Abstract
Background
Ceramides exhibit multiple biological activities that may influence the pathophysiology of heart failure. These activities may be influenced by the saturated fatty acid carried by the ceramide (Cer). However, the associations of different circulating Cer species, and their sphingomyelins (SM) precursors, with heart failure have received limited attention.
Methods and Results
We studied the associations of plasma Cer and SM species with incident heart failure in the Cardiovascular Health Study. We examined 8 species: Cer and SM with palmitic acid (Cer-16 and SM-16), species with arachidic acid (Cer-20 and SM-20), species with behenic acid (Cer-22 and SM-22) and species with lignoceric acid (Cer-24 and SM-24). During a median follow-up of 9.4 years, we identified 1179 cases of incident heart failure among 4249 study participants. In Cox regression analyses adjusted for risk factors, higher levels of Cer-16 and SM-16 were associated with higher risk of incident heart failure (Hazard ratio [HR] for one SD increase:1.25 [95% CI: 1.16–1.36] and 1.28 [1.18–1.40], respectively). In contrast, higher levels of Cer-22 were associated with lower risk of heart failure in multivariable analyses further adjusted for Cer-16 (HR: 0.85 [0.78–0.92]); and higher levels of SM-20, SM-22 and SM-24 were associated with lower risk of heart failure in analyses further adjusted for SM-16 (HRs: 0.83 [0.77–0.90], 0.81 [0.75–0.88], and 0.83 [0.77–0.90] respectively). No statistically significant interactions with age, sex, African American race, BMI or baseline coronary heart disease were detected. Similar associations were observed for heart failure with preserved (n=529) or reduced (n=348) ejection fraction.
Conclusions
This study shows associations of higher plasma levels of Cer-16 and SM-16 with increased risk of heart failure and higher levels of Cer-22, SM-20, SM-22, and SM-24 with decreased risk of heart failure.
Clinical Trial Registration
clinicaltrials.gov; unique identifier: NCT00005133.
Keywords: Lemaitre, Ceramides, Sphingomyelins, Heart Failure
INTRODUCTION
The pathophysiology of heart failure involves multiple cardiometabolic risk factors, including coronary disease, myocardial infarction, hypertension, diabetes 1, and complex compensatory processes that may result in remodeling of the heart and heart failure. Elucidation of novel determinants of risk of heart failure may lead to improved pathophysiologic understanding and new targets for the prevention of heart failure.
Ceramides (Cer) and sphingomyelins (SM) belong to the family of sphingolipids, lipid molecules composed of a sphingoïd backbone and a fatty acid, that are important constituents of cell membranes and participate in cell signaling. Cer are involved in several biological activities that may influence the pathophysiology of heart failure, including effects on apoptosis, oxidative stress, endothelial dysfunction, inflammation, lipotoxicity and insulin resistance 2–4. Furthermore, pharmacological inhibition of Cer biosynthesis with myriocin enhances cardiomyocyte survival in a mouse model of lipotoxic cardiomyopathy 5. However, Cer species differ depending on the fatty acid that is attached, and different species appear to have different biological properties. In cell and animal experiments, the Cer species that uniformly promotes apoptosis is Cer-16 (Cer with palmitic acid [16:0]), whereas Cer species with a very long-chain saturated fatty acid (VLSFA, with 20 carbons or more) appear to protect from apoptosis 6–8. For instance, heart-specific deletion of SPTLC2 (serine-palmitoyl transferase 2) in mice results in reduced Cer-20 (Cer with arachidic acid [20:0]) and Cer-24 (Cer with lignoceric acid [24:0]) and more apoptosis in the heart 9.
Sphingomyelins have the basic structure of Cer to which is added the head group phosphocholine. Cer species can be produced from SM species by action of sphingomyelinases, which remove the head group 10. While there is limited data on the potential role of SM in heart failure, indirect evidence comes from recent findings that plasma phospholipid VLSFA are associated with lower risk of incident heart failure in the Cardiovascular Health Study (CHS)11. The VLSFA are primarily components of sphingolipids, including SM and Cer, raising the possibility that the observed lower risk of HF was due to SM and Cer species with a VLSFA. Also suggesting that circulating VLSFA are connected to sphingolipids, the genetic variants associated with plasma phospholipid levels of VLSFA in genome-wide association studies are in key enzymes of the Cer biosynthesis pathway 12. These observations, together with the experimental evidence about Cer mentioned above, led us to hypothesize that Cer and SM species with a VLSFA are associated with reduced risk of heart failure while Cer and SM species with palmitic acid are associated with increased risk. We investigated this hypothesis in the CHS, a prospective cohort of older adults, where we measured plasma Cer and SM species with different saturated fatty acids in 4249 participants.
METHODS
The data and study materials will not be made available to other researchers for purpose of reproducing the results or replicating the procedure. The authors are not authorized to share CHS data. Information on CHS data requests can be found at https://chs-nhlbi.org.
Study Population
The CHS is a population-based cohort study of cardiovascular disease among older adults from four U.S. communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; Allegheny County, Pennsylvania) 13. The cohort consists of 5201 non-institutionalized men and women, aged ≥65 y at enrollment, recruited in 1989–1990, plus an additional 687 predominantly black participants recruited in 1992–93. The Cardiovascular Health Study was approved by the Institutional Review Board of the University of Washington and the participants provided informed written consent.
To measure sphingolipids, we used blood specimens from the 1994–1995 clinic exam, from all participants with available specimen at that visit (N=4026), and 586 specimens from the 1992–1993 clinic exam from additional participants without a specimen at the 94–95 visit. The 4249 CHS participants with sphingolipid data who were free of heart failure at the time of the blood draw for sphingolipid measurement were included in this report.
Ascertainment of heart failure
Study participants were followed by annual clinic examinations with interim phone contacts until 1999, telephone contacts twice a year thereafter, and another clinic visit in 2005–06. Cardiovascular events including heart failure were adjudicated by a centralized CHS committee based on information from outpatient and inpatient medical records, diagnostic tests and consultations, and interviews. Events have been adjudicated through 2014. Confirmation of definite heart failure required each of 3 criteria: 1) diagnosis of heart failure by a treating physician; 2) either heart failure symptoms (shortness of breath, fatigue, orthopnea, or paroxysmal nocturnal dyspnea) plus signs (edema, rales, tachycardia, gallop rhythm, or displaced apical impulse) or supportive clinical findings on echocardiography, contrast ventriculography, or chest radiography; and 3) medical therapy for heart failure, defined as diuretics plus either digitalis or a vasodilator (angiotensin-converting enzyme inhibitors, hydralazine, or long-acting nitrates) 14. Whenever possible, heart failure was subtyped into heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF) on the basis of findings from echocardiography and cardiac catheterization reports.
Measurement of sphingolipids
Sphingolipids were measured on a mix of fasting and non fasting EDTA plasma samples that had been stored at −70°C. Plasma lipids were extracted and sphingolipids quantified by liquid chromatography-tandem mass spectroscopy. The detailed methodology and quality control procedures have been reported 15. The sphingolipid concentrations were determined using a single point calibrator, made from a pooled EDTA plasma sample, used throughout the study and added to each batch in 5 replicates. Levels of sphingolipids were expressed as μM. This hypothesis-based investigation focused primarily on 8 species: Cer-16, Cer with arachidic acid (Cer-20), Cer with behenic acid (Cer-22), and Cer-24 (computed as the sum of two species of Cer-24 with distinct “d181” and “d182” sphingoïd backbones), and SM species with the same saturated fatty acids, including SM-16, SM-20, SM-22, and SM-24. Based on quality control over 52 batches of samples, the % coefficients of variation were 18.4% for Cer-16; 19.5% for Cer-20; 13.9% for Cer-22; 13.8% for Cer-24; 11.5% for SM-16; 12.2% for SM-20; 12.4% for SM-22; and 13.3% for SM-24. Correlations between the 8 primary species are shown in Table 1.
Table 1:
Plasma concentrations of ceramides and sphingomyelins carrying different saturated fatty acids and correlations between the species
| Cer-16 | Cer-20 | Cer-22 | Cer-24 | SM-16 | SM-20 | SM-22 | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD, μg/mL | Range | ------------------------Spearman Correlations------------------------ | |||||||
| Cer-16 | 0.27 ± 0.06 | 0.09 to 0.91 | 1 | ||||||
| Cer-20 | 0.08 ± 0.03 | 0.01 to 0.25 | 0.52 | 1 | |||||
| Cer-22 | 0.62 ± 0.19 | 0.18 to 1.90 | 0.60 | 0.60 | 1 | ||||
| Cer-24 | 4.62 ± 1.32 | 1.31 to 11.28 | 0.62 | 0.60 | 0.82 | 1 | |||
| SM-16 | 125.20±19.28 | 48.93 to 227.05 | 0.45 | 0.32 | 0.22 | 0.32 | 1 | ||
| SM-20 | 17.71 ± 3.57 | 6.19 to 36.35 | 0.29 | 0.36 | 0.46 | 0.43 | 0.48 | 1 | |
| SM-22 | 26.61 ± 5.88 | 9.60 to 63.23 | 0.33 | 0.30 | 0.57 | 0.43 | 0.56 | 0.79 | 1 |
| SM-24 | 14.27 ± 3.53 | 4.59 to 33.19 | 0.28 | 0.10 | 0.40 | 0.37 | 0.52 | 0.67 | 0.90 |
Abbreviations: Cer-16, Cer-20, Cer-22, Cer-24 = ceramide with palmitic, arachidic, behenic, lignoceric acid respectively. SM-16, SM-20, SM-22, SM-24 = sphingomyelin with palmitic, arachidic, behenic, lignoceric acid respectively.
Other risk factors
Information on a wide range of covariates was obtained during study visits, including medical history, lifestyle, and clinical risk factors 13. Information on age, sex, race, education, physical activity and smoking status was based on self-report. Usual walking habits, included average pace (gait speed) and distance walked. Weight, waist circumference, height, blood pressure were measured using standardized protocols. Plasma glucose was assessed on fasting blood samples using enzymatic methods 13. Atrial fibrillation included atrial flutter and was based on annual ECGs and hospital discharge ICD9 codes 16. Medications were ascertained by inventory of prescribed medications taken in the 2 weeks prior to each study visit. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Diabetes mellitus was defined by a fasting glucose level of ≥7 mmol/L (126 mg/dL) or use of insulin or oral hypoglycemic agents. Information on covariates was obtained at the same clinic exam from which their Cer and SM were measured except other laboratory measurements (low density lipoprotein [LDL] cholesterol, triglycerides, C-reactive protein, fibrinogen, estimated glomerular filtration rate [eGFR], cystatin C, albumin, troponin and N-terminal pro-B-type natriuretic peptide [NT -pro BNP]) were from all the 1992–93 exam.
Statistical analysis
Sphingolipid species concentrations were log-transformed to reduce skewness. Log sphingolipid concentrations were evaluated linearly as modeling of the log sphingolipid species using cubic splines did not show departure from linearity (not shown). To assess cross-sectional univariate associations of sphingolipid concentrations with baseline characteristics, we used linear regression for continuous variables and logistic regression for binary variables.
Cox proportional hazards regression was used to estimate the hazard ratio (HR) associated with incident heart failure. Associations are presented for one SD higher value. Three models with pre-specified adjustments were examined: a “minimal model” adjusted for age, sex, geographic area, and self-reported race (black vs white); a “multivariable model” additionally adjusted for baseline diabetes, coronary heart disease, atrial fibrillation, systolic blood pressure, treated hypertension, smoking, body mass index (BMI), physical activity, fasting glucose, and LDL cholesterol; and a “full model” with additional adjustment for one of the other species: for the associations of Cer-20, Cer-22 and Cer-24 with heart failure, the full model included Cer-16; for the associations of Cer-16, the full model included Cer-22; for the associations of SM-20, SM-22 and SM-24, the full model included SM-16, and for the associations of SM-16, it included SM-22. In secondary analyses, we examined separately the HRs of incident heart failure with reduced ejection fraction (HFrEF), defined as heart failure with <45% ejection fraction, and the HRs of incident heart failure with preserved ejection fraction (HFpEF) associated with the Cer and SM species. To correct the analyses for multiple comparisons, we applied a Bonferroni correction and used a significance threshold of 0.0063 (0.05/8 species).
We found departure from the proportional hazards assumption in the primary analyses of sphingolipids and heart failure. For this reason, we conducted sensitivity analyses stratified in quartiles of survival time.
Missing values of LDL (n=236), triglycerides (n=167), physical activity (n=48), glucose (n=16), BMI (n=7), hypertension medication use (n=2), and systolic blood pressure (n=1) were multiply imputed by chained equations using information on age, sex, race, height, weight, waist circumference, smoking, and prevalent diabetes. Twenty imputed datasets were generated and model fitting results were pooled using standard methods 17. In pre-specified analyses, we examined whether associations between sphingolipids and heart failure risk were modified by age, sex, African American race, BMI and CHD by adding product interaction terms to the models above. We used a threshold of significance of 0.05/5 interactions= 0.01 for the interaction analyses. Analyses were performed using Stata version 14.
RESULTS
At baseline, participant mean age was 76 ± 5 years, and 40% were male. Means and ranges of Cer and SM concentrations are shown in Table 1. The ranges of Cer species concentrations were comparable to those reported in other cohorts 18. The distributions of baseline characteristics across quartiles of Cer and SM species are shown in Figure 1 for Cer-16, Cer-22, SM-16 and SM-22 and in Table S1 for all 8 species. Multiple associations between participant characteristics and levels of Cer and SM were observed, with some associations observed across all 8 species. For example, higher LDL cholesterol, female sex, and higher systolic blood pressure were associated with higher levels of all or most species. Some of the associations diverged for Cer and SM species. For example, higher levels of HDL cholesterol were associated with lower levels of all Cer species but higher levels of SM species; baseline diabetes was associated with higher Cer species levels but not SM levels; higher left ventricular mass was associated with lower SM species levels, but not Cer levels. A few associations diverged for species with palmitic acid and species with a VLSFA. Older age and lower BMI were associated with lower levels of Cer and SM with a VLSFA. In addition, the prevalence of CHD and atrial fibrillation at baseline decreased across quartiles of SM species with a VLSFA.
Figure 1:
Baseline characteristics across quartiles of selected plasma ceramide and sphingomyelin species. The figure shows mean (SD) of baseline characteristics in the whole cohort and across quartiles of ceramides and sphingomyelins (trend), and mean levels in first (Q1) and fourth (Q4) quartiles. Red lines indicate statistically significant (p<0.0024) lower levels (or percentages) of the baseline characteristic at higher quartiles of sphingolipid species. Blue lines indicate statistically significant (p<0.0024) higher levels (or percentages) of characteristic at higher quartiles of sphingolipid species. Grey lines indicate no significant (p>=0.0024) association of sphingolipid with the baseline characteristic. Abbreviations: Cer-16 = ceramide with palmitic, Cer-22 = ceramide with behenic, SM-16 = sphingomyelin with palmitic, SM-22 = sphingomyelin with behenic.
During a median follow-up of 9.4 years, we identified 1179 incident heart failure. Of those 74% had data on ejection fraction, including 529 identified as HFpEF and 348 HFrEF. In multiple regression analyses adjusted for age, sex, site, race, baseline diabetes, CHD, atrial fibrillation, treated hypertension, systolic blood pressure, fasting glucose, LDL cholesterol, smoking, BMI and physical activity, higher levels of Cer-16 and SM-16 were associated with higher risk of incident heart failure (Table 2). In these analyses, Cer with VLSFA were not associated with risk and the associations of heart failure with SM containing VLSFA did not reach the pre-specified statistical significance. Because Cer-16 and SM-16 levels were positively correlated with levels of species with VLSFA (Table 1), Cer-16 and SM-16 may have masked the hypothesized opposite associations of Cer and SM species with a VLSFA. In analyses adjusted for Cer-16, Cer-22 was associated with lower risk of heart failure; and in analyses adjusted for SM-16, all 3 SM species with a VLSFA were associated with lower risk of incident heart failure (Table 2). In these analyses, higher Cer-16 and SM-16 levels showed stronger associations with higher heart failure risk than without adjustment for Cer-22 and SM-22, respectively.
Table 2:
Associations of plasma ceramides and sphingomyelins carrying saturated fatty acids with risk of incident heart failure
| Model | HR | 95% CI | P-value | |
|---|---|---|---|---|
| Cer-16 | Basic | 1.15 | 1.08–1.23 | 1.07×10−05 |
| Multi | 1.14 | 1.06–1.21 | 1.46×10−04 | |
| Multi + Cer-22 | 1.25 | 1.16–1.36 | 3.74×10−08 | |
| Cer-20 | Basic | 1.07 | 1.00–1.14 | 0.05 |
| Multi | 1.02 | 0.95–1.09 | 0.57 | |
| Multi + Cer-16 | 0.94 | 0.87–1.01 | 0.11 | |
| Cer-22 | Basic | 1.02 | 0.96–1.09 | 0.52 |
| Multi | 0.97 | 0.90–1.03 | 0.33 | |
| Multi + Cer-16 | 0.85 | 0.78–0.92 | 6.77×10−05 | |
| Cer-24 | Basic | 1.09 | 1.02–1.10 | 0.08 |
| Multi | 1.01 | 0.95–1.08 | 0.67 | |
| Multi + Cer-16 | 0.94 | 0.87–1.02 | 0.13 | |
| SM-16 | Basic | 1.07 | 1.01–1.14 | 0.02 |
| Multi | 1.15 | 1.07–1.23 | 1.75×10−04 | |
| Multi + SM-22 | 1.28 | 1.18–1.40 | 4.19×10−09 | |
| SM-20 | Basic | 0.9 | 0.84–0.95 | 4.83×10−04 |
| Multi | 0.92 | 0.86–0.98 | 0.01 | |
| Multi + SM-16 | 0.83 | 0.77–0.90 | 2.97×10−06 | |
| SM-22 | Basic | 0.92 | 0.86–0.97 | 6.06×10−03 |
| Multi | 0.93 | 0.86–0.99 | 0.03 | |
| Multi + SM-16 | 0.81 | 0.75–0.88 | 5.63×10−07 | |
| SM-24 | Basic | 0.9 | 0.85–0.96 | 1.12×10−03 |
| Multi | 0.94 | 0.88–1.01 | 0.07 | |
| Multi + SM-16 | 0.83 | 0.77–0.90 | 7.76×10−06 |
The table shows hazard ratio (HR) and 95% confidence interval (CI) of incident heart failure associated with an increase in plasma log-transformed sphingolipid species corresponding to one standard deviation. The hazard ratios were estimated from analyses using Cox regression. The basic model was adjusted for age, sex, African American race and study site. The “Multi” model was further adjusted for diabetes, CHD, atrial fibrillation, systolic blood pressure, treated hypertension, smoking, BMI, physical activity, fasting glucose, LDL cholesterol. Abbreviations: Cer-16, Cer-20, Cer-22, Cer-24 = ceramide with palmitic, arachidic, behenic, lignoceric acid respectively. SM-16, SM-20, SM-22, SM-24 = sphingomyelin with palmitic, arachidic, behenic, lignoceric acid respectively
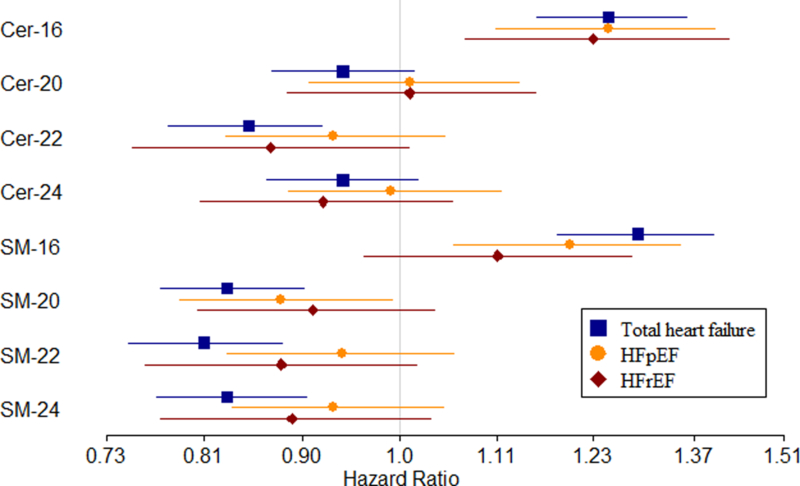
Further adjustments for fasting and time since eating, triglyceride levels, C-reactive protein, fibrinogen, eGFR, cystatin C, albumin and troponin did not change the study findings, while adjustment for NT-ProBNP moved the estimates slightly toward the null (Table S2). In addition, analyses with time-dependent myocardial infarction show no change in findings (Table S2) Sensitivity analyses showed the associations with heart failure risk were strongest in the time period closest to sphingolipid measurement, and moved toward the null in later follow-up time periods (Figure 2, Table S3), indicating that the primary analyses using the whole follow-up gave conservative estimates of the near-term hazard ratio. We found no statistically significant interaction of the Cer and SM species with age, sex, BMI, race or CHD on the risk of incident heart failure at the pre-specified threshold (Table S4). When examining the outcomes of HFpEF and HFrEF separately, we observed generally similar patterns of associations to those observed with total heart failure, although only the associations of Cer-16 reached statistical significance for all outcomes (Figure 3, Table S5).
Figure 2:
Associations of plasma ceramide and sphingomyelin species with incident heart failure in each 5-year period of follow-up time. The figure shows hazard ratios and 95% confidence intervals associated with an increase in plasma sphingolipid species of one standard deviation during 0–5 year follow-up period (blue), 5–10 year (red), 10–15 year (orange) and 15–20 year (yellow). The hazard ratios, obtained from Cox regression, were adjusted for age, sex, African American race, study site, diabetes, CHD, atrial fibrillation, systolic blood pressure, treated hypertension, smoking, BMI, physical activity, fasting glucose, LDL cholesterol; in addition, the analyses of Cer-20, Cer-22 and Cer-24 were adjusted for Cer-16; the analysis of Cer-16 was adjusted for Cer-22; the analyses of SM-20, SM-22 and SM-24 were adjusted for SM-16; and the analysis of SM-16 was adjusted for SM-22. Abbreviations: Cer-16, Cer-20, Cer-22, Cer-24 = ceramide with palmitic, arachidic, behenic, lignoceric acid respectively. SM-16, SM-20, SM-22, SM-24 = sphingomyelin with palmitic, arachidic, behenic, lignoceric acid respectively.
Figure 3:
Associations of plasma ceramide and sphingomyelin species with risks of total incident heart failure, heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. The figure shows hazard ratios and 95% confidence intervals associated with an increase in plasma sphingolipid species corresponding to one standard deviation obtained in analyses of total heart failure (blue), heart failure with preserved ejection fraction (HFpEF) (orange), and heart failure with reduced ejection fraction (HFrEF) (red). The hazard ratios, obtained from Cox regression, were adjusted for age, sex, African American race, study site, diabetes, CHD, atrial fibrillation, systolic blood pressure, treated hypertension, smoking, BMI, physical activity, fasting glucose, LDL cholesterol; in addition, the analyses of Cer-20, Cer-22 and Cer-24 were adjusted for Cer-16; the analysis of Cer-16 was adjusted for Cer-22; the analyses of SM-20, SM-22 and SM-24 were adjusted for SM-16; and the analysis of SM-16 was adjusted for SM-22. Abbreviations: Cer-16, Cer-20, Cer-22, Cer-24 = ceramide with palmitic, arachidic, behenic, lignoceric acid respectively. SM-16, SM-20, SM-22, SM-24 = sphingomyelin with palmitic, arachidic, behenic, lignoceric acid respectively.
A recent study reported an association of the ratio Cer-24/Cer-16 with lower risk of total mortality and incident heart failure risk 18. As a sensitivity analysis, we followed a similar approach, and found that higher values of both the ratios Cer-24/Cer-16 and Cer-22/Cer-16 were associated with lower risk of incident heart failure (HR for 1SD of ratio: 0.91 [0.86,0.97, p=0.005] and 0.85 [0.80,0.91, p=1.7 ×10−06] respectively). However, in models including both the ratio and the denominator Cer-16, Cer-16 remained independently associated with higher risk; furthermore Cer-24/Cer-16 was no longer significantly associated with risk when adjusted for Cer-16 (Table S6).
DISCUSSION
We have shown in a large biracial cohort of older adults that higher levels of Cer and SM species carrying palmitic acid are associated with higher risk of incident heart failure, while higher levels of Cer-22 and SM species that carry any VLSFA are associated with lower risk of heart failure. Associations did not significantly differ with BMI, age, sex, African American race and prevalent CHD. Associations appeared similar with the outcomes of HFpEF and HFrEF.
In a recent meta-analysis of the Framingham Heart Study and the Study of Health in Pomerania, the plasma ratios Cer-24/Cer-16 and Cer-22/Cer-16 showed a borderline significant association with lower risk of incident heart failure 18. Our findings, obtained with 5 times more incident heart failure, provide important evidence that Cer are associated with incident heart failure and that the direction of association depends upon the fatty acid attached to the sphingoïd backbone. In addition, in contrast to using only the Cer-22/Cer-16 ratio in the model which assumes that a higher level of Cer-22 is exactly equivalent to the same relative magnitude lower level of Cer-16, our study showed that higher levels of Cer-16 are associated with higher risk and higher levels of Cer-22 with lower risk independently. Finally, we showed parallel associations of SM-16 with higher risk, and SM-20, SM-22 and SM-24 with lower risk of incident heart failure.
Ceramides are involved in biological processes that may influence heart failure including apoptosis 19, oxidative stress and endothelial dysfunction 20, inflammation 20, lipotoxicity, insulin resistance 21, 22 and cardiomyopathy 2, 5. In heart failure, the heart shows remodeling with markedly increased fibrosis in the matrix 23, and in animal models of heart failure, myocyte loss by apoptosis occurs in parallel with the onset of fibrosis 24–27. Oxidative stress is also part of the pathogenesis of heart failure 28, 29. Therefore Cer may influence heart failure through multiple mechanisms.
Several lines of evidence suggest different Cer species have different effects, at least with regard to apoptosis. For instance, cell experiments show that Cer-16 is pro-apoptotic, and Cer-24 appears to be protect from apoptosis 6. In the worm C. elegans, Cer-16 promotes radiation-induced apoptosis 7, while Cer-20 and Cer-22 protect against hypoxia-induced apoptosis 8. Similarly, in mice, heart-specific deletion of SPTLC2, the rate limiting step of de novo synthesis of Cer, results in lower levels of Cer with a VLSFA concurrently with increased apoptosis in the heart 9. Our findings provide evidence from a large population study that circulating Cer with different saturated fatty acids show associations with incident heart failure that go in different directions.
The parallel associations of SM species and Cer species with heart failure may be due to the fact that Cer can be generated from SM by action of sphingomyelinases 30, in response to stress, cytokines or ischemia 10, 31. Because the SM fatty acid is conserved in the Cer that is produced, we hypothesize that SM fatty acid composition influences heart failure risk by influencing the biological activity of the released Cer.
Strengths of this study include the prospective design, the use of a well-phenotyped cohort with information on many potential confounders, the large number of heart failure events, the hypothesis-based approach and the use of an objective biomarker. Limitations include the observational nature of the study which precludes inferences of causality. The possibility of residual confounding from unmeasured or imprecisely-measured variables cannot be excluded. The associations of the single measurement of Cer and SM species with heart failure risk diminished over time and the hazard ratios in this report are an underestimate of early heart failure risk. Study results were obtained with an older, primarily white, population, and may not generalize to younger populations and different ethnicities. The purpose of the study was to test a priori hypotheses about associations of different Cer and SM with heart failure, and future studies will be needed to gauge the relative impact of Cer and SM compared to other soluble biomarkers 32.
In summary, we observed higher risk of incident heart failure associated with higher levels of Cer-16 and SM-16, and lower risk associated with higher levels of Cer-22 and higher levels of SM with a VLSFA. Future research investigating pharmacologic and nutritional factors that influence these species may help identify novel approaches to preventing heart failure.
Supplementary Material
Clinical Perspective.
What Is New?
In a large cohort of 4249 older adults, higher levels of plasma ceramide and sphingomyelin species that contain a palmitic acid are prospectively associated with higher risk of new heart failure, while several ceramide and sphingomyelin species with longer saturated fatty acids are associated with lower risk of heart failure
The associations appear similar for heart failure with preserved ejection fraction and heart failure with reduced ejection fraction
The associations are independent of major risk factors for heart failure and similar in subgroups defined by sex, African American race, baseline age, body mass index, and coronary heart disease
What Are the Clinical Implications?
Plasma ceramide and sphingomyelin species are related to heart failure risk in an epidemiologic study
If the associations prove to be causal, lowering levels of ceramide and sphingomyelin species with palmitic acid and raising levels of species with a very long-chain saturated fatty acid may help prevent heart failure
Next steps will be to determine life-style and pharmacologic determinants of plasma ceramide and sphingomyelin species with specific fatty acids to design future interventions.
Acknowledgments
Funding
This research was supported by grant R01HL128575 from the National Heart, Lung, and Blood Institute (NHLBI). The Cardiovascular Health Study is supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Disclosures
Dr. Mozaffarian reports research funding from the National Institutes of Health and the Gates Foundation; personal fees from GOED, Bunge, Indigo Agriculture, Amarin, Acasti Pharma, Cleveland Clinic Foundation, America’s Test Kitchen, and Danone; scientific advisory board, Elysium Health (with stock options), Omada Health, and DayTwo; and chapter royalties from UpToDate; all outside the submitted work.
REFERENCES
- 1.Rame JE, Ramilo M, Spencer N, Blewett C, Mehta SK, Dries DL and Drazner MH. Development of a depressed left ventricular ejection fraction in patients with left ventricular hypertrophy and a normal ejection fraction. Am J Cardiol. 2004;93:234–7. [DOI] [PubMed] [Google Scholar]
- 2.Jiang XC, Goldberg IJ and Park TS. Sphingolipids and cardiovascular diseases: lipoprotein metabolism, atherosclerosis and cardiomyopathy. Adv Exp Med Biol. 2011;721:19–39. [DOI] [PubMed] [Google Scholar]
- 3.Baranowski M and Gorski J. Heart sphingolipids in health and disease. Adv Exp Med Biol. 2011;721:41–56. [DOI] [PubMed] [Google Scholar]
- 4.Kolter T A view on sphingolipids and disease. Chem Phys Lipids. 2011;164:590–606. [DOI] [PubMed] [Google Scholar]
- 5.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED and Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. Journal of lipid research. 2008;49:2101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosch S, Schiffmann S and Geisslinger G. Chain length-specific properties of ceramides. Progress in lipid research. 2012;51:50–62. [DOI] [PubMed] [Google Scholar]
- 7.Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S and Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowder CM. Cell biology. Ceramides--friend or foe in hypoxia? Science. 2009;324:343–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SY, Kim JR, Hu Y, Khan R, Kim SJ, Bharadwaj KG, Davidson MM, Choi CS, Shin KO, Lee YM, Park WJ, Park IS, Jiang XC, Goldberg IJ and Park TS. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. The Journal of biological chemistry. 2012;287:18429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavoine C and Pecker F. Sphingomyelinases: their regulation and roles in cardiovascular pathophysiology. Cardiovasc Res. 2009;82:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemaitre RN, McKnight B, Sotoodehnia N, Fretts AM, Qureshi WT, Song X, King IB, Sitlani C, Siscovick DS, Psaty BM and Mozafarrian D. Circulating Very Long-Chain Saturated Fatty Acids and Heart Failure: The Cardiovascular Health Study. JAHA. 2018;7:e010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaitre RN, King IB, Kabagambe EK, Wu JH, McKnight B, Manichaikul A, Guan W, Sun Q, Chasman DI, Foy M, Wang L, Zhu J, Siscovick DS, Tsai MY, Arnett DK, Psaty BM, Djousse L, Chen YD, Tang W, Weng LC, Wu H, Jensen MK, Chu AY, Jacobs DR Jr., Rich SS, Mozaffarian D, Steffen L, Rimm EB, Hu FB, Ridker PM, Fornage M and Friedlander Y Genetic loci associated with circulating levels of very long-chain saturated fatty acids. Journal of lipid research. 2015;56:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP and Weiler PG. The Cardiovascular Health Study: design and rationale. Annals of epidemiology. 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 14.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE and Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. Journal of the American College of Cardiology. 2000;35:1628–37. [DOI] [PubMed] [Google Scholar]
- 15.Lemaitre RN, Yu C, Hoofnagle A, Hari N, Jensen P, Fretts AM, Umans JG, Howard BV, Sitlani CM, Siscovick DS, King IB, Sotoodehnia N and McKnight B. Circulating Sphingolipids, Insulin, HOMA-IR and HOMA-B: the Strong Heart Family Study. Diabetes. 2018;67:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD and Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–61. [DOI] [PubMed] [Google Scholar]
- 17.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 18.Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Volzke H, Felix SB, Jiang H, Sidhu R, Nauck M, Jiang X, Ory DS, Dorr M, Vasan RS and Schaffer JE. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. Journal of the American Heart Association. 2018;7:e007931. doi: 10.1161/JAHA.117.007931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettus BJ, Chalfant CE and Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochimica et biophysica acta. 2002;1585:114–25. [DOI] [PubMed] [Google Scholar]
- 20.Gulbins E and Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R11–26. [DOI] [PubMed] [Google Scholar]
- 21.Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in lipid research. 2006;45:42–72. [DOI] [PubMed] [Google Scholar]
- 22.Chavez JA and Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–94. [DOI] [PubMed] [Google Scholar]
- 23.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ and Paulus WJ. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73. [DOI] [PubMed] [Google Scholar]
- 24.Hanna N, Cardin S, Leung TK and Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc Res. 2004;63:236–44. [DOI] [PubMed] [Google Scholar]
- 25.Sharov VG, Sabbah HN, Shimoyama H, Goussev AV, Lesch M and Goldstein S. Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. The American journal of pathology. 1996;148:141–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Li XM, Ma YT, Yang YN, Liu F, Chen BD, Han W, Zhang JF and Gao XM. Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure. Clin Exp Pharmacol Physiol. 2009;36:1054–61. [DOI] [PubMed] [Google Scholar]
- 27.Sabbah HN, Sharov VG and Goldstein S. Cell death, tissue hypoxia and the progression of heart failure. Heart Fail Rev. 2000;5:131–8. [DOI] [PubMed] [Google Scholar]
- 28.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS and Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. Journal of the American College of Cardiology. 1998;31:1352–6. [DOI] [PubMed] [Google Scholar]
- 29.McMurray J, Chopra M, Abdullah I, Smith WE and Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. European heart journal. 1993;14:1493–8. [DOI] [PubMed] [Google Scholar]
- 30.Marchesini N and Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. [DOI] [PubMed] [Google Scholar]
- 31.Milhas D, Clarke CJ and Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS letters. 2010;584:1887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmad T, Fiuzat M, Pencina MJ, Geller NL, Zannad F, Cleland JG, Snider JV, Blankenberg S, Adams KF, Redberg RF, Kim JB, Mascette A, Mentz RJ, O’Connor CM, Felker GM and Januzzi JL. Charting a roadmap for heart failure biomarker studies. JACC Heart Fail. 2014;2:477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.