Abstract
Background:
Antiretroviral treatment (ART) adherence is often suboptimal in the perinatal period. We measured hair tenofovir (TFV) concentrations as a metric of adherence in postpartum women to understand patterns and predictors of adherence throughout this critical period. Additionally, we examined the association between hair TFV concentrations and virologic outcomes.
Methods:
Between 12/2012–09/2016, hair samples were collected longitudinally from delivery through breastfeeding from women on ART in the PROMISE study ( NCT01061151) in sub-Saharan Africa. Hair TFV levels were measured using validated methods. Using generalized estimating equations, we estimated the association between hair TFV levels and virologic suppression (<400 copies/mL) over time and assessed predictors of hair TFV levels.
Results:
Hair TFV levels were measured at 370 visits in 71 women from delivery through a median of 14 months (IQR 12–15) of breastfeeding. Levels ranged from below detection (0.002) to 1.067 nanograms/milligram (geometric mean: 0.047). After ≥90 days on ART, 69 women had at least one viral load measured (median 5 measures, range 1–9); 18 (26%) experienced viremia at least once. Each doubling of TFV level more than doubled odds of concurrent virologic suppression (OR 2.35, 95%CI: 1.44–3.84, p=0.0006) and was associated with 1.43 times the odds of future suppression (95%CI: 0.75–2.73, p=0.28). Relative to the first 3 months after delivery, hair levels were highest in months 6–12 (1.42 fold higher, 95%CI: 1.09–1.85, p=0.01).
Conclusion:
Hair TFV levels strongly predicted concurrent virologic suppression among breastfeeding women. Objective adherence metrics can supplement virologic monitoring to optimize treatment outcomes in this important transition period.
Keywords: HIV, adherence, pregnancy, breastfeeding, viral load, hair
INTRODUCTION
Among pregnant and breastfeeding women living with HIV, antiretroviral treatment (ART) optimizes maternal health [1–3] and virtually eliminates the risk of perinatal transmission [4–6]. Realization of these benefits requires satisfactory adherence to ART [7–9], which can be challenging. Identifying suboptimal adherence through self-report is often limited by over-reporting [10], and while viral load testing is increasingly available in sub-Saharan Africa, objective methods to detect suboptimal adherence could facilitate timely intervention to avert virologic failure.
Antiretroviral concentrations in hair reflect exposure to the medication over the prior weeks to months. Several antiretroviral classes (e.g. protease inhibitors, NNRTIs), when measured in hair, strongly predict virologic outcomes among persons living with HIV [11–19]. Tenofovir (TFV) disoproxil fumarate/emtricitabine (TDF/FTC) is used globally for both HIV treatment (in a multicomponent regimen) and prevention (as pre-exposure prophylaxis, PrEP). For persons on PrEP, hair TFV levels predict adherence [20–24] and toxicities [24, 25] but the relationship between hair TFV levels and virologic outcomes in persons living with HIV has not been evaluated.
The Promoting Maternal and Infant Survival Everywhere (PROMISE) studies examined optimal strategies for prevention of perinatal transmission and preservation of maternal health among pregnant and postpartum women living with HIV [1, 4, 6, 26]. In a sample of women enrolled in PROMISE taking TDF/FTC, we examined hair TFV levels as a predictor of concurrent and future virologic outcomes, and explored patterns and predictors of hair levels throughout breastfeeding.
METHODS
Study Population and Procedures
Data are from the antepartum [4] and postpartum [6] components of PROMISE 1077BF, which was conducted at 14 sites in sub-Saharan Africa and India, where breastfeeding is standard. Both components enrolled women between 2011 and 2014 who were not yet ART-eligible (by CD4 cell count per contemporaneous country guidelines). In the antepartum component, 3490 pregnant women were randomized to either zidovudine alone or one of two three-drug ART regimens, one of which contained TDF/FTC. In the postpartum component, 2431 women (95% from the antepartum component) were randomized 6–14 days postpartum to daily infant nevirapine or maternal combination ART (primarily ritonavir-boosted lopinavir with TDF/FTC) over the duration of breastfeeding, up to 18 months. Mothers were followed postpartum at weeks 1, 6, 14, then every 12 weeks. HIV status disclosure and food insecurity [27] were assessed at least every 24 weeks. Viral load was measured at weeks 1, 6, 14, 26, and every 24 weeks thereafter. Hair samples were collected at all visits in a sub-study within the postpartum component.
This analysis includes an initial subset of women with hair samples collected through the end of breastfeeding, selected among the 363 women in the Hair sub-study who were from sub-Saharan Africa, initiated ART in pregnancy, and continued postpartum. All study components were approved by site-specific institutional review boards; all participants provided written informed consent.
Hair collection and analyses
Hair samples of ~20–50 strands were cut from the scalp following published methods [28]. Hair grows approximately one centimeter per month and medications are incorporated from the systemic circulation into hair as it grows [29]. Thus, the centimeter of hair most proximal to the scalp reflects exposures in the month prior to collection, the next centimeter reflects the next prior month, and so on. Hair samples were shipped to and analyzed in the UCSF Hair Analytical Laboratory, where they were cut to 1 or 1.5 cm for those on TDF/FTC for 30–80 days or >80 days, respectively; a few samples had longer lengths assayed when the lab could not determine the proximal end. If samples included any portion unexposed to TDF/FTC, we adjusted concentrations with the following ratio: hair growth period (centimeters*30 days)/days of growth with exposure to TDF/FTC (assumed to start 6 days after initiation to allow TFV levels to build up in the systemic circulation and hair to grow out of the scalp). TFV levels were analyzed using validated liquid chromatography/tandem mass spectrometry (LC-MS/MS)-based methods as described previously [20, 30].
Statistical analysis
For all analyses, TFV levels were log2 transformed. Prior to transformation, those below the limit of detection (0.002 nanograms/milligrams (ng/mg)) were set to the lower limit, and a small constant (0.002) was added to all values to reduce the potential for undue influence from differences between very small values.
Hair TFV levels and viral suppression.
We defined viral suppression as HIV RNA <400 copies/mL, and only included virologic outcomes measured after ≥90 days on ART. The association between hair TFV levels and viral suppression over time was estimated with logistic regression using generalized estimating equations (GEE). Because log2 was used, the estimated odds ratios represent the relative odds of virologic suppression per 2-fold increase in TFV levels. In our primary analysis, we examined hair TFV levels as a predictor of concurrent viral suppression. In a secondary analysis, we examined hair TFV levels as a predictor of future suppression, including all hair samples from participants 1) known to be suppressed prior to hair collection, 2) without a viral load measured 14 days prior through 30 days after hair collection, and 3) with a viral load measured 31–180 days after hair collection. We adjusted for maternal age and time since delivery as potential confounders.
Predictors of hair TFV levels.
Predictors of hair TFV levels were assessed with linear regression using GEE. Because the outcome was log-transformed, we back-transformed coefficients to report fold-effects. Food insecurity and HIV status disclosure, which were not measured at every visit, were carried forward from the last visit collected. We built a multivariate model including all predictors with p<0.25 in univariate analysis.
All analyses were done in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 71 women from Malawi (n=13), South Africa (n=10), Tanzania (n=3), Uganda (n=30), Zambia (n=4), and Zimbabwe (n=11) who initiated ART in pregnancy and continued throughout breastfeeding were included. Baseline clinical and demographic characteristics in this subgroup were comparable to those in the parent PROMISE study at ART initiation [4] and delivery [6]. At enrollment during pregnancy, the median age was 26 years (interquartile range [IQR] 22–30), median CD4 count was 552 cells/mm3 (IQR 472–703), and median viral load was 7,097 copies/mL (IQR 1,317–26,794). At delivery, women had been on ART a median of 12 weeks (IQR 7–18), and during follow-up, women breastfed a median of 14 months (IQR 12–15).
Hair TFV levels across visits.
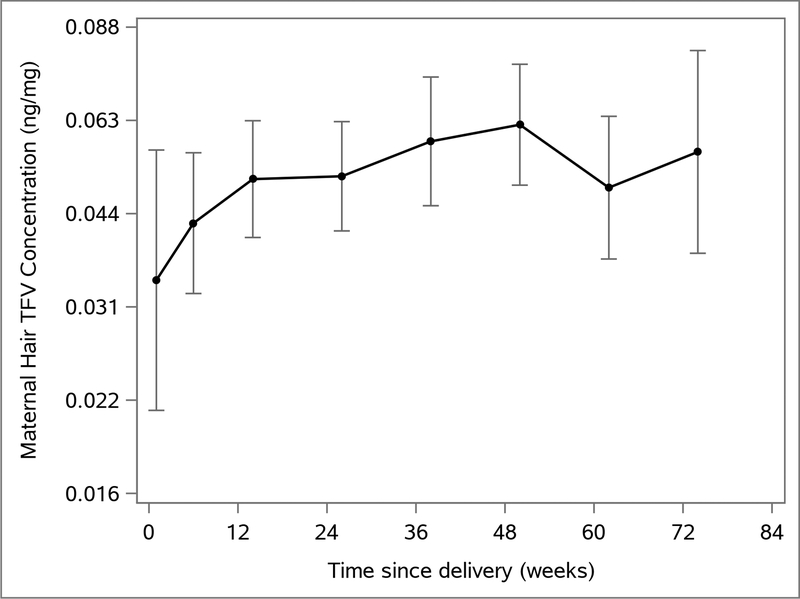
Hair TFV levels were measured at 370 visits (median 6 per woman, range 1–8) postpartum. The geometric mean TFV hair level across all visits was 0.047 (95% CI 0.043–0.052), with values ranging from below the limit of detection (0.002) to 1.067 ng/mg (Table 1). Since most women were switched to TDF/FTC with ritonavir-boosted lopinavir at the one-week postpartum visit, only 23 participants were on TDF/FTC long enough to have hair levels measured one-week postpartum, when levels were lowest (geometric mean 0.032 ng/mg, 95% CI 0.019–0.054; Figure 1). Hair levels were highest at the 50-week visit (geometric mean 0.060 ng/mg, 95% CI 0.047–0.075).
Table 1.
Hair TFV levels and predictors of hair TFV levels throughout breastfeeding in women who initiated ART in pregnancy in the PROMISE Study (N=71).
| Hair TFV levels (ng/mg) throughout breastfeeding (N=370 samples) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Geometric mean (95% CI) | 0.047 (0.043–0.052) | |||||||
| Median (IQR) | 0.052 (0.030–0.086) | |||||||
| Range | 0.002–1.067 | |||||||
| Predictors of hair TFV levels | ||||||||
| Unadjusted | Multivariable | |||||||
| Fold effect | 95% CI | p-value | Fold effect | 95% CI | p-value | |||
| Characteristics at ART initiation | ||||||||
| Maternal age, per year | 0.99 | 0.97 | 1.02 | 0.67 | ||||
| CD4 count, per 100 cells/mm3 | 0.98 | 0.93 | 1.03 | 0.44 | ||||
| HIV RNA, per log10 copies/mL | 1.05 | 0.92 | 1.20 | 0.45 | ||||
| Completed high school (vs. less) | 1.06 | 0.80 | 1.39 | 0.68 | ||||
| COUNTRY | ||||||||
| Uganda (reference) | Overall: 0.07 | Overall: 0.06 | ||||||
| Malawi | 0.76 | 0.48 | 1.19 | 0.23 | 0.78 | 0.47 | 1.25 | 0.30 |
| South Africa | 0.73 | 0.49 | 1.09 | 0.12 | 0.70 | 0.46 | 1.03 | 0.07 |
| Tanzania | 0.51 | 0.38 | 0.69 | <0.0001 | 0.51 | 0.38 | 0.71 | <0.0001 |
| Zambia | 0.61 | 0.39 | 0.97 | 0.03 | 0.62 | 0.38 | 1.01 | 0.05 |
| Zimbabwe | 1.26 | 0.88 | 1.81 | 0.21 | 1.27 | 0.86 | 1.85 | 0.21 |
| Time varying characteristics | ||||||||
| Time since delivery | ||||||||
| 0–90 days (reference) | Overall: 0.02 | Overall: 0.04 | ||||||
| 91–180 days | 1.27 | 0.99 | 1.63 | 0.06 | 1.22 | 0.95 | 1.58 | 0.12 |
| 181–365 days | 1.50 | 1.18 | 1.91 | 0.001 | 1.42 | 1.09 | 1.85 | 0.01 |
| >365 days | 1.28 | 0.97 | 1.69 | 0.09 | 1.30 | 0.89 | 1.61 | 0.24 |
| No longer breastfeeding (vs still) | 0.90 | 0.73 | 1.12 | 0.34 | ||||
| Disclosed to husband/partner | 0.99 | 0.73 | 1.34 | 0.92 | ||||
| Disclosed to someone else in home | 1.12 | 0.88 | 1.42 | 0.36 | ||||
| Food insecurity | ||||||||
| None | Overall: 0.22 | Overall: 0.59 | ||||||
| Moderate | 0.90 | 0.69 | 1.18 | 0.44 | 0.90 | 0.69 | 1.18 | 0.45 |
| Severe | 0.74 | 0.51 | 1.07 | 0.11 | 0.84 | 0.54 | 1.30 | 0.43 |
Figure 1.
Maternal hair TFV levels (geometric means and 95% confidence intervals) over time since delivery among 71 postpartum women in the PROMISE Study.
Hair TFV levels and viral suppression.
After ≥90 days on ART, 69 women had at least one viral load measured (median 5 measures, range 1–9); 18 (26%) experienced viremia at least once (median 8907 copies/mL, range 444–150,122); while 8 (12%) had >1 viral load ≥400 copies/mL. Each doubling of hair TFV level was associated with 2.35 times the odds of concurrent viral suppression (95% CI 1.44–3.84, p=0.0006). The median TFV hair level was 0.054 ng/mg (IQR 0.033–0.086) in those with concurrent virologic suppression and 0.026 ng/mg (IQR 0.007–0.041) in those without suppression (p=0.0006). Among 100 women suppressed prior to hair collection with no concurrent viral load, 4 experienced viremia 30–180 days after hair collection. As expected given this small number of events, hair TFV levels had a very wide confidence interval for prediction of subsequent viral suppression (OR 1.43 per doubling, 95% CI 0.75–2.73, p=0.28).
Predictors of hair TFV levels.
Compared to the first three months postpartum, hair TFV levels were marginally higher in months 3–6 (1.22 fold, 95% CI 0.95–1.58, p=0.12) and highest in months 6–12 (1.42 fold higher, 95% CI 1.09–1.85, p=0.01) in multivariable analysis (Table 1). Relative to participants from Uganda, we observed lower hair TFV levels in women from South Africa (0.70 fold lower, 95% CI 0.46–1.03), Tanzania (0.51 fold, 95% CI 0.38–0.71) and Zambia (0.62 fold, 95% CI 0.38–1.01), though the number of observations from each country was small. Other characteristics did not have substantial associations with hair levels.
DISCUSSON
In this longitudinal study nested within a large multi-site clinical trial primarily conducted in sub-Saharan Africa, we found that hair TFV levels were strongly associated with virologic suppression among postpartum breastfeeding women on TDF/FTC-based ART. The median hair TFV level in suppressed participants was twice that among participants with unsuppressed viral loads. This is the first report to examine the association between hair TFV levels and virologic outcomes in persons living with HIV, building on prior evidence that hair TFV levels reflect adherence to PrEP [20–24].
We observed the lowest hair TFV levels in the early postpartum period, with increases over the duration of breastfeeding. This result is consistent with a study from Malawi, which found lower adherence in the first 3 months postpartum based on pharmacy refill data [31]. The event of childbirth and associated life changes could present barriers to adherence, including disruption of routine and post-delivery time spent with family members where fear of disclosure could interfere with pill-taking. Viremia has been observed in a number of cohorts of postpartum women living with HIV across sub-Saharan Africa [1, 32–35], so early identification of suboptimal adherence during this major transition period could facilitate timely adherence support.
Although pharmacologic adherence metrics are often incorporated into PrEP demonstration projects, the most common way to objectively assess inadequate adherence to ART is by diagnosing virologic failure. Objectively measuring adherence with concurrent viremia could help distinguish between virologic failure due to non-adherence versus resistance [36], avoid switches to more expensive 2nd and 3rd line ART regimens, and facilitate adherence support. Of note, although TDF is still widely used worldwide, tenofovir alafenamide (TAF) is replacing TDF in many settings and its use can also be monitored by measuring hair TFV levels [37].
Our analysis of hair levels as a predictor of future (not concurrent) virologic suppression was limited by a small number of events but suggest a potential association between hair levels and future viremia among previously-suppressed women, warranting further research on the use of objective metrics to predict future suppression. Hair assays using LC-MS/MS are expensive but lower-cost assays are under development [38], and low-cost point-of-care urine-based immunoassays for TFV are now validated [39–41]. Further research is needed to inform the clinical utility and cost-effectiveness of objective adherence metrics, including metrics of both recent and long-term medication use, to supplement virologic monitoring in resource-limited settings.
In conclusion, hair TFV levels were strongly associated with virologic suppression in this sample of postpartum women living with HIV on TDF/FTC-based ART in the PROMISE 1077BF trial. Objective adherence metrics can help distinguish virologic failure due to inadequate adherence from failure due to viral resistance. Low-cost approaches to objective adherence monitoring are urgently needed to optimize maternal health and prevent HIV transmission during breastfeeding.
Funding:
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. Additional funding by NIAID/NIH 2RO1AI098472 (P.I. Gandhi) and NIMH/NIH T32 MH19105–30 (supporting PMM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Study products were provided free of charge by AbbVie, Gilead Sciences, Boehringer Ingelheim, and ViiV/GlaxoSmithKline.
REFERENCES
- 1.Currier JS, Britto P, Hoffman RM, Brummel S, Masheto G, Joao E, et al. Randomized trial of stopping or continuing ART among postpartum women with pre-ART CD4 >/= 400 cells/mm3. PLoS One 2017,12:e0176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.TEMPRANO ANRS Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015,373:808–822. [DOI] [PubMed] [Google Scholar]
- 3.INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015,373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016,375:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet 2012,379:2449–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn PM, Taha TE, Cababasay M, Fowler MG, Mofenson LM, Owor M, et al. Prevention of HIV-1 transmission through breastfeeding: efficacy and safety of maternal antiretroviral therapy versus infant nevirapine prophylaxis for duration of breastfeeding in HIV-1-infected women with high CD4 cell count (IMPAACT PROMISE): A randomized, open-label, clinical trial. J Acquir Immune Defic Syndr 2018,77:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boussari O, Subtil F, Genolini C, Bastard M, Iwaz J, Fonton N, et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Med Res Methodol 2015,15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiwuwa-Muyingo S, Oja H, Walker A, Ilmonen P, Levin J, Mambule, et al. Dynamic logistic regression model and population attributable fraction to investigate the association between adherence, missed visits and mortality: a study of HIV-infected adults surviving the first year of ART. BMC Infect Dis 2013,13:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis NL, Miller WC, Hudgens MG, Chasela CS, Sichali D, Kayira D, et al. Maternal and breastmilk viral load: impacts of adherence on peripartum HIV infections averted-The Breastfeeding, Antiretrovirals, and Nutrition Study. J Acquir Immune Defic Syndr 2016,73:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcaide ML, Ramlagan S, Rodriguez VJ, Cook R, Peltzer K, Weiss SM, et al. Self-report and dry blood spot measurement of antiretroviral medications as markers of adherence in pregnant women in rural South Africa. AIDS Behav 2017,21:2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi M, Ameli N, Bacchetti P, Gange SJ, Anastos K, Levine A, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS 2009,23:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011,52:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011,56:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxi SM, Greenblatt RM, Bacchetti P, Jin C, French AL, Keller MJ, et al. Nevirapine Concentration in Hair Samples Is a Strong Predictor of Virologic Suppression in a Prospective Cohort of HIV-Infected Patients. PLoS One 2015,10:e0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koss CA, Natureeba P, Mwesigwa J, Cohan D, Nzarubara B, Bacchetti P, et al. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS 2015,29:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pintye J, Bacchetti P, Teeraananchai S, Kerr S, Prasitsuebsai W, Singtoroj T, et al. Lopinavir hair concentrations are the strongest predictor of viremia in HIV-infected Asian children and adolescents on second-line antiretroviral therapy. J Acquir Immune Defic Syndr 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chawana TD, Gandhi M, Nathoo K, Ngara B, Louie A, Horng H, et al. Defining a cut-off for atazanavir in hair samples associated with virological failure among adolescents failing second-line antiretroviral treatment. J Acquir Immune Defic Syndr 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasitsuebsai W, Kerr SJ, Truong KH, Ananworanich J, Do VC, Nguyen LV, et al. Using Lopinavir Concentrations in Hair Samples to Assess Treatment Outcomes on Second-Line Regimens Among Asian Children. AIDS Res Hum Retroviruses 2015,31:1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi M, Ofokotun I, Bacchetti P, Jin C, Ribaudo HJ, Haas DW, et al. Antiretroviral concentrations in hair strongly predict virologic response in a large HIV treatment-naive clinical trial. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr 2015,68:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, et al. Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States. Clin Infect Dis 2018,66:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abaasa A, Hendrix C, Gandhi M, Anderson P, Kamali A, Kibengo F, et al. Utility of different adherence measures for PrEP: patterns and incremental value. AIDS Behav 2018,22:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen SE, Sachdev D, Lee SA, Scheer S, Bacon O, Chen MJ, et al. Acquisition of tenofovir-susceptible, emtricitabine-resistant HIV despite high adherence to daily pre-exposure prophylaxis: a case report. Lancet HIV 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandhi M, Murnane PM, Bacchetti P, Elion R, Kolber MA, Cohen SE, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS 2017,31:2245–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV 2016,3:e521–e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taha TE, Brummel S, Angelidou K, Fowler MG, Flynn PM, Mukuzunga C, et al. PROMISE trial: results of continued vs discontinued ART after end of breastfeeding. In: Conference on Retroviruses and Opportunistic Infections Boston, MA; 2018. [Google Scholar]
- 27.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Food Access: Indicator Guide (Version 3) In. Washington, D.C: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [Google Scholar]
- 28.Hickey MD, Salmen CR, Tessler RA, Omollo D, Bacchetti P, Magerenge R, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr 2014,66:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beumer JH, Bosman IJ, Maes RA. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract 2001,55:353–357. [PubMed] [Google Scholar]
- 30.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014,9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas AD, Msukwa MT, Egger M, Tenthani L, Tweya H, Jahn A, et al. Adherence to antiretroviral therapy during and after pregnancy: cohort study on women receiving care in Malawi’s Option B+ Program. Clin Infect Dis 2016,63:1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myer L, Dunning L, Lesosky M, Hsiao NY, Phillips T, Petro G, et al. Frequency of viremic episodes in HIV-infected women initiating antiretroviral therapy during pregnancy: a cohort study. Clin Infect Dis 2017,64:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chetty T, Newell ML, Thorne C, Coutsoudis A. Viraemia before, during and after pregnancy in HIV-infected women on antiretroviral therapy in rural KwaZulu-Natal-South Africa, 2010–2015. Trop Med Int Health 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffman RM, Warshaw M, Amico KR, Pilotto J, Masheto G, Achalapong J, et al. Predictors of virologic failure in postpartum women on ART in PROMISE 1077HS. In: Conference on Retroviruses and Opportunistic Infections Boston, MA; 2018. [Google Scholar]
- 35.Odayar J, Abrams EJ, Zerbe A, Phillips TK, Myer L. Early clinical events after ART initiation in pregnancy influence viral load outcomes In: Conference on Retroviruses and Opportunistic Infections. Boston, MA; 2018. [Google Scholar]
- 36.Hermans LE, Steegen K, ter Heine R, Schuurman R, Tempelman H, Moraba R, et al. PI drug-level testing as a screening tool for drug resistance in 2nd-line ART failure In: Conference on Retroviruses and Opportunistic Infections. Seattle, WA; 2019. [Google Scholar]
- 37.Okochi H, Louie A, Kuncze K, Koss C, Benet L, Phung N, et al. A Method to Analyze Tenofovir Alafenamide (TAF) in Hair for Adherence Monitoring (Abstract A-899-0113-01232) In: 22nd International AIDS Conference (AIDS 2018) Amsterdam, The Netherlands. [Google Scholar]
- 38.Gandhi M, Yang Q, Bacchetti P, Huang Y. A low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses 2014,30:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandhi M, Bacchetti P, Rodrigues WC, Spinelli M, Koss CA, Drain PK, et al. Development and validation of an immunoassay for tenofovir in urine as a real-time metric of antiretroviral adherence. EClinicalMedicine 2018,2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gandhi M, Bacchetti P, Spinelli IM, Okochi H, Baeten JM, Siriprakaisil O, et al. Validation of a Urine Tenofovir Immunoassay for Adherence Monitoring to PrEP and ART and Establishing the Cut-Off for a Point-of-Care Test. J Acquir Immune Defic Syndr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spinelli MA, Glidden DV, Rodrigues W, Wang G, Vincent M, Okochi H, et al. Low tenofovir level in urine by a novel immunoassay is associated with seroconversion in a PrEP demonstration project. AIDS (in press) 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]