Abstract
Injury-induced axon degeneration in model organisms and cell culture has emerged as an area of growing interest due to its experimental tractability and to the promise of identifying conserved mechanisms that mediate axon loss in human disease. Injury-induced axon degeneration is also observed within the well-studied process of Wallerian degeneration, a complex phenomenon triggered by axon injury to peripheral nerves in mammals. Recent studies have led to the identification of key molecular components of injury-induced axon degeneration. Axon survival factors, such as NMNAT2, act to protect injured axons from degeneration. By contrast, factors such as SARM1, MAPK and PHR1 act to promote degeneration. The coordinated activity of these factors determines axon fate after injury. Since axon loss is an early feature of neurodegenerative diseases, it is possible that understanding the molecular mechanism of injury-induced degeneration will lead to new treatments for axon loss in neurodegenerative disease. Here we discuss the critical pathways for injury-induced axon degeneration across species with an emphasis on their interactions in an integrated signaling network.
Introduction:
Axon loss is a prominent feature of various neurological disorders, including neuropathy, injury and degenerative diseases. For example, axon degeneration is an early stage in the dying-back degeneration observed in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis [1–3]. Traumatic brain injury also causes progressive axon loss, which accelerates the onset of other neurological disorders [4], highlighting a central role of axon degeneration. Therefore, it is essential to understand the mechanism of axon degeneration and develop therapies to promote axon survival.
Injury-induced axon degeneration is an excellent model to study axon degeneration. In injury-induced axon degeneration, axons are disconnected from the cell body by experimentally-provoked injury and then degenerate [5]. Unlike disease models, in which degeneration is often gradual and unsynchronized, injury-induced axon degeneration can be induced at a defined time and site for all axons. These features have enabled substantial progress towards the identification of multiple axonal survival factors in both injury and disease conditions.
Injury-induced axon degeneration was first observed by Augustus Waller as part of a larger physiological program triggered by axon injury, now termed Wallerian degeneration [5]. During Wallerian degeneration, the distal axons remain physically intact for about 36hr in mice [6]. After this latent period, the axonal cytoskeleton disintegrates and the axons fragment abruptly. The degeneration is followed by myelin degradation and infiltration of macrophages which clear the debris [7]. Discovery of a spontaneous mouse mutant, WldS, provided the first insight that Wallerian degeneration—and in particular injury-induced axon degeneration in that context—depended on specific cellular mechanisms functioning in neurons. Analysis of this mutant led to discovery of the key survival factor NMNAT. More recently, injury-induced axon degeneration has also been studied in models such as Drosophila and cultured neurons. Work in these models enabled discovery of a key degeneration factor, SARM1. Impressively, elevation of NMNAT and deletion of SARM1 can delay Wallerian degeneration by 2 weeks or longer [8–10]. Further characterization of these genes has begun to describe the molecular mechanisms that mediate degeneration, and revealed that pro- and anti-degenerative programs act in concert to determine the life and death of axons.
Nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) is a crucial axon survival factor
The axon protective WLDS gene product is a chimeric protein comprising an N-terminal fragment of the ubiquitination factor Ube4b fused to the enzyme NMNAT1 [11,12]. Although analysis of WLDS function indicates that its NMNAT1 component mediates axon protection [13–15], endogenous NMNAT1 is localized to the nucleus and—unlike NMNAT1 contained in the WLDS fusion—is not thought to affect axon survival [11]. Fusion with the N-terminal fragment of Ube4b in WLDS confers axonal localization to NMNAT1, and it is the axonal NMNAT1 contained in WLDS that protects axons from degeneration [16]. Consistently, direct transduction of NMNAT1 into early transected axons delays Wallerian degeneration [17]. In fact, overexpression of any of the three mammalian NMNAT isoenzymes can protect axons [18]. Of these three enzymes, it is NMNAT2, a labile axonal protein that undergoes anterograde axonal transport, that is the endogenous axon survival factor [10]. Knock down of NMNAT2 induces axon degeneration in cultured mouse neurons and Drosophila neurons even without injury [10,19]. Low levels of NMNAT2 in mice compromise axon development and increase axon vulnerability to stress [20], consistent with an important axonal function of NMNAT2. Thus, it is likely that the gain of axonal NMNAT1 in WLDS acts by replacing the labile NMNAT2 to protect axons after injury.
NMNAT2 levels in axons are regulated by the balance between continuous anterograde transport from the cell body, and degradation in the axon. The E3 ubiquitin ligase Highwire (in D. melanogaster) or PHR1 (in mouse) promotes NMNAT2 turnover in both normal and injury conditions [21,22]. Loss of Highwire/PHR1 thus leads to increased NMNAT2 levels and prevents axons from degeneration. Similarly, loss of the E3 ubiquitin ligase component SkpA/Skp1a delays Wallerian degeneration by increasing axonal NMNAT2 levels [23,24]. In addition, MAPK signaling speeds the turnover of NMNAT2 and promotes axon degeneration [25]. These degradation pathways establish a surveillance system that ensures healthy axons with abundant NMNAT2 are maintained, while injured axons undergo degeneration rapidly due to NMNAT2 loss.
At the molecular level, NMNAT enzymes catalyze nicotinamide adenine dinucleotide (NAD+) formation from nicotinamide mononucleotide (NMN) and ATP [26] (Figure 1). NMNAT1 enzymatic activity is required for the axon protective role of WLDS [13–15], suggesting that NAD+ or a related metabolite mediates NMNAT function in axon degeneration. As discussed below, the relationship of NAD+ metabolism to axon degeneration is an area of active research.
Figure 1. The NAD+ synthesis and breakdown pathways.
NAD+ can be synthesized from NMN by NMNAT and WLDS, or from NaAD by NAD synthetase. NRK converts NR to NMN and NAR to NaMN. NMN deamidase (NMN DD) is a bacterial enzyme that converts NMN to NaMN. NaMN can further be converted to NaAD by NMNAT. Axon injury activates SARM1, which then cleaves NAD+ into Nam, ADPR and cADPR. Nam is converted to the NAD+ precursor NMN by NAMPT, which can be inhibited by FK866.
SARM1 is an intrinsic trigger of axon self-destruction
SARM1 (sterile alpha and TIR motif–containing 1) was identified in genetic screens for loss-of-function mutants that block injury-induced axon degeneration in Drosophila and cultured mouse neurons [9,27]. SARM1 knockout (KO) in mice leads to robust protection against axon degeneration for over two weeks. SARM1 is also required for vincristine-induced neuropathy and axon degeneration following trophic factor withdrawal in cultured mouse neurons [27]. Recent studies show that SARM1 KO blocks the development of vincristine-induced peripheral neuropathy in mice in vivo [28], but is not sufficient to suppress motor neuron degeneration in a mouse ALS model [29]. Together these studies suggest that SARM1 represents a conserved pro-degenerative program that can be activated by multiple insults.
Structure-function analyses have shed light on the molecular function of SARM1. The SARM1 protein consists of three regions: An N-terminal region containing a mitochondria localization signal and Heat/Armadillo (ARM) domains, two central sterile α motifs (SAM), and a C-terminal Toll/interleukin-1 receptor (TIR) domain [9] (Figure 2). Overexpression of the SAM-TIR region but not full-length SARM1 is sufficient to induce degeneration, indicating that the N-terminal domain is autoinhibitory [27]. Within the SAM-TIR region, both SAM and TIR domains are required for SARM1 activity in degeneration. SAMs typically mediate multimerizations, and TIR domains in other Toll-like receptors dimerize to transmit signals [30]. This suggests that SARM1 function as a multimer. Indeed, pharmacologically induced dimerization of the TIR domains in SARM1 (SARM1-TIR) is sufficient to induce axon degeneration [31]. These findings together lead to a model of SARM1 activation (Figure 2). SARM1 is normally present in the axon in an inactive state. The SAM domains mediate SARM1 multimerization, but dimerization of the TIR domains is blocked, likely due to direct physical interactions with the autoinhibitory N-terminal region [32]. Upon injury, autoinhibition of the TIR domains is released. This allows TIR dimerization and triggers the downstream pro-degenerative program.
Figure 2. SARM1 structure and function.
A. The autoinhibitory SARM1 N terminus contains a mitochondria localization signal and ARM domains. The two SAM domains mediate multimerization. The C terminal TIR domain possesses intrinsic NAD+ cleavage activity.
B. SARM1 forms multimers. The TIR domains are inhibited in normal conditions, possibly through direct interactions with the N terminus. Axon injury activates SARM1 by releasing the auto-inhibition on the TIR domains. Activated TIRs then cleave NAD+ into Nam, ADPR and cADPR and trigger axon degeneration.
How does SARM1 activation lead to axon degeneration? TIR dimerization locally within injured axons causes dramatic and rapid NAD+ loss, followed by ATP depletion, cytoskeletal degradation and axon destruction [31,33]. Degeneration can be completely blocked by expression of NMNAT1 and NAMPT (nicotinamide phosphoribosyltransferase), which together synthesize NAD+ [31]. These results suggest that NAD+ loss triggered by SARM1 activation is pro-degenerative. Since well-characterized TIR domains in Toll-like receptors act as scaffolds to recruit other cytosolic adaptor proteins [30], it has been proposed that dimerized SARM1-TIRs may activate NAD+ consuming enzymes to deplete NAD+. Surprisingly, Essuman et al. recently demonstrated that SARM1-TIR possesses intrinsic NAD+ cleavage activity [34]. Using both purified proteins and a cell-free protein translation system, they showed that SARM1-TIR domains alone are capable of cleaving NAD+ into ADP-ribose (ADPR), cyclic ADPR and nicotinamide (Nam), and this enzymatic activity functions in axons to promote axonal degeneration. Phylogenetic analysis showed that SARM1 is more closely related to bacterial proteins with TIR domains than to other metazoan TIR-containing proteins [35]. Like SARM1, multiple bacterial and archaeal TIR domains have the enzymatic activity to cleave NAD+, suggesting that TIR domain proteins are an ancient family of NAD+ consuming enzymes [36]. SARM1 leverages this primordial function of the TIR domain to trigger axon degeneration.
How injury activates SARM1 is still unclear. Given the importance of NMNAT2 depletion in axon degeneration (discussed above), an appealing hypothesis is that SARM1 activation results from NMNAT2 depletion. Loss of SARM1 rescues the embryonic lethality and axon defects in NMNAT2 knockout mice [37], indicating that SARM1 is required for degeneration due to NMNAT2 loss. A recent study that characterized NAD+ metabolism after axon injury showed that overexpression of cytoplasmic NMNAT1 (cytNMNAT1) abolishes SARM1-dependent NAD+ consumption [38]. Whether NMNAT2 directly or its related metabolites such as NAD+ inhibit SARM1 is unknown. Further studies that examine the relationship between NMNAT2 and SARM1 may help elucidate the mechanism of SARM1 activation.
Multiple axon protective manipulations converge on NAD+ metabolism
Both NMNAT and SARM1 implicate NAD+ metabolism as a critical factor in axon survival. Moreover, other manipulations that affect the NAD+ synthesis pathways also have axon protective activity (Figure 1). Expression of an E. coli NMN deamidase (NMN DD) that converts NMN into NaMN (nicotinic acid mononucleotide) protects axons as well as WLDS [39,40]. Overexpression of the NMN biosynthetic enzyme NAMPT delays degeneration, possibly by facilitating NAD+ synthesis [38]. However, application of the NAMPT inhibitor FK866 also delays degeneration, albeit to a lesser extent [38,40]. Supplying NR (nicotinamide riboside) or NAR (nicotinic acid riboside) to NRK (nicotinamide riboside kinase)-expressing neurons confers mild axon protection as well [38,41]. All of these studies highlight the role of NAD+ metabolism in axon degeneration and survival. The precise mechanisms by which changes in NAD+ metabolism affect axon degeneration are an area of active research.
While many experiments point to NAD+ metabolism as critical for axon survival, a key finding is that steady-state levels of NAD+ before injury do not correlate with axon protection. Manipulations that lead to lower, normal and higher steady-state NAD+ levels are all able to protect axons [11,38]. For example, WLDS mice have unaltered levels of NAD+, but increased protection. Similarly, overexpression of cytNMNAT1 confers protection, but does not increase baseline NAD+. Expression of NMN DD decreases steady-state NAD+, while expression of NAMPT dramatically increases steady-state NAD+; both these manipulations protect axons. In contrast to the lack of correlation with steady-state NAD levels, analysis of NAD+ flux indicates that degeneration is well-correlated with rapid loss of NAD+ after axotomy. These data indicate that NAD+ consumption—presumably by activated SARM—is the key event in triggering degeneration [38].
Besides NAD+, the closely related metabolite NMN may also be a key factor in degeneration. NMNAT2 loss in the distal axon after injury leads not only to NAD+ depletion, but also to accumulation of its substrate, NMN [38]. It has been proposed that increased NMN levels are toxic and can promote axon degeneration. In support of this hypothesis, FK866, an inhibitor of NAMPT (which synthesizes NMN), delays Wallerian degeneration [38,40,42]. Further, converting NMN into NaMN by NMN DD confers axon protection [40]. More recently, NMN DD overexpression was shown to delay injury-induced axon degeneration in zebrafish larvae and transgenic mice by reducing NMN accumulation [39]. NMN DD also rescues axonal outgrowth defects and perinatal lethality caused by lack of NMNAT2 in mice. These studies strongly suggest that accumulation of NMN is toxic and pro-degenerative. However, Sasaki et al. examined multiple NAD+ related metabolites after axon injury and found that NMN levels do not correlate with axon degeneration [38]. At present, while questions remain about the precise relationship between NAD+ metabolism and axon survival, it is clear that NMN and NAD+ are key factors. Time-dependent measurements of NMN and other metabolites after axotomy may help determine the precise relationship between the flux of additional metabolites and axon survival.
MAPK and other pathways that regulate axon degeneration
MAPK signaling in axon degeneration was first indicated by the finding that the MAPKKK DLK/MAP3K12 functions in injury-induced degeneration in cultured neurons [43]. Later, two additional MAPKKKs, MEKK4/MAP3K4 and MLK2/MAP3K10, were found to act redundantly with DLK [44]. These three MAP3Ks converge on the JNK pathway, which is also inhibited by AKT. One target of JNKs is the microtubule binding protein SCG10 (stathmin 2) [45]. Phosphorylation by JNKs targets SCG10 for degradation, leading to axon dismantling. These studies suggest that SCG10 acts as another axon survival factor negatively regulated by MAPK signaling.
The position of the MAPK pathway in relation to SARM1 and NMNAT2 has been investigated. In cultured mouse neurons, phosphorylation of MKK4 and JNKs downstream of the MAP3Ks depends on SARM1 [44]. Axon degeneration induced by dimerization of SARM1-TIRs is also partially blocked by MKK4/7 depletion [44]. These data place MAPK downstream of SARM1. However, a recent study suggests that MAPK signaling occurs upstream of SARM1 in cultured DRG neurons by speeding the turnover of NMNAT2 [25]. Surprisingly, in Drosophila, null alleles of many MAPK genes, including the single JNK ortholog Bsk, do not suppress degeneration induced by axotomy or dSARM dimerization [19]. This study argues against the requirement of JNK signaling in axon degeneration in vivo. Further studies are needed to elucidate the role of the MAPK signaling pathway in mouse in vivo.
Recent genetic studies have also identified other genes involved in axon degeneration [19,46,47]. Their functions and their relationships to the NMNAT2/SARM1/MAPK program are currently under exploration. One exciting example is Axed (Axundead) in Drosophila [19]. Loss of Axed is able to block axon degeneration induced by axotomy, NMNAT knockdown and SARM1-TIR dimerization in vivo, suggesting it is the downstream convergence point of multiple axon degeneration pathways. Axed encodes a BTB (bric-a-brac, tramtrack, broad complex)/BACK (BTB and C-terminal Kelch) domain-containing protein. How Axed is activated and how it mediates degeneration remain unclear. Further investigation of Axed may provide insights on axon degeneration mechanisms.
Axon degeneration after laser axotomy in C. elegans relies on an unknown mechanism
Robust injury-induced axon degeneration of severed axons is observed in flies, zebrafish, mice, rats and humans. Degeneration in these organisms shares both morphological and mechanistic features, suggesting a common and ancient mechanism for degeneration [18]. Surprisingly, in C. elegans, axon degeneration induced by laser axotomy appears largely distinct from Wallerian degeneration. First, degeneration is developmentally regulated. Degeneration of sensory and motor neurons after laser axotomy is prominent in L1 (early larval) animals, but is greatly reduced in L4 (late larval) animals and adulthood [48]. Second, even when axon degeneration occurs in L1 animals, axon protective manipulations that are effective in other organisms fail to prevent axon degeneration after laser axotomy [48]. These include overexpression of WldS and C. elegans Nmnat genes, as well as knockout of the C. elegans homologs of Dlk, Sarm1 and Highwire. Thus, axon degeneration after laser axotomy in C. elegans suggests that an unidentified pro-degenerative program exists.
Another unusual feature of axon degeneration in C. elegans is the role of RIC-7. RIC-7 is a nematode-specific protein that functions in neuropeptide secretion and mitochondria trafficking [49,50]. In animals that lack RIC-7, axons degenerate robustly after laser axotomy, and axons of a few neuron types degenerate even without experimental injury. Experiments that manipulate mitochondrial localization suggest that the primary driver of altered degeneration in ric-7 mutants is mitochondria mislocalization [50], but the link between mitochondria and axon degeneration is not known. Understanding the precise mechanism of axon degeneration in neurons that lack RIC-7 could help decipher the C. elegans axon degeneration program.
Why is axon degeneration normally so slow in C. elegans? One intriguing possibility is that preventing degeneration may support the ability of C. elegans to repair injured axons by fusion. In C. elegans, an injured axon can fuse with its detached distal axon segment and completely restore circuit function [51– 53]. Axon fusion is mediated by the C. elegans specific fusogen EFF-1 [52], and so far, no evidence of axon fusion in mammalian systems has been reported. Functionally, fusion is an efficient way to reconnect the circuit after injury, since the injured axon does not need to regenerate or to rebuild synapses. By contrast, when measured at the single neuron level, conventional regeneration fails to restore normally-functioning circuits [54]. Thus, limiting degeneration may allow C. elegans to bias regenerative outcomes toward fusion, rather than regrowth.
Conclusions and future directions
Significant progress has been made recently in understanding injury-induced axon degeneration. While many gaps remain, the identification of key factors—such as NMNAT2, SARM1 and MAPK—establishes a framework for understanding the molecular mechanisms operating in injured neurons (Figure 3). Describing the precise relationship of these and other factors to each other, analyzing how these factors impinge on NAD metabolism, and deciphering how NAD metabolism in turn affects axons, are key questions for future study. In the long term, a better understanding of injury-induced axon degeneration will help answer the broader question of how the mechanisms of injury-induced axon degeneration relate or even contribute to axon pathology observed in disease.
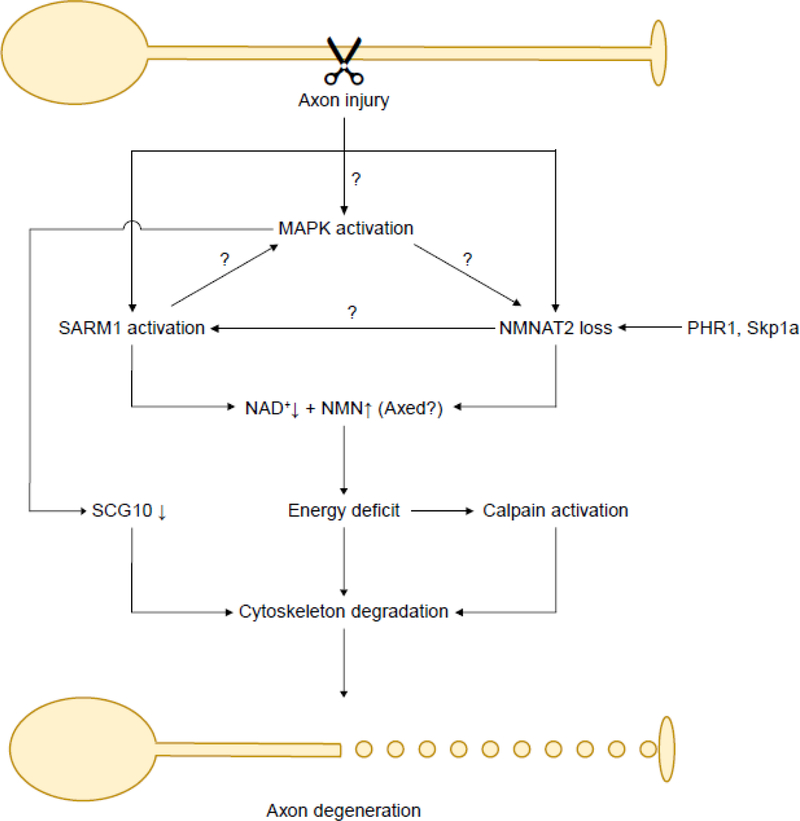
Figure 3. An integrated model of axon degeneration pathways.
Axon injury triggers NMNAT2 loss, SARM1 activation and MAPK activation in the axon. PHR1 and Skp1a also speed NMNAT2 turnover. Depletion of NMNAT2 may lead to SARM1 activation. The position of MAPK pathway in relation to SARM1 and NMNAT2 is still controversial. Nevertheless, MAPK activation results in SCG10 loss. SARM1 activation and NMNAT2 loss together lead to NAD+ depletion and NMN accumulation in the axon, which then causes ATP depletion and energy deficit. Axed is a convergence point downstream of SARM1 and NMNAT2 in flies, but its exact function is still unclear. Energetic failure leads to calpain activation. These pathways then culminate in cytoskeleton degradation and eventually lead to irreversible fragmentation of the injured axon.
Highlights:
Injury-induced axon degeneration is a powerful model for discovery of mechanisms regulating axon fate.
Axon fate is determined by opposing activities that promote axon survival and self-destruction.
These mechanisms converge on NAD+ metabolism to determine axon survival and death.
Promoting axon survival might be an effective therapy for neurological diseases.
Footnotes
Declarations of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Annotated references
• of special interest
•• of outstanding interest
References
- 1.Kanaan NM, Pigino GF, Brady ST, Lazarov O, Binder LI, Morfini GA: Axonal degeneration in Alzheimer’s disease: when signaling abnormalities meet the axonal transport system. Exp Neurol 2013, 246:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dadon-Nachum M, Melamed E, Offen D: The “dying-back” phenomenon of motor neurons in ALS. J Mol Neurosci 2011, 43:470–477. [DOI] [PubMed] [Google Scholar]
- 3.Tagliaferro P, Burke RE: Retrograde Axonal Degeneration in Parkinson Disease. J Parkinsons Dis 2016, 6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson VE, Stewart W, Smith DH: Axonal pathology in traumatic brain injury. Exp Neurol 2013, 246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waller A: Experiments on the Section of the Glossopharyngeal and Hypoglossal Nerves of the Frog, and Observations of the Alterations Produced Thereby in the Structure of Their Primitive Fibres. Philosophical Transactions of the Royal Society of London 1850, 140:423–429. [Google Scholar]
- 6.Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP: The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci 2005, 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas ME, Barres BA: Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci 2007, 30:153–179. [DOI] [PubMed] [Google Scholar]
- 8.Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S: Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. European Journal of Neuroscience 1989, 1:27–33. [DOI] [PubMed] [Google Scholar]
- 9.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. : dSarm/Sarm1 Is Required for Activation of an Injury-Induced Axon Death Pathway. Science 2012, 337:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilley J, Coleman MP: Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol 2010, 8:e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, et al. : Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci 2001, 4:1199–1206. [DOI] [PubMed] [Google Scholar]
- 12.Conforti L, Tarlton A, Mack TGA, Mi W, Buckmaster EA, Wagner D, Perry VH, Coleman MP: A Ufd2/D4Cole1 chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proceedings of the National Academy of Sciences 2000, 97:11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araki T, Sasaki Y, Milbrandt J: Increased Nuclear NAD Biosynthesis and SIRT1 Activation Prevent Axonal Degeneration. Science 2004, 305:1010. [DOI] [PubMed] [Google Scholar]
- 14.Avery MA, Sheehan AE, Kerr KS, Wang J, Freeman MR: WldS requires Nmnat1 enzymatic activity and N16–VCP interactions to suppress Wallerian degeneration. The Journal of Cell Biology 2009, 184:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conforti L, Wilbrey A, Morreale G, Janeckova L, Beirowski B, Adalbert R, Mazzola F, Di Stefano M, Hartley R, Babetto E, et al. : WldS protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. The Journal of Cell Biology 2009, 184:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Ghosh AK, Kim HJ, Jeon NL, Jaffrey SR: Chemical genetic-mediated spatial regulation of protein expression in neurons reveals an axonal function for WldS. Chem Biol 2012, 19:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki Y, Milbrandt J: Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J Biol Chem 2010, 285:41211–41215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conforti L, Gilley J, Coleman MP: Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci 2014, 15:394–409. [DOI] [PubMed] [Google Scholar]
- ••19.Neukomm LJ, Burdett TC, Seeds AM, Hampel S, Coutinho-Budd JC, Farley JE, Wong J, Karadeniz YB, Osterloh JM, Sheehan AE, et al. : Axon Death Pathways Converge on Axundead to Promote Functional and Structural Axon Disassembly. Neuron 2017, 95:78–91 e75.This study identifies axundead (axed) in a forward genetic screen using sensory neurons of the Drosophila wing. Axed is a BTB/BACK domain-containing protein that localizes to neurites and synapses. Axed is required for axon degeneration induced by injury, loss of NMNAT and overexpression of constitutively active dSarm. The study also shows that loss of Bsk, the only JNK ortholog in Drosophila, does not block axon degeneration after injury or overexpression of active dSarm. This result argues against a central role of JNK signaling in axon degeneration in vivo.
- 20.Gilley J, Mayer P, Yu G, Coleman MP: Low levels of NMNAT2 compromise axon development and survival. Human Molecular Genetics 2018:ddy356–ddy356. [DOI] [PubMed]
- 21.Xiong X, Hao Y, Sun K, Li J, Li X, Mishra B, Soppina P, Wu C, Hume RI, Collins CA: The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol 2012, 10:e1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A: The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep 2013, 3:1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamagishi Y, Tessier-Lavigne M: An Atypical SCF-like Ubiquitin Ligase Complex Promotes Wallerian Degeneration through Regulation of Axonal Nmnat2. Cell Rep 2016, 17:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brace EJ, Wu C, Valakh V, DiAntonio A: SkpA restrains synaptic terminal growth during development and promotes axonal degeneration following injury. J Neurosci 2014, 34:8398–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •25.Walker LJ, Summers DW, Sasaki Y, Brace EJ, Milbrandt J, DiAntonio A: MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 2017, 6.This study demonstrates that MAPK promotes the turnover of NMNAT2. Knockdown of MKK4/7 increases NMNAT2 levels. The study also suggests that MAPK is upstream of SARM1, as knockdown of MKK4/7 does not delay axon degeneration and NAD+ loss induced by constitutively active SARM1.
- 26.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S: Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci 2004, 61:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J: Sarm1-Mediated Axon Degeneration Requires Both SAM and TIR Interactions. The Journal of Neuroscience 2013, 33:13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geisler S, Doan RA, Strickland A, Huang X, Milbrandt J, DiAntonio A: Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 2016, 139:3092–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters OM, Lewis EA, Osterloh JM, Weiss A, Salameh JS, Metterville J, Brown RH, Freeman MR: Loss of Sarm1 does not suppress motor neuron degeneration in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Hum Mol Genet 2018. [DOI] [PMC free article] [PubMed]
- 30.Narayanan KB, Park HH: Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis 2015, 20:196–209. [DOI] [PubMed] [Google Scholar]
- ••31.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J: SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 2015, 348:453.This study describes engineered SARM1 that can be activated or inhibited by drugs. Using these tools, it is found that SARM1 activation is required for axon degeneration, and that dimerization of the SARM1-TIR domains is sufficient to induce axon degeneration. Further, axon degeneration caused by SARM1-TIR dimerization correlates with NAD+ loss.
- 32.Summers DW, Gibson DA, DiAntonio A, Milbrandt J: SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injury-induced SARM1 activation. Proc Natl Acad Sci U S A 2016, 113:E6271–E6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M: Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron 2013, 80:1175–1189. [DOI] [PubMed] [Google Scholar]
- ••34.Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J: The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD+ Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron 2017, 93:1334–1343 e1335.This study shows that the TIR domain of SARM1 is an enzyme that can cleave NAD+ into Nam, ADPR and cADPR. This enzymatic activity is required for axon degeneration.
- 35.Zhang Q, Zmasek CM, Cai X, Godzik A: TIR domain-containing adaptor SARM is a late addition to the ongoing microbe-host dialog. Dev Comp Immunol 2011, 35:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Essuman K, Summers DW, Sasaki Y, Mao X, Yim AKY, DiAntonio A, Milbrandt J: TIR Domain Proteins Are an Ancient Family of NAD+-Consuming Enzymes. Curr Biol 2018, 28:421–430 e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilley J, Orsomando G, Nascimento-Ferreira I, Coleman MP: Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep 2015, 10:1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••38.Sasaki Y, Nakagawa T, Mao X, DiAntonio A, Milbrandt J: NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD+ depletion. Elife 2016, 5.This study measures NAD+ metabolites in healthy and injured axons using both steady-state and flux analysis. Absolute values of NAD+ and NMN are shown not to correlate with axon degeneration. Rather, degeneration correlates with NAD+ consumption. In addition, the study suggests that NMNAT and NMN DD likely inhibit SARM1 induced NAD+ loss after injury.
- •39.Di Stefano M, Loreto A, Orsomando G, Mori V, Zamporlini F, Hulse RP, Webster J, Donaldson LF, Gering M, Raffaelli N, et al. : NMN Deamidase Delays Wallerian Degeneration and Rescues Axonal Defects Caused by NMNAT2 Deficiency In Vivo. Curr Biol 2017, 27:784–794.This study shows that bacterial NMN DD can delay Wallerian degeneration in vivo in zebrafish and mice. NMN DD also rescues axonal outgrowth defects and lethality in mice lacking NMNAT2. This study supports a pro-degenerative effect of accumulating NMN in axons.
- 40.Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, et al. : A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ 2015, 22:731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu HW, Smith CB, Schmidt MS, Cambronne XA, Cohen MS, Migaud ME, Brenner C, Goodman RH: Pharmacological bypass of NAD+ salvage pathway protects neurons from chemotherapy-induced degeneration. Proc Natl Acad Sci U S A 2018. [DOI] [PMC free article] [PubMed]
- 42.Sasaki Y, Vohra BP, Lund FE, Milbrandt J: Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci 2009, 29:5525–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A: A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 2009, 12:387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •44.Yang J, Wu Z, Renier N, Simon DJ, Uryu K, Park DS, Greer PA, Tournier C, Davis RJ, Tessier-Lavigne M: Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 2015, 160:161–176.This study examines the role of MAPK signaling in Wallerian degeneration using cultured neurons. MAPK is found to be active in the early phase of axon degeneration. MAPK activation requires SARM1 and is inhibited by cytNMNAT1, suggesting that MAPK is downstream of SARM1 and NMNAT.
- 45.Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A: SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci U S A 2012, 109:E3696–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farley JE, Burdett TC, Barria R, Neukomm LJ, Kenna KP, Landers JE, Freeman MR: Transcription factor Pebbled/RREB1 regulates injury-induced axon degeneration. Proc Natl Acad Sci U S A 2018, 115:1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karney-Grobe S, Russo A, Frey E, Milbrandt J, DiAntonio A: HSP90 is a chaperone for DLK and is required for axon injury signaling. Proc Natl Acad Sci U S A 2018, 115:E9899–E9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •48.Nichols ALA, Meelkop E, Linton C, Giordano-Santini R, Sullivan RK, Donato A, Nolan C, Hall DH, Xue D, Neumann B, et al. : The Apoptotic Engulfment Machinery Regulates Axonal Degeneration in C. elegans Neurons. Cell Rep 2016, 14:1673–1683.This study demonstrates that laser-induced axon degeneration in L1 C. elegans cannot be delayed by overexpression of WLDS, NMNATs or loss of the SARM1 ortholog tir-1. Axon degeneration is regulated by the apoptotic engulfment machinery. However, the axon intrinsic self-destruction program is still unknown.
- 49.Hao Y, Hu Z, Sieburth D, Kaplan JM: RIC-7 promotes neuropeptide secretion. PLoS Genet 2012, 8:e1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rawson RL, Yam L, Weimer RM, Bend EG, Hartwieg E, Horvitz HR, Clark SG, Jorgensen EM: Axons degenerate in the absence of mitochondria in C. elegans. Curr Biol 2014, 24:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abay ZC, Wong MY, Teoh JS, Vijayaraghavan T, Hilliard MA, Neumann B: Phosphatidylserine save-me signals drive functional recovery of severed axons in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2017, 114:E10196–E10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumann B, Coakley S, Giordano-Santini R, Linton C, Lee ES, Nakagawa A, Xue D, Hilliard MA: EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature 2015, 517:219–222. [DOI] [PubMed] [Google Scholar]
- 53.Neumann B, Nguyen KCQ, Hall DH, Ben-Yakar A, Hilliard MA: Axonal regeneration proceeds through specific axonal fusion in transected C. elegans neurons. Developmental Dynamics 2011, 240:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding C, Hammarlund M: Aberrant information transfer interferes with functional axon regeneration. eLife 2018, 7:e38829. [DOI] [PMC free article] [PubMed] [Google Scholar]