | -- | -- |
0.47 (0.41, 0.54) |
| Education | -- | -- | -- | -- |
0.98 (0.97, 0.99) |
| Wealth | -- | -- | -- | -- | 1.00 (1.00, 1.00) |
| Post-estimation marginal odds ratio comparisons for race/ethnic groups by interview language (Model 5) | |||||
| OR (95% CI) | |||||
|
Latino-Spanish vs. White-English |
1.34 (1.17, 1.51) | ||||
|
Latino-Spanish vs. Black-English |
1.35 (1.15, 1.55) | ||||
|
Latino-Spanish vs. Latino-English |
1.18 (0.93, 1.43) | ||||
|
Latino-English vs. Black-English |
1.14 (0.93, 1.35) | ||||
|
Latino-English vs. White-English |
1.14 (0.95, 1.33) | ||||
|
Black-English vs. White-English |
0.99 (0.90, 1.08) | ||||
Notes: Significant odds ratios in bold. Continuous variables were grand-mean centered.
A small number of non-Latino respondents provide interviews in Spanish (n=36) and finding no plausible basis to assume that this response group is error, we do not omit these from the analysis. Retaining such a small number of cases does not substantially affect the results; however, given the small numbers and large confidence intervals we opted not to present or interpret these data.
RESULTS
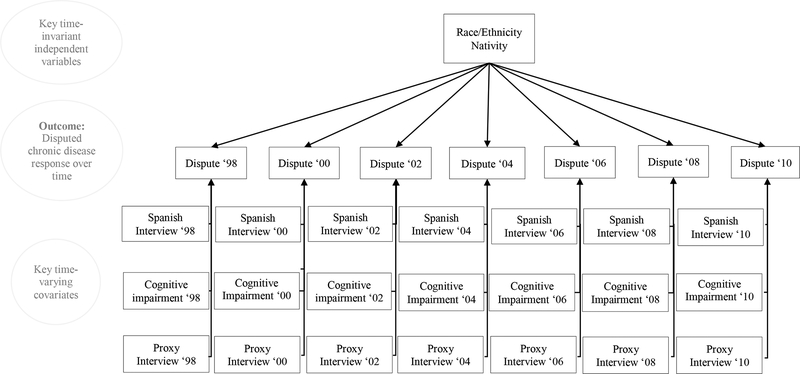
Table 1 provides baseline descriptive characteristics for the study sample. Of the 23,593 interviewed, 76.3% were non-Latino White; 14.9%, non-Latino Black, and 8.8%, Latino. Fifty-four per cent of Latino respondents provided interviews in Spanish. Non-Latino white respondents had 12.6 mean years of education, in comparison to 10.9 years for non-Latino black and 8.4 years for Latino respondents. Approximately 22% of respondents disputed prior chronic disease self-reports during the study period: 21% of non-Latino white, 20.5% of non-Latino black, and 28% of Latino respondents disputed. This proportion includes respondents who disputed in one and more than one wave. Approximately 14% of respondents disputed in multiple waves. Figure 1 provides a descriptive schematic of the analytic model for key time-invariant and time-varying independent variables on the odds of disputing chronic disease reports from 1998–2010.
Table 1.
Characteristics of the Study Population, Health and Retirement Study
| Variable | Total | White | Black | Latino |
|---|---|---|---|---|
| Study population, N (%) | 23,593 (100) |
18,002 (76.3) |
3,513 (14.9) |
2,078 (8.8) |
| Spanish interview at baseline1, n (%) | 1,118 (4.7) |
22 (0.1) |
1 (0.0) |
1,095 (52.7) |
| Age at baseline, mean (SD) [range] | 66.2 (10.7) [50–106] |
66.7 (10.8) [50–104] |
65.0 (10.7) [50–106] |
63.7 (10.0) [51–102] |
| Foreign-born, n (%) | 2,171 (9.2) |
826 (4.6) |
194 (5.5) |
1,151 (55.4) |
| Female, n (%) | 12,946 (54.9) |
9,728 (54.0) |
2,085 (59.3) |
1,133 (54.5) |
| Married at baseline, n (%) | 15,725 (66.7) |
12,611 (70.1) |
1,735 (49.4) |
1,379 (66.4) |
| Education level, mean (SD) [range] | 12.0 (3.4) [0–17] |
12.6 (2.8) [0–17] |
10.9 (3.6) [0–17] |
8.4 (4.8) [0–17] |
| Cognitively impaired at baseline, n (%) | 1,699 (7.2) |
987 (5.5) |
494 (14.1) |
218 (10.5) |
| Proxy interview at baseline, n (%) | 2,321 (9.8) |
1,623 (9.0) |
411 (11.7) |
287 (13.8) |
| Wealth at baseline, mean (SD) [range] | 308.0 (1,081.1) [−3,636.7 –86,210] |
375.2 (1,221.9) [−1,999.2 –86,210] |
85.5 (244.0) [−178.2 –10,644.5] |
101.8 (264.0) [−3,636.8 −3,162.5] |
| Arthritis at baseline, n (%) | 12,165 (51.6) |
9,274 (51.6) |
1,936 (55.2) |
955 (50.0) |
| Cancer at baseline, n (%) | 2,533 (10.8) |
2,098 (11.7) |
287 (8.1) |
148 (7.1) |
| Diabetes at baseline, n (%) | 3,433 (14.6) |
2,187 (12.2) |
807 (23.0) |
439 (21.1) |
| Heart Disease at baseline, n (%) | 5,236 (22.2) |
4,218 (23.5) |
711 (20.3) |
307 (14.8) |
| Hypertension at baseline, n (%) | 11,134 (47.2) |
7,959 (44.2) |
2,234 (63.7) |
941 (45.3) |
| Lung at baseline, n (%) | 2,078 (8.8) |
1,732 (9.6) |
241 (6.9) |
105 (5.1) |
| Stroke at baseline, n (%) | 1,532 (6.5) |
1,124 (6.3) |
301 (8.6) |
107 (5.2) |
| Ever dispute any disease during the study period, n (%)2 | 5,201 (22.0) |
3,898 (21.7) | 721 (20.5) |
582 (28.0) |
| Dispute in one wave only, n (%) |
4,463
(86) |
4,463
(86) |
3,368
(86) |
605
(84) |
| Dispute in multiple waves, n (%) |
738
(14) |
738
(14) |
530
(14) |
116
(16) |
Notes:
Baseline is defined as the first wave of available data during the 1998–2010 study period (i.e. the 1998 study wave for respondents who entered the HRS on or before 1998 and the entry wave for respondents entering after 1998).
The reported numbers reflect respondents who dispute prior chronic disease self-reports at any point during the study period and include respondents who dispute in one and more than one data collection wave.
Figure 1.
Illustration of Modeling Key Time-Invariant and Time-Varying Covariates on the Odds of Disputing Chronic Disease Responses over Time, HRS 1998–2010
Table 2 details descriptive characteristics for time-varying variables at each follow-up interview wave. Wave-by-wave descriptive statistics reflect the expected small slight study population attrition as well as the HRS refresh of the sample in 2004. Overall, sample characteristics were stable over time. Respondents were consistent over time in their chosen language of interview; only 1–2% switched from Spanish to English or vice versa in a wave-by-wave analysis. The proportion of respondents disputing a prior chronic disease response at each wave was also stable (4.3% - 5.9%).
Table 2:
Descriptive Characteristics for Time-Varying Covariates at Follow-Up, Health and Retirement Study Data 2000–2010
| Characteristics/Variables | Year | |||||
|---|---|---|---|---|---|---|
| 2000 | 2002 | 2004 | 2006 | 2008 | 2010 | |
| Study population, N | 17,585 | 16,204 | 17,594 | 16,064 | 14.286 | 12,509 |
| Spanish interview, n (%) | 779 (4.4) |
691 (4.3) |
860 (4.9) |
752 (4.7) |
695 (4.9) |
575 (4.6) |
| Language interview change, n (%) | 265 (1.5) |
237 (1.5) |
246 (1.4) |
211 (1.3) |
167 (1.2) |
134 (1.1) |
| Age, mean (SD) [range] | 69.29 (9.9) [50–108] |
70.46 (9.5) [52–110] |
68.92 (10.7) [51–108] |
70.38 (10.2) [53–106] |
71.49 (9.8) [55–108] |
72.44 (9.4) [52–109] |
| Married, n (%) | 11,322 (64.4) |
10,201 (63.0) |
11,145 (63.4) |
10,042 (62.5) |
8,750 (61.3) |
7,511 (60.0) |
| Cognitive impairment, n (%) | 1,477 (8.4) |
1,307 (8.1) |
1,300 (7.4) |
1,318 (8.2) |
1,061 (7.4) |
1,083 (8.7) |
| Proxy interview, n (%) | 1,872 (10.7) |
1,843 (11.4) |
1,591 (9.0) |
1,129 (7.0) |
1,031 (7.2) |
1,062 (8.5) |
| Wealth, mean (SD) [range] | 343.42 (931.7) [−355–53200.2] |
354.19 (910.3) [−480.9–41640] |
413.58 (1436.4) [−2245.5–77225] |
535.82 (2388.1) [−2453–100790] |
493.72 (1279.5) [−1064–38050] |
434.42 (927.3) [−1190–26901] |
| Dispute prior response1, n (%) | 1,043 (5.9) |
702 (4.3) |
832 (4.7) |
812 (5.1) |
833 (5.8) |
705 (5.6) |
Note:
The reported numbers reflect the wave-by-wave numbers of respondents who dispute prior chronic disease self-reports.
Table 3 reports the results from the multilevel random effects logistic models estimated with sequentially-added covariates in Models 1–5 and the post-estimation marginal odds ratio comparisons for race/ethnic groups by interview language using Model 5 estimates. The odds of disputing a prior chronic disease report did not vary over time, nor did it vary with increasing numbers of waves participated in, in any of the model specifications. Latinos had 24% greater odds of disputing in the race/ethnicity-adjusted model (Model 2, OR=1.24, 95% CI [1.11, 1.37]). Once Spanish language of interview was included in Model 3, the association between Latino ethnicity and dispute was no longer significant. However, providing a Spanish language interview increases the odds of dispute by 46% (Model 3, OR=1.46, 95% CI [1.21, 1.78]). Foreign-born respondents had a 16% increase in odds of dispute (Model 5, OR=1.16, 95% CI [1.01, 1.33]).
The fully-adjusted model (Model 5) indicates that the odds of disputing a previous response to a chronic disease question among Latinos, relative to non-Latinos, was significantly related to Spanish language of interview (OR=5.53, 95% CI [1.75, 17.46]). Subgroup comparison of model-predicted odds using post-estimation commands revealed that after adjusting for covariates in Model 5, Latinos interviewed in Spanish had 34% greater odds of disputing a previous response relative to non-Latino whites who were interviewed in English (OR=1.34, 95% CI [1.17, 1.51]). Similarly, Latinos interviewed in Spanish had 35% greater odds of dispute relative to non-Latino blacks interviewed in English (OR=1.35, 95% CI [1.15, 1.55]). No other racial/ethnic by interview language subgroup comparisons were statistically significant.
Additional covariates demonstrated significant associations in the fully-adjusted model (Model 5). Older study participants and cognitively impaired individuals had 3% and 31% higher odds of disputing previous chronic disease diagnoses, respectively. Participants who had interview responses provided by a proxy had 53% lower odds of dispute over the 12 years of observation.
DISCUSSION
This study examined the odds of disputing prior self-reports of chronic disease over time, and whether individuals who dispute are more likely to be of minority racial/ethnic backgrounds, foreign-born, or interviewed in Spanish. We found that language of interview is a strong driver of chronic disease disputes over time. The odds of disputing a prior chronic disease self-report were higher for middle-aged and older respondents of Latino backgrounds interviewed in Spanish compared with non-Latino whites interviewed in English. Further, higher odds of dispute for Latinos interviewed in Spanish persisted even after accounting for other sociodemographic factors, cognitive declines, and time-in-sample considerations. Interestingly, we found that proxy interviews had 53% lower odds of dispute over the study period, suggesting that proxy respondents may be unwilling or hesitant to dispute a respondent’s prior report.
In examining the interaction effects between ethnicity and language of interview, we used post-estimation commands to calculate the odds and odds ratios of disputing a chronic disease report. Only Latinos who provided their interviews in Spanish had significantly greater odds of dispute when compared with non-Latino whites interviewed in English, or compared with non-Latino blacks interviewed in English. There were no statistically significant differences between Latinos who provided their interviews in English and non-Latino whites or non-Latino blacks. Interestingly, there were also no significant differences in the odds of dispute between Latinos who provided their interviews in Spanish compared with Latinos who provided their interviews in English. These findings suggest that Spanish language of interview—a marker of low acculturation—is a strong and important driver of disputed chronic disease reports.
Our findings highlight several important racial and ethnic dynamics in the self-reporting of important health indicators. We find that Latinos who provide their interviews in Spanish have greater odds of dispute relative to non-Latino whites or non-Latino blacks who are interviewed in English. These differences in reporting being diagnosed with a chronic disease, and then subsequently disputing these reports of a diagnosis at a follow-up interview may reflect important differences understanding or interpreting important health information. It is also possible that access to a trusted and reliable usual source of care in high-quality healthcare settings may be difficult to attain for foreign-born Latinos27. For instance, Spanish-speaking Latinos may be less likely to seek care in primary care settings, where clinicians are more likely to engage patients in diagnosing and managing their chronic health problems28,29. Where and how middle-aged and older Latinos seek care could manifest in uncertainty about their own chronic disease diagnoses.
In addition, there may be important survey methodology and translation considerations implicit in our findings. Language of interview may simply signal a discrepancy between cultural or linguistic processes occurring when survey instruments are translated into Spanish30. Latent constructs subsumed in survey questions may not be shared across languages, or there may be ambiguities and lack of clarity after translation that give rise to participant confusion about how to answer health interview questions. Prior literature suggests that for certain commonly-used questions in health interview surveys the translated questions or response categories—for example in the translation of the self-rated health response categories of excellent, very good, good, fair, and poor—and may not be equivalent between the source and target languages28,31,32. Indeed, valid interview instruments hinge on the assumption that questionnaires can be framed and phrased in such a way that they are interpreted as intended between survey participants, and not interpreted differently by subgroups of participants33. Still, the commonly used set of chronic disease questions, “has a doctor told you that you have X” are phrased from the point of view of whether a conversation in a clinical encounter occurred and not from the perspective of an internalized or societal health construct. Therefore, it seems likely that Spanish-speaking Latinos are interpreting the concept of receiving a disease diagnosis from a clinician similarly to respondents interviewed in English. While we found language of interview to be a strong driver of longitudinal chronic disease disputes, this may be indicative of acculturative processes that signal different cultural beliefs, attitudes, and behaviors and may interact with and contribute to low health literacy rather than differences in translation12,34.
Cultural perceptions of illness and wellbeing as well as health literacy issues may also explain our findings of greater odds of disputing prior chronic disease reports. Latino study participants may vary in their readiness to admit to health problems, or may have a less comprehensive understanding of their health conditions30,35. Inadequate health literacy, marked by having difficulties understanding important health information that is necessary to acknowledge and appropriately managing chronic health conditions, may reflect poor self-disease knowledge36.
This study draws from many strengths. First, the HRS is an ongoing nationally-representative longitudinal survey that spans over two decades and includes the oldest-old and a diverse population of middle-aged and older adults. In addition, because the HRS represents such a large population-based sample and oversamples racial and ethnic middle-aged and older adults, this is an ideal data source to examine racial/ethnic differences in disputes of longitudinal self-reported disease diagnoses. Second, the prospective, longitudinal study design allows us to explore the time-varying nature of correlates to disputed chronic disease responses, a unique attribute of the HRS. Finally, the HRS data represent a long-running data source complemented by a robust set of sociodemographic information that is well-suited to understanding the issues involved in racial/ethnic differences in longitudinal inconsistencies of chronic disease self-reports.
Several study limitations should be noted. The data rely on self-report of chronic disease diagnoses. However, disputes of self-reports are a key feature of the analysis and allow us to explore how clinically-inconsistent longitudinal responses are associated with study participant factors and dynamics. In addition, self-reported data on chronic disease is an important dimension to take into consideration as a direct reflection of which diseases respondents believe they do or do not have and self-management behaviors they may or may not be engaging in. Second, while the HRS oversamples racial and ethnic subpopulations, there are insufficient numbers of a heterogeneous group of respondents that identify as other racial/ethnic groups—such as Native American or Asian study participants.
CONCLUSION
Our findings highlight the importance of being aware of clinically-implausible inconsistencies in longitudinal self-reports of chronic diseases, particularly for study participants from Latino backgrounds interviewed in Spanish. Latino Spanish speakers may be unclear about whether or not they have been diagnosed with a chronic disease, and consequently, may be less likely to engage in chronic disease self-management efforts. We acknowledge that survey data collection is a difficult and complex endeavor and remains a critical source of important patient reported outcomes. Our findings point toward the leveraging of multiple sources of data (e.g., self-reported, administrative, clinical) to triangulate information from different perspectives. In this way, self-reported information can be supplemented with information from prescription lists or records of procedures that may help substantiate diagnoses. In addition, it may be helpful to gauge health literacy or acculturation level at the outset so that interviewers may be alerted to potential issues or trigger the use of a bilingual interviewer. As we begin to understand the complex interrelationship between respondent-level factors and survey administration factors that contribute to data quality problems we can better devise solutions to address these issues.
ACKNOWLEDGEMENTS
1. Contributors: There were no contributors beyond the listed authors.
2. Prior presentations: An early version of these research findings was presented at the 2015 Annual Meeting of the Gerontological Society of America, 2015.
Funders: This work was supported by: the National Institute on Aging at the National Institutes of Health (grant number R03AG048852 to A.R.Q and C.T.C; R01AG055681 to A.R.Q.; 5K08AG031837 to C.T.C.); the American Diabetes Association Career Development Award (grant number ADA 7–13-CD-08 to A.R.Q.); the Ann Arbor VA Geriatric Research, Education and Clinical Center (GRECC) to C.T.C.; the University of Michigan Claude D. Pepper Older Americans Independence Center to C.T.C.; and the National Hartford Centers of Gerontological Nursing Excellence to C.L.N.
Footnotes
CONFLICT OF INTEREST
The authors do not have any conflicts of interest to disclose.
REFERENCES
- 1.Ferraro KF. Self-ratings of health among the old and the old-old. J Health Soc Behav. 1980;377–83. [PubMed] [Google Scholar]
- 2.Ferraro KF, Farmer MM. Utility of health data from social surveys: Is there a gold Standard for measuring morbidity? Am Sociol Rev. 1999;303–15. [Google Scholar]
- 3.Hodes RJ, Suzman R. Growing older in America: The Health and Retirement Study. Bethesda Natl Inst Aging Natl Inst Health US Dep Health Hum Serv; 2007; [Google Scholar]
- 4.Beckett M, Weinstein M, Goldman N, Yu-Hsuan L. Do Health Interview Surveys Yield Reliable Data on Chronic Illness among Older Respondents? Am J Epidemiol. 2000;151(3):315–23. [DOI] [PubMed] [Google Scholar]
- 5.Cigolle CT, Nagel CL, Blaum CS, Liang J, Quiñones AR. Inconsistency in the Self-report of Chronic Diseases in Panel Surveys: Developing an Adjudication Method for the Health and Retirement Study. J Gerontol Ser B. 2018. June 14;73(5):901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher GG, Faul JD, Weir DR, Wallace RB, Herzog AR, Ofstedal MB, et al. Documentation of Chronic Disease Measures in the Heath and Retirement Study [Internet]. Ann Arbor, Michigan: Institute for Social Research, University of Michigan; 2005. [cited 2017 Jun 29]. Available from: https://hrs.isr.umich.edu/sites/default/files/biblio/dr-009.pdf [Google Scholar]
- 7.Leventhal EA, Crouch M. Are there differences in perceptions of illness across the lifespan? In: Petrie KJ, Weinman JA, editors. Perceptions of health and illness. New York, NY: Routledge; 1997. p. 77–102. [Google Scholar]
- 8.Halm EA, Mora P, Leventhal H. No Symptoms, No Asthma: The Acute Episodic Disease Belief Is Associated With Poor Self-Management Among Inner-City Adults With Persistent Asthma. Chest. 2006. March 1;129(3):573–80. [DOI] [PubMed] [Google Scholar]
- 9.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016. April;12(4):459–509. [DOI] [PubMed] [Google Scholar]
- 10.Haaksma ML, Vilela LR, Marengoni A, Calderón-Larrañaga A, Leoutsakos J-MS, Rikkert MGMO, et al. Comorbidity and progression of late onset Alzheimer’s disease: A systematic review. PLOS ONE. 2017. May 4;12(5):e0177044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nutbeam D The evolving concept of health literacy. Soc Sci Med. 2008. December 1;67(12):2072–8. [DOI] [PubMed] [Google Scholar]
- 12.Shaw SJ, Huebner C, Armin J, Orzech K, Vivian J. The Role of Culture in Health Literacy and Chronic Disease Screening and Management. J Immigr Minor Health. 2009. December 1;11(6):460–7. [DOI] [PubMed] [Google Scholar]
- 13.Hayward MD, Miles TP, Crimmins EM, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. Am Sociol Rev. 2000;910–30. [Google Scholar]
- 14.Link BG, Phelan JC. Social Conditions as Fundamental Causes of Disease. J Health Soc Behav. 1995;36:80–94. [PubMed] [Google Scholar]
- 15.Woolf SH, Braveman P. Where health disparities begin: the role of social and economic determinants—and why current policies may make matters worse. Health Aff (Millwood). 2011;30(10):1852–9. [DOI] [PubMed] [Google Scholar]
- 16.Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the US health care workforce do the job? Health Aff (Millwood). 2009;28(1):64–74. [DOI] [PubMed] [Google Scholar]
- 17.Smedley BD, Stith AY, Nelson AR. Unequal treatment: confronting racial and ethnic disparities in health care (with CD). Washington D.C: National Academies Press; 2009. [PubMed] [Google Scholar]
- 18.Thomas SB, Quinn SC, Butler J, Fryer CS, Garza MA. Toward a fourth generation of disparities research to achieve health equity. Annu Rev Public Health. 2011;32:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heeringa SG, Connor JH. Technical Description of the Health and Retirement Survey Sample Design. 1995. Report No.: http://hrsonline.isr.umich.edu/sitedocs/userg/HRSSAMP.pdf.
- 20.Ofstedal MB, Fisher GG, Herzog AR. Documentation of cognitive functioning measures in the Health and Retirement Study. Ann Arbor, Michigan: Institute for Social Research, University of Michigan; 2005. [Google Scholar]
- 21.Cigolle CT, Kabeto MU, Lee PG, Blaum CS. Clinical complexity and mortality in middle-aged and older adults with diabetes. J Gerontol A Biol Sci Med Sci. 2012;67(12):1313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of Cognition Using Surveys and Neuropsychological Assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol Ser B. 2011. July 1;66B(suppl_1):i162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winship C, Radbill L. Sampling weights and regression analysis. Sociol Methods Res. 1994;23(2):230–57. [Google Scholar]
- 24.Hox JJ, Moerbeek M, Schoot R van de. Multilevel Analysis: Techniques and Applications, Second Edition. Routledge; 2010. 393 p. [Google Scholar]
- 25.Ai C, Norton EC. Interaction terms in logit and probit models. Econ Lett. 2003. July 1;80(1):123–9. [Google Scholar]
- 26.Buis ML. Stata tip 87: Interpretation of interactions in non-linear models. Stata J. 2010;10(2):305–308. [Google Scholar]
- 27.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in Quality: Addressing Socioeconomic, Racial, and Ethnic Disparities in Health Care. JAMA. 2000. May 17;283(19):2579–84. [DOI] [PubMed] [Google Scholar]
- 28.DuBard CA, Gizlice Z. Language Spoken and Differences in Health Status, Access to Care, and Receipt of Preventive Services Among US Hispanics. Am J Public Health. 2008. November 1;98(11):2021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiscella K, Franks P, Doescher MP, Saver BG. Disparities in Health Care by Race, Ethnicity, and Language ... : Medical Care. Med Care. 2002;40(1):52–9. [DOI] [PubMed] [Google Scholar]
- 30.Hunt SM, Bhopal R. Self report in clinical and epidemiological studies with non-English speakers: the challenge of language and culture. J Epidemiol Community Health. 2004. July 1;58(7):618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bzostek S, Goldman N, Pebley A. Why do Hispanics in the USA report poor health? Soc Sci Med. 2007. September 1;65(5):990–1003. [DOI] [PubMed] [Google Scholar]
- 32.Dowd JB, Todd M. Does Self-reported Health Bias the Measurement of Health Inequalities in U.S. Adults? Evidence Using Anchoring Vignettes From the Health and Retirement Study. J Gerontol Ser B. 2011. July 1;66B(4):478–89. [DOI] [PubMed] [Google Scholar]
- 33.Suchman L, Jordan B. Interactional Troubles in Face-to-Face Survey Interviews. J Am Stat Assoc. 1990;85(409):232–41. [Google Scholar]
- 34.Lopez-Class M, Castro FG, Ramirez AG. Conceptions of acculturation: A review and statement of critical issues. Soc Sci Med. 2011. May 1;72(9):1555–62. [DOI] [PubMed] [Google Scholar]
- 35.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low Health Literacy and Health Outcomes: An Updated Systematic Review. Ann Intern Med. 2011. July 19;155(2):97. [DOI] [PubMed] [Google Scholar]
- 36.Lee S-YD, Stucky BD, Lee JY, Rozier RG, Bender DE. Short Assessment of Health Literacy—Spanish and English: A Comparable Test of Health Literacy for Spanish and English Speakers. Health Serv Res. 2010. August 1;45(4):1105–20. [DOI] [PMC free article] [PubMed] [Google Scholar]