Abstract
Citrus macroptera (Rutaceae) has long been used in folk medicine in Bangladesh. Considering the folkloric context, this study was aimed to scrutinize anti-proliferative activity of C. macroptera fruit pulp juice (CMFPJ) against Ehrlich’s ascites carcinoma (EAC). The anti-proliferative capacity of CMFPJ was investigated and confirmed primarily using MTT assay. In vivo anti-proliferative aptitude of CMFPJ was investigated with 25, 50, and 100 mg/kg/day intraperitoneal (i.p.) treatment. Anti-proliferative efficacy of CMFPJ was assessed based on EAC growth inhibition. CMFPJ inhibited EAC growth in vitro in a dose-dependent manner. And the percentages of in vivo EAC growth inhibition were 19.53, 49.2, and 68.9% at 25, 50, and 100 mg/kg CMFPJ respectively. CMFPJ significantly induced expression of apoptosis regulatory genes caspase-8, caspase-9, cytochrome-c, and caspase-3. This considerable anti-cancer activity was perhaps due to combinatorial effect of lectin, polyphenols, and flavonoids present in CMFPJ.
Keywords: Citrus macroptera, Extrinsic pathway, Intrinsic pathway, Apoptosis
INTRODUCTION
Cancer is a disease in which constant clonal expansion of somatic cells kill by invading, subverting and eroding normal tissues (1). The treatment methods to fight against cancer comprise chemotherapy, radiotherapy, hormone therapy, and surgery (2); however, each of them has its own disadvantages and severely affects patient’s normal cell. Therefore, the use of natural products nowadays has been contemplated of outstanding value in controlling cancer. Plant extracts can cause apoptosis through induction of caspase pathway (3). Apoptosis is a programmed cell death and a highly arranged manner to eradicate cancerous cells and plays a key role in tissue homeostasis and maintenance (4). Proteolytic enzymes such as caspases are central effector molecules in apoptosis and their activation or inactivation is vital in controlling cancer (5).
Citrus macroptera, also known as “wild orange” (6), is an indigenous fruit of Malaysia, Melanesia (7) and Bangladesh (8). C. macroptera is available in the hilly areas of north-eastern Bangladesh (Sylhet Division). Locally it is known as “Satkara” and has long been used to treat hypertension, stomach pain, alimentary disorders (9) and fever (10). C. macroptera fruit is significantly cytotoxic (11). The fruit pulp was reported to contain 2.89 ± 0.32 mg proteins and antioxidants including 291.06 ± 10.14 mg polyphenols, 145.02 ± 0.36 mg flavonoids, 526.08 ± 3.32 mg tannin, and 120.83 ± 0.0019 mg ascorbic acids per 100 g dry weight and significant radical scavenging activity was confirmed by an in vitro study (12). Epidemiological studies showed that many of these antioxidants possess anti-inflammatory, anti-atherosclerotic, anti-cancer, anti-mutagenic, anti-carcinogenic, anti-bacterial, and anti-viral activities (13). Considering the information mentioned above, this current study was undertaken to investigate the anti-proliferative activity of C. macroptera fruit pulp juice (CMFPJ) against Ehrlich’s ascites carcinoma (EAC) in mice.
MATERIALS AND METHODS
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and 3-[4, 5-dimethylthiazol-2-yl]-2, 5- diphenyltetrazolium bromide (MTT) were bought from Sigma-Aldrich (St. Louis, MO, USA). Hemagglutination buffer was prepared as 20 mM Tris-HCl buffer, pH 7.8 containing 1% NaCl and 10 mM CaCl2. Trypan blue was bought from Invitrogen (Carlsbad, CA, USA). M-MLV (Moloney Murine Leukemia Virus) reverse-transcriptase was purchased from Tiangen (Beijing, China), primers were custom synthesized from IDT (Integrated DNA Technologies), Seri Kembangan, Selangor, Malaysia. GoTaq® qPCR Master Mix (2X) was collected from Promega (Madison, WI, USA). All other chemicals and reagents used were of analytical grade.
Plant sample
The fruits of C. macroptera were collected in mid-October, 2015 from a local market of Sylhet, Bangladesh. The collected fruits were authenticated by a taxonomist of the Department of Botany, University of Rajshahi (Rajshahi, Bangladesh); where a voucher specimen has been preserved under accession number 00212 for future reference.
Preparation of C. macroptera fruit pulp juice
The thick rind of C. macroptera fruit was removed by using a sharp knife with gloved hand to collect pulp. The pulp was cut into small pieces and juice was collected by squeezing the pieces of pulp with gloved hand. The juice was dried with VirTis BenchTop Pro Freeze Dryer (SP Scientific, Stone Ridge, NY, USA) and dissolved in distilled water at 1 g/10 mL concentration. Juice was filtered by using Glass Fiber Filter paper (Macherey NAGEL GmBH, Düren, Germany) with DURAN® Filtering Apparatus (Mainz, Germany) at room temperature. Finally, the juice filtrate was again dried with VirTis BenchTop Pro Freeze Dryer and stored at −20°C for further use.
Experimental animal and EAC cell
Male Swiss albino mice (25–30 g) were bought from the Department of Pharmacy of Jahangirnagar University (Dhaka, Bangladesh). The mice were housed in polypropylene cage (Tarson, Bangalore, India) and kept in the animal house of Institute of Biological Sciences (IBSc), University of Rajshahi under standard condition (12 hr light/dark cycle, 25 ± 2°C temperature and ~70% humidity). The animals were allowed to acclimatize for 1 week before beginning the experiment. Food and water were supplied ad libitum. Methodologies followed in this research work along with handling of experimental animal were approved by the Institutional Animal, Medical Ethics, Biosafety and Biosecurity Committee (IAMEBBC) for Experimentations on Animal, Human, Microbes and Living Natural Sources (license no: 31/320-IAMEBBC/IBSc), Institute of Biological Sciences (IBSc), University of Rajshahi. EAC cells used in this study were kindly provided by the Department of Pharmacy of Jahangirnagar University.
In vitro cytotoxicity test
In vitro cytotoxicity of CMFPJ was performed as described previously with a few modifications (14). Cytotoxicity of CMFPJ on the growth of EAC cell was determined by measuring the metabolic capacity using the 3-[4, 5-dimethylthiazol-2-yl]-2, 5- diphenyltetrazolium bromide (MTT) assay. The MTT assay was carried out as follows: EAC cells (100 μL cells from 1 × 106 cells/mL) were treated for 24 hr with five different doses of CMFPJ (500, 250, 125, 62.5, and 31.25 μg/mL in DMEM medium). After incubation at 37°C for 24 hr, 0.1 mg (50 μL of a 2 mg/mL solution) MTT was added to each well and the cells were again incubated at 37°C for 8 hr. The aliquot was carefully removed and 100 μL dimethylsulfoxide (DMSO) was then added to each well to dissolve the formazan crystal. The plate was read immediately at 540 nm on a multi-well plate reader (Multiskan™ FC Microplate Photometer, Thermo Scientific, Waltham, MA, USA). All the experiments were performed in triplicate and the mean absorbance values were calculated. The results were articulated as the percentage of inhibition by comparing to the untreated control. The following equation was used to calculate the cell proliferation inhibition percentage:
where A is the OD540 nm of the cellular homogenate without CMFPJ (control) and B is the OD540 nm of the cellular homogenate with CMFPJ.
Hemagglutination assay
To check the presence of lectin protein in CMFPJ, hemagglutination assay was performed with minor modification as described previously (15). A final volume of 100 μL was prepared in 96-well flat-bottomed microtiter plate containing 50 μL of CMFJ (100 mg/mL) serially diluted with 50 μL hemagglutination buffer (20 mM Tris-HCl buffer, pH 7.8 containing 1% NaCl and 10 mM CaCl2) and 50 μL of 2% red blood cells (RBCs) suspension previously washed with 1% NaCl. PBS was used as a control. After a gentle shaking, the plate was set aside at room temperature for 30 min. The visual agglutination titer of the maximum dilution giving the positive agglutination was noted.
EAC inoculation and treatment procedure
EAC cells (200 μL) adjusted 106 cells/mL in saline water (0.9% NaCl) were pre-inoculated into the i.p. of mice on the 1st day (16). The mice were divided randomly into four groups (n = 6). CMFPJ at 25, 50, and 100 mg/kg/day dosage was administrated to the treatment groups in a total volume of 200 μL with distilled water for 7 consecutive days at a regular interval of 24 hr starting from 24 hr of post inoculation of EAC cells (17). Control mice received 200 μL of saline solution each day.
In vivo cell growth inhibition assay. Cell growth
inhibition assay was carried out as described previously (16,18). After 7 consecutive days of treatment, 5 mL saline water (0.9% NaCl) was injected into the peritoneum of mice of both control and treated groups and then they were anesthetized using chloroform. Then cells from peritoneum were harvested and diluted 10 times (100 μL: 900 μL) in saline. Thereafter, diluted cells were stained with Trypan blue dye for quantification of viable cells. Then 10 μL cell suspension was placed on hemocytometer and viable cells were counted by using Optika inverted microscope (Italy). Cell growth inhibition percentage was calculated by the following formula:
where, T = mean number of EAC cells of CMFPJ treated group and C = mean number of EAC cells of control.
RNA isolation, reverse transcription and real time-PCR
Total RNA from EAC of control and CMFPJ treated mice were isolated after 24 hr of last treatment. RNA was extracted using RNAsimple Total RNA Kit (Tiangen, Beijing, China) according to the manufacturer’s protocol. RNA quality was checked on 1% agarose gel stained with 10 μg/mL ethidium bromide and visualized via a gel documentation system (Alphaimager mini, New Taipei City, Taiwan). Concentration and purity of isolated RNA were measured by spectrometry at 260 and 280 nm. Isolated RNA was then converted to cDNA using TIANscript M-MLV reverse-transcriptase. For each reaction (20 μL), 1 μL of 10 mM oligo (dT), 1 μL dNTPs (10 mM), 1 μg RNA and nuclease-free water up to 15 μL were taken in PCR tube and incubated at 70°C for 5 min. The mixture was immediately chilled on ice for 3 min. 1 μL M-MLV reverse transcriptase, 4 μL (5X) of 1st strand buffer was added to each tube and incubated at 45°C for 50 min. Further, the mixture was incubated at 95°C for 5 min to inactivate the M-MLV reverse-transcriptase enzyme. Prepared cDNA was stored at −20°C for further use. The reverse transcription products were used as templates for real-time PCR. PCR amplification was performed using specific primers (Table 1). Each reaction was performed in triplicate in 10 μL volume containing 5 μL GoTaq® qPCR Master Mix (2X) (Promega, Madison, WI, USA), 0.5 μL (10 mM) of each primer, 3 μL nuclease free water and 1 μL template. Thermal cycling was performed for each gene on cDNA samples in 48-well reaction plates by using the Eco™ Real- Time PCR System (Illumina®, San Diego, CA, USA). Realtime PCR was conducted with the following cycling conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 30 sec, 50°C for 30 sec and 72°C for 25 sec. PCR reaction specificity was confirmed by melt curve analysis at 95°C for 15 sec, 60°C for 15 sec, 95°C for 15 sec. The quantitation of gene expression was carried out using GAPDH as an endogenous control and relative to the calibrator sample using the ΔΔCq method.
Table 1.
Primers used for PCR amplification
| Gene | Primer | Sequence |
|---|---|---|
| GAPDH | Forward | 5′-GTGGAAGGACTCATGACCACAG-3′ |
| Reverse | 5′-CTGGTGCTCAGTGTAGCCCAG-3′ | |
| Caspase-8 | Forward | 5′-CTGCTGGGGATGGCCACTGTG-3′ |
| Reverse | 5′-TCGCCTCGAGGACATCGCTCTC-3′ | |
| Cytochrome-c | Forward | 5′-CCAGGTATACAAGCAGGTGTGCTC-3′ |
| Reverse | 5′-CATCATTAGGGCCATCCTGGAC-3′ | |
| Caspase-9 | Forward | 5′-ATGGACGAAGCGGATCGG-3′ |
| Reverse | 5′-CCCTGGCCTTATGATGTT-3′ | |
| Caspase-3 | Forward | 5′-GCAGCAAACCTCAGGGAAAC-3′ |
| Reverse | 5′-GGTTTCCCTGAGGTTTGCTG-3′ |
Statistical analysis
Data presented as mean ± SD (Standard Deviation) and graphs were prepared by using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA). Analysis of variance (ANOVA) was performed using SPSS (IBM, version 16; SPSS Inc., Chicago, IL,
RESULTS
In vitro cytotoxicity test
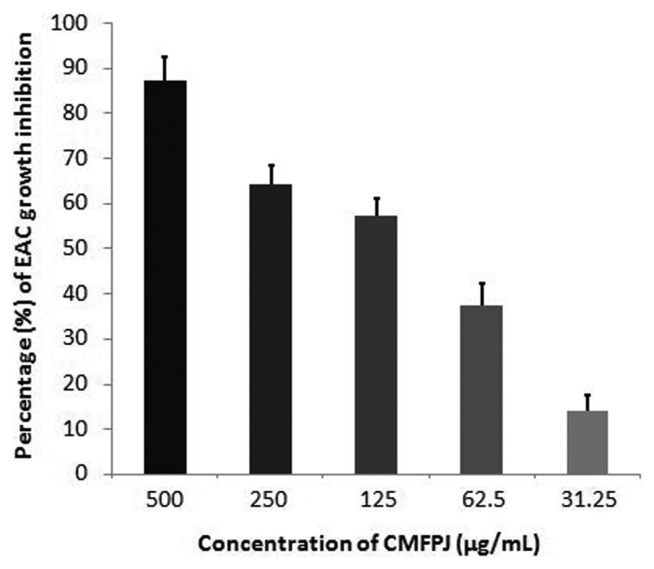
The in vitro cytotoxic effect of CMFPJ on EAC cells was in a dose-dependent manner (Fig. 1). The CMFPJ inhibited 87.3% EAC cells at 500 μg/mL concentration. The cytotoxic effect was in decreasing trend with the reduction of CMFPJ concentration and it reached to 13.9% at 31.25 μg/mL.
Fig. 1.
Effect of CMFPJ on EAC growth in vitro. CMFPJ slowed down EAC cell growth in a dose-dependent manner. The results are represented as mean ± SD (n = 3).
Hemagglutination assay
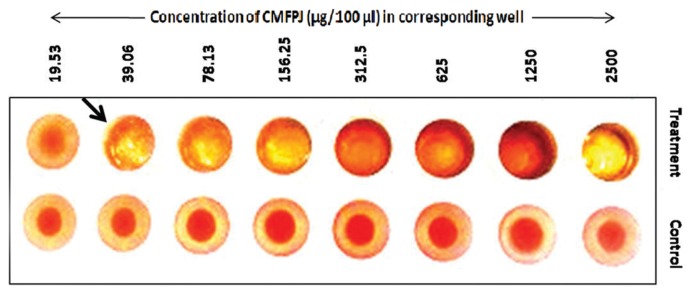
Result regarding the hemagglutination assay demonstrated that CMFPJ has considerable agglutination activity on mouse RBCs (Fig. 2). CMFPJ showed hemagglutination activity on mouse RBCs at the very last concentration of 39.06 μg/100 μL. Thereafter, the presence of lectin in CMFPJ was confirmed from the well-established agglutination activity on mouse RBCs.
Fig. 2.
Hemagglutination activity of CMFPJ. CMFPJ showed hemagglutination activity on mouse RBCs (upper row) but the control wells did not show hemagglutination at any concentration of PBS (lower row).
In vivo cell growth inhibition assay
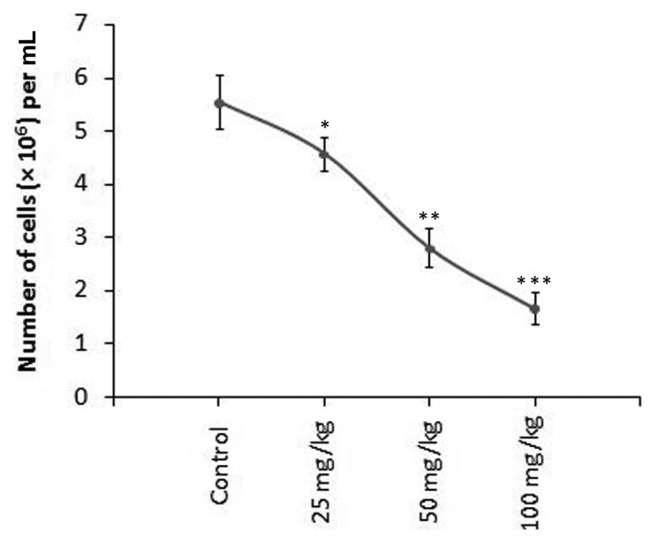
Result regarding cell growth inhibition revealed that CMFPJ significantly slowed down EAC growth in vivo in a dose-dependent manner (Fig. 3). The number of EAC counted in the control group were 5.53 × 106 cells/mL, whereas 4.45 × 106, 2.81 × 106, and 1.72 × 106 cells/mL were observed in the 25, 50, and 100 mg/kg CMFPJ treated group respectively (Fig. 3). According to the number of EAC cells, we found that CMFPJ reduced growth of EAC cells by 19.53, 49.2, and 68.9% at 25, 50, and 100 mg/kg CMFPJ respectively.
Fig. 3.
In vivo effect of CMFPJ on EAC growth inhibition. CMFPJ significantly reduced EAC growth compared to control. Significance levels were determined and set at *p< 0.05, **p< 0.01, and ***p< 0.001 in respect to the control. The results are represented as mean ± SD (n = 6).
Real time-PCR
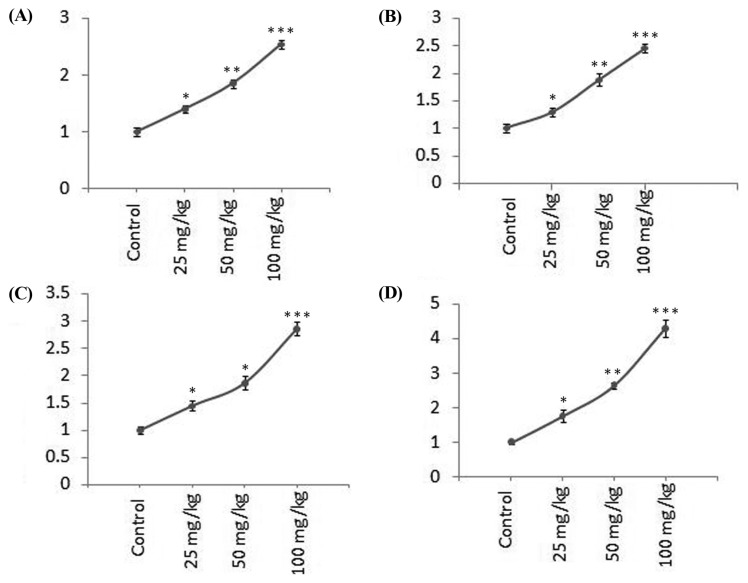
Molecular investigation demonstrated significant alteration in the expression of apoptosis regulatory genes caspase-8, cytochrome-c, caspase-9, and caspase- 3 in EAC grown in all CMFPJ treated groups (Fig. 4). The expression of the genes was in rising trend with the increasing amount of CMFPJ. CMFPJ increased the expression of caspase-8 in a dose-dependent manner by 1.42, 1.79, and 2.58 fold with 25, 50, and 100 mg/kg, respectively (Fig. 4). As shown in Fig. 4, at 25, 50, and 100 mg/kg dose of CMFPJ increased expression of cytochrome- c by 1.29, 1.90, and 2.49 fold respectively. The expression of caspase-9 was amplified by 1.45, 1.83, and 2.87 fold at 25, 50, and 100 mg/kg CMFPJ respectively (Fig. 4). And the expression of caspase-3 was increased by 1.77, 2.63, and 4.21 fold at 25, 50, and 100 mg/kg CMFPJ respectively (Fig. 4).
Fig. 4.
Effect of CMFPJ on expression of apoptotic markers (A) caspase-8, (B) cytochrome-c, (C) caspase-9, and (D) caspase-3 in EAC. CMFPJ significantly increased the expression of caspase-8, cytochrome-c, caspase-9, and caspase-3 compared to control. Significance levels were determined and set at *p< 0.05, **p < 0.01, and ***p < 0.001. The results are represented as mean ± SD (n = 3).
DISCUSSION
Nowadays, phytochemicals are of greater interest in alternative cancer therapy from the points of less toxicity and cost benefit. The CMFPJ contained elevated amount of lectin, flavonoids, and polyphenols. However, in this study, we showed in vitro and in vivo cytotoxic activity of the CMFPJ in dose-dependent manner.
Lectins are ubiquitous protein with similar biochemical properties performing different functions and are profusely found in seeds and fruits pulp. Lectin was attached to the carbohydrate moieties on the surface of mouse erythrocytes and agglutinated them (19). The affinity of lectins towards cancer cell is higher than that of healthy cells (20). Thereafter, lectin exhibited EAC growth inhibition by altering cell cycle, inducing non-apoptotic G1-phase accumulation mechanisms, G2/M phase cell cycle arrest, and apoptosis (21). According to a previous study, lectin from Pisum sativum seed reduced the EAC cell growth by 44 and 63% at 1.4 mg/kg/day and 2.8 mg/kg/day respectively (22).
Moreover, plants rich in polyphenols are known to be linked to decrease risk of developing cancer through various mechanisms, including apoptosis (23). The phenolic compound of CMFPJ induced oxidative stress on EAC, leading to cell cycle arrest and apoptosis (22). Besides, the flavonoids compacted cellular viability through activation of caspase mediated pathways (24). A similar study reported that fraction of Morus alba rich in polyphenols and flavonoids showed anti-proliferation of EAC by 70.20% at 100 mg/kg/day (17). In our case, we found EAC growth inhibition by 19.53, 49.2, and 68.9% at 25, 50, and 100 mg/kg CMFPJ respectively.
We found increased expression of caspase-8, cytochrome- c, caspase-9, and caspase-3 genes. According to the results of altered gene expression, both of the extrinsic pathway and the intrinsic pathway are the probable pathways through which CMFPJ induced apoptosis in EAC cell. Usually, due to ligand binding to exact receptors, the death-inducing signalling complex (DISC) forms and activates caspase-8 in the extrinsic pathway (25). In the intrinsic pathway, mitochondria discharge cytochrome-c which participate in the formation of apoptosome and lead to caspase-9 activation (25). The activated caspase-8 either directly cleaves effector caspase or cleaves Bid which finally provoke mitochondria to release cytochrome-c (25). Then cytochrome-c participates in apoptosome formation with the accumulation of procaspase-9 and apoptotic protease activating factor 1 (APAF1) to activate caspase-9 (25). Subsequently, caspase-8 and caspase-9 induce activation of downstream caspase-3 (25), the key effector in apoptosis which acts by constrained proteolysis of the structural proteins, including cytokeratins, PARP, and nuclear protein NuMA, and leads to apoptosis (16).
However, the inhibition of EAC growth and alteration of caspase pathways possibly were due to the combinatorial effect of lectin, flavonoids, and polyphenols of CMFPJ.
ACKNOWLEDGMENTS
We are thankful to Mr. Ruhul Amin [Senior Scientific Officer, Bangladesh Council of Scientific and Industrial Research (BCSIR), Rajshahi Division, Rajshahi, Bangladesh] as he gave us an opportunity to measure RNA quantity in his laboratory.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest.
REFERENCES
- 1.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Choi KC. Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol Res. 2013;29:229–234. doi: 10.5487/TR.2013.29.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fingrut O, Flescher E. Plant stress hormones suppress the proliferation and induce apoptosis in human cancer cells. Leukemia. 2002;16:608–616. doi: 10.1038/sj.leu.2402419. [DOI] [PubMed] [Google Scholar]
- 4.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer DL, Huey PF. Coumarins of Citrus macroptera. Phytochemistry. 1973;12:3011–3013. doi: 10.1016/0031-9422(73)80536-9. [DOI] [Google Scholar]
- 7.Abbott IA, Leakey RR, Elevitch CR. Traditional Trees of Pacific Islands: Their Culture, Environment, and Use (1st edition) Permanent Agriculture Resources; Holualoa, Hawaii, USA: 2006. pp. 283–317. [Google Scholar]
- 8.Paul S, Islam M, Tanvir EM, Ahmed R, Das S, Rumpa NE, Hossen MS, Parvez M, Gan SH, Khalil MI. Satkara (Citrus macroptera) fruit protects against acetaminophen-induced hepatorenal toxicity in rats. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/9470954. 9470954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik SK, Chaudhury R. The cryopreservation of embryonic axes of two wild and endangered Citrus species. Plant Genet Resourc. 2006;4:204–209. doi: 10.1079/PGR2006124. [DOI] [Google Scholar]
- 10.Rahmatullah M, Khatun MA, Morshed N, Neogi PK, Khan SUA, Hossan S, Mahal MJ, Jahan R. A randomized survey of medicinal plants used by folk medicinal healers of Sylhet Division, Bangladesh. Adv Nat Appl Sci. 2010;4:52–62. [Google Scholar]
- 11.Uddin N, Hasan MR, Hossain MM, Hasan MM, Roy A, Islam T, Hossain MM, Faruque A, Rana MS. Antioxidant, brine shrimp lethality and antimicrobial activities of methanol and ethyl-acetate extracts of Citrus macroptera Montr. fruit using in vitro assay models. Br J Pharm Res. 2014;4:1725–1738. doi: 10.9734/BJPR/2014/11235. [DOI] [Google Scholar]
- 12.Paul S, Hossen MS, Tanvir EM, Islam MA, Afroz R, Ahmmed I, Saha M, Gan SH, Khalil MI. Antioxidant properties of Citrus macroptera fruit and its in vivo effects on the liver, kidney and pancreas in wistar rats. Int J Pharmacol. 2015;11:899–909. doi: 10.3923/ijp.2015.899.909. [DOI] [Google Scholar]
- 13.Sala A, Recio MDC, Giner RM, Máñez S, Tournier H, Schinella G, Ríos JL. Anti-inflammatory and antioxidant properties of Helichrysum italicum. J Pharm Pharmacol. 2002;54:365–371. doi: 10.1211/0022357021778600. [DOI] [PubMed] [Google Scholar]
- 14.Hyun JH, Kang JI, Kim SC, Kim E, Kang JH, Kwon JM, Park DB, Lee YJ, Yoo ES, Kang HK. The effects of Crinum asiaticum on the apoptosis induction and the reversal of multidrug resistance in HL-60/MX2. Toxicol Res. 2008;24:29–36. doi: 10.5487/TR.2008.24.1.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correia MT, Coelho LC. Purification of a glucose/mannose specific lectin, isoform 1, from seeds of Cratylia mollis mart. (Camaratu bean) Appl Biochem Biotechnol. 1995;55:261–273. doi: 10.1007/BF02786865. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mamun MA, Husna J, Khatun M, Hasan R, Kamruzzaman M, Hoque KM, Reza MA, Ferdousi Z. Assessment of antioxidant, anticancer and antimicrobial activity of two vegetable species of Amaranthus in Bangladesh. BMC Complement Altern Med. 2016;16:157. doi: 10.1186/s12906-016-1130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam AK, Hossain AS, Khan MA, Kabir SR, Reza MA, Rahman MM, Islam MS, Rahman MAA, Rashid M, Sadik MG. The antioxidative fraction of white mulberry induces apoptosis through regulation of p53 and NFκB in EAC cells. PLoS ONE. 2016;11:e0167536. doi: 10.1371/journal.pone.0167536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sa DJ, Lee EJ, Yoo BS. Apoptosis induction by menadione in human promyelocytic leukemia HL-60 cells. Toxicol Res. 2009;25:113–118. doi: 10.5487/TR.2009.25.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam SK, Ng TB. Lectins: production and practical applications. Appl Microbiol Biotechnol. 2011;89:45–55. doi: 10.1007/s00253-010-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZT, Peng H, Li CY, Liu JJ, Zhou TT, Yan YF, Li Y, Bao JK. Polygonatum cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis via a caspase-dependent pathway as compared to Ophiopogon japonicus lectin. Phytomedicine. 2010;18:25–31. doi: 10.1016/j.phymed.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 21.De Mejía EG, Prisecaru VI. Lectins as bioactive plant proteins: a potential in cancer treatment. Crit Rev Food Sci Nutr. 2005;45:425–445. doi: 10.1080/10408390591034445. [DOI] [PubMed] [Google Scholar]
- 22.Kabir SR, Nabi MM, Haque A, Zaman RU, Mahmud ZH, Reza MA. Pea lectin inhibits growth of Ehrlich ascites carcinoma cells by inducing apoptosis and G2/M cell cycle arrest in vivo in mice. Phytomedicine. 2013;20:1288–1296. doi: 10.1016/j.phymed.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Islam S, Nasrin S, Khan MA, Hossain AS, Islam F, Khandokhar P, Mollah MN, Rashid M, Sadik G, Rahman MA, Alam AH. Evaluation of antioxidant and anticancer properties of the seed extracts of Syzygium fruticosum Roxb. growing in Rajshahi, Bangladesh. BMC Complement Altern Med. 2013;13:142. doi: 10.1186/1472-6882-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pai KSR, Srilatha P, Suryakant K, Setty MM, Nayak PG, Rao CM, Baliga MS. Anticancer activity of Berberis aristata in Ehrlich ascites carcinoma-bearing mice: a preliminary study. Pharm Biol. 2012;50:270–277. doi: 10.3109/13880209.2011.599035. [DOI] [PubMed] [Google Scholar]
- 25.Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V. Role of apoptosis in disease. Aging (Albany NY) 2012;4:330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]