Abstract
The epithelium of the kidney collecting duct (CD) is composed mainly of two different types of cells with distinct and complementary functions. CD principal cells (PCs) traditionally have been considered to have a major role in Na+ and water regulation, while intercalated cells (ICs) were thought to largely modulate acid-base homeostasis. In recent years, our understanding of IC function has significantly improved due to new research findings. Thus, we now have a new model for CD transport that integrates mechanisms of salt and water reabsorption, K+ homeostasis and acid-base status between PCs and ICs. There are three main types of IC (Types A, B and “non-A, non-B”), which first appear in the late distal convoluted tubule (DCT) or in the connecting segment (CNT) in a species-dependent manner. ICs can be detected in CD from cortex to the initial part of the inner medulla, although some transport proteins that are key components of ICs are also present in medullary CD, cells considered inner medullary (IMCDs). Of the three types of ICs, each has a distinct morphology and expresses different complements of membrane transport proteins that translate into very different functions in homeostasis and contributions to CD luminal pro-urine composition. This review includes recent discoveries in IC intracellular and paracrine signaling that contributes to acid-base regulation as well as Na+, Cl−, K+ and Ca2+ homeostasis. Thus, these new findings highlight the potential role of ICs as targets for potential hypertension treatments.
1. Introduction
Kidney ICs in the CD were first identified by their morphology which is reminiscent of other epithelial acid-secreting cells. Unfortunately, ICs were difficult to purify in culture initially, as compared to the neighboring PCs, and as a result knowledge of IC function s considerably less than that of other kidney epithelial cell types. The CD and CNT (or “connecting segment”, depending on the species) arise from the mesonephric kidney or Wolffian duct, which also gives origin to the male excurrent duct (31). Initially, the CD and CNT were not considered part of the classical nephron, and were thought of as not having a real function (85, 124). As the transport processes in the distal nephron were characterized and with the identification of individual membrane transport proteins in ICs, such as the vacuolar H+-ATPase (V-ATPase), the kidney isoform of anion exchanger 1 (kAE1) and the Cl−/HCO3−pendrin (SLC26A4), the critical role of ICs in acid-base regulation was confirmed. A salient feature of the CD “salt and pepper” epithelium is that it contains three types of ICs that coordinate their function with PCs (72, 115). As in any epithelium, the plasma membrane of PCs and ICs organize into apical and basolateral functional domains that develop distinct membrane lipid and transport protein compositions. Recently identified paracrine signals make it possible for the different CD cell types to coordinate their function into coordinated CD transport processes (49). Within the mammalian kidney parenchyma, ICs are detected in the CNT, cortical CD (CCD) as well as in the outer medulla (OMCD), and in some species in the outermost inner medullary CD (IMCD)(reviewed in (75)).
ICs are indispensable to the proper functioning of whole organism acid-base balance and K+ homeostasis. These cells are able to eliminate non-volatile acid (also called “fixed acid”) generated by metabolism and dietary intake (reviewed in (4)). The fixed acid equivalents represent chemical forms of acid that are not able to be excreted via the lungs. In a human consuming an omnivore diet, the kidney contributes to the net secretion of approximately 70 mEq of non-volatile acid per day (54). The majority of the kidney’s contributions to acid-base homeostasis takes place in the proximal tubule with the recovery of filtered HCO3− using a favorable Na+ gradient (via the Na+/H+ exchanger NHE3)(54). On the other hand, in the CD of animals consuming an omnivore diet, ICs are able to adapt and generate new HCO3−. This base-equivalent generation takes place thanks to the hydration of CO2 by carbonic anhydrase II (CAII). This reaction generates H2CO3 that rapidly results in the generation of H+, which is in turn pumped across the apical plasma membrane, while the HCO3− is transported from the IC cytosol into the interstitial fluid/blood (53). This ATP-mediated apical acid secretion into the CD lumen is greatly facilitated by the presence of luminal buffer such as NH3 (4). Therefore, the CNT and CD rely mostly on ICs to reabsorb residual luminal HCO3−. In addition, ICs participate in the coordinated luminal excretion of NH3/ NH4+, a topic reviewed in detail elsewhere (140). We address the interaction of acid-transport proteins and NH3/ NH4+ transport proteins below.
Dysfunction of ICs was later found be an important cause of distal (type 1), type 4, and mixed renal tubular acidoses (RTAs)(75). Of note, in clinical practice, IC dysfunction leading to metabolic acidosis is not as immediately evident as dysregulation of PCs. This is exemplified by the fact that clinicians have identified clear work-up pathways in patients suspected of having diabetes insipidus (DI) or the syndrome of inappropriate anti-diuretic hormone (SIADH) secretion (33). However, laboratory work-ups of IC disorders of metabolic acidosis are rarely performed in the clinic. Perhaps this lack of clinical interest results due to the masking compensatory effects of lung and bone in preventing acidemia, as well as the increased buffering capacity in the CD lumen generated by proximal kidney segments.
Sensors of acid-base status and of other electrolytes have been characterized in ICs. For example, our group and others found evidence of the role of the HCO3− -activated soluble adenylyl cyclase (sAC) in the regulation of H+-secreting pathways in ICs (47, 99), while others revealed that the G-protein coupled receptor 4 (GPR4) is also needed for IC acid-mediated secretion (126). In addition, kinases such as PKA or the metabolic sensor AMP-activated protein kinase (AMPK) have been identified as key regulators of IC function (47). Moreover, ICs are now also recognized as key participants in not only Cl− but also Na+ reabsorption. That ICs are important for intravascular volume preservation was established with the identification first of the HCO3−/Cl− exchanger pendrin and later of the Na+-dependent CI7HCO3− exchanger (NDCBE) in B-ICs (80) which work in concert to absorb NaCl in an electroneutral fashion. It is truly fascinating that it has been shown that that ICs regulate their intracellular volume via the V-ATPase, a mechanism independent of Na+/K+-ATPase (Na,K-ATPase) function (24). Moreover, the functional integrity of the CD epithelium depends on V-ATPase function in these unusual cells (29). This concept was clearly demonstrated relatively recently when V-ATPase function to provide the necessary driving force for CD NaCl transepithelial transport and for the protection of intravascular volume in concert with PCs (Fig. 1; reviewed by Eladari et al. (35)).
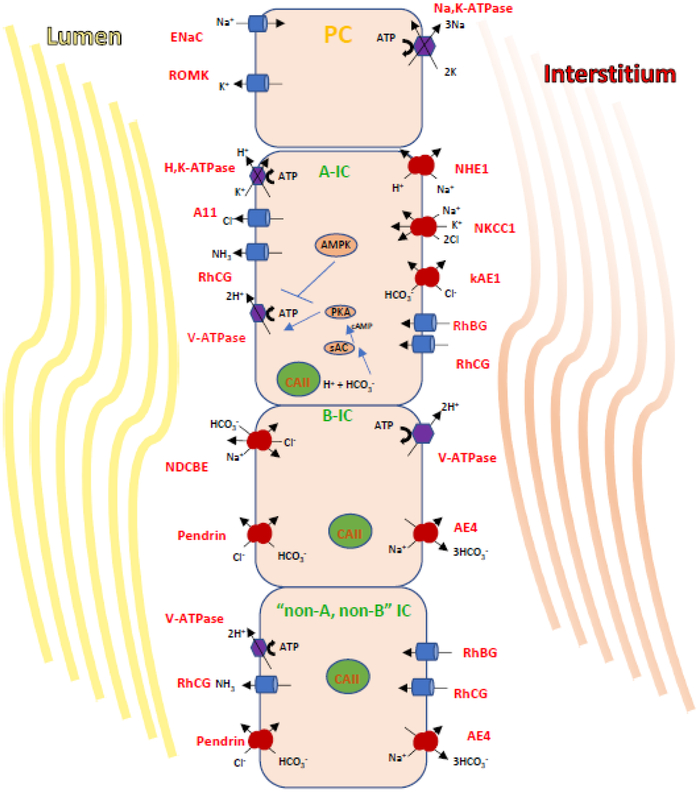
Figure 1. Transport processes and regulatory mechanisms in A- and B- and non-A, non-B IC.
A) This diagram illustrates the major transport proteins that define three CD cell types: PCs which expresse ENaC and ROMK; the acid-secreting A-IC; the HCO3--secreting Cl--reabsorbing type B-IC. In cortical and OMCD, A-ICs express apically the V-ATPase and the H+/K+-ATPase and AE1 (kidney isoform) at their basolateral membrane. The B-ICs express pendrin apically as well a Na+-driven Cl–/HCO3–exchanger called NDCBE. Together NDCBE and pendrin reabsorb NaCL in an electroneutral mode, while Na+ exits the B-Ics via AE4, and the pathway of basolateral Cl- exit is still unclear. In B-ICs the basolateral V-ATPase rather than the Na,K-ATPase, generates the potential to reabsorb luminal NaCl.
B) The HCO3- sensor sAC and its downstream effector PKA work in concert to activate V-ATPase at the apical membrane. This activation is counteracted by the metabolic sensor AMPK, an enzyme upregulated in response to ischemia or metabolic or increased cellular [AMP]/[ATP]. AMPK then mediates the downregulation of V-ATPase.
In this review we address the types, morphology, and function of the different types of ICs, as well as new and recently identified IC protein “markers” described in recent publications. We will address ICs role in Na+ reabsorption, and the potential role of ICs in the regulation of blood pressure as well as K+ secretion and reabsorption. The coordinated kidney segment responses to acid-base disturbances in health and disease are reviewed by Wagner in another chapter of this Seminars in Nephrology issue. In addition, ICs are important for the innate immune response and they express multiple defensins, including neutrophil gelatinase-associated lipocalin (NGAL) and RNAse7, and for example, patients with RNAse7 deficiency have a with higher incidence of urinary tract infections (88, 125) (reviewed in (15)). For example, a 2015 study showed a novel role for ICs in recruiting neutrophils to the medulla in response to UDP-glucose a damage associated molecule (10). The upregulation of inflammatory cytokines was shown to be mediated via the ERK pathway using MDCK-C11 cells in culture as a model for ICs. We hope that our compilation will encourage continued constructive discussions on these still understudied yet fascinating cells.
2. Intercalated Cells Types, their Plasticity and Novel Markers
As opposed to the uniformity of a single characteristic cell type per kidney tubule segment, the CD has both PC and three main types of ICs (64, 85). It follows that up until relatively recently an obstacle to the study of ICs has been their heterogeneity and the relative difficulty of isolation within the CD epithelium as they are dispersed in the CD epithelium amongst the more abundant PCs. The ratio of PCs to ICs varies among species and individuals and ranges from 2:1 to 3:1 in cortical CD (81, 86). Three types of ICs are traditionally identified in the CNT/CD (reviewed in (74)): Type A (A-ICs or “α“), Type B (B-ICs or “β“), and the “non-A, non-B” ICs (Figure 1). In some studies A-ICs are referred to as “α”- and “β”-IC, especially in studies using rabbit CD (86). More elegant and complete morphological descriptions of IC types are presented elsewhere (74, 86). This morphological classification was upheld when functional studies showed that each IC type had specific transport characteristics.
Classically, cells expressing IC markers can be detected in the mammalian cortical CNT, CD (CCD) and the outer medullary CD (OMCD) as well as in the outermost inner medullary CD (IMCD). More specifically, B-IC cells are predominantly detected in the CCD while A-ICs are more abundant in the CNT and OMCD (27, 90). This tubular histological composition matches the functional processes. For example, while the CCD can secrete or reabsorb HCO3−, the OMCD with a majority of A-ICs functions to reabsorb the HCO3− (reviewed in (75)).
At present, experts in the field classify ICs based on subcellular localization of the kidney isoform of kAE1, the multisubunit V-ATPase (7, 21, 74, 138), and expression of pendrin (138). The A-ICs function in urinary acidification, lack pendrin expression, and express V-ATPase at their apical membrane and kAE1 at their basolateral membrane. In contrast, ICs responsible for urinary HCO3− secretion (which are more abundant in herbivore animals) express pendrin at their apical membrane. These HCO3−-secreting cells are further classified as B-ICs and “non-A, non-B” ICs. The B-ICs express basolateral V-ATPase while non-A, non-B ICs express both the V-ATPase and pendrin apically and are located in the CNT (67, 86). All IC types have abundant cytosolic carbonic anhydrase II (CAII), an enzyme that facilitates the rapid, reversible hydration of CO2 (118). CA helps generate HCO3− and H+. Table I summarizes the transport protein markers that are classically used to classify ICs types.
TABLE I. Relevant Proteins Expressed in Kidney Intercalated Cells (adapted from (74)).
The differential localization patterns pertaining to the transport proteins listed in bold and italics identify a combination of key markers that aid in the classification of the different intercalated cell subtypes.
| Intercalated Cell Type | Type A | Type B | Non-A, non-B | |
|---|---|---|---|---|
| Protein Expressed | ||||
| Carbonic anhydrase II | apical | cytoplasmic | cytoplasmic | |
| V-ATPase | apical | basolateral | apical and diffuse vesicular | |
| AE1 | basolateral | |||
| c-Kit | basolateral | |||
| Pendrin (Slc26a4) | apical | apical | ||
| H+,K+-ATPase | apical | apical ? | ||
| AE4 | basolateral | |||
| RhBG | basolateral | basolateral | ||
| RhCG | apical | apical | ||
| NDCBE (Slc4a8) | apical | |||
| Slc4a11 | apical |
A further difficulty in IC research has been the paucity of immortalized cell lines that replicate all the different ICs phenotypes in culture. Cell models that have been used successfully by several groups include the rabbit Clone-C cells, the mouse OMCDis, and the canine MDCK.C11 (34, 44, 50). Clone-C cells are particularly relevant because they can be induced to switch IC phenotype (from B-to A-IC) by changing the density of the cell culture conditions (3, 129). Of note, many very relevant studies that have uncovered important regulatory pathways in ICs, such as pH sensing or the regulated V-ATPase cellular traffic have been performed in cells of IMCD origin such as the mIMCD cell line 3 (119, 127). With the detection of novel IC markers, primary preparations of ICs are now possible (26).
• Type A ICs:
The A-IC type is most abundant in the OMCD (outer stripe of the OMCD, specifically) in most species (7, 85, 86). The number of ICs, and the expression of kAE1 and the V-ATPase are upregulated during metabolic acidosis in A-ICs (13). These cells lack a cilium and display numerous apical microplicae, which become more abundant when stimulated by various agonists such as cAMP (101). By electron microscopy it is also easy to identify A-ICs by the position of their mitochondria near the apical pole (78, 86). These cells secrete H+ into the pro-urine and lack pendrin expression. The secretion of H+ equivalents into the urine can occur via either the V-ATPase or the H+/K+-ATPase (H,K-ATPase), both pumps being expressed at the apical membrane (21, 57, 83). In addition, A-ICs express the anion exchanger kAE1 (solute carrier family 4 member 1 (SLC4A1)) basolaterally (Figure 1)(7). An important fact about these cells is that by secreting H+ into the pro-urine downstream of CAII, they generate intracellular HCO3−, which is reabsorbed into the interstitium by kAE1, in exchange for Cl−. In these cells, the V-ATPase can be detected in subapical vesicles or tubulovesicular structures. These V-ATPase complexes are so abundant in this cell type that they form the cytosolic vesicular coat and have been described as “rod-shaped” particles (20, 22).
The second pathway to H+ extrusion in A-ICs in the CD is the H,K-ATPase. These pumps can have two different alpha subunit isoforms: HKα1 (gastric) or HKα2 (colonic). The A-ICs from HKα1,2– null mice had significantly slower H+ extrusion when compared to A-ICs cells from HKα1-null or HKα2-null mice (83), although the lack of either α subunit had an effect on the rate of H+ extrusion from both A- and B-ICs but not the acid-base status of the animal (48). The A-ICs actively reabsorb K+ via H,K-ATPases, and undergo hypertrophy when animals are fed a low K+ diet. However, the contribution of each of the α isoforms in kidney K+ and H+ handling remains to be determined, as the null mice for either the α-1 or α-2 subunits did not have significant changes in acid-base status or K+ excretion (83). A relatively new finding is the expression of the Cl−/HCO3− exchanger SLC26A7 basolaterally in A-ICs (12, 144) where it co-localizes with kAE1. The functional relevance of having this second anion exchanger at the same domain as kAE1 may be that SLC26A7 is targeted to the basolateral A-IC membrane in the setting of hypertonicity and K+ depletion (144).
• Type A ICs: K+ secretion
Changes in plasma [K+] also influence A-ICs via high conductance BK (or maxi-K) channels. The BK channel is expressed in A-ICs (92), especially in animals fed a high K+ diet. These BK channels are activated by depolarization, stretch via increased luminal flow, rises in intracellular Ca2+ increases, and cell swelling triggered by hypoosmotic stress (reviewed in (142)). Mineralocorticoids may also regulate K+ secretion in ICs. With hyperkalemia, aldosterone is produced and activates the epithelial Na+ channel (ENaC) via the mineralocorticoid receptor (MR). This ENaC activation decreases the luminal voltage, which promotes K+ secretion via the kidney outer medullary small-conductance K+ channel (ROMK) in PCs, and perhaps the BK channel in ICs (41, 96). One study in rabbits showed that aldosterone did not contribute to BK channel expression and/or activity in response to high K+ diet (36). BK channels are regulated by the with-no-lysine kinase 4 (WNK4) in a phosphorylation-dependent manner (123, 148). In a later study performed in a cell line with characteristics of ICs, WNK4 was shown to downregulate BK channel activity in part by increasing channel ubiquitination and degradation (139). Please, see the “Endocrine regulation of ICs” below for further discussion of MR regulation of CD and IC function.
• B-ICs and Non-A non-B ICs: base excretion in alkalosis and NaCl reabsorption
The B-ICs are responsible for the secretion of OH− equivalents into the pro-urine and are especially important in settings of chronic dietary alkali-load or metabolic alkalosis. Traditionally, B-ICs were identified by the expression of the Cl−/HCO3− exchanger pendrin at their apical membrane and the V-ATPase at their basolateral membrane (21, 113)(Table I). Pendrin is also expressed in the epithelium of the inner ear (38) and thyroid gland (112, 113). Pendrin does not transport some anions, such as sulfate (37), but can transport I− (121), and ICs it acts as a Cl−/HCO3− exchanger (38, 112). Pendred described the clinical presentation of this disorder as the combination of deafness and goiter (91, 104). Curiously, under baseline conditions these patients and pendrin-null mice do not have any obvious kidney pathologies (71, 113), thus highlighting the differential roles that pendrin plays in the different organs. Upon HCO3− administration, which enhances pendrin expression in wild-type mice, the pendrin-null mice developed a severe metabolic alkalosis, thus suggesting a critical role for this transport protein in the protection against metabolic alkalosis (131, 136).
Although the mechanisms of Na+ reabsorption in the kidney CD showed a role of ENaC (42) the reabsorption of Cl− in this segment was originally attributed to a paracellular pathway, although there were some studies that indicated the presence of a transcellular Cl− absorption pathway (116) (reviewed in (35, 138)). However once pendrin was identified in the B-ICs their critical role in this transcellular pathway reabsorption became clear when pendrin was identified in these cells (113), especially when aldosterone and angiotensin II were shown to increase its levels (90, 103). Given it’s regulation by the RAAS system and involvement in salt resorption this transport protein is now considered a potential culprit in the pathogenesis of hypertension and also a potential candidate target for the treatment of this highly prevalent disease (76, 131). This role is illustrated in pendrin-null mice that were resistant to mineralocorticoid-induced hypertension and in turn became hypotensive when fed a low Na+ diet (71, 113). Pendrin levels inversely related to urinary [Cl−] in various clinical situations, such as in the use of loop diuretics (131). On the other hand, metabolic acidosis induced by acetazolamide or NH4SO4 reduced the levels of pendrin despite low Cl− levels in the urine (51). Recently, some studies have shown that this exchanger may contribute to the renal regulation of blood pressure via a kidney-specific mechanism that does not include circulating aldosterone (69), with angiotensin II-mediated pendrin up-regulation and subcellular localization changes occurring via the angiotensin 1a receptor (130). It is now clear that pendrin-mediated Cl− uptake leads to vascular volume expansion (69), and more importantly, its deletion in B-ICs decreased ENaC function in PCs (69, 102). (69, 102). Some studies have also shown a role for luminal [HCO3−] and/or pH changes as contributors to this signaling (102).
Both B-IC and non-A, non-B ICs express pendrin at their apical pole, although in two distinct cellular domains: at the membrane and in sub-apical intracellular vesicles (113, 135). Higher levels of pendrin at the apical plasma membrane is characteristic of non-A-non-B ICs under basal conditions, suggesting higher levels of anion exchange under baseline conditions in these cells that are more abundant in the CNT (68). The specificity in subcellular pendrin distribution suggests that it is likely regulated by the flux of vesicular trafficking between the two apical domains, involving signaling by NO and cAMP-dependent mechanisms (120), as well as glycosylation (6, 11). It is also interesting that IC-expressed pendrin controls I− homeostasis (70).
In the mouse kAE4 is localized to the basolateral membrane of CCD B-ICs, although in other species it has been detected in A-ICs and in other subcellular domains (reviewed in (75)). In mice with disruption of this exchanger an overt phenotype was not detected and the B-IC function was not reported (73).
• Plasticity of IC types
The variety of IC cell subtypes remains surprising. For example, there are “non-A, non-B” ICs and other epithelial CNT/CD cells types that express IC markers and PC markers as well. These varied IC types were thought to represent a series of cellular intermediate forms, that could give rise to more differentiated cell types as an adaption to changes in acid-base status. For example, a B-IC could transform into a A-IC as a response to an increased acid load. This hypothetical “intermediate” IC type might express the V-ATPase diffusely in the cytoplasm or both at the apical and basolateral membrane domains (7, 21). The group of Al-Awqati and others have shown evidence that A- and B-ICs may represent different states of cellular differentiation during kidney development, in the setting of maintaining homeostasis, or after tubular injury (3). To illustrate this possibility it was proposed that adaptation to acidosis was not accompanied by increases in total IC number but rather via type-switching of A-ICs to B-ICs (4, 108). Researchers showed that transition is not reversible. The A-ICs population is thus “terminally” differentiated due to the deposition by A-ICs of basolateral extracellular matrix proteins such as hensin (DMBT1) and galectin 3 ((43); reviewed in (4)).
Notch signaling regulates development of primitive epithelia such as that of the CD (62, 115). This cascade is responsible for the “lateral inhibition” process in development, where cells undergo a final differentiation which ultimately leads to drastically different phenotypes from their neighbors. In fact, disruption of Notch signaling increases ICs numbers and leads to nephrogenic DI, due to lack of PCs. Therefore this Notch cascade is required to differentiate PCs from ICs, while IC would develop due to inactivation of this pathway (62). In other studies, rodent IC development was tracked by following the expression of several IC-specific V-ATPase subunits and other markers (19, 40), such as a4 and B1 appear early in the embryonic medulla after the detection of the expression of Foxi1, a forkhead family transcription factor specific to ICs (133). Pendrin-positive epithelial cells are detected in mouse kidney at E14. Moreover, there is uncertainty on whether ICs develop from PCs or vice versa, or whether ICs or PCs can give rise to each other after CD epithelial injury. A mouse strain lacking Foxi1 (16) did not have ICs per se (no V-ATPase or pendrin expression in CD). Their CD epithelial cells instead displayed an undifferentiated phenotype with both PC and IC traits, suggesting that these two cell types arise from the same cell precursor. This proposed undifferentiated cell type expressed aquaporin-2, which is usually detected in PCs exclusively, and CAII, an enzyme abundant in IC cytosol.
Insights into epithelial type-switching can be obtain from studying Li+ toxicity in CD (106). Li+ treatment increases both ratios of ICs to PCs and the expression of cell proliferation markers in both cell types. One mechanism of Li+ toxicity involves reduction of adenylyl cyclase (AC) activity and aquaporin-2 expression and thus leading to nephrogenic DI (89). Indeed one study found that mice deficient in one of the AC isoforms (AC6) had a higher IC-to-PC ratio compared with wild-type mice (106). Yet AC6-deficient mice did not display IC cell proliferation after Li+ treatment. Therefore, the increase in IC-to-PC ratio in AC6-null mice was likely due to conversion of PCs to ICs and thus introduces AC6 as a possible regulator of CD cell type plasticity after injury.
• Novel markers of ICs
Two recent studies of primary collecting duct cells have shed light on potential ways to study primary ICs in vitro. Using single cell digestion, flow cytometry, and single cell RNAseq, Chen et al characterized differential expression in PCs, A-ICs, and B-ICs (26). These authors identified the proto-oncogene c-Kit (tyrosine protein kinase Kit or CD117) as a selective, novel membrane-bound marker for flow cytometric enrichment of A-ICs. They also confirmed the use of Dolichos biflorus agglutinin (DBA) lectin for isolation of PCs in mice (in rats, DBA binds to both proximal tubular cells and PCs)(56), and employed negative selection followed by positive selection with peanut agglutinin (PNA) for B-ICs. This study was the first to demonstrate the differential transcriptome of the different IC populations using single cell RNAseq (26). Bioinformatic analysis of these cell-specific transcriptomes revealed pairings of signaling pathways between PCs and ICs. For example: 1) the receptor c-Kit was predominantly expressed in A-ICs, whereas its ligand, Kitl, was expressed chiefly in PCs; 2) Notch2, was detected chiefly in PCs, whereas its ligand Jagged1 (Jag1) was expressed largely in ICs; 3) Nephronectin (Npnt), a functional ligand of integrin α-8/β-1 in kidney development, was expressed predominantly in PCs and its ligand was expressed predominantly in B-ICs. Whether the expression of the ligand-receptor pairs occurs in the same epithelial domain (apical vs basolateral) remains to be studied. These studies also detected mRNA expression of additional GPRs, transcription factors, and transport proteins in IC types, although the expression of the actual protein products needs to be confirmed.
In the earlier of the two studies by Labarca et al. (77), the DBA lectin and a less stringent digestion protocol were used to isolate mouse cortical cells containing PCs and ICs. After seven days, these cultured primary cells retained functional characteristics of PCs. Although the functions of ICs were not studied, markers of ICs were present and 25% of primary cells at 7 days of culture did not have markers for DCT, CNT, or PCs. These primary cell preparations required a defined media to maintain aldosterone-dependent, amiloride-sensitive Na+ current, a key property of PCs attributable to ENaC function. Taken together, these studies laid the groundwork to test the viability of primary A-ICs and B-ICs in a co-culture system and tested the need for interaction with PCs. Another possible method for primary IC cultures would be supplementation of media with specific PC ligands to maintain IC viability and differentiation.
3. Paracrine signaling in the CD
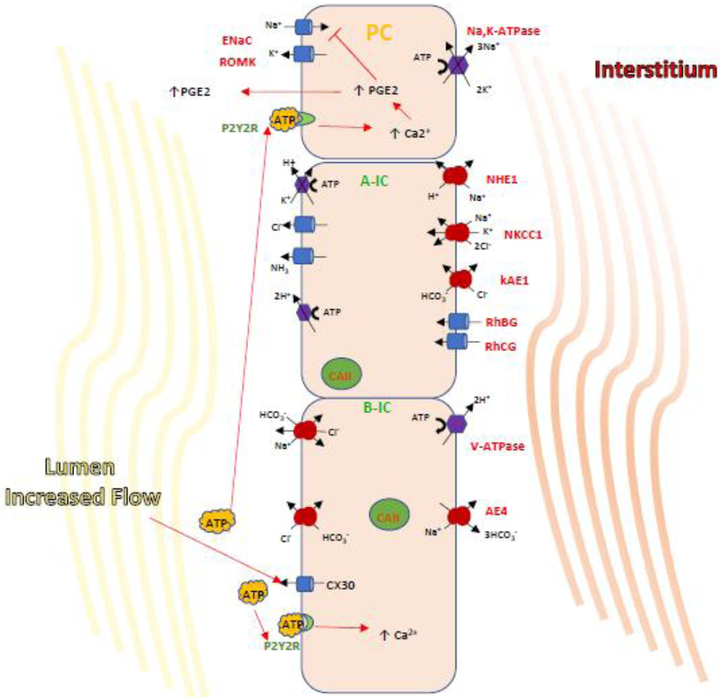
As mentioned earlier, recently identified paracrine signals make it possible for the different CD cell types to coordinate their function (49). ICs help regulate the Na+ reabsorption pathway in PC via ENaC by secreting paracrine factors, such as prostaglandin-E2 (PGE2) (49, 114). This prostaglandin is relevant in Na+ and volume regulation as mPGES-1 (mRNA for PGE2) expression is significantly increased in CD in response to a high-Na+ diet. Moreover, knockout of mPGES-1 impaired urinary PGE2 excretion, decreased the ability to excrete a NaCl load, and the resulted in the subsequent development of hypertension (63). It has also been demonstrated that a second paracrine factor ATP is released by kidney epithelial cells. ATP in CD inhibits salt reabsorption by binding to the purinergic receptor P2Y2, causing a subsequent increase in intracellular Ca2+ and inhibition of ENaC-dependent Na+ absorption in microperfused CCD (Fig. 2)(105). ENaC may be also inhibited through direct interaction of ATP with this channel (84, 105). Release of ATP from B-IC into the CD lumen likely occurs via the ATP permeable hemi-channel connexin-30 (Cx30) in response to mechanical forces generated by increased luminal flow. This hypothesis was confirmed when the Cx30 deficient mice did not release ATP in response to high urine flow generated by a salt-deficient diet and instead developed hypertension when salt loaded (87). Moreover, PGE2 release by isolated microperfused CCDs in response to inhibition of basolateral V-ATPase (localized to B-ICs) required activation of luminal GPR purinergic (ATP) receptors (49). It is likely that this paracrine ATP/PGE2 downregulation of salt absorption by ENaC in PCs is fast acting and helps achieve homeostasis during times of NaCl overload. In this scenario increased NaCl delivery to the CD induces the B-ICs to release ATP into the lumen, thus rapidly inhibiting further salt absorption by the PCs (25). The relevance of these paracrine pathways in blood pressure regulation was confirmed when mice with knock out of P2Y2 developed hypertension (110).
Figure 2. A cascade of paracrine regulation of epithelial transport proteins in the CD.
Paracrine regulation by B-IC occurs via the release of ATP which then binds to purinergic receptors expressed both in IC and PCs to regulate salt and water balance. It has been shown that a high NaCl intake, increasing luminal flow triggers ATP release into the urine by ATP permeable hemi-channel Connexin 30. ATP then binds and activates the luminal purinergic receptor P2Y2, leading to increases in intracellular [Ca2+]and inhibition of ENaC-dependent Na+ absorption. This action could be directly or achieved by triggering PGE2 release which is well established to decreased ENaC activity and abundance. These findings may indicate a pathway for B-IC-mediated inhibition of Na+ reabsorption by PCs in the presence of sudden salt loading leading to increased luminal flow.
4. Acid-base and other electrolyte sensors in ICs and their regulatory cascades
Acid-base transport proteins in ICs respond quickly to pH or [HCO3−] changes in the body. Thus there has been much focus on the characterization of sensing mechanisms for these changes within ICs and the kidney as a whole. One of the pH/HCO3− pathways that we have studied involves the regulation of the V-ATPase. We found that in minutes to hours, the V-ATPase in kidney cells is regulated by kinases such as PKA and the metabolic sensor AMPK-activated protein kinase (AMPK) (8, 9, 47). These findings link regulation of acid-base transporters by metabolic stress such as under ischemia, a regulation that is present in other kidney-like epithelia (5, 52). Therefore V-ATPase phosphorylation has been identified and characterized by our group as a regulatory mechanism downstream of PKA activation, such as with the activation of G-protein coupled receptors (8, 9, 46). The V-ATPase is regulated by a HCO3−sensor, the soluble adenylyl cyclase (sAC), thus linking acid-base status to V-ATPase cellular traffic and changes in IC morphology (100). Acute inhibition of sAC prevented PKA-mediated apical V-ATPase accumulation in ICs (47). Also in ICs V-ATPase and sAC co-localized apically in response to chronic luminal HCO3− delivery to the CD (99). Recently, the Na+-independent SO42− anion transporter SLC26A11 expressed in A-ICs has been identified as a regulator of V-ATPase function (143).
Proton receptors are G protein-coupled receptors (GPR), and GPR4 was first identified and characterized in cell lines with some IC characteristics such as mOMCD1 cells (127). Its relevance to pH sensing was confirmed when GPR4-null mice were shown to have decreased net acid secretion from the kidney. Lack of GPR4 thus resulted in a “non-gap” metabolic acidosis. Thus GPR4 is an important player in linking changes in kidney pH sensing coupling to actions such as H+ extrusion in the CD. Moreover, the non-receptor tyrosine kinase Pyk2 was studied as a potential sensor of pH changes. Pyk2 is expressed in kidney is autophosphorylated by several stimuli including decreased pH (39). Studies also performed in mOMCD1 cells revealed that Pyk2 and downstream effectors increased V-ATPase but not H,K-ATPase in this IC model. Whether these signaling cascades are relevant in B-ICs or other cells expressing the V-ATPase in CD remains to be studied.
Regulation of V-ATPase function in CD ICs is complex and depends not only on urinary buffers but apparently also requires the presence of NH3/NH4+ transporters. NH3 via its ability to become protonated as NH4+ is a critical urinary buffer. As NH4+ remains trapped in the CD tubule lumen a, H+ equivalents are thus excreted in the urine. This H+ extrusion depends on the dual action of the apical V-ATPase or H,K-ATPase especially in CD, where A-ICs express high levels of these pumps. The NH3 transporter RhCG is expressed in a majority of mouse CCD cells, including ICs of the OMCD and IMCD (18)(reviewed in(141)). This transporter is a type of Rh glycoprotein belonging to solute transporter family SLC42. Similarly, another NH3 transporter of the same family called RhBG was detected via immunolabeling in all mouse CNT cells and in cortical CD. RhBG was detected in in A-ICs but not B-ICs intercalated cells in outer and inner medullary CD and early IMCD only ICs. Interestingly, RhBG and RhCG are expressed in basolateral and apical domains of A-ICs in rodent kidney (93, 109)(Fig. 1).
• ICs and sensing Ca2+
The role of the Ca2+-sensing receptor (CaSR) in kidney and the parathyroid glands is relatively well studied. However, the localization of this receptor in kidney CD has been under debate. In a recent study the CaSR was detected in mouse kidney using a combination of in situ hybridization and immunohistochemistry (146). In this study, the CaSR colocalized with AE4 along the basolateral membrane of B-ICs while it was not detected in PCs or A-ICs. A later study confirmed B-IC-specific expression of CaSR transcripts (26). Yasuoka and colleagues also detected that the CaSR in B-ICs increased when the mice were treated with alkali diets, while it decreased when mice were received acidic diets. Perhaps more interestingly, when the mice were treated with neomycin, a CaSR agonist, urinary Ca2+ excretion increased (via action in the basolateral CaSR in the thick ascending limb) and the urine became significantly more acidic. The expression of CaSR was upregulated by dietary alkali loading and downregulated by acid loading. The authors concluded that CaSR signaling may be involved in maintaining acid-base homeostasis after an alkali load by contributing to producing a more alkaline urine. Interestingly, CaSR expression nearly doubled in kidneys of GPR4-null mice, a model afflicted with spontaneous metabolic acidosis, although these animals did not display any disturbances in Ca2+ homeostasis (127). This increase in the CaSR in GPR4 null mice was probably involved in the blunted response of their isolated CD from to additional acid loads. In summary, ICs sensing of Ca2+ and pH are likely synergistic mechanisms contributing to the regulation of acid-base homeostasis.
5. Update on ICs responses to dietary changes and detection of IC-derived exosomes
The Cl− channel CIC-K2/b allows absorption of Cl− into ICs, and its dysfunction leads to the salt-wasting disorder of Bartter's syndrome (type 3). In recent studies performed in isolated mouse CD the activity of CIC-K2/b decreased downregulated in animals fed a high Cl− diet (128). In contrast, the activity of this channel in ICs was not affected by changes in dietary K+ alone, or changes in aldosterone activity. The demonstration that diet changes alone can alter activity of this channel brings CIC-K2/b to the forefront as a potential target for pharmacologic tools to control blood pressure via ICs.
A recent study compared wild-type mice placed on diets with either normal protein levels or with a 70% reduction in protein content to understand the effects of protein restriction on how NH3 is handled in the kidney (79). The protein-restricted mice had decreased NH3 excretion, and yet there was an unexpected increase RhBG expression in OMCD ICs. However, protein restriction in mice with CD-specific RhBG deletion had no effect on NH3 excretion, indicating that this transport protein may be involved in processes other than NH3 handling. Indeed, global RhCG knock-out mice had many adaptive changes to maintain normal NH3 excretion while on a normal protein diet. However, during a protein restriction period NH3 excretion was not altered by RhCG deletion. It appears that RhCG expression is necessary for normal NH3 excretion during basal conditions but is not required for adaptation to a protein restricted diet.
Studies of changes in IC characteristics in response to diets are routinely performed in rodents, and often mouse transgenic models do not replicate human disease. A recent study analyzed pendrin expression in the humans following either acute 1) acid (NH4Cl), 2) alkali (NaHCO3), or NaCl dietary loads by analyzing urinary exosomes from healthy human individuals (97). The study results showed that after alkali loading pendrin expression was rapidly upregulated, within 1 hour, and then normalized by 3 hours. Salt loading induced downregulation of pendrin by 2 hours (nadir at 4 hours). Conversely, after acid loading pendrin levels were rapidly down regulated by 3 hours. Interestingly, patients with inherited dRTA showed reduced levels of pendrin at baseline that did not change with acid loading. These data show that exosomal pendrin is potentially useful as a marker for acute acid-base and volume status changes in humans. Additionally, a similar study from the same group revealed that some V-ATPase subunits (ATP6V1B1 and B2) detected in urinary exosomes can also be correlated to the acid-base status changes, with B1 abundance significantly increased 2-6 hours after acid loading (98).
6. Basolateral membrane biology in ICs.
An interesting recent study showed by immunohistochemistry that albumin can be detected in A-ICs and interstitial kidney cells of mice (61). In addition, albumin abundance in these cells decreased in response to escalating aldosterone levels. Interestingly, by co-localization studies, albumin was not detected in early endosomes, late endosomes, lysosomes, or recycling endosomes. By electron microscopy, albumin was found in round membrane-associated structures in ICs. Assays with labeled albumin revealed that type I MDCK cells were permeable to albumin only on the basolateral side. This study’s results suggest that albumin produced within the kidney interstitium is taken up from the basolateral pole by A-ICs via clathrin- and dynamin-independent pathways. Their findings are also consistent with the clinically-accepted role of albumin as a carrier of non-water soluble substances across the tubule.
The basolateral pole of A-ICs is populated by the NH3/NH4+ transporter RhBG and kAE1(7). A recent study showed that the erythrocyte form of AE1 (eAE1) exists in a metabolically-relevant complex to consisting of RhBG, the spectrin-based skeleton linker ankyrin-G, and the eAE1 cytoplasmic N terminus (23, 95). The presence of kAE1 in a similar complex was confirmed by a recent publication in heterologous expression systems (such as HEK293 cells)(45). Using stop flow spectrofluorometry the authors showed that kAE1 was not functional when the binding site for ankyrin G was modified. In addition, changes in that kAE1 sequence prevented its traffic to the membrane. These findings indicate that a functional RhBG-kAE1 ·ankyrin-G complex akin to the complex found in erythrocytes is also present in ICs. While the exact role of this complex is unclear, it is possible that it could participate in IC responses that maintain acid-base homeostasis.
7. Endocrine Regulation of ICs
The renin-angiotensin-aldosterone system (RAAS) has key regulators of K+, Na+ and volume homeostasis (132), as well as cell metabolism (66, 122). In this section, we will review the findings of several studies that have highlighted the role of the RAAS in ICs, including the role of pro-renin, renin, and angiotensin II, as well as the role of aldosterone and cortisol in Cl− secretion in ICs via MR (123).
The (pro)renin receptor [(P)RR] is also referred to as the ATP6AP2 (ATPase, H+ transporting, lysosomal accessory protein 2)(82). Initially, this protein was identified as an accessory subunit of the V-ATPase. Subsequently, this receptor was studied as a potential signaling molecule in ICs, which express orders of magnitude higher V-ATPase as compared to PCs. Similarly to the classical V-ATPase pump complex, the (P)RR is ubiquitously expressed and yet both are detected at much higher levels in ICs compared to PCs.
The (P)RR receptor ligands are both renin and pro-renin, and binding occurs via a large (P)RR extracellular domain (14, 60, 94). Moreover (P)RR cleavage generates a soluble form of this receptor (s(P)RR)(28, 147). The catalytic efficiency of pro-renin (from PCs) and renin to (P)RR (more abundant in A-ICs) increases with their binding to the (P)RR, and thus increases the conversion of angiotensinogen to angiotensin by four-fold (107, 111). Moreover, in cell culture (P)RR interaction with the V-ATPase is required for pro-renin-induced activation of Erk1/2 (2) and vasopressinmediated V-ATPase activity and cAMP accumulation. However, a recent study examined the association and potential regulatory roles of (P)RR and pro-renin on the V-ATPase (30). This study showed in a mouse model that both Atp6ap2 mRNA and protein co-localized with the V-ATPase in ICs. In addition, when mice were treated with a variety of acid-base and fluid/electrolyte challenges the effects the (P)RR response was not co-regulated with that of the V-ATPase. In the same study, the microperfusion of CCDs with luminal pro-renin did not increase V-ATPase activity. These findings indicated that in this system pro-renin did not play a role in the regulation of the interaction between P(RR) and the V-ATPase. (P)RR dysfunction has been implicated in the pathogenesis of kidney disease induced by systemic disorders such as diabetic nephropathy, hypertension, albuminuria, and preeclampsia. However, pharmacological agents targeting the (P)RR may cause systemic side effects through effects on V-ATPase regulation (32, 58, 59, 65).
The RAAS can stimulate Cl− absorption by several possible mechanisms. Angiotensin II increases net transcellular Cl− absorption across B-ICs via direct stimulation of pendrin and V-ATPase activation (103). Recent work suggests that angiotensin II stimulation of pendrin may require aldosterone, and aldosterone may stimulate pendrin in the setting of hypokalemia (55, 145). The MR receptor is expressed in ICs (1, 123), but in contrast to PCs, ICs lack the enzyme 11 β hydroxysteroid dehydrogenase (11βHSD2), an enzyme that rapidly converts cortisol to the less active cortisone (17). Thus, in PCs the MR is primarily activated by aldosterone, and in ICs, MR activation is predicted to be dominated by cortisol. However, several studies have implicated aldosterone directly or indirectly in IC signaling, e.g. aldosterone-mediated stimulation of pendrin (55, 145). Thus, the ligand for IC MR in a specific physiologic context may be different.
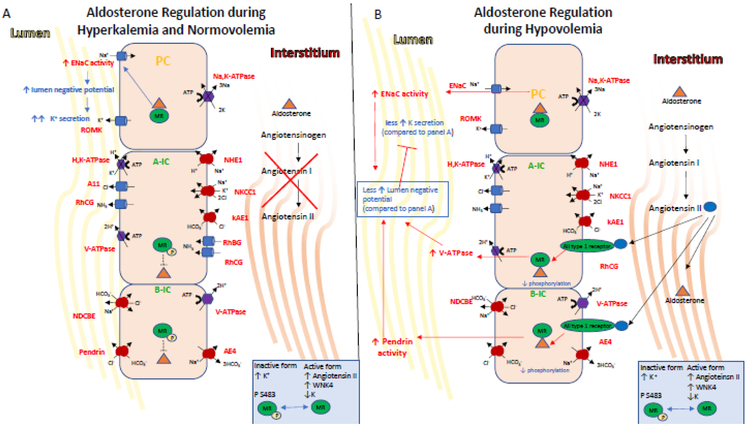
A recent study by Shibata, et al. demonstrated an important physiologic context for MR activation in ICs to explain the aldosterone paradox (123). This paradox refers to the ability of aldosterone and MR activation to respond to two disparate stimuli: volume depletion or hyperkalemia, and yet appropriately respond with either Na+ reabsorption or K+ secretion, respectively. Several potential explanations exist, but ICs may play a role in resolving this paradox (Figure 3). MR in ICs stimulate the V-ATPase and pendrin, and thus, can mediate Cl− reabsorption, an important component of volume repletion. Phosphorylation of the MR can occur at Ser-843, and can inhibit activation by its natural ligands (aldosterone or cortisol). Phosphoryation of MR Ser-843 in kidney is exclusive to ICs. Importantly, while angiotensin II and WNK4 signaling decrease phosphorylated MR, hyperkalemia increases this phosphorylation. It follows that as volume depletion is accompanied by angiotensin II production, aldosterone can activate IC-MR, and can thereby couple aldosterone-mediated Na+ reabsorption by ENaC in PCs with aldosterone/cortisol-mediated Cl− reabsorption by pendrin in B-ICs (Fig. 3). The reabsorption of Cl− limits the negative lumen potential at the apical membrane of PCs, and thereby limits K+ secretion. On the other hand, hyperkalemia promotes phosphorylation of Ser-843 in ICs, and thus aldosterone’s effect on ENaC (without angiotensin II) is primarily to create a driving force for ROMK-mediated K+ secretion rather than a coordinated PC/IC, Na+/CI− reabsorption.
Figure 3. Endocrine regulation of IC transport.
The mineralocorticoid receptor is one of the end targets of the RAAS. These hormones elicit signaling events in the kidney that regulate K+ and salt homeostasis, intravascular volume and cell metabolism.
A) Indirectly during hyperkalemia, aldosterone is secreted and its stimulation of ENaC via MR leads to lumen electronegativity, which promotes K+ secretion via ROMK expressed in PCs and the BK channel (not shown here), which is expressed in ICs. Aldosterone also indirectly increases acid excretion by the kidney as the net lumen-negative transepithelial voltage makes secretion of H+ by the ICs more favorable. Aldosterone, facilitated by the MR receptor, also regulates activity in ICs. Aldosterone action on the MR receptor in ICs is modulated by phosphorylation at Ser-483, a modification that reduces affinity and activation of the receptor for aldosterone. Importantly, hyperkalemia also induces MR phosphorylation at Ser-483, thus inhibiting aldosterone-stimulated V-ATPase in A-ICs and the pendrin in B-ICs. This diminished Cl− reabsorption limits net NaCl reabsorption, and thus allows for aldosterone to promote K+ secretion while limiting volume expansion.
B) On the other hand, Angiotensin II, WNK4 activity and hypokalemia induce decreased levels of phosphorylated MR, allowing aldosterone binding to MR which increases the coordinated action of the V-ATPase and pendrin. This coordinated action increases plasma volume while inhibiting K+ secretion via increase of electroneutral NaCl reabsorption and limiting the lumennegative potential, the driving force for K+ secretion.
Finally, metabolic acidosis, which can be regulated by IC response, increases RAAS signaling (117), while RAAS blockade inhibits H+ excretion even in the setting of acidosis (122)(reviewed in (134)). States of aldosterone excess also create a driving force for H+ excretion via increased ENaC activity and a lumen negative potential (137). It has also been shown that MR dephosphorylation in volume depletion causes aldosterone-dependent increases of transport proteins in ICs such as pendrin and the apical V-ATPase (123). One could envision that even in the setting of acidosis and ammoniagenesis, the lack of these transport proteins would prevent CD IC-mediated acid excretion.
8. Conclusions
ICs were first identified by their morphology and slowly their function as acid-base regulatory cells in the kidney has been established. Individual membrane transport proteins in ICs, such as the V-ATPase, kAE1 and pendrin have helped decipher the molecular mechanisms of CD transport processes. A number of cellular sensors in ICs such as sAC and GPR4 have been uncovered, and their role in kinase cascades such as those of PKA or AMPK have been elucidated. Moreover, the role of ICs critical role as regulators of salt reabsorption and intravascular volume preservation was solidified with the identification of NDCBE in B-ICs and paracrine signaling tying ATP produced by ICs to the inhibition of Na+ reabsorption by PCs. Moreover, the primacy of the V-ATPase in the regulation of IC volume has been firmly accepted. Emerging areas in the study of these interesting cells are their role in the innate immune system, their transport protein responses to dietary changes, and how the ICs function is regulated by their basolateral membrane
Acknowledgments:
This work was supported by the Dean’s Pilot Program of the Keck School of Medicine and the USC/UKRO Kidney Research Center (N.P.S.) and VB received support from the NIH (1R01 DK091565).
Footnotes
We thank Dr. Ken Hallows for his critical review of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, Rossier BC, and Loffing J. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. American journal of physiology Renal physiology 299: F1473–1485, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, and Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Al-Awqati Q Plasticity in epithelial polarity of renal intercalated cells: targeting of the H(+)- ATPase and band 3. Am J Physiol 270: C1571–1580, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Al-Awqati Q Terminal differentiation in epithelia: the role of integrins in hensin polymerization. Annu Rev Physiol 73: 401–412, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Al-bataineh MM, Gong F, Marciszyn AL, Myerburg MM, and Pastor-Soler NM. Regulation of proximal tubule vacuolar H(+)-ATPase by PKA and AMP-activated protein kinase. American journal of physiology Renal physiology 306: F981–995, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alesutan I, Daryadel A, Mohebbi N, Pelzl L, Leibrock C, Voelkl J, Bourgeois S, Dossena S, Nofziger C, Paulmichl M, Wagner CA, and Lang F. Impact of bicarbonate, ammonium chloride, and acetazolamide on hepatic and renal SLC26A4 expression. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 28: 553–558, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Alper SL, Natale J, Gluck S, Lodish HF, and Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proceedings of the National Academy of Sciences of the United States of America 86: 5429–5433, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzamora R, Al-Bataineh MM, Liu W, Gong F, Li H, Thali RF, Joho-Auchli Y, Brunisholz RA, Satlin LM, Neumann D, Hallows KR, and Pastor-Soler NM. AMP-activated protein kinase regulates the vacuolar H+-ATPase via direct phosphorylation of the A subunit (ATP6V1A) in the kidney. American journal of physiology Renal physiology 305: F943–956, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alzamora R, Thali RF, Gong F, Smolak C, Li H, Baty CJ, Bertrand CA, Auchli Y, Brunisholz RA, Neumann D, Hallows KR, and Pastor-Soler NM. PKA regulates vacuolar H+- ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J Biol Chem 285: 24676–24685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azroyan A, Cortez-Retamozo V, Bouley R, Liberman R, Ruan YC, Kiselev E, Jacobson KA, Pittet MJ, Brown D, and Breton S. Renal intercalated cells sense and mediate inflammation via the P2Y14 receptor. PloS one 10: e0121419, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azroyan A, Laghmani K, Crambert G, Mordasini D, Doucet A, and Edwards A. Regulation of pendrin by pH: dependence on glycosylation. The Biochemical journal 434: 61–72, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Barone S, Amlal H, Xu J, Kujala M, Kere J, Petrovic S, and Soleimani M. Differential regulation of basolateral Cl-/HCO3- exchangers SLC26A7 and AE1 in kidney outer medullary collecting duct. Journal of the American Society of Nephrology: JASN 15: 2002–2011, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Bastani B, Purcell H, Hemken P, Trigg D, and Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest 88: 126–136, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJ, Burckle CA, Muller DN, Bader M, Nguyen G, and Danser AH. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. Journal of hypertension 25: 2441–2453, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Becknell B, Schwaderer A, Hains DS, and Spencer JD. Amplifying renal immunity: the role of antimicrobial peptides in pyelonephritis. Nat Rev Nephrol 11: 642–655, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, and Enerback S. Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113: 1560–1570, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bostanjoglo M, Reeves WB, Reilly RF, Velazquez H, Robertson N, Litwack G, Morsing P, Dorup J, Bachmann S, and Ellison DH. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. Journal of the American Society of Nephrology: JASN 9: 1347–1358, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeois S, Bounoure L, Christensen EI, Ramakrishnan SK, Houillier P, Devuyst O, and Wagner CA. Haploinsufficiency of the ammonia transporter Rhcg predisposes to chronic acidosis: Rhcg is critical for apical and basolateral ammonia transport in the mouse collecting duct. J Biol Chem 288: 5518–5529, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breton S and Brown D. Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda) 28: 318–329, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown D, Gluck S, and Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol 105: 1637–1648, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown D, Hirsch S, and Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature 331: 622–624, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Brown D, Weyer P, and Orci L. Nonclathrin-coated vesicles are involved in endocytosis in kidney collecting duct intercalated cells. The Anatomical record 218: 237–242, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, and Tanner MJ. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101: 4180–4188, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, Hentschke M, Zdebik AA, Schwartz GJ, Hubner CA, and Eladari D. Renal intercalated cells are rather energized by a proton than a sodium pump. Proceedings of the National Academy of Sciences of the United States of America 110: 7928–7933, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambrey R and Trepiccione F. Relative roles of principal and intercalated cells in the regulation of sodium balance and blood pressure. Curr Hypertens Rep 17: 538, 2015. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Paunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, and Knepper MA. Reply to Edemir: Physiological regulation and single-cell RNA sequencing. Proceedings of the National Academy of Sciences of the United States of America 115: E351–E352, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clapp WL, Madsen KM, Verlander JW, and Tisher CC. Intercalated cells of the rat inner medullary collecting duct. Kidney international 31: 1080–1087, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, and Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Crayen ML and Thoenes W. Architecture and cell structures in the distal nephron of the rat kidney. Cytobiologie 17: 197–211, 1978. [PubMed] [Google Scholar]
- 30.Daryadel A, Bourgeois S, Figueiredo MF, Gomes Moreira A, Kampik NB, Oberli L, Mohebbi N, Lu X, Meima ME, Danser AH, and Wagner CA. Colocalization of the (Pro)renin Receptor/Atp6ap2 with H+-ATPases in Mouse Kidney but Prorenin Does Not Acutely Regulate Intercalated Cell H+-ATPase Activity. PloS one 11: e0147831, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies JA and Davey MG. Collecting duct morphogenesis. Pediatr Nephrol 13: 535–541, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Deinum J, Tarnow L, van Gool JM, de Bruin RA, Derkx FH, Schalekamp MA, and Parving HH. Plasma renin and prorenin and renin gene variation in patients with insulin-dependent diabetes mellitus and nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 14: 1904–1911, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Dhondup T and Qian Q. Acid-Base and Electrolyte Disorders in Patients with and without Chronic Kidney Disease: An Update. Kidney Dis (Basel) 3: 136–148, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards JC, van Adelsberg J, Rater M, Herzlinger D, Lebowitz J, and al-Awqati Q. Conditional immortalization of bicarbonate-secreting intercalated cells from rabbit. Am J Physiol 263: C521–529, 1992. [DOI] [PubMed] [Google Scholar]
- 35.Eladari D, Chambrey R, and Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, and Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. American journal of physiology Renal physiology 295: F780–788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, and Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nature genetics 17: 411–422, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Everett LA, Morsli H, Wu DK, and Green ED. Expression pattern of the mouse ortholog of the Pendred';s syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc Natl Acad Sci U S A 96: 9727–9732, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher KD, Codina J, Petrovic S, and DuBose TD Jr. Pyk2 regulates H+-ATPase-mediated proton secretion in the outer medullary collecting duct via an ERK1/2 signaling pathway. American journal of physiology Renal physiology 303: F1353–1362, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forgac M Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nature reviews Molecular cell biology 8: 917–929, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Frindt G and Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol 256: F143–151, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Fuller CM, Awayda MS, Arrate MP, Bradford AL, Morris RG, Canessa CM, Rossier BC, and Benos DJ. Cloning of a bovine renal epithelial Na+ channel subunit. Am J Physiol 269: C641–654, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Gao X, Eladari D, Leviel F, Tew BY, Miro-Julia C, Cheema FH, Miller L, Nelson R, Paunescu tG, McKee M, Brown D, and Al-Awqati Q Deletion of hensin/DMBT1 blocks conversion of beta- to alpha-intercalated cells and induces distal renal tubular acidosis. Proceedings of the National Academy of Sciences of the United States of America 107: 21872–21877, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gekle M, Wunsch S, Oberleithner H, and Silbernagl S. Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflugers Archiv: European journal of physiology 428: 157–162, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Genetet S, Ripoche P, Le Van Kim C, Colin Y, and Lopez C. Evidence of a structural and functional ammonium transporter RhBG.anion exchanger 1.ankyrin-G complex in kidney epithelial cells. J Biol Chem 290: 6925–6936, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gidon A, Al-Bataineh MM, Jean-Alphonse FG, Stevenson HP, Watanabe T, Louet C, Khatri A, Calero G, Pastor-Soler NM, Gardella TJ, and Vilardaga JP. Endosomal GPCR signaling turned off by negative feedback actions of PKA and v-ATPase. Nature chemical biology 10: 707–709, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong F, Alzamora R, Smolak C, Li H, Naveed S, Neumann D, Hallows KR, and Pastor- Soler NM. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. American journal of physiology Renal physiology 298: F1162–1169, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenlee MM, Lynch IJ, Gumz ML, Cain BD, and Wingo CS. The renal H,K-ATPases. Current opinion in nephrology and hypertension 19: 478–482, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, and Chambrey R. Renal beta-intercalated cells maintain body fluid and electrolyte balance. The Journal of clinical investigation 123: 4219–4231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guntupalli J, Onuigbo M, Wall S, Alpern RJ, and DuBose TD Jr. Adaptation to low-K+ media increases H(+)-K(+)-ATPase but not H(+)-ATPase-mediated pHi recovery in OMCD1 cells. Am J Physiol 273: C558–571, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Hafner P, Grimaldi R, Capuano P, Capasso G, and Wagner CA. Pendrin in the mouse kidney is primarily regulated by Cl- excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295: C1658–1667, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, and Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. American journal of physiology Cell physiology 296: C672–681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamm LL, Hering-Smith KS, and Vehaskari VM. Control of bicarbonate transport in collecting tubules from normal and remnant kidneys. Am J Physiol 256: F680–687, 1989. [DOI] [PubMed] [Google Scholar]
- 54.Hamm LL, Nakhoul N, and Hering-Smith KS. Acid-Base Homeostasis. Clin J Am Soc Nephrol 10: 2232–2242, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirohama D, Ayuzawa N, Ueda K, Nishimoto M, Kawarazaki W, Watanabe A, Shimosawa T, Marumo T, Shibata S, and Fujita T. Aldosterone Is Essential for Angiotensin II-Induced Upregulation of Pendrin. Journal of the American Society of Nephrology: JASN 29: 57–68, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holthofer H Lectin binding sites in kidney. A comparative study of 14 animal species. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 31: 531–537, 1983. [DOI] [PubMed] [Google Scholar]
- 57.Holtzclaw JD, Grimm PR, and Sansom SC. Intercalated cell BK-alpha/beta4 channels modulate sodium and potassium handling during potassium adaptation. Journal of the American Society of Nephrology: JASN 21: 634–645, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ichihara A, Kaneshiro Y, and Suzuki F. Prorenin receptor blockers: effects on cardiovascular complications of diabetes and hypertension. Expert opinion on investigational drugs 15: 1137–1139, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, and Itoh H. Renin, prorenin and the kidney: a new chapter in an old saga. Journal of nephrology 22: 306–311, 2009. [PubMed] [Google Scholar]
- 60.Jan Danser AH, Batenburg WW, and van Esch JH. Prorenin and the (pro)renin receptor--an update. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 22: 1288–1292, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Jensen TB, Cheema MU, Szymiczek A, Damkier HH, and Praetorius J. Renal type a intercalated cells contain albumin in organelles with aldosterone-regulated abundance. PloS one 10: e0124902, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeong HW, Jeon US, Koo BK, Kim WY, Im SK, Shin J, Cho Y, Kim J, and Kong YY. Inactivation of Notch signaling in the renal collecting duct causes nephrogenic diabetes insipidus in mice. J Clin Invest 119: 3290–3300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jia Z, Zhang A, Zhang H, Dong Z, and Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Kaissling B and Kriz W. Structural analysis of the rabbit kidney. Advances in anatomy, embryology, and cell biology 56: 1–123, 1979. [DOI] [PubMed] [Google Scholar]
- 65.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, and Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney international 70: 641–646, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Karim Z, Szutkowska M, Vernimmen C, and Bichara M. Renal handling of NH3/NH4+: recent concepts. Nephron Physiology 101: p77–81, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Kim YH, Cha JH, Tisher CC, and Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. Journal of the American Society of Nephrology: JASN 10: 1–12, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, and Nielsen S. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. American journal of physiology Renal physiology 283: F744–754, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin W, Verlander JW, Sutliff RL, and Wall Sm. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. American journal of physiology Renal physiology 293: F1314–1324, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Kim YH, Pham TD, Zheng W, Hong S, Baylis C, Pech V, Beierwaltes WH, Farley DB, Braverman LE, Verlander JW, and Wall SM. Role of pendrin in iodide balance: going with the flow. American journal of physiology Renal physiology 297: F1069–1079, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim YH, Verlander JW, Matthews SW, Kurtz I, Shin W, Weiner ID, Everett LA, Green ED, Nielsen S, and Wall SM. Intercalated cell H+/OH- transporter expression is reduced in Slc26a4 null mice. American journal of physiology Renal physiology 289: F1262–1272, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Kleyman TR, Satlin LM, and Hallows KR. Opening lines of communication in the distal nephron. J Clin Invest 123: 4139–4141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, and Ishibashi K. AE4 is a DIDS-sensitive CI(−)/HCO(−)(3) exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283: C1206–1218, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Kriz Wand Kaissling B Structural Organization of the Mammalian Kidney In: SELDIN AND GIEBISCH’S THE KIDNEY: PHYSIOLOGY AND PATHOPHYSIOLOGY, edited by Alpern RJ, Caplan MJ and Moe OW: Elsevier, 2013, p. 595–691. [Google Scholar]
- 75.Kurtz I Molecular mechanisms and regulation of urinary acidification. Compr Physiol 4: 1737–1774, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurtz TW, Al-Bander HA, and Morris RC Jr. "Salt-sensitive" essential hypertension in men.Is the sodium ion alone important? The New England journal of medicine 317: 1043–1048, 1987. [DOI] [PubMed] [Google Scholar]
- 77.Labarca M, Nizar JM, Walczak EM, Dong W, Pao AC, and Bhalla V. Harvest and primary culture of the murine aldosterone-sensitive distal nephron. American journal of physiology Renal physiology 308: F1306–1315, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Latta H, Maunsbach AB, and Madden SC. Cilia in different segments of the rat nephron. The Journal of biophysical and biochemical cytology 11: 248–252, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee HW, Osis G, Handlogten ME, Guo H, Verlander JW, and Weiner ID. Effect of dietary protein restriction on renal ammonia metabolism. American journal of physiology Renal physiology 308: F1463–1473, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, and Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. The Journal of clinical investigation 120: 1627–1635, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu W, Xu S, Woda C, Kim P, Weinbaum S, and Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, and Schagger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998. [DOI] [PubMed] [Google Scholar]
- 83.Lynch IJ, Rudin A, Xia SL, Stow LR, Shull GE, Weiner ID, Cain BD, and Wingo CS. Impaired acid secretion in cortical collecting duct intercalated cells from H-K-ATPase-deficient mice: role of HKalpha isoforms. American journal of physiology Renal physiology 294: F621–627, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Ma HP, Li L, Zhou ZH, Eaton DC, and Warnock DG. ATP masks stretch activation of epithelial sodium channels in A6 distal nephron cells. American journal of physiology Renal physiology 282: F501–505, 2002. [DOI] [PubMed] [Google Scholar]
- 85.Madsen KM and Tisher CC. Structural-functional relationship along the distal nephron. Am J Physiol 250: F1–15, 1986. [DOI] [PubMed] [Google Scholar]
- 86.Madsen KM, Verlander JW, and Tisher CC. Relationship between structure and function in distal tubule and collecting duct. J Electron Microsc Tech 9: 187–208, 1988. [DOI] [PubMed] [Google Scholar]
- 87.Mironova E, Peti-Peterdi J, Bugaj V, and Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem 286: 1054–1060, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, and Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. Journal of the American Society of Nephrology: JASN 14: 2534–2543, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Moeller HB, Rittig S, and Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev 34: 278–301, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohebbi N, Perna A, van der Wijst J, Becker HM, Capasso G, and Wagner CA. Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PloS one 8: e55286, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morgans ME and Trotter WR. Association of congenital deafness with goitre; the nature of the thyroid defect. Lancet 1: 607–609, 1958. [DOI] [PubMed] [Google Scholar]
- 92.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, and Satlin LM. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. American journal of physiology Renal physiology 289: F922–932, 2005. [DOI] [PubMed] [Google Scholar]
- 93.Nakhoul NL and Hamm LL. Non-erythroid Rh glycoproteins: a putative new family of mammalian ammonium transporters. Pflugers Archiv: European journal of physiology 447: 807–812, 2004. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, and Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nicolas V, Le Van Kim C, Gane P, Birkenmeier C, Cartron JP, Colin Y, and Mouro- Chanteloup I. Rh-RhAG/ankyrin-R, a new interaction site between the membrane bilayer and the red cell skeleton, is impaired by Rh(null)-associated mutation. J Biol Chem 278: 25526–25533, 2003. [DOI] [PubMed] [Google Scholar]
- 96.Palmer LG and Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. American journal of physiology Renal physiology 292: F966–973, 2007. [DOI] [PubMed] [Google Scholar]
- 97.Pathare G, Dhayat N, Mohebbi N, Wagner CA, Cheval L, Neuhaus TJ, and Fuster DG. Acute regulated expression of pendrin in human urinary exosomes. Pflugers Archiv: European journal of physiology, 2017. [DOI] [PubMed] [Google Scholar]
- 98.Pathare G, Dhayat NA, Mohebbi N, Wagner CA, Bobulescu IA, Moe OW, and Fuster DG. Changes in V-ATPase subunits of human urinary exosomes reflect the renal response to acute acid/alkali loading and the defects in distal renal tubular acidosis. Kidney international, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paunescu TG, Da Silva N, Russo LM, McKee M, Lu HA, Breton S, and Brown D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol 294: F130–138, 2008. [DOI] [PubMed] [Google Scholar]
- 100.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, and Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. American journal of physiology Renal physiology 298: F643–654, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, and Brown D. Cyclic AMP stimulates apical V-ATPase accumulation, microvillar elongation and proton extrusion in kidney collecting duct A-intercalated cells. American journal of physiology Renal physiology, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, and Wall SM Pendrin modulates ENaC function by changing luminal HCO3. Journal of the American Society of Nephrology: JASN 21: 1928–1941, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pech V, Zheng W, Pham TD, Verlander JW, and Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. Journal of the American Society of Nephrology: JASN 19: 84–91, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pendred V DEAF-MUTISM AND GOITRE. The Lancet 148: 532, 1896. [Google Scholar]
- 105.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, and Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poulsen SB, Kristensen TB, Brooks HL, Kohan DE, Rieg T, and Fenton RA. Role of adenylyl cyclase 6 in the development of lithium-induced nephrogenic diabetes insipidus. JCI Insight 2: e91042, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prieto-Carrasquero MC, Botros FT, Kobori H, and Navar LG. Collecting Duct Renin: A major player in Angiotensin II-dependent Hypertension. Journal of the American Society of Hypertension : JASH 3: 96–104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Purkerson JM, Tsuruoka S, Suter DZ, Nakamori A, and Schwartz GJ. Adaptation to metabolic acidosis and its recovery are associated with changes in anion exchanger distribution and expression in the cortical collecting duct. Kidney international 78: 993–1005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, and Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. Journal of the American Society of Nephrology: JASN 14: 545–554, 2003. [DOI] [PubMed] [Google Scholar]
- 110.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, and Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007. [DOI] [PubMed] [Google Scholar]
- 111.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, and Lalouel JM. Elements of a paracrine tubular reninangiotensin system along the entire nephron. Hypertension 34: 1265–1274, 1999. [DOI] [PubMed] [Google Scholar]
- 112.Royaux IE, Suzuki K, Mori A, Katoh R, Everett LA, Kohn LD, and Green ED. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141: 839–845, 2000. [DOI] [PubMed] [Google Scholar]
- 113.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, and Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proceedings of the National Academy of Sciences of the United States of America 98: 4221–4226, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sakairi Y, Jacobson HR, Noland TD, and Breyer MD. Luminal prostaglandin E receptors regulate salt and water transport in rabbit cortical collecting duct. Am J Physiol 269: F257–265, 1995. [DOI] [PubMed] [Google Scholar]
- 115.Sampogna RV and Al-Awqati Q. Salt and pepper distribution of cell types in the collecting duct. Journal of the American Society of Nephrology: JASN 24: 163–165, 2013. [DOI] [PubMed] [Google Scholar]
- 116.Sansom SC, Weinman EJ, and O’Neil RG. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol 247: F291–302, 1984. [DOI] [PubMed] [Google Scholar]
- 117.Schambelan M, Sebastian A, Katuna BA, and Arteaga E. Adrenocortical hormone secretory response to chronic NH4Cl-induced metabolic acidosis. Am J Physiol 252: E454–460, 1987. [DOI] [PubMed] [Google Scholar]
- 118.Schwartz GJ. Physiology and molecular biology of renal carbonic anhydrase. Journal of nephrology 15 Suppl 5: S61–74, 2002. [PubMed] [Google Scholar]
- 119.Jh Schwartz, Li G, Yang Q, Suri V, Ross JJ, and Alexander EA Role of SNAREs and H+-ATPase in the targeting of proton pump-coated vesicles to collecting duct cell apical membrane. Kidney international 72: 1310–1315, 2007. [DOI] [PubMed] [Google Scholar]
- 120.Scott DA and Karniski LP. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol 278: C207–211, 2000. [DOI] [PubMed] [Google Scholar]
- 121.Scott DA, Wang R, Kreman TM, Sheffield VC, and Karniski LP. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nature genetics 21: 440–443, 1999. [DOI] [PubMed] [Google Scholar]
- 122.Sebastian A, Sutton JM, Hulter HN, Schambelan M, and Poler SM. Effect of mineralocorticoid replacement therapy on renal acid-base homeostasis in adrenalectomized patients. Kidney international 18: 762–773, 1980. [DOI] [PubMed] [Google Scholar]
- 123.Shibata S, Rinehart J, Zhang J, Moeckel G, Castaneda-Bueno M, Stiegler AL, Boggon TJ, Gamba G, and Lifton RP. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith H The Kidney: Structure and function in Health and Disease: Oxford University Press, 1951. [Google Scholar]
- 125.Spencer JD, Schwaderer AL, Dirosario JD, McHugh KM, McGillivary G, Justice SS, Carpenter AR, Baker PB, Harder J, and Hains DS. Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney international 80: 174–180, 2011. [DOI] [PubMed] [Google Scholar]
- 126.Sun X, Stephens L, DuBose TD Jr., and Petrovic S Adaptation by the collecting duct to an exogenous acid load is blunted by deletion of the proton-sensing receptor GPR4. American journal of physiology Renal physiology 309: F120–136, 2015. [DOI] [PubMed] [Google Scholar]
- 127.Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, and Petrovic S. Deletion of the pH sensor GPR4 decreases renal acid excretion. Journal of the American Society of Nephrology: JASN 21: 1745–1755, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tomilin VN, Zaika O, Subramanya AR, and Pochynyuk O. Dietary K(+) and Cl(−) independently regulate basolateral conductance in principal and intercalated cells of the collecting duct. Pfiugers Archiv: European journal of physiology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van Adelsberg J, Edwards JC, Takito J, Kiss B, and al-Awqati Q. An induced extracellular matrix protein reverses the polarity of band 3 in intercalated epithelial cells. Cell 76: 1053–1061, 1994. [DOI] [PubMed] [Google Scholar]
- 130.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, and Wall SM. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. American journal of physiology Renal physiology 301: F1314–1325, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, and Wall SM. Dietary Cl(−) restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. American journal of physiology Renal physiology 291: F833–839, 2006. [DOI] [PubMed] [Google Scholar]
- 132.Verrey F, Hummler E, Schild L, and Rossier B. Control of Na+ transport by aldosterone In: The Kidney: Physiology and Pathophysiology, edited by Seldin DW and Giebisch G Philadelphia: Williams & Wilkins, 2000, p. 1441–1471. [Google Scholar]
- 133.Vidarsson H, Westergren R, Heglind M, Blomqvist SR, Breton S, and Enerback S. The forkhead transcription factor Foxi1 is a master regulator of vacuolar H-ATPase proton pump subunits in the inner ear, kidney and epididymis. PloS one 4: e4471, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wagner CA, Devuyst O, Bourgeois S, and Mohebbi N. Regulated acid-base transport in the collecting duct. Pfiugers Archiv: European journal of physiology 458: 137–156, 2009. [DOI] [PubMed] [Google Scholar]
- 135.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, and Verlander JW. Localization of pendrin in mouse kidney. American journal of physiology Renal physiology 284: F229–241, 2003. [DOI] [PubMed] [Google Scholar]
- 136.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, and Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl- conservation. Hypertension 44: 982–987, 2004. [DOI] [PubMed] [Google Scholar]
- 137.Wall SM and Pech V. The interaction of pendrin and the epithelial sodium channel in blood pressure regulation. Current opinion in nephrology and hypertension 17: 18–24, 2008. [DOI] [PubMed] [Google Scholar]
- 138.Wall SM and Weinstein AM. Cortical distal nephron Cl(−) transport in volume homeostasis and blood pressure regulation. American journal of physiology Renal physiology 305: F427–438, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang Z, Subramanya AR, Satlin LM, Pastor-Soler NM, Carattino MD, and Kleyman TR. Regulation of large-conductance Ca2+-activated K+ channels by WNK4 kinase. American journal of physiology Cell physiology 305: C846–853, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weiner ID, Mitch WE, and Sands JM. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clinical journal of the American Society of Nephrology: CJASN, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weiner ID and Verlander JW. Molecular physiology of the Rh ammonia transport proteins. Current opinion in nephrology and hypertension 19: 471–477, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, and Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. American journal of physiology Renal physiology 285: F629–639, 2003. [DOI] [PubMed] [Google Scholar]
- 143.Xu J, Barone S, Li H, Holiday S, Zahedi K, and Soleimani M. Slc26a11, a chloride transporter, localizes with the vacuolar H(+)-ATPase of A-intercalated cells of the kidney. Kidney international 80: 926–937, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu J, Worrell RT, Li HC, Barone SL, Petrovic S, Amlal H, and Soleimani M. Chloride/bicarbonate exchanger SLC26A7 is localized in endosomes in medullary collecting duct cells and is targeted to the basolateral membrane in hypertonicity and potassium depletion. Journal of the American Society of Nephrology: JASN 17: 956–967, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, Uchida S, and Shibata S. Hypokalemia and Pendrin Induction by Aldosterone. Hypertension 69: 855–862, 2017. [DOI] [PubMed] [Google Scholar]
- 146.Yasuoka Y, Sato Y, Healy JM, Nonoguchi H, and Kawahara K. pH-sensitive expression of calcium-sensing receptor (CaSR) in type-B intercalated cells of the cortical collecting ducts (CCD) in mouse kidney. Clin Exp Nephrol 19: 771–782, 2015. [DOI] [PubMed] [Google Scholar]
- 147.Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, and Senbonmatsu T. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertension research : official journal of the Japanese Society of Hypertension 34: 599–605, 2011. [DOI] [PubMed] [Google Scholar]
- 148.Zhuang J, Zhang X, Wang D, Li J, Zhou B, Shi Z, Gu D, Denson DD, Eaton DC, and Cai H. WNK4 kinase inhibits Maxi K channel activity by a kinase-dependent mechanism. American journal of physiology Renal physiology 301: F410–419, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]