Abstract
Food addiction (FA) describes a group of disordered eating behaviors. Childhood trauma has been associated with adult FA and trauma has known effects on the endocrine system, but it is unclear whether FA is associated with insulin resistance. We hypothesized that severity of childhood trauma will be associated with FA and higher insulin resistance (HOMA-IR) in a sample of obese women with type 2 diabetes mellitus (T2DM), and that FA will mediate the association between childhood trauma and HOMA-IR. Women with a diagnosis of T2DM (N=73; MBMI=35.86, SDBMI=7.72; Mage=50.59, SDage=9.72) were recruited from a diabetes clinic at a county hospital. Participants completed the Childhood Trauma Questionnaire and the Yale Food Addiction Scale. Fasting blood samples were obtained from 64 participants to assess plasma hemoglobin A1c (HbA1c), insulin and glucose (used to calculate HOMA-IR); Oral Glucose Tolerance Test (OGTT) was performed to measure change in glucose and insulin secretion. 48% of the sample met diagnostic criteria for FA. Women with FA reported significantly higher HOMA-IR (F=25.692, p<0.001, df=1,62), HbA1c (F=4.358, p=0.041, df=1,62), and OGGT glucose (F=5.539, p=0.022, df=1,62) as well as severity of childhood trauma (F=10.453, p=0.002, df=1,71). In a hierarchical linear regression controlling for BMI, income level, and T2DM treatment, the severity of childhood trauma did not contribute to the prediction of HOMA-IR (β=−0.011, p=0.942) whereas FA did (β=0.422, p=0.007). In a bootstrapped mediation analysis, the association between childhood trauma and HOMA-IR was mediated by FA severity (b=0.596, p=0.020). Understanding the psychological factors that contribute to HOMA-IR in an underserved population of African American women may lead to more effective diabetes management and prevention strategies.
Keywords: Food addiction, disordered eating, insulin resistance, diabetes, childhood trauma, childhood abuse
1. Introduction
Childhood trauma encompasses a range of maltreatment behaviors, including sexual abuse, physical abuse and neglect, and emotional abuse and neglect (Butchart, Harvey, Mian, & Furniss, 2006; Norman et al., 2012). Other traumatic events common in childhood include exposure to community and domestic violence, bullying, and motor vehicle accidents (De Bellis & Zisk, 2014). Childhood trauma is associated with adverse behavioral health outcomes later in life (Lindert et al., 2014; Nemeroff & Binder, 2014) as well as higher rates of anxiety disorders, mood disorders, and PTSD in adulthood (Brewin, Andrews, & Valentine, 2000; Infurna et al., 2016; Lindert et al., 2014). Additionally, individuals who experience childhood trauma are at higher risk for disordered eating behaviors as well as substance use disorders (Fischer, Stojek, & Hartzell, 2010; Michopoulos et al., 2015; Simpson & Miller, 2002; Wonderlich et al., 2001) that are linked to obesity and cardiometabolic risk (Kessler et al., 2013; Micali et al., 2015; Succurro et al., 2015).
Type 2 diabetes mellitus (T2DM) and associated insulin resistance (IR) contribute to risk for cardiovascular disease and increased mortality (Huxley, Barzi, & Woodward, 2006; Pollare, Lithell, & Berne, 1990). For instance, the risk of fatal coronary artery disease has been found to be significantly higher in women with T2DM (Huxley et al., 2006). African American (AA) women are almost twice as likely to have T2DM compared to Caucasian women and the prevalence of T2DM in AA women is one of the highest out of all racial groups (Centers for Disease Control, 2017). While susceptibility to T2DM and the metabolic syndrome is in part explained by obesity, stressful life events have also been associated with, and predictive of, increased insulin concentration and IR (Mooy, de Vries, Grootenhuis, Bouter, & Heine, 2000). Given the known effects of lifetime trauma on neurobiological and endocrine systems (such as the hypothalamic-pituitary-adrenal axis) (Heim, Shugart, Craighead, & Nemeroff, 2010; Nemeroff & Seligman, 2013), it is possible that there is a link between the experience of childhood trauma and impaired insulin sensitivity. For example, PTSD is associated with greater systemic inflammation which may be one mechanism that confers increased risk for metabolic syndrome (Michopoulos, Vester, & Neigh, 2016; Rohleder & Karl, 2006; Spitzer et al., 2010). While childhood trauma appears to be associated with obesity, the evidence for the association between childhood trauma and T2DM is minimal and equivocal (Norman et al., 2012).
In recent years, calorie dense (i.e., high-fat, high-sugar) food has been conceptualized as having behavioral reinforcement properties similar to those observed with substances of abuse (Gearhardt et al., 2011; Gearhardt, Corbin, & Brownell, 2009). Food addiction (FA) describes a group of disordered eating behaviors, such as binge eating, that are phenomenologically similar to behaviors conventionally associated with substance dependence (Gearhardt et al., 2009). A cross-sectional study found a positive relationship between severity of childhood physical and sexual abuse and the presence of FA in adult women (Mason, Flint, Field, Austin, & Rich-Edwards, 2013). Lifetime presence of at least one PTSD symptom and exposure to traumatic events have also been associated with higher prevalence of FA in multiple studies (Hardy, Fani, Jovanovic, & Michopoulos, 2018; Mason et al., 2014). More specifically, PTSD is more strongly related to FA when exposure to trauma occurred at an earlier age in childhood (Mason et al., 2014). As expected, FA is associated with body mass index (BMI) and binge eating disorder (Meule & Gearhardt, 2014; Murphy, Stojek, & MacKillop, 2014; Pursey, Stanwell, Gearhardt, Collins, & Burrows, 2014), but it remains unclear whether FA contributes to metabolic dysfunction.
The current study aimed to clarify the associations between severity of childhood trauma exposure, adult FA severity and IR in a highly traumatized sample of AA women with T2DM. We hypothesized that participants with FA will exhibit significantly higher insulin resistance compared to participants without FA in a sample of obese AA women with T2DM. We hypothesized that women with current FA will report greater childhood trauma severity compared to those without FA. We also hypothesized that both, childhood trauma and FA would be associated with higher IR. Our exploratory aim was to examine the indirect association between childhood trauma severity and current IR via current FA severity.
2. Methods and Materials
2.1. Participants and Procedure
Participants were drawn from a study of PTSD risk factors in a low socioeconomic, urban minority sample of women. Participants were recruited from waiting rooms in the diabetes, gynecology, and primary care medical clinics at a publicly funded hospital in Atlanta, Georgia. We did not narrow recruitment to specific criteria but approached randomly any woman in the waiting room between 9AM and 4PM Monday through Friday. Eligibility requirements for the study included the ability to give informed consent, having a phone number by which they could be contacted, and the ability to speak and understand English. Additionally, participants had to be between the ages of 18 and 65 (see Gillespie et al., 2009 for full details regarding study procedures). Written and verbal informed consent was obtained for all participants. Women who consented completed a number of self-report measures (described below) that took between one and two hours to complete, depending on the participant’s self-report of their trauma history and psychiatric symptoms.
Study participants with T2DM were offered the opportunity to participate further in a separate associated study by providing a fasted blood sample and undergoing a structured clinical interview. Exclusion criteria included developmental delay, bipolar or psychotic disorder, treatment for an autoimmune disorder, cancer, or HIV, treatment with prescription oral non-steroidal anti-inflammatory, corticosteroid, anticonvulsant (other than gabapentin’{1), antipsychotic drugs, or benzodiazepine class pharmaceuticals. T2DM status was determined for each participant by electronic medical record review. The Emory Institutional Review Board and the Research Oversight Committee of Grady Memorial Hospital, Atlanta, GA approved all study procedures.
2.2. Measures
Body mass index (BMI).
BMI was calculated based on participant’s height and weight measured at the time of study interview and calculated using the standard equation for females (kg)/(height (m))2. Participants were weighed without their shoes on, using the same clinic scale. The clinic scales are inspected and calibrated annually. All participants were weighed in the morning, before consuming glucose.
Demographics and medication.
Level of education, household monthly income, and employment status were assessed using a demographics form. Electronic medical record review was conducted to assess whether participants were receiving T2DM treatment. The following medications for the management of T2DM were included in the medical record review: metformin, insulin, bromocriptine, nateglinide, glimepride, glipizide, glyburide, and pioglitazone.
Yale Food Addiction Scale (YFAS; Gearhardt, Corbin, & Brownell, 2009).
The YFAS is a 25-item measure which assesses addictive eating behavior such as reduced involvement in social, occupational and recreational pursuits due to addictive eating (e.g., there have been times when I avoided professional or social situations because I was not able to consume certain foods there) and food tolerance (e.g., over time, I have found that I need to eat more and more to get the feeling I want, such as reduced negative emotions or increased pleasure). Responses are used to calculate a symptom count of FA symptoms ranging from zero to seven. A diagnosis of FA can be made if an individual endorses three or more symptoms and indicates clinically significant impairment. The YFAS has good internal consistency, convergent validity with related measures of eating behavior, and discriminant validity with measures of substance use (Gearhardt et al., 2009).
Childhood Trauma Questionnaire (CTQ; Bernstein & Fink, 1998).
The CTQ is a 28-item measure used to assess childhood traumatic experiences. Scale scores are calculated for sexual abuse, emotional abuse, physical abuse, and emotional and physical neglect, and range from “minimal” to “moderate to severe.” A total CTQ score is also calculated based on all types of childhood abuse. CTQ was validated in clinical and community samples of adolescents and adults (Bernstein, Ahluvalia, Pogge, & Handelsman, 1997; Scher, Stein, Asmundson, McCreary, & Forde, 2001), and demonstrated good internal consistency, and good convergent validity with clinician-administered interview of traumatic experiences (Bernstein et al., 1997).
Insulin resistance.
Fasting blood samples were obtained for measurement of plasma glucose (mg/dL) and serum insulin (μIU/mL). Glucose concentrations were measured by colorimetric method using Sekisui Diagnostics. Insulin concentrations were determined using an immunoturbidometric using reagents and calibrators from Kamiya (Sekisui Diagnostics, Exton, PA). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as the product of fasting plasma glucose concentration (mg/dL) and fasting serum insulin concentration (μIU/mL) divided by 405 (Matthews et al., 1985).
Hemoglobin A1c (HbA1c).
Whole blood samples were stored at −80°C until the time of assay. HbA1c was measured using high performance liquid chromatography by ARUP laboratories (Salt Lake City, Utah).
Oral Glucose Tolerance Test (OGTT).
Plasma glucose and insulin response during the two hour OGTT reflect the ability of pancreatic β-cells to secrete insulin (DeFronzo, 1988; Matsuda & DeFronzo, 1999). During the OGTT, blood samples were obtained at 0, 15, 30, 60, 90, and 120 minutes following oral administration of 75-gram glucose solution for measurement of plasma glucose and insulin. Patients had to consume the glucose in less than 5 minutes. Area under the curve ground (AUCg) (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) was calculated to quantify the change in secretion of plasma glucose and insulin over time during the OGTT.
2.3. Statistical Analysis
All analyses were conducted using SPSS for Windows version 24 (SPSS, Inc., Chicago, IL). Data were examined for normality. To satisfy assumptions of normality, HOMA-IR and Childhood Trauma Questionnaire (CTQ) total score and subscales were log (base 10) transformed. Unless otherwise specified, all subsequent analyses utilize transformed data. To maximize statistical power, cases were deleted pairwise if there were missing data (Peugh & Enders, 2004). Mean age, BMI, level of education, household monthly income, unemployment status, and current T2DM treatment with any of the medications identified during the medical chart review (i.e., metformin, insulin, bromocriptine, nateglinide, glimepride, glipizide, glyburide, pioglitazone, or a combination of these medications) were examined for participants with and without FA, and chi squared analysis for the categorical variables (i.e., level of education, household monthly income, unemployment status, current T2DM treatment) or t-tests for the continuous variables (i.e., age and BMI) were conducted to determine whether groups differed at baseline on any demographic variables. To examine between-group differences in childhood trauma severity, HOMA-IR, baseline HbA1c and glucose and insulin secretion AUCg during the OGTT, one-way ANOVAs were conducted with FA diagnosis status (FA vs. non-FA) as the between-group factor. To examine the relative contributions of childhood trauma and FA to the prediction of current HOMA-IR, hierarchical linear regression was conducted. BMI, income, and current T2DM treatment (combined metformin and insulin treatment2) were entered in the first step of the model to statistically control for their potential effects on insulin resistance, severity of childhood trauma was entered in the second step of the model, and the FA symptom count was entered in the third step of the model.
To evaluate the indirect contribution of childhood trauma to HOMA-IR, a mediation analysis was conducted using the PROCESS macro version 2.13 developed by Hayes (2013). Mediation analyses examine the hypotheses about whether there may be an intervening variable between a proposed cause and a proposed outcome. We examined a mediation model where the proposed cause was childhood trauma exposure measured via the total CTQ score, the outcome variable was the severity of IR measured via HOMA-IR, and the proposed mediator was severity of FA measured via the YFAS total score (Model 4 in PROCESS); we included BMI, income and current T2DM treatment as covariates. We used a percentile bootstrap confidence interval with 5,000 resamples to make inferential decisions about the significance of these indirect effects (Hayes & Scharkow, 2013; Preacher & Hayes, 2004).
3. Results
3.1. Descriptive Statistics
Of the 73 women with T2DM who completed all the surveys, 35 women (48%) met diagnostic criteria for FA. Descriptive statistics for the total sample, for the subsample of women with the FA diagnosis and a subsample of women without the FA diagnosis are reported in Table 1. The FA group had a statistically significantly higher BMI than the non-FA group (p=0.010); therefore, BMI was entered as a covariate into the subsequent analyses. There were no other statistically significant differences between groups on the sociodemographic variables (Table 1). In the subsequent analyses, variables were deleted pairwise when there were missing data. Therefore, sample sizes are reported for each analysis.
Table 1.
Descriptive statistics for a sample of African American women with type 2 diabetes mellitus (T2DM) with and without food addiction (FA).
| Total Sample (N=73) |
Women with FA (n=35) |
Women without FA (n=38) |
p values | |
|---|---|---|---|---|
| Age, Mean (SD) | 50.589 (9.764) | 50.200 (9.964) | 50.947 (9.695) | 0.746 |
| ≥12th grade education or GED, n (%) |
36 (49%) | 14 (40%) | 22 (58%) | 0.248 |
| Household monthly income, <$1,000 US, n (%) | 37 (51%) | 19 (56%) | 18 (47%) | 0.789 |
| Currently unemployed, n (%) | 55 (75%) | 29 (83%) | 26 (68%) | 0.153 |
| Body Mass Index, Mean (SD) | 35.863 (7.717) | 38.243 (8.400) | 33.671 (6.383) | 0.010 |
| Current T2DM treatment with metformin*, n (%) | 41 (66%) | 16 (46%) | 25 (66%) | 0.182 |
| Current T2DM treatment with insulin*, n (%) | 20 (32%) | 9 (26%) | 11 (29%) | 0.986 |
| Current T2DM treatment with other medications, n (%)+ | 11 (17%) | 3 (11%) | 8 (24%) | 0.189 |
Total n=62; women with FA n=28; women without FA n=34.
The medications other than metformin and insulin collected via medical chart review included bromocriptine, nateglinide, glimepride, glipizide, glyburide, and pioglitazone.
Note: FA=food addiction; T2DM=type 2 diabetes mellitus.
p-values are reported for the Chi-squared analyses and for the t-tests conducted to assess baseline group differences.
3.2. Differences in Metabolic Indices Based on FA Diagnosis
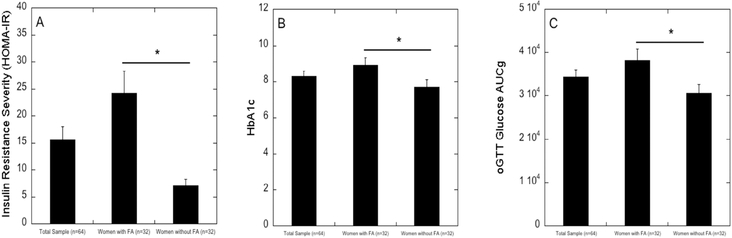
One-way ANOVA indicated that, compared to women without FA (n=32), women with FA (n=32) had significantly higher HOMA-IR (F=25.692, p<0.001, df=1, 62) and HbA1c (F=4.358, p=0.041, df=1, 62), and demonstrated significantly greater change in plasma glucose secretion during the OGTT (F=5.539, p=0.022, df=1, 59) (all presented in Figure 1). However, there was no significant difference based on FA on the change in insulin secretion during the OGTT (F=2.570, p=0.114, df=1, 59; data not shown).
Figure 1.
The severity of metabolic dysregulation as measured by HOMA-IR (Panel A), hemoglobin A1c (Panel B) and change in glucose (AUCg) during the oral Glucose Tolerance Test (OGTT; Panel C) in a sample of African-American women with type 2 diabetes mellitus (N=64; n=32 women with FA; n=32 women without FA; for the OGTT test [panel C], N=61; n=30 women with FA; n=31 women without FA). Women with FA, compared to those without FA, demonstrated higher severity of insulin resistance (p<0.001), higher levels of hemoglobin A1c (p=0.041) and greater change in glucose during the OGTT (p=0.022).
3.3. Differences in Childhood Trauma Exposure on FA Diagnosis
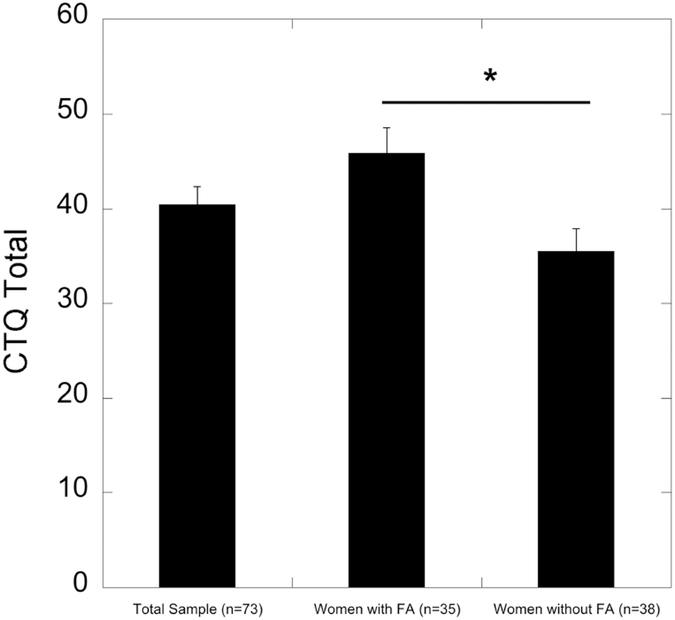
Compared to women without FA (n=38), women with FA (n=35) reported significantly higher severity of overall childhood trauma (F=10.453, p=0.002, df=1, 71; Figure 2). When considering different types of childhood trauma, women with FA reported significantly higher levels of physical abuse (F=5.378, p=0.023, df=1, 71), emotional abuse (F=11.609, p=0.001, df=1, 71), and emotional neglect (F=7.027, p=0.010, df=1, 71) than women without FA (data not shown). There were no significant differences between women with and without FA on the levels of childhood sexual abuse (F=1.463, p=0.230, df=1, 71) and physical neglect (F=2.976, p=0.089, df=1, 71).
Figure 2.
The level of childhood trauma in a sample of African-American women with type 2 diabetes mellitus. Women with food addiction, compared to those without food addiction, have higher severity of overall childhood trauma (p=0.002).
3.4. The Impact of Childhood Trauma on FA and IR
A total of 57 participants provided the YFAS scores, information regarding T2DM treatment, and the blood samples required for these analyses, therefore the regression and mediation models are based on 57 participants (n=27 women with FA; n=30 women without FA). A hierarchical linear regression was conducted to examine the relative contributions of childhood trauma and FA to the prediction of current HOMA-IR in this sample (Table 2). BMI, income, and T2DM treatment were entered in the first step of the model to statistically control for their potential effects on HOMA-IR. To reduce the number of variables in the prediction equation, only the severity of overall childhood trauma (CTQ Total) was included in the second step of the model as it captures all trauma types. The number of FA symptoms endorsed by participants was entered in the third step of the model. The severity of childhood trauma did not contribute variance to the prediction of HOMA-IR (β=−0.011, p=0.942) while FA contributed significant variance to the prediction of HOMA-IR (β=0.422, p=0.007).
Table 2.
Hierarchical linear regression of homeostatic model assessment of insulin resistance (HOMA-IR) on the severity of overall childhood trauma and food addiction, statistically controlling for body mass index (BMI), income, and current type 2 diabetes mellitus (T2DM) treatment (metformin, insulin, bromocriptine, nateglinide, glimepride, glipizide, glyburide, pioglitazone, or a combination of these medications). Note: N=57 participants provided YFAS scores, information regarding T2DM treatment, and blood samples for calculating HOMA-IR. Therefore, the model is based on 57 participants: n=27 women with FA, and n=30 women without FA.
| R2 | F | B | t | p | |
|---|---|---|---|---|---|
| Step 1 | 0.032 | 0.576 | 0.634 | ||
| Constant | 2.342 | 0.023 | |||
| BMI | 0.093 | 0.687 | 0.495 | ||
| Income | −0.081 | −0.582 | 0.563 | ||
| T2DM Treatment | −0.151 | −1.084 | 0.283 | ||
| Step 2 | 0.066 | 0.920 | 0.460 | ||
| Constant | 0.156 | 0.877 | |||
| BMI | 0.069 | 0.511 | 0.611 | ||
| Income | −0.112 | −0.801 | 0.427 | ||
| T2DM Treatment | −0.153 | −1.107 | 0.273 | ||
| CTQ Total | 0.190 | 1.386 | 0.172 | ||
| Step 3 | 0.193 | 2.434 | 0.047 | ||
| Constant | 1.1.093 | 0.280 | |||
| BMI | 0.016 | 0.123 | 0.903 | ||
| Income | −0.038 | −0.280 | 0.638 | ||
| T2DM Treatment | −0.067 | −0.506 | 0.615 | ||
| CTQ Total | −0.011 | −0.073 | 0.942 | ||
| FA symptom count | 0.422 | 2.828 | 0.007 |
Note: BMI=Body Mass Index; CTQ=Childhood Trauma Questionnaire; CTQ Total=severity of all childhood traumatic events; FA=food addiction.
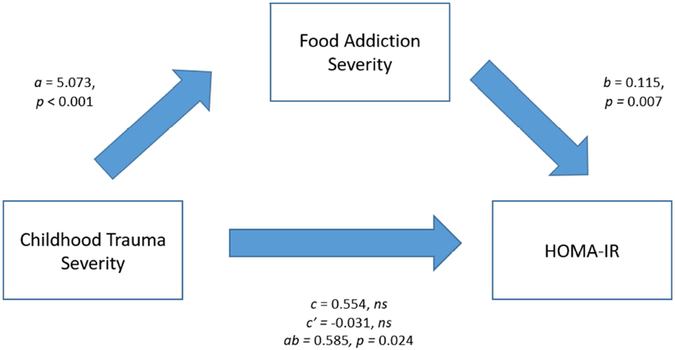
To examine whether the severity of childhood trauma exposure contributed to the variance in HOMA-IR through FA symptoms, a mediation analysis was conducted (Figure 3). There was a statistically significant effect of childhood trauma severity on FA severity (B = 5.073, p < 0.001) and of FA severity on HOMA-IR (B = 0.115, p = 0.007). There was no significant direct effect of childhood trauma severity on HOMA-IR (B = 0.554, ns). However, there was a significant indirect effect of childhood trauma severity on HOMA-IR via FA severity (B = 0.585, p = 0.024).
Figure 3.
Mediation Model: Association between Childhood Trauma and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) by Way of Food Addiction Severity, controlling for BMI, income, and T2DM treatment (metformin, insulin, bromocriptine, nateglinide, glimepride, glipizide, glyburide, pioglitazone, or a combination of these medications) as covariates. Unstandardized regression coefficients for each path.
Note: a = Effect of childhood trauma severity on food addiction severity; b = Effect of food addiction severity on HOMA-IR; c = Total effect of childhood trauma severity on HOMA-IR; c’ = Direct effect of childhood trauma severity on HOMA-IR adjusting for the mediator; ab = Indirect effect of childhood trauma severity on HOMA-IR via food addiction severity. BMI, household income, and current treatment with metformin were entered as covariates. The model is based on 57 participants (n=27 women with FA, and n=30 women without FA).
3.5. Post-Hoc Power Analysis
A post-hoc power analysis was conducted using the G*Power software (Faul, Erdfelder, Buchner, & Lang, 2009; Faul, Erdfelder, Lang, & Buchner, 2007) for both the ANOVA and the regression analysis for the main outcome variable of interest (HOMA-IR). For the first hypothesis (that those with FA will exhibit significantly higher insulin resistance compared to those without FA), the effect size f was 0.68. Thus, with a total sample size of 64, our power to detect differences at the α level set to 0.05 was 99%. For the second hypothesis (that childhood trauma and FA would be associated with higher IR), the R2 effect size was 0.214. Therefore, with sample size of 57, our power to detect significant effects was 92%.
4. Discussion
The goal of the current study was to clarify the associations between childhood trauma exposure, FA, and IR in a sample of obese AA women with T2DM. As hypothesized, women with current FA symptoms demonstrated significantly higher insulin resistance as measured by HOMA-IR; they also demonstrated higher HbA1c, plasma glucose secretion in response to the OGTT, and BMI compared to those without FA. The data also showed that, as hypothesized, women who currently display FA symptoms experienced higher severity of childhood trauma compared to those without current FA symptoms. Contrary to our hypothesis, childhood trauma was not directly associated with current HOMA-IR, while FA was, consistent with our hypothesis. Exploratory analysis showed that FA severity mediated the relationship between childhood trauma and current HOMA-IR, indicating that childhood trauma may be related to T2DM and associated IR through the behavioral mechanism of eating dysregulation. Although the current study was cross-sectional, our study tested the associations between variables with a conceptual sequence, with childhood trauma as the distal event, and eating dysregulation as an intermediate variable that may result from childhood trauma (Mason et al., 2013, 2014) and which may contribute to impairment in insulin sensitivity. Understanding psychological pathways that contribute to dysregulated eating, and by extension, IR, should inform T2DM prevention and intervention efforts in women. To our knowledge, this is the first study to demonstrate that FA is associated with higher HOMA-IR, HbA1c, plasma glucose change during the OGTT, and BMI in women with T2DM. These are important findings, as increased IR and associated metabolic indices have serious health consequences for those with T2DM, including elevated risk of cardiovascular disease and increased mortality. In the current study, childhood trauma was not directly related to HOMA-IR but it was related to severity of FA, which in turn was related to IR. Rodent models have demonstrated that in rats with diabetes, glucocorticoids and insulin interact to promote the intake of high-calorie foods (Warne et al., 2007). Social stress exposure over time leads to attenuation in availability of dopamine D2 receptors in the striatum of healthy rodents and monkeys, thus contributing to overconsumption of calorically dense foods (Grant et al., 1998; Nader et al., 2012). Administration of glucocorticoids increases caloric intake in healthy-weight men (Tataranni et al., 1996).
From a psychological perspective, consuming high-calorie foods that stimulate the reward neurocircuitry may be a powerful emotion regulation strategy in response to increased stress (Adam & Epel, 2007). Past studies have shown that women with FA endorse higher emotion dysregulation, including decreased impulse control and difficulties in goal directed behavior (Hardy et al., 2018). These data indicate the path from childhood trauma to FA is likely multifaceted and warrants empirical testing. It is possible that women with more severe stressful childhood experiences may have elevated levels of cortisol, which contributes to increased food-seeking behaviors (Epel, Lapidus, McEwen, & Brownell, 2001; Newman, O’Connor, & Conner, 2007; Warne et al., 2007). Additionally, this HPA dysregulation may contribute to decreased availability of dopamine D2 receptors in the reward system, contributing to increased consumption of calorically dense foods. Behaviorally, pathological consumption of calorically dense foods has been shown to be a learned emotion regulation strategy (Brockmeyer et al., 2014; Heatherton & Wagner, 2011)., It is possible that through incentive sensitization, women with FA anticipate an emotional relief from calorically dense foods given their known effects on dopamine release in the reward neurocircuitry (Gearhardt et al., 2011). Once women start consuming calorically dense foods, they may experience difficulties with impulse control, leading to overconsumption (Gearhardt et al., 2011). Acting impulsively in the face of negative emotions is also a mediator between FA and BMI (Murphy, Stojek, & MacKillop, 2014). As expected and demonstrated in the present study, in women exposed to traumatic stress in childhood, dysregulated eating patterns associated with FA may contribute to the development and/or maintenance of IR.
It is important to note the systematic health disparities that were not tested in the current study but that are likely contributing to the indirect pathway from childhood trauma to IR through FA. Past research has consistently demonstrated that in minority and low-income neighborhoods, poor quality retail food environment and limited economic resources are associated with obesity (Chen, Jaenicke, & Volpe, 2016; Ford & Dzewaltowski, 2008). Research on targeted marketing of high-calorie foods and beverages demonstrated that African Americans are consistently exposed to food promotion and distribution patterns with relatively greater potential adverse health outcomes than Whites (Grier & Kumanyika, 2008). Factors that further contribute to more prevalent adverse health outcomes in minority populations include less access to physical activity facilities in their high-minority low-income neighborhoods compared to more affluent areas (Gordon-Larsen, Nelson, Page, & Popkin, 2006) and barriers in access to healthcare ranging from healthcare providers’ implicit biases (Hall et al., 2015) to structural racism in healthcare settings (Bailey et al., 2017). Therefore, in order to address the psychological factors contributing factors to T2DM in minority populations, it is also imperative to consider this host of environmental and sociopolitical factors beyond individual’s control that impact adverse health outcomes.
4.1. Strengths and Limitations
Strengths of the current study include the use of a trauma-exposed sample of AA women with T2DM and systematic collection of biomarkers related to glycemic regulation. African American women are underrepresented in research despite the fact that prevalence of diabetes among them is over twice that of white women (Centers for Disease Control, 2017). Therefore, understanding the potential mechanisms that may be at play in putting AA women at higher risk for insulin resistance may contribute to more effective treatment and prevention strategies. Limitations of our study include a relatively small sample size, cross-sectional nature of the data, and lack of inclusion of potential confounders in our analyses (i.e. diet and exercise). The use of fasting measures of insulin and glucose, rather than criterion measure like the euglycemic hyperinsulinemic clamp, to estimate IR is also a potential limitation. Some studies also suggest that anthropometric proxies for central obesity (such as waist circumference) as opposed to total obesity (measured by BMI) are slightly better predictors of health risk factors and mortality (Guasch-Ferré et al., 2012; Janssen, Katzmarzyk, & Ross, 2004; Schneider et al., 2010). In the current study, we measured exclusively total obesity. However, it is important to note that some studies found that anthropometric indicators for total and central obesity did not differ in their predictive validity (Stevens et al., 2001; The Emerging Risk Factors Collaboration, 2011; Tulloch-Reid, Williams, Looker, Hanson, & Knowler, 2003). In recruitment, we approached women of all ages in the waiting rooms of several clinics on all days of the week during the operating hours of the clinic. However, it is possible that some participants were less likely to be approached due to environmental factors, such as seeming visibly upset or being accompanied by someone. Similarly, our exclusion criteria included a current prescription of benzodiazepine class pharmaceuticals, thus potentially excluding participants with more severe trauma who manage their symptoms using these drugs. While we statistically controlled for the prescription of T2DM medication, we were unable to control for whether participants were actually adherent to their medication regimen. Additionally, FA severity, the primary variable of interest, was obtained via a self-report and childhood trauma was measured via retrospective self-report. While the Childhood Trauma Questionnaire has good psychometric properties (Bernstein & Fink, 1998; Spinhoven et al., 2014) and measures a range of maltreatment behaviors considered traumatic, it does not measure all traumatic events (e.g., exposure to domestic or community violence, bullying, motor vehicle accidents, parental abandonment or impairment, separation or loss of caregivers). A clinical interview, such as the Childhood Maltreatment Interview Schedule (Briere & Runtz, 1990; Cloitre, Cohen, Edelman, & Han, 2001) or the Childhood Trauma Interview (Fink, Bernstein, Handelsman, Foote, & Lovejoy, 1995) would have been more comprehensive tools for the assessment of childhood trauma.
Future studies should examine the relationship between early life trauma, FA, and IR longitudinally, particularly in AA women. Additionally, more studies examining the mechanistic relationships, using both neurobiological and psychological markers, would be useful in elucidating points at which interventions could be administered. Dysregulated eating has been linked to difficulties in emotion regulation (Brockmeyer et al., 2014; Heatherton & Wagner, 2011) and dispositional mindfulness has been associated with lower odds of binge eating in adolescent girls at risk for T2DM (Pivarunas et al., 2015). A brief course of group mindfulness training has been shown to be effective in decreasing insulin resistance and fasting insulin in adolescent girls at risk for T2DM at the end of treatment (Shomaker et al., 2017) and at 1-year follow up (Shomaker et al., 2019). Therefore, testing brief interventions in adult populations targeted at increasing those skills, such as emotion regulation skills training (Svaldi, Tuschen-Caffier, Trentowska, Caffier, & Naumann, 2014) or mindfulness training (Godfrey, Gallo, & Afari, 2015; Shomaker et al., 2017), that can be delivered in a primary care setting or a diabetes clinic would also be a target for future research.
4.2. Conclusions
In conclusion, African American women with T2DM who endorse dysregulated eating patterns also have higher metabolic dysfunction indices and report higher severity of childhood trauma. Additionally, early life trauma, indirectly contributes to insulin resistance in African American women, operating through dysregulation of eating patterns. . As increased insulin resistance and associated metabolic indices have serious health consequences for those with T2DM, these findings point to the need for screening and interventions related to dysregulated eating and trauma symptoms in that population, preferably in the primary care or a diabetes clinic setting. Understanding the psychological factors that contribute to insulin resistance in African American women with diabetes is especially important as the prevalence of diabetes in this population is one of the highest out of all racial groups (Centers for Disease Control, 2017). Therefore, investigating behavioral interventions for eating dysregulation in African American women with T2DM and trauma histories has the potential of having a cascade impact on improving their physical health through more effective diabetes management skills.
Acknowledgments
Source of Funding
The current work was primarily supported by the National Institute of Mental Health (MH099211; PI: Gillespie). Support was also received from HD085850 (PI: Michopoulos), PHS Grant UL1RR025008 from the Clinical and Translational Science Award Program, and M01 RR000039, National Institutes of Health, National Centers for Research Resources.
Footnotes
Declaration of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
22% of the study sample reported having a prescription of gabapentin, and there were no between-group differences based on the food addiction diagnosis status in rates of gabapentin use (chi squared=2.618, p=0.106). Data on pregabalin prevalence were not available.
There were no between-group differences in rates of participants on metformin and insulin (chi squared=0, p=0.99).
References
- Adam TC, & Epel ES (2007). Stress, eating and the reward system. Physiology & Behavior, 91(4), 449–458. https://doi.Org/10.1016/j.physbeh.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, & Bassett MT (2017). Structural racism and health inequities in the USA: evidence and interventions. The Lancet, 389(10077), 1453–1463. 10.1016/S0140-6736(17)30569-X [DOI] [PubMed] [Google Scholar]
- Bernstein DP & Fink L (1998). Childhood Trauma Questionnaire manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bernstein David P., Ahluvalia T, Pogge D, & Handelsman L (1997). Validity of the Childhood Trauma Questionnaire in an Adolescent Psychiatric Population. Journal of the American Academy of Child & Adolescent Psychiatry, 36(3), 340–348. 10.1097/00004583-199703000-00012 [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Valentine JD (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology, 68(5), 748–766. 10.1037/0022-006X.68.5.748 [DOI] [PubMed] [Google Scholar]
- Briere J, & Runtz M (1990). Differential adult symptomatology associated with three types of child abuse histories. Child Abuse & Neglect, 14(3), 357–364. 10.1016/0145-2134(90)90007-G [DOI] [PubMed] [Google Scholar]
- Brockmeyer T, Skunde M, Wu M, Bresslein E, Rudofsky G, Herzog W, & Friederich H-C (2014). Difficulties in emotion regulation across the spectrum of eating disorders. Comprehensive Psychiatry, 55(3), 565–571. 10.1016/J.COMPPSYCH.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Butchart A, Harvey AP, Mian M, & Furniss T (2006). Preventing child maltreatment: a guide to taking action and generating evidence. Geneva Switzerland: World Health Organization [WHO] 2006. Retrieved from https://www.popline.org/node/181981 [Google Scholar]
- Centers for Disease Control. (2017). National Diabetes Statistics Report. Atlanta, GA: Retrieved from https://www.cdc.gov/diabetes/data/statistics/statistics-report.html [Google Scholar]
- Chen D, Jaenicke EC, & Volpe RJ (2016). Food Environments and Obesity: Household Diet Expenditure Versus Food Deserts. American Journal of Public Health, 106(5), 881–888. 10.2105/AJPH.2016.303048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloitre M, Cohen LR, Edelman RE, & Han H (2001). Posttraumatic Stress Disorder and Extent of Trauma Exposure as Correlates of Medical Problems and Perceived Health Among Women with Childhood Abuse. Women & Health, 34(3), 1–17. 10.1300/J013v34n03_01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, & Zisk A (2014). The biological effects of childhood trauma. Child and Adolescent Psychiatric Clinics of North America, 23(2), 185–222, vii. 10.1016/j.chc.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA (1988). Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes, 37(6), 667–687. 10.2337/DIAB.37.6.667 [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, & Brownell K (2001). Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology, 26(1), 37–49. 10.1016/s0306-4530(00)00035-4 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang AG (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Fink LA, Bernstein D, Handelsman L, Foote J, & Lovejoy M (1995). Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. American Journal of Psychiatry, 152(9), 1329–1335. 10.1176/ajp.152.9.1329 [DOI] [PubMed] [Google Scholar]
- Fischer S, Stojek M, & Hartzell E (2010). Effects of multiple forms of childhood abuse and adult sexual assault on current eating disorder symptoms. Eating Behaviors, 11(3), 190–192. Retrieved from http://www.ncbi.nlm.nih.gov/pubmedf20434068 [DOI] [PubMed] [Google Scholar]
- Ford PB, & Dzewaltowski DA (2008). Disparities in obesity prevalence due to variation in the retail food environment: three testable hypotheses. Nutrition Reviews, 66(4), 216–228. 10.1111/j.1753-4887.2008.00026.x [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Corbin WR, & Brownell KD (2009). Food addiction: an examination of the diagnostic criteria for dependence. Journal of Addiction Medicine, 3(1), 1. [DOI] [PubMed] [Google Scholar]
- Gearhardt Ashley N, Corbin WR, & Brownell KD (2009). Preliminary validation of the Yale Food Addiction Scale. Appetite, 52(2), 430–436. 10.1016/j.appet.2008.12.003 [DOI] [PubMed] [Google Scholar]
- Gearhardt Ashley N, Yokum S, Orr PT, Stice E, Corbin WR, & Brownell KD (2011). Neural correlates of food addiction. Archives of General Psychiatry, 68(8), 808–816. 10.1001/archgenpsychiatry.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, … Ressler KJ (2009). Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry, 31(6), 505–514. 10.1016/J.GENHOSPPSYCH.2009.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Gallo LC, & Afari N (2015). Mindfulness-based interventions for binge eating: a systematic review and meta-analysis. Journal of Behavioral Medicine, 38(2), 348–362. 10.1007/s10865-014-9610-5 [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Nelson MC, Page P, & Popkin BM (2006). Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics, 117(2), 417–424. 10.1542/peds.2005-0058 [DOI] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, … Mach RH (1998). Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse, 29(1), 80–83. [DOI] [PubMed] [Google Scholar]
- Grier SA, & Kumanyika SK (2008). The context for choice: health implications of targeted food and beverage marketing to African Americans. American Journal of Public Health, 98(9), 1616–1629. 10.2105/AJPH.2007.115626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch-Ferré M, Bulló M, Martínez-González MÁ, Corella D, Estruch R, Covas M-I,… Salas-Salvadó J (2012). Waist-to-Height Ratio and Cardiovascular Risk Factors in Elderly Individuals at High Cardiovascular Risk. PLoS ONE, 7(8), e43275 10.1371/journal.pone.0043275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, … Coyne-Beasley T (2015). Implicit Racial/Ethnic Bias Among Health Care Professionals and Its Influence on Health Care Outcomes: A Systematic Review. American Journal of Public Health, 105(12), e60–76. 10.2105/AJPH.2015.302903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R, Fani N, Jovanovic T, & Michopoulos V (2018). Food addiction and substance addiction in women: Common clinical characteristics. Appetite, 120, 367–373. 10.1016/J.APPET.2017.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: Guilford Press. [Google Scholar]
- Hayes AF, & Scharkow M (2013). The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychological Science, 24(10), 1918–1927. 10.1177/0956797613480187 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, & Wagner DD (2011). Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences, 15(3), 132–139. 10.1016/J.TICS.2010.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, & Nemeroff CB (2010). Neurobiological and psychiatric consequences of child abuse and neglect. Developmental Psychobiology, 52(7), 671–690. 10.1002/dev.20494 [DOI] [PubMed] [Google Scholar]
- Huxley R, Barzi F, & Woodward M (2006). Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ (Clinical Research Ed.), 332(7533), 73–78. 10.1136/bmj.38678.389583.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna MR, Reichl C, Parzer P, Schimmenti A, Bifulco A, & Kaess M (2016). Associations between depression and specific childhood experiences of abuse and neglect: A meta-analysis. Journal of Affective Disorders, 190, 47–55. 10.1016/J.JAD.2015.09.006 [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, & Ross R (2004). Waist circumference and not body mass index explains obesity-related health risk. The American Journal of Clinical Nutrition, 79(3), 379–384. 10.1093/ajcn/79.3.379 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, … Xavier M (2013). The Prevalence and Correlates of Binge Eating Disorder in the World Health Organization World Mental Health Surveys. Biological Psychiatry, 73(9), 904–914. 10.1016/J.BIOPSYCH.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindert J, von Ehrenstein OS, Grashow R, Gal G, Braehler E, & Weisskopf MG (2014). Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: systematic review and meta-analysis. International Journal of Public Health, 59(2), 359–372. 10.1007/s00038-013-0519-5 [DOI] [PubMed] [Google Scholar]
- Mason SM, Flint AJ, Field AE, Austin SB, & Rich-Edwards JW (2013). Abuse victimization in childhood or adolescence and risk of food addiction in adult women. Obesity, 21(12), E775–E781. 10.1002/oby.20500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SM, Flint AJ, Roberts AL, Agnew-Blais J, Koenen KC, & Rich-Edwards JW (2014). Posttraumatic Stress Disorder Symptoms and Food Addiction in Women by Timing and Type of Trauma Exposure. JAMA Psychiatry. 10.1001/jamapsychiatry.2014.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, & DeFronzo RA (1999). Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care, 22(9), 1462–1470. 10.2337/DIACARE.22.9.1462 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, & Turner RC (1985). Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia, 28(7), 412–419. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- Meule A, & Gearhardt AN (2014). Five years of the Yale Food Addiction Scale: Taking stock and moving forward. Current Addiction Reports, 1(3), 193–205. 10.1007/s40429-014-0021-z [DOI] [Google Scholar]
- Micali N, Solmi F, Horton NJ, Crosby RD, Eddy KT, Calzo JP, … Field AE (2015). Adolescent Eating Disorders Predict Psychiatric, High-Risk Behaviors and Weight Outcomes in Young Adulthood. Journal of the American Academy of Child and Adolescent Psychiatry, 54(8), 652–659.e1. 10.1016/j.jaac.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Moore C, Villarreal S, Ressler KJ, & Bradley B (2015). The mediating role of emotion dysregulation and depression on the relationship between childhood trauma exposure and emotional eating. Appetite, 91, 129–136. 10.1016/j.appet.2015.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Vester A, & Neigh G (2016). Posttraumatic stress disorder: A metabolic disorder in disguise? Experimental Neurology, 284(Pt B), 220–229. https://doi.Org/10.1016/j.expneurol.2016.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooy JM, de Vries H, Grootenhuis PA, Bouter LM, & Heine RJ (2000). Major stressful life events in relation to prevalence of undetected type 2 diabetes: the Hoorn Study. Diabetes Care, 23(2), 197–201. 10.2337/DIACARE.23.2.197 [DOI] [PubMed] [Google Scholar]
- Murphy CM, Stojek MK, & MacKillop J (2014). Interrelationships among impulsive personality traits, food addiction, and Body Mass Index. Appetite, 73 10.1016/j.appet.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy Cara M., Stojek MK, & MacKillop J (2014). Interrelationships among impulsive personality traits, food addiction, and Body Mass Index. Appetite, 73, 45–50. 10.1016/j.appet.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, … Reboussin BA (2012). Social Dominance in Female Monkeys: Dopamine Receptor Function and Cocaine Reinforcement. Biological Psychiatry, 72(5), 414–421. 10.1016/J.BIOPSYCH.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, & Binder E (2014). The preeminent role of childhood abuse and neglect in vulnerability to major psychiatric disorders: toward elucidating the underlying neurobiological mechanisms. Journal of the American Academy of Child and Adolescent Psychiatry, 53(4), 395–397. 10.1016/j.jaac.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, & Seligman F (2013). The pervasive and persistent neurobiological and clinical aftermath of child abuse and neglect. The Journal of Clinical Psychiatry, 74(10), 999–1001. 10.4088/JCP.13com08633 [DOI] [PubMed] [Google Scholar]
- Newman E, O’Connor DB, & Conner M (2007). Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology, 32, 125–132. [DOI] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, & Vos T (2012). The Long-Term Health Consequences of Child Physical Abuse, Emotional Abuse, and Neglect: A Systematic Review and Meta-Analysis. PLoS Medicine, 9(11), e1001349 10.1371/journal.pmed.1001349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peugh JL, & Enders CK (2004). Missing Data in Educational Research: A Review of Reporting Practices and Suggestions for Improvement. Review of Educational Research, 74(4), 525–556. 10.3102/00346543074004525 [DOI] [Google Scholar]
- Pivarunas B, Kelly NR, Pickworth CK, Cassidy O, Radin RM, Shank LM, … Shomaker LB (2015). Mindfulness and eating behavior in adolescent girls at risk for type 2 diabetes. The International Journal of Eating Disorders, 48(6), 563–569. 10.1002/eat.22435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollare T, Lithell H, & Berne C (1990). Insulin resistance is a characteristic feature of primary hypertension independent of obesity. Metabolism: Clinical and Experimental, 39(2), 167–174. Retrieved from http://www.ncbi.nlm.nih.gov/pubmedf2405235 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36(4), 717–731. 10.3758/BF03206553 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. Retrieved from http://www.ncbi.nlm.nih.gov/pubmedf12892658 [DOI] [PubMed] [Google Scholar]
- Pursey K, Stanwell P, Gearhardt A, Collins C, & Burrows T (2014). The Prevalence of Food Addiction as Assessed by the Yale Food Addiction Scale: A Systematic Review. Nutrients, 6(10), 4552–4590. 10.3390/nu6104552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, & Karl A (2006). Role of endocrine and inflammatory alterations in comorbid somatic diseases of post-traumatic stress disorder. Minerva Endocrinologica, 31(4), 273–288. Retrieved from http://www.ncbi.nlm.nih.gov/pubmedf17213794 [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJG, McCreary DR, & Forde DR (2001). The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress, 14(4), 843–857. 10.1023/A:1013058625719 [DOI] [PubMed] [Google Scholar]
- Schneider HJ, Friedrich N, Klotsche J, Pieper L, Nauck M, John U, … Wittchen H-U (2010). The Predictive Value of Different Measures of Obesity for Incident Cardiovascular Events and Mortality. The Journal of Clinical Endocrinology & Metabolism, 95(4), 1777–1785. 10.1210/jc.2009-1584 [DOI] [PubMed] [Google Scholar]
- Shomaker LB, Bruggink S, Pivarunas B, Skoranski A, Foss J, Chaffin E, … Bell C (2017). Pilot randomized controlled trial of a mindfulness-based group intervention in adolescent girls at risk for type 2 diabetes with depressive symptoms. Complementary Therapies in Medicine, 32, 66–74. 10.1016/j.ctim.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Pivarunas B, Annameier SK, Gulley L, Quaglia J, Brown KW, … Bell C (2019). One-Year Follow-Up of a Randomized Controlled Trial Piloting a Mindfulness-Based Group Intervention for Adolescent Insulin Resistance. Frontiers in Psychology, 10, 1040 10.3389/fpsyg.2019.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, & Miller WR (2002). Concomitance between childhood sexual and physical abuse and substance use problems: A review. Clinical Psychology Review, 22(1), 27–77. 10.1016/S0272-7358(00)00088-X [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Penninx BW, Hickendorff M, van Hemert AM, Bernstein DP, & Elzinga BM (2014). Childhood Trauma Questionnaire: Factor structure, measurement invariance, and validity across emotional disorders. Psychological Assessment, 26(3), 717–729. 10.1037/pas0000002 [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, … Grabe HJ (2010). Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: Evidence from the general population. Journal of Psychiatric Research, 44(1), 15–21. 10.1016/J.JPSYCHIRES.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Stevens J, Couper D, Pankow J, Folsom AR, Duncan BB, Nieto FJ, … Tyroler HA (2001). Sensitivity and Specificity of Anthropometrics for the Prediction of Diabetes in a Biracial Cohort. Obesity Research, 9(11), 696–705. 10.1038/oby.2001.94 [DOI] [PubMed] [Google Scholar]
- Succurro E, Segura-Garcia C, Ruffo M, Caroleo M, Rania M, Aloi M, … Arturi F (2015). Obese Patients With a Binge Eating Disorder Have an Unfavorable Metabolic and Inflammatory Profile. Medicine, 94(52), e2098 10.1097/MD.0000000000002098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaldi J, Tuschen-Caffier B, Trentowska M, Caffier D, & Naumann E (2014). Differential caloric intake in overweight females with and without binge eating: Effects of a laboratory-based emotion-regulation training. Behaviour Research and Therapy, 56, 39–46. https://doi.Org/10.1016/J.BRAT.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson DA, Snitker S, Young JB, Flatt JP, & Ravussin E (1996). Effects of glucocorticoids on energy metabolism and food intake in humans. The American Journal of Physiology, 271, E317–E325. [DOI] [PubMed] [Google Scholar]
- The Emerging Risk Factors Collaboration. (2011). Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. The Lancet, 377(9771), 1085–1095. 10.1016/S0140-6736(11)60105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch-Reid MK, Williams DE, Looker HC, Hanson RL, & Knowler WC (2003). Do measures of body fat distribution provide information on the risk of type 2 diabetes in addition to measures of general obesity? Comparison of anthropometric predictors of type 2 diabetes in Pima Indians. Diabetes Care, 26(9), 2556–2561. 10.2337/DIACARE.26.9.2556 [DOI] [PubMed] [Google Scholar]
- Warne JP, Foster MT, Horneman HF, Pecoraro NC, Ginsberg AB, Akana SF, & Dallman MF (2007). Hepatic Branch Vagotomy, Like Insulin Replacement, Promotes Voluntary Lard Intake in Streptozotocin-Diabetic Rats. Endocrinology, 148(7), 3288–3298. 10.1210/en.2007-0003 [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Crosby RD, Mitchell JE, Thompson KM, Redlin J, Demuth G, … Haseltine B (2001). Eating disturbance and sexual trauma in childhood and adulthood. International Journal Of Eating Disorders, 30(4), 401–412. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=mnh&AN=11746301&site=ehost-live [DOI] [PubMed] [Google Scholar]