Abstract
Nerium oleander (N. oleander) is a well-known poisonous shrub that is frequently grown in gardens and public areas and contains numerous toxic compounds. The major toxic components are the cardiac glycosides oleandrin and neriin. The aim of our study was to evaluate the toxic effects of an ethanolic N. oleander leaf extract on haematological, cardiac, inflammatory, and serum biochemical parameters, as well as histopathological changes in the heart. N. oleander extract was orally administered for 14 and 30 consecutive days at doses of 100 and 200 mg of dried extract/kg of body weight in 0.5 mL of saline. The results showed significant increases in mean corpuscular volume, white blood cell counts, platelet counts, interleukins (IL-1 and IL-6), tumour necrosis factor alpha, C reactive protein, alanine aminotransferase, lactate dehydrogenase, creatine kinase and creatine kinase MB, especially at high doses. Marked pathological changes were perceived in the heart tissue. Thus, it can be concluded that exposure to N. oleander leaf extract adversely affects the heart and liver.
Keywords: Subacute toxicity, Nerium oleander, Ethanolic extract, Cardiac glycosides, Mice
INTRODUCTION
Nerium oleander (N. oleander) is an evergreen shrub that is frequently grown as an ornamental plant in gardens and public areas. N. oleander has linear and leathery leaves that come in various colours, from dark green to grey green with distinct light yellowish veins. Its flowers are fragrant, funnel-shaped and arranged in clusters at the tip of twigs, with white to pink to deep red colours. The fruit is a narrow pod containing many silky-haired seeds (Fig. 1). This plant is native to Mediterranean regions of Africa and Europe (1).
Fig. 1.
Nerium oleander (common oleander).
N. oleander is well known for its toxicity, as all parts of the plant contain numerous toxic compounds. The major toxic components are the cardiac glycosides oleandrin and neriin. Plants with red flowers produce more cardiac glycosides than plants with white flowers, especially in the flowering stage (2). Commonly, animal poisoning occurs due to accidental contamination of food or, in some cases, due to consumption of toxic plants by hungry animals (3). Cardiac glycosides inactivate the Na+/K+ ATPase pump on the cytoplasmic membrane of cardiac cells (4).
Despite its toxicity, N. oleander has been used as an abortifacient and for the treatment of heart failure, leprosy, malaria, ringworm and indigestion (5). N. oleander has insecticidal, molluscicidal, rodenticidal and antibacterial effects (6).
The lethal doses of N. oleander leaves were found to differ among animal species, such as sheep and rats (250 and 4,000 mg/kg body weight, respectively) (7,8). It has been reported that consuming minute amounts of oleander leaves, even 0.005% of the animal’s body weight, is fatal in cattle and horses (3).
The aim of this study was to evaluate the toxic effects of an ethanolic N. oleander leaf extract on haematological, cardiac, inflammatory, and serum biochemical parameters, as well as histopathological changes in the heart, after oral administration for 14 and 30 days.
MATERIALS AND METHODS
Plant material and preparation of the ethanolic extract
Fresh mature green leaves from N. oleander bushes with pink flowers were collected from 5 different locations on the Suez Canal University (Ismailia, Egypt) campus during February 2015. The leaves were washed with distilled water, dried in a hot-air oven at 40°C and then ground to minute pieces. Exactly 500 g of ground leaves was extracted several times with 90% ethanol using a separating funnel. The extract was filtered through filter paper (No. 1) and then evaporated under reduced pressure at 42°C using a rotary evaporator (RE111, BUCHI Corporation, DE, USA). The extract was stored in a 5°C refrigerator for further phytochemical analysis and toxicological study (9).
Phytochemical analysis
Moisture, ash and crude fibre content of the dried leaves were determined. Moisture and ash contents were estimated by subjecting 100 g of dried leaves to 90°C and 450°C for 12 hr and for 5 min, respectively. The percentage of crude fibre was measured according to the standards of the Association of Official Analytical Chemists (10).
In addition, glycosides and oleandrin (the most important bioactive constituents) in the extract were determined and calculated as percentages in 100 g of dried leaves. The total glycoside content was evaluated using Baljet’s reagent according to the method described by El-Olemy et al (11). After purification of the extract by lead acetate, Na2HPO4 and filtration, Baljet’s reagent was added, and the intensity of the produced reddish colour was then measured at 495 nm using a Shimadzu UV-2600 spectrophotometer. The concentration was calculated using a digitoxin standard curve (0.1–1.6 mg/mL). Oleandrin was extracted from N. oleander ethanolic extract with 1-naphthoyl chloride and quantified using HPLC (Agilent Infinity 1260) with a fluorescence detector (excitation = 220 nm and emission = 345 nm) equipped with a C18 reverse column according to the method described by Tor et al (12). The oleandrin standard was purchased from Sigma Chemical Co (St. Louis, MO, USA).
Acute oral toxicity (LD50 evaluation)
The median lethal dose (LD50) of the N. oleander ethanolic extract was determined using 12 BALB/c male mice. The animal management protocol and that experimental design were accepted by the Research Ethics Committee of the Faculty of Veterinary Medicine, Suez Canal University (Approval number 2016090). Graduated doses between 0.5 and 3.0 g of dried extract/kg b.w. were used after reconstitution in 0.5 mL of saline and administered orally by stomach gavage. The animals were observed for 24 hr for apparent signs of toxicity and mortality. The LD50 for the extract was determined according to the modified up-and-down technique (13), and its value was calculated using the acute oral toxicity statistical program (AOT425, version 1.0, USEPA, Washington DC, USA).
Animals and experimental design
A total of 30 male mice with body weights of 20–25 g were purchased from the Laboratory Animal Centre of the Faculty of Veterinary Medicine, Suez Canal University. The mice were housed in polypropylene cages (10 mice/cage) in a ventilated room with a controlled light (12 hr): dark (12 hr) cycle and temperature (25 ± 2°C). Food and water were provided ad libitum. The animal management protocol and the experimental design were accepted by the Research Ethics Committee of the Faculty of Veterinary Medicine, Suez Canal University (Approval number 2016090).
After one week of acclimatization, mice were randomly divided into 3 different groups: 10 animals in each group. Of these 10 mice, 5 were used for the 14-day study, and the other 5 were used for the 30-day experiment. The mice in the 1st group were administered normal saline and kept as controls. The animals in the 2nd and 3rd groups were administered N. oleander ethanolic extract using intragastric gavage at doses of 100 and 200 mg dried extract/kg body weight, respectively, in 0.5 mL of saline. These doses were determined to be less than one tenth (1/10) of the calculated LD50 in this study (14) to avoid any possible toxin accumulation in the mice. Nerium extract was administered daily for 14 and 30 consecutive days from the beginning of the treatment.
Blood collection, serum and tissue preparation
After 14 and 30 days of oral administration of N. oleander, blood samples were collected from the postorbital plexus 24 hr after the last treatment. The blood samples were collected in EDTA tubes to investigate the haematological parameters and in non-EDTA-containing tubes to prepare serum for biochemical analysis.
The mice were sacrificed by decapitation, and heart samples were rapidly excised for histopathological evaluation.
Blood and serum biochemical analysis
Blood parameters, including red blood cells (RBCs), haemoglobin concentration (Hb), haematocrit value (HCT), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), white blood cells (WBCs), lymphocytes, and platelet count (PLT), were estimated. Alanine aminotransferase (ALT), creatinine, and inflammatory markers, including interleukin 1 (IL-1), interleukin 6 (IL-6), tumour necrosis factor α (TNF-α) and C reactive protein (CRP), were measured in serum. Myocardial damage markers, including lactate dehydrogenase (LDH), creatine kinase (CK) and creatine kinase MB (CK-MB), were also evaluated. ALT, creatinine, CRP, CK, CK-MB and LDH were measured using a Roche/Hitachi Cobas C311 automatic analyzer. Each parameter was analysed according to the method and the instructions provided by the manufacturer of the automated analyzer. The CBC was analysed using an automated Sysmex KX-21N haematology analyzer according to the manufacturer’s instructions. TNF, IL-1 and IL-6 were estimated by an ELISA reader in accordance with the manufacturer’s directions. All machines and equipment were calibrated and standardized periodically before running the tests.
Histopathological evaluation
Heart muscles were washed with normal saline solution (0.9% NaCl in distilled water) and then fixed in 10% buffered formalin. After standard processing of the tissue, 5 μm sections were prepared and stained with haematoxylin and eosin. The slides were then examined under a light microscope to investigate the histopathological changes. All images were captured with a calibrated standard digital microscope camera (ISH1000 digital microscope camera, Fuzhou Tucsen Photonics Co., Fujian, China) using an CX21 microscope (Olympus Co., Tokyo, Japan), with “IS Capture” software (Olympus Co.) for image capture and enhancement. A semi-quantitative scoring system for myocardial damage was applied based on the severity and extent of the lesions observed in each mouse (15). Briefly, for each myocardial slide, histopathological signs of inflammation and/or myocarditis were scored on a five-degree scale ranging from 0 to 4+. A score of zero indicated no or doubtful presence of lesions in each category. While a score of 1+ described a limited focal distribution of lesions, scores of 2+ to 3+ denoted intermediate rigorousness with multiple lesions, and a score of 4+ indicated the presence of coalescing and extensive lesions throughout the entire examined myocardial tissue. A maximum score of 16 was possible using this scoring system.
Statistical analysis
Data were examined using one-way ANOVA followed by Duncan’s post hoc test to detect significant differences at a probability level of 5% (p ≤ 0.05). All data were expressed as the mean ± SE.
RESULTS
Phytochemical analysis
Moisture, ash, crude fibre, total glycoside and oleandrin contents were estimated in dried leaves of N. oleander and are presented in Table 1. The total glycoside and oleandrin contents were 1,944 and 82 mg/100 gm dried leaves, respectively.
Table 1.
Phytochemical analysis of Nerium oleander dried leaves
| Consistent of dried leaves | Percentage |
|---|---|
| Moisture | 5.13 |
| Ash | 9.06 |
| Crude fiber | 20.66 |
| Glycosides | 1.94 |
| Oleandrin | 0.0082 |
LD50 experiment results
Oral administration of N. oleander ethanolic extract up to a dose of 3 g/kg b.w. revealed neither toxic signs nor death within 24 hr of extract administration. Furthermore, there were no apparent signs of delayed toxicity when the mice were observed for an additional 14 days.
Blood parameter estimation results
After 14 days of oral treatment with N. oleander alcoholic extract, blood parameters showed a significant increase in MCH and a decrease in WBCs in the high-dose group, with a significant decrease in lymphocytes (%) in both the low- and high-dose groups. After 30 days of oral administration, blood parameters revealed significant elevations of MCV, WBCs and PLT in the high-dose group, with significant decreases in lymphocytes (%) in the low- and high-dose groups compared to those in the control group (Table 2, 3).
Table 2.
Blood parameters at 14 days post oral treatment with Nerium oleander ethanolic extract
| Parameter/Group | Control | Low dose | High dose |
|---|---|---|---|
| RBCs (106)/μL | 7.90 ± 0.20a | 7.52 ± 0.24a | 7.53 ± 0.30a |
| Hb g/dL | 10.78 ± 0.31a | 10.64 ± 0.21a | 10.52 ± 0.22a |
| HCT% | 38.50 ± 0.77a | 37.44 ± 0.46a | 36.80 ± 0.95 a |
| MCV fL | 48.38 ± 0.27a | 47.86 ± 0.48a | 47.98 ± 0.75a |
| MCH pg | 12.22 ± 0.23b | 12.76 ± 0.56b | 13.90 ± 0.55a |
| MCHC g/dL | 27.12 ± 0.45b | 27.72 ± 0.46ab | 28.86 ± 0.63a |
| WBC (103)/μL | 10.36 ± 0.34a | 10.12 ± 0.23a | 9.12 ± 0.49b |
| Lymphocyte % | 95.60 ± 0.97a | 93.20 ± 1.11b | 91.00 ± 0.91b |
| PLT (103)/μL | 955.60 ± 15.95a | 1031.00 ± 70.03a | 1059.20 ± 89.06a |
Data are expressed as means ± SEM, n = 5. Values having different alphabetic superscripts within the same row are significantly different (p ≤ 0.05).
Table 3.
Blood parameters on 30 days post oral treatment with Nerium oleander ethanolic extract
| Parameter/Group | Control | Low dose | High dose |
|---|---|---|---|
| RBCs (106)/μL | 8.13 ± 0.53a | 8.17 ± 0.25a | 7.86 ± 0.33a |
| Hb g/dL | 11.72 ± 0.58a | 11.68 ± 0.53a | 11.14 ± 0.79a |
| HCT% | 41.02 ± 2.07a | 40.68 ± 0.77a | 42.28 ± 0.90 a |
| MCV fL | 49.14 ± 0.69b | 48.78 ± 0.94b | 53.78 ± 0.39a |
| MCH pg | 14.02 ± 0.37a | 14.28 ± 0.29a | 14.90 ± 0.47a |
| MCHC g/dL | 28.30 ± 0.34a | 29.82 ± 0.56a | 28.80 ± 0.91a |
| WBC (103)/μL | 10.70 ± 0.88b | 9.04 ± 1.04b | 14.14 ± 1.09a |
| lymphocyte % | 91.00 ± 2.12b | 95.00 ± 1.08a | 94.60 ± 0.88a |
| PLT (103)/μL | 771.00 ± 88.69b | 789.60 ± 66.51b | 1082.40 ± 129.07a |
Data are expressed as means ± SEM, n = 5. Values having different alphabetic superscripts within the same row are significantly different (p ≤ 0.05).
Serum biochemical parameter estimation results
IL-1, IL-6, TNF-α, CK, and CK-MB were significantly increased in the high-dose group after 14 days of treatment, while CRP and LDH were significantly increased in both the low- and high-dose groups. After 30 days of administration, IL-6, TNF-α, CRP, ALT, LDH, CK and CK-MB levels were significantly increased in both the low- and high-dose groups, while IL-1 was only significantly increased in the high-dose group compared to those in the control group (Table 4, 5).
Table 4.
Serum biochemical parameters after 14 days from oral treatment with Nerium oleander alcoholic extract
| Parameter/Group | Control | Low dose | High dose |
|---|---|---|---|
| IL1 pg/mL | 2.72 ± 0.26b | 2.76 ± 0.139b | 5.12 ± 0.440a |
| IL6 pg/mL | 9.46 ± 0.30b | 9.34 ± 0.320b | 15.42 ± 0.886a |
| TNF α pg/mL | 8.70 ± 0.24b | 9.12 ± 0.214b | 12.36 ± 0.594 a |
| CRP | 3.74 ± 0.37c | 5.72 ± 0.599b | 8.24 ± 0.315a |
| ALT | 44.20 ± 5.56a | 33.40 ± 2.221b | 47.80 ± 3.795a |
| Creatinine | 0.48 ± 0.05a | 0.48 ± 0.048a | 0.50 ± 0.041a |
| LDH | 54.00 ± 3.24c | 63.60 ± 2.183b | 84.20 ± 4.990a |
| CK | 237.20 ± 11.60b | 259.20 ± 15.489b | 426.40 ± 47.646a |
| CK Mb | 16.40 ± 1.20b | 20.20 ± 0.753b | 116.20 ± 7.973a |
Data are expressed as means ± SEM, n = 5. Values having different alphabetic superscripts within the same row are significantly different (p ≤ 0.05).
Table 5.
Serum biochemical parameters after 30 days from oral treatment with Nerium oleander ethanolic extract
| Parameter/Group | Control | Low dose | High dose |
|---|---|---|---|
| IL1 pg/mL | 2.92 ± 0.29b | 3.66 ± 0.211b | 8.08 ± 0.511a |
| IL6 pg/mL | 9.44 ± 0.30c | 12.40 ± 0.623b | 18.74 ± 0.631a |
| TNF α pg/mL | 8.64 ± 0.28c | 11.24 ± 0.446b | 16.66 ± 0.290a |
| CRP | 3.84 ± 0.43c | 6.44 ± 0.431b | 9.00 ± 0.922a |
| ALT | 47.20 ± 4.03c | 58.20 ± 2.955b | 72.00 ± 5.745a |
| Creatinine | 0.50 ± 0.06ab | 0.40 ± 0.058b | 0.58 ± 0.048a |
| LDH | 53.80 ± 3.66c | 95.20 ± 1.844b | 116.40 ± 5.141a |
| CK | 239.80 ± 14.60c | 357.80 ± 29.786b | 550.40 ± 75.563a |
| CK Mb | 16.80 ± 1.18c | 26.00 ± 1.472b | 42.00 ± 4.546a |
Data are expressed as means ± SEM, n = 5. Values having different alphabetic superscripts within the same row are significantly different (p ≤ 0.05).
Histopathological results
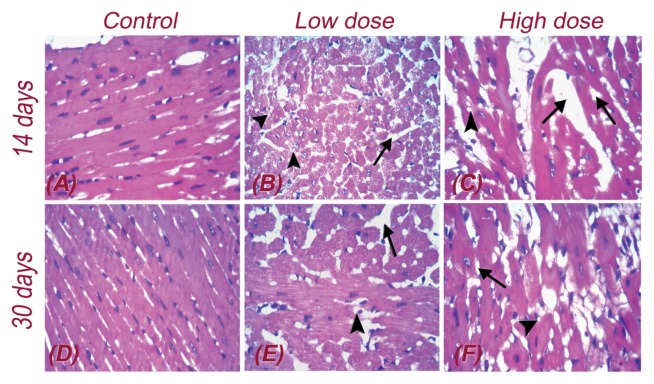
In the control group, heart muscle formed of myocytes arranged into fascicles with striations and peripheral oval nuclei separated by loose connective tissue and thin vessels (Fig. 2A, 2D).
Fig. 2.
Histopathological results on 14 (A, B, C) and 30 days (D, E, F) post oral treatment with Nerium oleander ethanolic extract. All slides were stained with Hematoxylin and Eosin dyes and captured with high power field of 400×. Arrows and arrowheads point out the pathological changes such as inter-fascicular edema (black arrow) and degenerated myocytes with vacuolation of myofibrils (arrow head).
After 14 days of oral administration of a low dose of N. oleander alcoholic extract, heart muscles showed diffuse mild inter-fascicular oedema with mildly congested vessels. In addition, many degenerated myocytes showed fragmentation of myofibrils (Fig. 2B). In contrast, in the high-dose group, the heart muscles showed moderate inter-fascicular oedema with dilated congested vessels and few degenerated myocytes with focal striation loss and focal vacuolar degeneration (Fig. 2C).
After 30 days of oral administration of a low dose of N. oleander extract, the heart muscles showed focal mild inter-fascicular oedema with mildly congested vessels and very few degenerated myocytes (Fig. 2E). In contrast, in the high-dose group, the heart muscles showed focal marked inter-fascicular oedema with dilated congested vessels (black arrow) and moderately degenerated myocytes with vacuolation of the muscle. Additionally, a few myofibrils showed striation loss (arrow head) (Fig. 2F). Table 6 shows semi-quantitative histopathological scoring of mouse cardiac lesions at 14 and 30 days after N. oleander extract administration.
Table 6.
Semi-quantitative histopathological scoring of myocardial damage after 14 and 30 days from oral treatment with Nerium oleander alcoholic extract in mice
| Groups | 14 days | 30 days |
|---|---|---|
| Control | 0.0 ± 0.00b | 0.2 ± 0.26c |
| Low dose | 2.0 ± 0.41a | 2.6 ± 0.32b |
| High dose | 3.2 ± 0.75a | 4.6 ± 0.66a |
Data are expressed as means ± SEM, n = 5. Values having different alphabetic superscripts within the same column are significantly different (p ≤ 0.05).
DISCUSSION
Oleander poisoning is a common problem in many parts of the world. Oleander toxicity is due to oleandrin (the main cardiac glycoside of oleander) and neriin, which cause damage by inhibiting the plasmalemmal Na+/K+-ATPase. Na+/K+-ATPase is responsible for maintaining the membrane potential of cardiac muscle cells, and its inhibition leads to necrosis of myocytes (16). In the current study, we examined the haematological, biochemical and histopathological changes that occur after administration of an N. oleander leaf ethanolic extract in mice. Haematological analysis showed a significant increase in MCV, WBCs and PLT, and these results are consistent with the results of other researchers (17,18). The increase in MCV is an indication of a pathological condition called “macrocytosis”, which is due to liver injury that may be caused by free radicals resulting from N. oleander administration (18). The liberation of free radicals into the blood may also influence the circulating cells and significantly altera their numbers (19). Significant decreases in RBC, Hb and PCV were recorded in female mice treated with N. oleander alkaloid extracts (20). The author attributed these declines to the disruption of factors that influence erythropoiesis or to the rupture of the RBC membrane. However, the effect of N. oleander on blood parameters remains controversial among researchers (18,20). Higher platelet and white blood cell counts may act as markers of pro-inflammatory conditions (21).
These results revealed significant increases in IL-6, TNF-α, CRP, ALT, LDH, CK and CK-MB levels in both the low- and high-dose groups, while IL-1 was significantly increased only in the high-dose group compared to the control group. Increased serum concentrations of the inflammatory marker CRP and pro-inflammatory cytokines (IL-1, IL-6 and TNF-α) are observed during chronic heart failure and mediate both immune and inflammatory responses, inducing apoptosis, in myocardial cells (22) (23). Toxic substances induce pro-inflammatory cytokines through the activation of macrophages (24). The pro-inflammatory cytokine TNF-α is involved in the initiation and progression of the inflammatory response and the production of inflammatory mediators, such as IL-1 and IL-6 (25–27). Our findings are consistent with the results obtained by other investigators (28,29).
The liver enzyme (ALT) was elevated in the serum, especially after 30 days of N. oleander treatment, indicating liver injury and disruption of hepatocyte membranes, which may be due to the liberation of free radicals by N. oleander (18,30). LDH levels were also significantly increased, which could represent a good marker along with ALT, of tissue and cellular damage, especially hepatic cells.
CK is an essential enzyme in cellular energy transfer, helping in the transfer of high-energy phosphoryl groups in the mitochondria to generate ATP (31). The MB isoform of creatine kinase (CK-MB) is considered to be a specific indicator of myocyte injury and is elevated in myocardial damage (32). Jortani et al. (33) reported that oleander plants containing the cardiac glycoside oleandrin exert toxic effects via inhibition of Na+/K+-ATPase pump activity. In this study, CK and CK-MB were significantly elevated, indicating severe myocardial damage that may be due to cardiac glycoside inhibition of the Na+/K+-ATPase pump in the cytoplasmic membrane of cardiac cells, leading to positive inotropic effects and spontaneous depolarization in cardiac muscles (34). The inhibition of Na+/K+-ATPase leads to an increase in the intracellular sodium concentration, which in turn induces an increase in the intracellular calcium concentration via the sodium-calcium exchanger. The sodium-calcium exchanger is particularly active in the myocardium and in vascular smooth muscles. The increase in intracellular calcium increases the force of contraction of the heart and vascular smooth muscles (35).
Our results were confirmed by histopathological examinations, which indicated several degrees of myocardial vacuolar degeneration, the loss of myofibril striation and inter-fascicular oedema. These findings indicate some degree of myocyte damage and myocardial injury after treatment with N. oleander, and these results are consistent with those observed by several researchers (1,36,37).
Finally, we can conclude that exposure to N. oleander leaf extract adversely affects the heart and liver. The results showed significant adverse changes in haematological, biochemical and inflammatory parameters, as well as histopathological alterations. As this plant is used in the medical field, extra effort must be made to purify the effective chemical constituents to avoid toxicity.
Footnotes
CONFLICT OF INTEREST
All authors declare that there are no conflicts of interest related to this manuscript. This research is self-funded; we had no funding grants from any governmental or non-governmental organizations or institutes.
REFERENCES
- 1.Taheri S, Solati A, Moradi P, Tavassoly A, Yadi J. Toxic effects of Nerium oleander aqueous leaf extract on haematological parameters and histopathological changes of the lungs and heart in rabbits. Comp Clin Pathol. 2013;22:1189–1193. doi: 10.1007/s00580-012-1548-9. [DOI] [Google Scholar]
- 2.Karawya MS, Balbaa SI, Khayyal SE. Estimation of cardenolides in Nerium oleander. Planta Med. 1973;23:70–73. doi: 10.1055/s-0028-1099414. [DOI] [PubMed] [Google Scholar]
- 3.Galey FD, Holstege DM, Pulmee KH, Tor E, Johnson B, Anderson ML, Blanchard PC, Brown F. Diagnosis of oleander poisoning in livestock. J Vet Diagn Investig. 1996;8:358–364. doi: 10.1177/104063879600800314. [DOI] [PubMed] [Google Scholar]
- 4.Demiryurek AT, Demiryurek S. Cardiotoxicity of digitalis glycosides: roles of autonomic pathways, autacoids and ion channels. Auton Autacoid Pharmacol. 2005;25:35–52. doi: 10.1111/j.1474-8673.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Osterloh J, Herold S, Pond S. Oleander interference in the digoxin radioimmunoassay in a fatal ingestion. JAMA. 1982;247:1596–1597. doi: 10.1001/jama.1982.03320360046030. [DOI] [PubMed] [Google Scholar]
- 6.Oji O, Okafor QE. Toxicological studies on stem bark, leaf and seed kernel of yellow oleander (Thevetia peruviana) Phytother Res. 2000;14:133–135. doi: 10.1002/(SICI)1099-1573(200003)14:2<133::AID-PTR598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Haeba MH, Mohamed AI, Mehdi AW, Nair GA. Toxicity of Nerium oleander leaf extract in mice. J Environ Biol. 2002;23:231–237. [PubMed] [Google Scholar]
- 8.Aslani MR, Movassaghi AR, Mohri M, Abbasian A, Zarehpour M. Clinical and pathological aspect of experiment oleander (Nerium oleander) toxicosis in sheep. Vet Res Commun. 2004;28:609–616. doi: 10.1023/B:VERC.0000042870.30142.56. [DOI] [PubMed] [Google Scholar]
- 9.Tittel G, Wagner H. Qualitative und quantatitve Analyse von Herzglykosiddrogen durch HPLC-Verfahren. Planta Med. 1981;43:252–262. doi: 10.1055/s-2007-971506. [DOI] [PubMed] [Google Scholar]
- 10.AOAC. Official Methods of Analysis. Association of Official Analytical Chemists (AOAC); Washington, DC: 1990. [Google Scholar]
- 11.El-Olemy MM, Al-Muhtadi FJ, Afifi AFA. Experimental Phytochemistry: A Laboratory Manual. King Saud University Press; Saudi Arabia: 1994. pp. 21–27. [Google Scholar]
- 12.Tor ER, Holstege DM, Galey FG. Determination of oleander glycosides in biological matrices by High-Performance liquid chromatography. J Agric Food Chem. 1996;44:2716–2719. doi: 10.1021/jf950818h. [DOI] [Google Scholar]
- 13.Bruce RD. A confirmatory study of the up-and-down method for acute oral toxicity testing. Fundam Appl Toxicol. 1987;8:97–100. doi: 10.1016/0272-0590(87)90104-7. [DOI] [PubMed] [Google Scholar]
- 14.Singhal KG, Gupta GD. Hepatoprotective and antioxidant activity of methanolic extract of flowers of Nerium oleander against CCl4-induced liver injury in rats. Asian Pac J Trop Med. 2012;5:677–685. doi: 10.1016/S1995-7645(12)60106-0. [DOI] [PubMed] [Google Scholar]
- 15.Rahman A, More N, Schein PS. Doxorubicin-induced chronic cardiotoxicity and its protection by liposomal administration. Cancer Res. 1982;42:1817–1825. [PubMed] [Google Scholar]
- 16.Barbosa RR, Fontenele-Neto JD, Soto-Blanco B. Toxicity in goats caused by oleander (Nerium oleander) Res Vet Sci. 2008;85:279–281. doi: 10.1016/j.rvsc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Altaee MF. In vivo toxicity study of Nerium oleander’s leaves and flowers aqueous extracts in mice (cytogenetic, biochemical and hematological Study) Baghdad Sci J. 2011;8:366–372. doi: 10.21123/bsj.8.1.366-372. [DOI] [Google Scholar]
- 18.Akhtar T, Sheikh N, Abbasi MH. Clinical and pathological features of Nerium oleander extract toxicosis in Wistar rats. BMC Res Notes. 2014;7:947. doi: 10.1186/1756-0500-7-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doi K, Kurabe S, Shimazu N, Inagaki M. Systemic histopathology of rats with CCl4-induced hepatic cirrhosis. Lab Anim. 1991;25:21–25. doi: 10.1258/002367791780808121. [DOI] [PubMed] [Google Scholar]
- 20.Hussein AM. The study of alkaloid extracts effect of Nerium oleander and Apium graveolens in the body Weight and blood parameters in laboratory mice females Mus Musculus L. Int J Agric Sci Res. 2016;6:87–96. [Google Scholar]
- 21.Jesri A, Okonofua EC, Egan BM. Platelet and white blood cell counts are elevated in patients with the metabolic syndrome. J Clin Hypertens (Greenwich) 2005;7:705–713. doi: 10.1111/j.1524-6175.2005.04809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M, Han CH, Lee MY. NADPH oxidase and the cardiovascular toxicity associated with smoking. Toxicol Res. 2014;30:149–157. doi: 10.5487/TR.2014.30.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology. 2012;122:23–35. doi: 10.1159/000338166. [DOI] [PubMed] [Google Scholar]
- 24.Kang GJ, Kang NJ, Han SC, Koo DH, Kang HK, Yoo BS, Yoo ES. The Chloroform fraction of Carpinus tschonoskii leaves inhibits the production of inflammatory mediators in HaCaT keratinocytes and RAW264.7 macrophages. Toxicol Res. 2012;28:255–262. doi: 10.5487/TR.2012.28.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huong PTT, Jeon YJ. Macrophage activation by glycoprotein isolated from Dioscorea batatas. Toxicol Res. 2011;27:167–172. doi: 10.5487/TR.2011.27.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu A, Sen T, Ray RN, Chaudhuri AK. Hepatoprotective effects of Calotropis procera root extract on experimental liver damage in animals. Fitoterapia. 1992;63:507–514. [Google Scholar]
- 27.Wang H, Wei W, Wang NP, Gui SY, Wu L, Sun WY, Xu SY. Melatonin ameliorates carbon tetrachloride-induced hepatic fibrogenesis in rats via inhibition of oxidative stress. Life Sci. 2005;77:1902–1915. doi: 10.1016/j.lfs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Lange GL, Schreiner GF. Immune mechanisms of cardiac disease. N Engl J Med. 1994;330:1129–1135. doi: 10.1056/NEJM199404213301607. [DOI] [PubMed] [Google Scholar]
- 29.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.CIR.93.4.704. [DOI] [PubMed] [Google Scholar]
- 30.Madkour FF, Khalil WF, Dessouki AA. Protective effect of ethanol extract of Sargassum Dentifolium (Phaeophyceae) in carbon tetrachloride induced hepatitis in rats. Int J Pharm Pharm Sci. 2012;4:637–641. [Google Scholar]
- 31.Saks V, Dzeja P, Schlattner U, Vendelin M, Terzic A,, Wallimann T. Cardiac system bioenergetics: metabolic basis of the Frank-Starling law. J Physiol. 2006;571:253–273. doi: 10.1113/jphysiol.2005.101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christenson RH, Azzazy HEM. Biochemical markers of the acute coronary syndromes. Clin Chem. 1998;44:1855–1864. [PubMed] [Google Scholar]
- 33.Jortani SA, Helm RA, Valdes R. Inhibition of Na, K-ATPase by oleandrin and oleandrigenin, and their detection by digoxin immunoassays. Clin Chem. 1996;42:1654–1658. [PubMed] [Google Scholar]
- 34.Heard K. In: Digoxin and therapeutic cardiac glycosides in Medical Toxicology (3rd edition) Dart RC, editor. Lippincott Williams & Wilkins; 2004. pp. 700–701. [Google Scholar]
- 35.Meyer-Lehnert H, Bäcker A, Kramer HJ. Inhibitors of Na-K-ATPase in human urine: effects of ouabain-like factors and of vanadium-diascorbate on calcium mobilization in rat vascular smooth muscle cells: comparison with the effects of ouabain, angiotensin II, and arginine-vasopressin. Am J Hypertens. 2000;13:364–369. doi: 10.1016/S0895-7061(99)00197-1. [DOI] [PubMed] [Google Scholar]
- 36.Yahaya MA, AL-Farhan AH, Adam SE. Preliminary toxicity study on the individual and combined effects of Citrullus colocynthis and Nerium oleander in rats. Fitoterapia. 2000;71:385–391. doi: 10.1016/S0367-326X(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 37.Aslani MR, Movassaghi AR, Janati-Pirouz H, Karazma M. Experimental oleander (Nerium oleander) poisoning in goats: a clinical and pathological study. Iran J Vet Res. 2007;8:58–63. [Google Scholar]