Abstract
The physiological barriers of the eye pose challenges to the delivery of the array of therapeutics for the effective medication of ocular diseases as well as patient compliance. While the innovations in drug encapsulation and release mechanisms, biocompatibility, and treatment duration have become highly sophisticated, the challenge of widespread application of hydrogel formulations in the clinic is still apparent. This article reviews the latest hydrogel formulations and their associated chemistries for use in ocular therapies, spanning from external anterior to internal posterior regions of the eye in order to evaluate the state of recent research. This article discusses the utility of hydrogels in soft contact lens, wound dressings, intraocular lens, vitreous substitutes, vitreous drug release hydrogels, and cell-based therapies for regeneration. Additional focus is placed on the pre-formulation, formulation, and manufacturing considerations of the hydrogels based on individual components (polymer chains, linkers, and therapeutics), final hydrogel product, and required preparations for clinical/commercial applications, respectively.
Keywords: hydrogel, topical, injectable, drug delivery, sterilization
Graphical Abstract

1. Introduction
Ocular diseases and visual impairment are highly prevalent across the globe and can be debilitating to the affected patient. These diseases include infection and inflammation like conjunctivitis, age-related macular degeneration (AMD), glaucoma, and diabetic retinopathy as the leading causes of worldwide blindness (Wong et al., 2014, Tham et al., 2014, Ting et al., 2016). Despite deviating in the affected area and tissue of the eye, these diseases share a worst-case scenario outcome: total vision loss. The variability of onset and severity is also significant, making diagnosis and treatment highly individualistic. Additionally, the structure of the eye and its tissues and barriers make the design of treatment modalities challenging. The orb itself is composed of three concentric coats – the fibrous (sclera and cornea) layer, the choroid, and the nervous layer (retina) – separated into anterior and posterior segments. The anterior chamber includes the cornea, iris, ciliary body, crystalline lens, and aqueous humor, while the posterior chamber holds the vitreous humor, retina, retinal pigment epithelium (RPE), and optic disk.
Current treatment modalities for ocular diseases include approaches ranging in degree of invasiveness to reach the afflicted tissues, from conventional liquid drops to direct injection into the vitreous, as well as surgical procedures to remove damaged areas and to implant devices (Yang et al., 2012, Xing et al., 2014, Abell et al., 2015, Chuang et al., 2014). However, each treatment technique comes with its own set of advantages and drawbacks. Eye drop solutions, like those to treat the symptoms of elevated intraocular pressure (IOP) in glaucoma, only have a bioavailability of 5% due to the low corneal penetration exacerbated by blinking and lacrimation (Farkouh et al., 2016). As a result, frequent administration is required, but this is often met with poor patient compliance (Robin and Grover, 2011). Additionally, topically applied pharmaceutics are not suitable for treatment of posterior tissues of the eye. Systemic delivery of drugs for the treatment of ophthalmic diseases can also present difficulties in low ocular targeting and therapeutic levels in the relevant tissues due to the selectivity of the blood-retinal barrier (BRB). Adverse off-target effects also accompany this treatment route (Kim et al., 2014). Local treatment to the vitreous is an approach to circumvent the bioavailability issue; however, this technique is more invasive. Intravitreal injection (IVI) uses a small gauge needle to puncture through the fibrous and vascular tunics of the eye. While deemed relatively safe, complications such as retinal detachment and endophthalmitis can arise (Kang Derwent and Mieler, 2008). Another aspect to consider for treatment delivery is the drug itself. Small hydrophobic and hydrophilic drug molecules, proteins, and nucleic acids encompass some of the ocular therapeutics and differ in their mode of action, half-life, toxicity, and minimum therapeutic level.
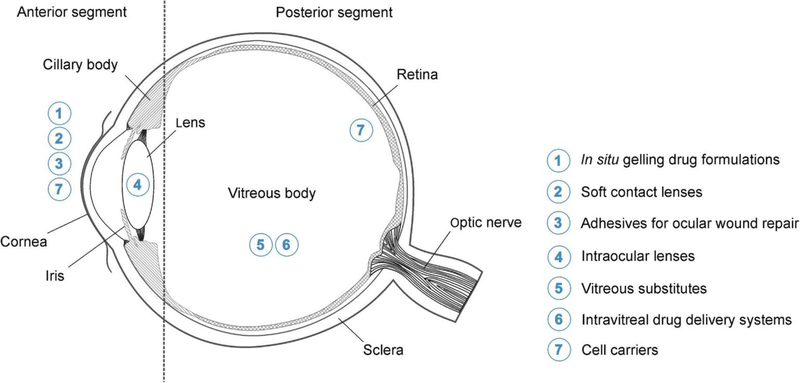
Given the physiological barriers of the eye, challenges in the effective delivery of the array of therapeutics, and overall patient compliance with treatment options, innovative delivery methods are required to enhance the field of ophthalmic medicine. In addition to ocular drug delivery systems such as colloids (Fangueiro et al., 2014, Costa et al., 2015), and polymeric implants (Yasin et al., 2014), hydrogels are another class of material that offer favorable properties for sustained drug release (Kirchhof et al., 2015). Composed of a three-dimensional network of hydrophilic polymer chains, hydrogels have a high capacity for water retention. Their properties such as stimuli-responsiveness to heat, pH, and light (Lim et al., 2014) can be altered with monomers and crosslinkers employed. These properties are particularly useful for drug loading with controllable release mechanisms, as well as in situ gelation for ease of delivery of liquid-gel formulations. Hydrogels have already found their way into clinical ophthalmology in the form of soft contact lenses (SCLs) and intraocular lenses (IOLs), as well as some drug-eluting hydrogels. These examples mainly treat the anterior segments of the eye while research into posterior applications is being widely investigated. Figure 1 demonstrates the current and potential applications of hydrogels of ophthalmic treatment.
Figure 1.
Regions of the eye where hydrogels are currently used. Reprinted with permission from (Kirchhof et al., 2015). Copyright 2015 Elsevier Inc.
This article reviews the latest hydrogel formulations and their associated chemistries for use in ocular therapies, spanning from external anterior to internal posterior regions of the eye in order to evaluate the state of recent research. This article also includes discussion of SCLs, wound dressings from trauma or surgery, IOLs, vitreous substitutes, vitreous drug release hydrogels, and cell-based therapies for regeneration. Additional focus is placed on the pre-formulation, formulation, and manufacturing considerations of the hydrogels based on the individual components (polymer chains, linkers, and therapeutics), the final hydrogel product, and required preparations for clinical/commercial applications, respectively.
2. Hydrogel preparation and therapeutic loading considerations
Designing the ideal ocular treatment or drug delivery platform requires an understanding of not only the mode of action of the therapeutic but also the components that make up the vehicle. As alluded to previously, hydrogels are a network of monomers and multifunctional linkers that react to form a flexible, water-laden structure. The network formation is achieved with hydrophilic monomers that would otherwise be solubilized in non-crosslinked form. Crosslinkers are what make the overall hydrogel structure insoluble but retain a high water fraction (Ahmed, 2015). This basic definition of hydrogels allows users a high degree of tuning: by altering the crosslinking density one can control for porosity, swelling, mechanical strength, and degradation rates. Additionally, specific functionalized hydrogel components can be used that respond to a biological stimulus. This ability to change based on the surrounding environment has major implications for in situ forming hydrogels – those that crosslink when temperature increases from room temperature to body temperature – as well as controlled drug release as a result of pH or photostimulation.
Hydrogels can be composed of natural or synthetic monomers. Natural hydrogels have been extensively researched for tissue engineering purposes for their biocompatibility and degradation into non-toxic constituents. Examples of natural polymers include hyaluronic acid, chitosan, and collagen. The vitreous humor itself is a hydrogel composed of collagen fibers and the glycosaminoglycans (GAGs), hyaluronic acid, chondroitin sulfate, and heparan sulfate (Alovisi et al., 2017). Naturally-derived hydrogels can also interact with biomacromolecules, which could represent an advantage in the case of attracting vitreous seeds from escaping through needle injection tracks, or a disadvantage in cases of protein adsorption on IOL replacements. Compared to synthetic polymers, the limitations of naturally-derived hydrogels, however, are their relatively weak mechanical strength, difficulty in reproducing accurate formulation and dosage loading amounts, and immunogenic risks (Kirchhof et al., 2015). Synthetic polymers are those not found in nature, with common ones being poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and poly(hydroxyethyl methacrylate) (PHEMA) (Yu et al., 2014, Jiang et al., 2014, Galante et al., 2015). Hydrogels made of synthetic polymers can be more finely tuned for specific applications over natural polymer hydrogels, have higher batch-to-batch reproducibility, and longer stability. However, the disadvantages of synthetically-derived hydrogels include clearance and break down into toxic by-products.
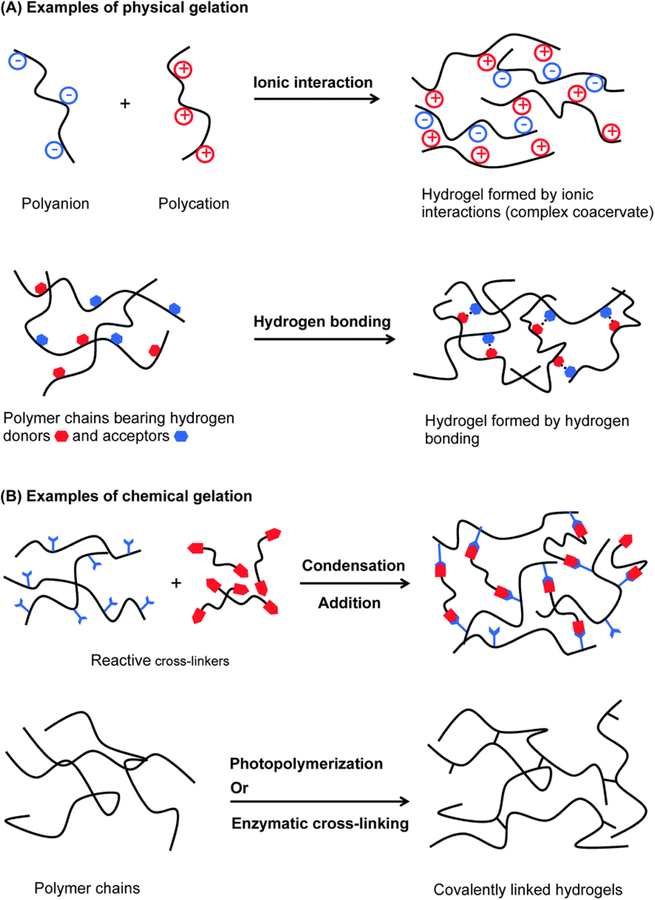
Polymer crosslinking can be subdivided into two approaches: physical and chemical (Figure 2). The former can occur via chain entanglement, hydrogen bonding, hydrophobic interactions, complexation and crystalline formation (Hoare and Kohane, 2008, Wang et al., 2018). Though stable enough to avoid aqueous solubilization, degradation of physically crosslinked hydrogels can occur through some stimulus, such as heat, pH, ionic strength, and mechanical forces, as this type of crosslinking is reversible. Chemical interactions, on the other hand, produce permanent hydrogels through covalent interactions of polymer and crosslinker functional groups. Polymerization into a hydrogel network can proceed through chain growth, addition, or condensation reactions for producing finely-tuned hydrogels. Other chemistries associated with chemical crosslinking are click chemistry via azide-alkyne cycloaddition, Michael-addition, and Diels-Adler reactions (Xu et al., 2017, Wang et al., 2017a).
Figure 2.
Examples of physical and chemical hydrogel formation. Reprinted with permission from (Ghobril and Grinstaff, 2015). Copyright 2015 The Royal Society of Chemistry.
3. Hydrogel developmental stages – from bench to bedside
It is essential to consider the final application scenario when developing hydrogels in the laboratory. The process of designing an ocular hydrogel, synthesizing the formulation, and preparing it for clinical use can be divided into three developmental steps: 1) pre-formulation step, which is centered on the selection of polymer and crosslinking constituents, the reaction type, and specifications; 2) formulation step, which includes the hydrogel itself and the method of action, payload encapsulation, and its release are collectively defined as the formulation; and 3) manufacturing step, which includes hydrogel scale-up, shelf-life of the hydrogel and therapeutic agent, sterilization techniques, and the delivery method and associated tools and equipment. Table 1 summarizes the associated properties and test parameters used to characterize hydrogels at each of these three developmental stages.
Table 1.
Properties of hydrogels and associated evaluation parameters at each formulation stage.
| Development Stage | Property | Evaluation Parameters |
|---|---|---|
| Pre-formulation | Solubility | • pKa |
| • pH range solubility | ||
| • Temperature range solubility | ||
| • Dissolution | ||
| Stability | • Payload at varying temperatures and pH | |
| • Monomers and crosslinkers at varying temperatures and pH | ||
| • Drug-polymer interaction | ||
| Drug crystallinity | ||
| Drug polymorphism | ||
| Formulation | Mechanical properties | • Rheology |
| • Compression/Extension | ||
| • Crosslinking density evaluation | ||
| Morphology | • Porosity | |
| • Topography | ||
| Swelling | ||
| Drug content | ||
| Drug release | • Dissolution | |
| Biosafety | • In vitro and in vivo biocompatibility | |
| Bioactivity | ||
| Manufacturing | Stability | • Post-sterilization |
| • Shelf-life | ||
| • Bulk stability | ||
| Administration | • Tool compatibility | |
| • Pre-injection mixing |
3.1. Pre-formulation
In pharmaceutical science, pre-formulation refers to the assessment of the physiochemical properties of drugs and other therapeutic agents with or without excipients for a better understanding of dosage form design (Sigfridsson and Carlsson, 2017). In this section, the previous definition for the assessment of active pharmaceutical ingredients will be extended to encompass the characterization techniques of hydrogels and their components: polymers, crosslinkers, and payload. Commonly characterized physiochemical properties for drugs include solid state thermal analysis, ionization constant (pKa), solubility and stability, along with the associated changes based on temperature and pH (Barich et al., 2005). A major aspect of pre-formulation is the interaction of the payload with the components of the hydrogel, and how it changes the drug’s pure form behavior. This can be particularly problematic when the drug is added to the gelation reaction. Hammer et al. studied the modification of lysozymes during gelation of different functionalized PEG chains (maleimide, vinyl sulfone, and acrylamide groups). They found that Michael-type additions occurred between nucleophilic amino acid residues and vinyl groups based on pH, with detection of 96% unmodified lysozymes with PEG-acrylamide at pH 4, and more than 80% PEG-modified (number of PEG chain modifications: 1–5) lysozymes from physiologic pH to pH 9 with PEG-vinyl sulfone (Hammer et al., 2015). While simultaneous drug incorporation and in situ drug gelation allows for exact loading estimates and loading reproducibility, these modifications can cause the therapeutic to lose its functionality and prevent the effective release from the hydrogel. However, there are some approaches to avoiding this loss of activity during loading. The first is to functionalize for covalent conjugation during the crosslinking process and allowing it to incorporate into the gel network whereby it can be released through an environmental stimulus in the body (Lv et al., 2014). This method maintains a good control over drug loading amount while minimizing the loss of bioactivity and incorporating a controlled release mechanism. Another approach is to load the hydrogel by submerging the crosslinked hydrogel in a concentrated solution of the drug. This approach minimizes the possibility of altering the therapeutic but can lead to batch-to-batch variable drug content. The release of the drug can be aided by diffusion in response to a concentration gradient in the target tissue or by stimulus-activated hydrogel degradation, such as ion-, pH-, light-, and temperature-sensitivity (Fernandez-Ferreiro et al., 2015, Kharkar et al., 2015, Luo et al., 2016).
3.2. Formulation
While the pre-formulation section discussed the characterization of the individual hydrogel components and their interactions, this section will focus on the properties of the drug-loaded hydrogel in terms of its network and mechanical properties, stability and drug release kinetics, and the overall safety of the hydrogel and the degradation products. The eye is a unique organ of physiologically distinct tissues and layers. Its dynamic and static barriers, ion containing films, and high turn-over aqueous humor versus viscous vitreous humor mean that hydrogel optimization is required for each targeted region of the eye. Generally, the resulting hydrogel should be transparent, flexible, and biocompatible, as well as retain a large fraction of water, however more specific properties are required depending on the precise application. A hydrogel for topical application should be flexible, mucoadhesive, and minimally viscous, while a sustained drug release hydrogel for vitreous delivery should have a viscosity matching that of the vitreous gel. Besides the favorable properties of the hydrogels with regards to ocular treatment, it must also possess the proper interaction with the therapeutic so that it is available in proper dosage amounts and stable over the intended delivery duration. Depending on hydrogel crosslinking (physical or chemical), loading method (simultaneous or post-crosslinking), drugs can be passively delivered out of the hydrogels via diffusion, displacement as a result of hydrogel swelling, or through the disintegration of the hydrogel network naturally over time or in response to a physiological stimulus. Factors that affect these passive drug release approaches include polymer concentration in the hydrogel, crosslinking density, and the overall affinity of the payload for the hydrogel constituents (Kirchhof et al., 2015). An alternative is to covalently conjugate the drug to the polymer via cleavable functional groups to create a polymer-therapeutic prodrug hydrogel. The release of the drug and associated degradation of the hydrogel could then be triggered in response to temperature, pH, ions, enzymes, or light (Casolaro et al., 2012, Yu et al., 2017). A drawback to relying solely on chemically conjugated drugs to the polymer is similar to the loss of activity as described above, as well as limiting the amount of the payload within the hydrogel.
3.3. Manufacturing
The advantages hydrogels offer over other materials for ocular drug delivery are apparent through the growth of research in the field to treat an array of ocular diseases. However, many hydrogel formulations do not make it to the clinic for several reasons, with most being used for SCLs, IOLs, or topically applied in situ hydrogels. Manufacturing of hydrogels into commercial and clinical products requires the following considerations: sterilization, shelf-life evaluation of the hydrogel (whole and individual constituents) and payload, and delivery tool compatibility with the hydrogel formulation for least administration (Kirchhof et al., 2015).
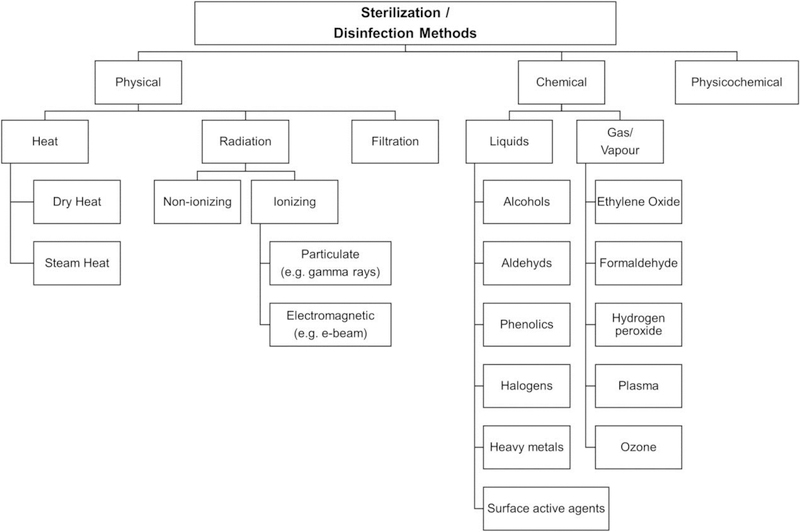
Sterilization is required to prevent infection of the target tissue upon vehicle delivery. Sterilization falls into two major categories, aseptic and terminal (Galante et al., 2018). Aseptic refers to the sterilization of the components separately before combination, but this requires extreme care to avoid downstream contamination. Terminal sterilization refers to the sterilization of the final formulation and its readiness for subsequent use. Materials can be sterilized via physical and chemical methods (Figure 3). Steam heat (SH) and dry heat (DH) are two physical approaches that require timed exposure to very high temperatures (SH – 15 min at 121–124 °C; DH – 30 min at ~180 °C). These temperatures and durations are sufficient to reduce microbial presence to acceptable levels but can lead to degradation of the hydrogel and the therapeutic payload.
Figure 3.
Sterilization and disinfection methods during hydrogel manufacturing. Reprinted with permission from (Galante et al., 2018). Copyright 2017 Wiley Periodicals, Inc.
Ionizing radiation represents the second type of physical sterilization. It is achieved through energizing electrons through an electron accelerator, or by gamma irradiation (GI). The latter is commonly used to disinfect pharmaceutics and medical equipment, due to its ability to sterilize at low temperatures, lack of chemical residue, and immediate availability post-sterilization. However, as with heat sterilization, alteration of the irradiated formulations can occur. Chemical sterilization approaches include the use of gases and liquids, most commonly with ethylene oxide and alcohols, aldehydes, and phenols, respectively. Again, these approaches remove the disadvantage of altering the formulation integrity when exposed to high temperatures; however, lengthy aeration and multiple rinses are required for gas and liquid sterilized materials. The most significant issue with all these disinfection methods is the risk of altering the expected function of the formulation and its payload. Hydrogels are susceptible to degradation, decomposition, further crosslinking, and inducing toxic effects as a result of the sterilization as mentioned above techniques. The presence of water in hydrogels also exacerbates some of the destructive effects of sterilization. Galante et al. provide an extensive review of the relatively narrow field of different sterilization techniques on natural and synthetic polymer hydrogels and those containing active drug compounds (Galante et al., 2018). They also touch on the unchartered territory of researchers performing sterilization during the hydrogel formation, to utilize the unfavorable sterilization-induced polymerization for hydrogel formation. Not only would it produce a final sterile hydrogel but would also remove the need for a polymerization initiator. Several labs have produced sterilized polymer via SH, GI, and ethylene oxide (Ji and Shi, 2012, Zhang et al., 2016, Ferraz et al., 2014).
Stability of the drug-loaded hydrogel, or the shelf-life of the constituents in a form for optimal user compliance, represents another challenge in the hydrogel manufacturing process. In most cases, final hydrogel formulations do not remain stable for very long due to the hydrolysis of crosslinking interactions. The polymers and crosslinkers themselves lose their ability to interact over time especially when stored in liquid form. If the formulation is considered in situ and can gel rapidly, separate storage of the individual components may extend shelf life. The next important consideration is the tools and devices used to deliver the formulations, specifically as it pertains to the type of ocular administration. If the hydrogel is too viscous, then the possibility to inject the formulation through a small gauge needle is removed. For in situ forming hydrogels, dual chamber syringes to store and then mix components during the injection can promote homogenous gelation and increase ease of administration (Kirchhof et al., 2015).
4. Hydrogels in Ocular Disease Treatment
4.1. SCLs and other hydrogels for topical drug delivery
By far the most common polymer hydrogel formulations for ocular delivery are in the form of vision correcting SCL, likely due to the ease of applying it on the surface of the eye and not containing any therapeutic payload. The first contact lens material was formulated out of PHEMA, with today’s formulations having evolved into PHEMA-hydrophilic/hydrophobic monomer hybrids, poly(dimethyl siloxane) (PDMS), and silicone based SCLs. Improvements have been made to the oxygen permeability of the hydrogels to increase comfort and duration of wear. Silicone polymer, whose backbones consist of siloxane bonds, enable greater gas permeability relative to other synthetic monomers and are often combined with monomers whose flexibilities are appropriate to fit the curvature of the cornea(Lin et al., 2014). Additionally, surface-modified hydrogels have been extensively studied to prevent protein and macromolecule adsorption on the lens surface to maintain optical clarity and reduce the risk of infection (Wang and Liu, 2012). These hydrogels used as vision-correcting contact lenses, with improved biocompatibility, extended wear, durability, and repeated use provide a vast potential to act as drug eluting formulations simultaneously. While no such SCL is commercially available for the delivery of drugs across the cornea, many researchers are exploring hydrogel formulations for this purpose. The first approach is finding ways to load commercially available SCLs by passively soaking the formulations in concentrated drug solutions, molecular imprinting, liposome loading, and surface modification with ionic ligands or nanoparticles (Kirchhof et al., 2015). The drawback to post-modifying SCLs is the limited drug loading and potential alteration of optimal properties since the original purpose of the lens is not optimized for modification or drug-carrying. The second approach under consideration is developing SCLs with the purpose of drug loading and sustained drug release. Maulvi et al. developed a PHEMA hydrogel containing timolol maleate-loaded cellulose nanoparticles as a ring implant sandwiched between two PHEMA lenses for sustained drug release at therapeutic rates to treat glaucoma (Maulvi et al., 2016). They found that the formulation successfully and safely delivered timolol-maleate in vivo for 168 hours (at a drug to nanoparticle ratio of 1:3) with therapeutic IOP reduction for 192 hours. Although they reported a high loss of drug during sterilization (radiation) and poor shelf-life in hydrated form, the formulation successfully delivered drugs without significant alteration to the ideal physical properties of SCLs (optically transparent, proper thickness, biocompatibility) – a major challenge to drug-eluting lenses.
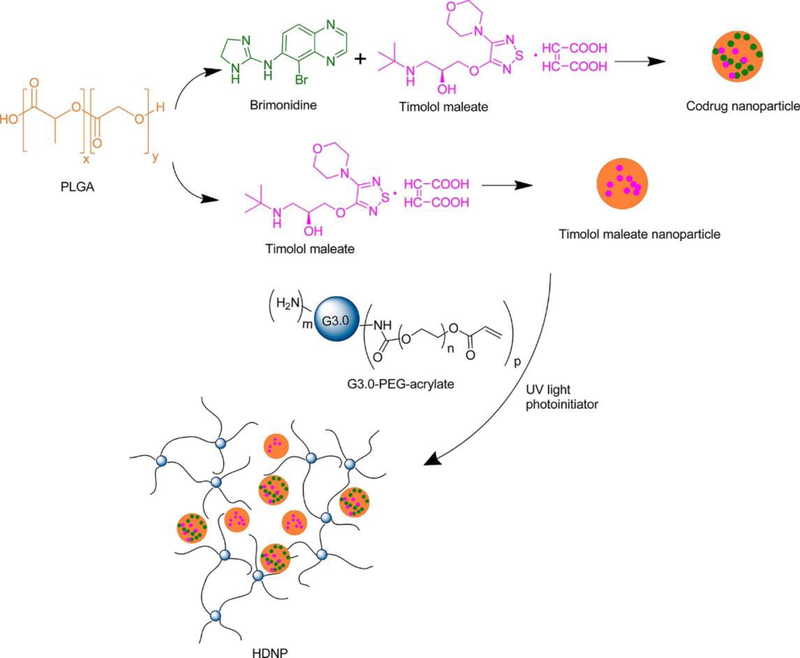
Another approach to the delivery of therapeutics across the cornea or sclera is the use of hydrogels that degrade over time. The significant difference between these hydrogel formulations and those used in SCLs is the long-term stability. While contact lenses are typically designed with repeat use in mind, making drug loading and potential re-loading – especially at appropriate levels – particularly challenging, hydrogels for one-time use can take advantage of the degradation profiles to ensure complete delivery of the therapeutic. Yang et al. developed a hybrid hydrogel composed of generation 3 poly(amidoamine) (PAMAM) dendrimers and PLGA nanoparticles for the co-delivery of two anti-glaucoma drugs, timolol maleate and brimonidine (Figure 4) (Yang et al., 2012). This hydrogel combines the biocompatibility and biodegradation of the PLGA particles for controlled drug release, and the photo-initiated crosslinking of the dendrimer and PEG-acrylate, to produce a liquid mucoadhesive hydrogel. Their results show that IOP reduction could be achieved for 4 days by sustained release of the drugs, with corneal, aqueous humor, and conjunctiva drug residence up to 7 days in a normotensive rabbit model. These mucoadhesive hydrogel formulations offer the potential of reducing administration frequency to tackle the issue of patient non-compliance with glaucoma treatment. Polycationic dendrimers (generation 5) were also used by Wang et al. to develop topical in situ-forming hydrogels via mild crosslinking with PEG-diacrylate for the sustained delivery of brimonidine tartrate and enhanced corneal permeation relative to free drug (Wang et al., 2017b).
Figure 4.
Hybrid dendrimer/PLGA hydrogel for the codelivery of brimonidine and timolol maleate Reprinted with permission from (Yang et al., 2012). Copyright 2012 American Chemical Society.
Although conventional eye drops or other surface-applied drug delivery formulations are rarely used to achieve posterior treatment, due to the physiological barriers that make bioavailability through this route challenging, Shikamura et al. reported on scleral/corneal contact lenses and subconjunctival rings to deliver ofloxacin to the posterior segment of the eye (Shikamura et al., 2016). Their contact lens and ring hydrogels were made of PHEMA crosslinked with ethylene glycol dimethacrylate, polymerized with 2,2′-azobisisobutyronitrile, and soaked in concentrated ofloxacin solution. One of the transparent contact lenses covered only the cornea, another the cornea and sclera. Both ring formulations were designed to contact the sclera and conjunctiva only, with ring 2 being shape optimized for the in vivo study in rabbits. In order of increasing drug availability to the retina/choroid, ofloxacin eye drops, corneal lens, corneal/scleral lens, ring 1, and ring 2 – successful delivery of ofloxacin to the posterior segment of the eye was achieved after 8-hour continuous wear of the hydrogel. While more study of the pharmacokinetics and bioactivity of the delivered drugs is required, these researchers have demonstrated and corroborated other research of transscleral and transconjunctival delivery for posterior drug delivery.
4.2. Wound healing – surgery and trauma
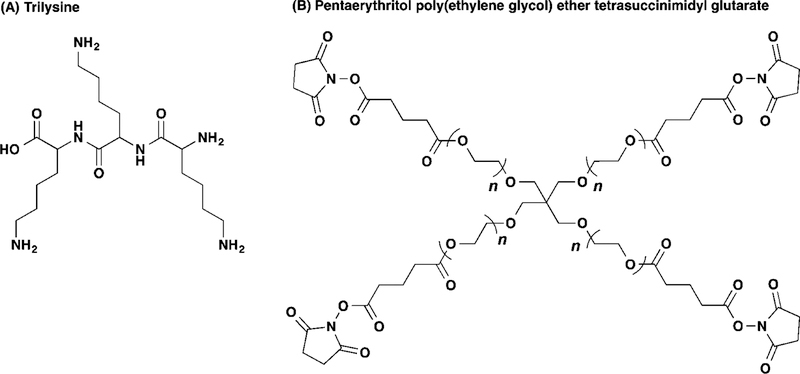
Wound healing represents another use for hydrogels after trauma or surgical procedures. Typically, nylon sutures are used to close wounds, but these can hinder normal healing, lead to infection, cause neovascularization, and require surgical removal (Tong et al., 2018). The desirable mucoadhesive properties used in other topical applications, like SCLs and drug-eluting hydrogels, can be further modified for wound healing. A standard surgical procedure that requires wound closure is the insertion of IOLs. While the properties of these lenses have made its administration easier and require ever smaller incisions in the cornea, problems such as non-tight sealing can cause several adverse effects after cataract surgery: the ingress of ocular surface fluid can lead to infection; the egress of anterior chamber fluids can lead to anterior chamber collapse and IOL rotation. Current wound closing measures, like sutures or stromal hydration, may not adequately seal the incision (Matossian et al., 2015). Hydrogels represent an alternative to ophthalmic adhesives, assuming they meet the appropriate requirements for leak pressure (> 80 mm Hg), cross-linking time (< 30 seconds), mechanical properties (5–200 kPa), swelling (< 200% w/w), adhesions strength (> 0.1 kPa), and degradation and residence time (1 week – 6 months) (Oelker and Grinstaff, 2008). Currently, there is only one FDA approved ocular healing hydrogel, called ReSure® sealant, while a similar formulation called DuraSeal™ approved for cranial adhesion has also been explored for ocular wound sealing (Trujillo-de Santiago et al., 2019, Ghobril and Grinstaff, 2015). Composed mostly of 4-armed NH-capped PEG crosslinked trilysine amine (Figure 5), these hydrogels are sloughed off the surface of the eye during re-epithelialization (Nallasamy et al., 2017). ReSure® hydrogel is explicitly approved for corneal application, but other researchers have investigated similar PEG-based formulations for use retinal reattachment and vitrectomies (Hoshi et al., 2018, Barliya et al., 2018).
Figure 5.
Chemical structures of the individual components of DuraSealTM hydrogel, (A) trilysine crosslinker and (B) tetrafunctional PEG capped with N-Hydroxysuccinimide. Reprinted with permission from (Ghobril and Grinstaff, 2015). Copyright 2015 The Royal Society of Chemistry.
While these formulations act to seal wounds, restore and maintain intraocular pressure, promote biocompatible response by maintaining normal corneal healing solute diffusion, avoid the formation of astigmatism, and are removed with the healing and regeneration process, drug-eluting ocular adhesives are actively being explored to enhance the healing process. The addition of epithelial growth factors (EGF) and antibiotics like doxycycline and vancomycin to wound healing hydrogel formulations has been shown to enhance the healing process, reduce neovascularization, and limit infection (Schultz and Morck, 2010, Anumolu et al., 2010, Carreira et al., 2014).
4.3. Intraocular lenses
Cataracts and myopia require the replacement of the natural crystalline lens with an artificial IOL to restore unhindered vision. While cataracts are clouding of the natural lens, myopia refers to the near-sightedness of an individual due to an increase in the axial length of the eye whereby light is focused in front of the retina. IOLs can address both issues by replacing the physically altered crystalline lens, as well as providing refractive power to correct for the vision impairment. IOL surgery is the top performed ocular surgery worldwide. The first IOL was synthesized in 1949 by Harold Ridley from poly(methyl methacrylate) (PMMA). While the optical properties and inertness of PMMA made these IOLs the gold standard for early cataract treatment, drawbacks such as its rigidity, large incision requirement, iris atrophy, and glaucoma ushered in research for more flexible IOL alternatives. Using hydrogels to make flexible and foldable IOLs allowed the incision size to decrease, thereby reducing the wound healing time touched on in section 4.2. The next generation of IOLs were siloxane-based hydrogels that could be folded for simpler insertion, followed by hydrophilic hydrogels and hydrophobic acrylate polymer lenses (Tetz and Jorgensen, 2015). Despite some improvements to foldable IOL compatibility, other adverse effects, such as posterior capsule opacification (PCO) still present challenges to IOL designs. PCO occurs when remaining epithelial cells from the original lens proliferate on the posterior end of the IOL – clouding the new lens. Although PCO capsulotomy can be safely treated by non-invasive YAG laser procedure performed by an eye doctor, this treatment can be expensive and cause further complications. Researchers are developing IOL material modifications to prevent PCO altogether. One approach is to graft polymers on the surface that prevent cell adhesion and proliferation. Bozukova et al. observed non-fouling properties by chemically coating IOL surfaces with varying molecular weight PEG-chains to prevent cell or protein adsorption (Bozukova et al., 2007). Lin et al. modified silicone hydrogel IOLs with hyaluronic acid/chitosan polyelectrolyte multilayers, to inhibit primary epithelial cell attachment and proliferation while preserving optimal optical properties (Lin et al., 2015).
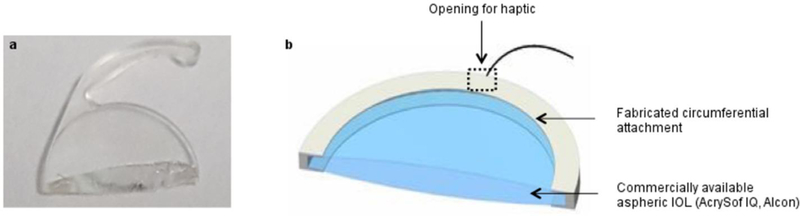
Incorporation of drugs into the IOL or the haptics can also prevent post-operative PCO. Like the SCLs discussed previously, IOLs can be submerged in high drug concentration solutions, impregnated with supercritical fluids, chemically coated with polymer/drug layers, or covalently attach drug molecules to the surface (Kirchhof et al., 2015). Unlike SCLs, IOLs have additional components that keep the IOL in place in the capsular bag. They can also be employed for drug delivery and have the added benefit of not needing to adhere to the same optical properties as the lens itself. This drug-eluting IOL approach can reduce the risk of endophthalmitis and postoperative infection related to cataract surgery, but also deliver drugs to other regions of the eye (Gonzalez-Chomon et al., 2011). However, there are currently no drug-eluting IOLs on the market. Kleinmann et al. demonstrated the affinity of hydrophilic acrylate lenses with fourth-generation fluoroquinolones, namely moxifloxacin or gatifloxacin. Their results suggest that the drug-loaded IOL did not cause any adverse reaction, and that sustained drug release was achieved at minimum inhibitory concentrations to prevent infections like endophthalmitis (Kleinmann et al., 2006). Another group loaded moxifloxacin and levofloxacin to a degradable poly(L-lactide-co-ε-caprolactone) polymer multilayer film that acted as a depot that could be attached to a commercially available IOL and haptic (Tan et al., 2016). They found that levoflaxin could be released over 14 days within an appropriate therapeutic window to reduce the risk of infection (Figure 6).
Figure 6.
Diagram of drug eluting IOL by incorporating drug-loaded poly(L-lactide-co-ɛ-caprolactone) (PLC) polymer depot between the IOL and haptic (Tan et al., 2016).
4.4. Vitreous substitute
A vitrectomy is the removal of the vitreous gel that makes up most of the volume in the posterior chamber of the eye. This procedure can be necessary for a variety of issues and conditions: diabetic vitreous hemorrhage, retinal detachment, proliferative vitreoretinopathy, endophthalmitis, and intraocular foreign body removal. Designing an optimal replacement for the natural vitreous humor is challenging, as the physical and chemical requirements are quite extensive. It must be transparent, possess the proper refractive index, density, and mechanical rigidity, be hydrophilic, non-absorbable and non-biodegradable, biologically and chemically inert, and allow the transfer of metabolites (Kirchhof et al., 2015). Current practices use gases and liquids as vitreous substitutes, but these do not provide adequate replacement for the original vitreous. Sulfur hexafluoride and perfluoropropane gases are used to treat vitreoretinal disease, for their favorable nontoxic and ocular properties, as well as for their ability to double or quadruple in injected volume and to remain in the vitreous cavity for 2 to 8 weeks, respectively (Alovisi et al., 2017). Drawbacks to these gases are the required face-down positioning for a few days after injection for proper positioning of the retina against the RPE, as well as associated IOP increases during and after injection, and gas-induced cataracts. Liquids such as semifluorinated alkanes, silicone oils, and heavy silicone oils have also been used in the clinic for optical and biocompatible properties but vary in their abilities to provide complete retinal tamponades. They can also cause adverse problems with elevated IOP, choroidal thinning, and anterior segment inflammation (Alovisi et al., 2017).
Hydrogels represent a unique alternative to vitreous substitutes, especially in-situ forming ones for more comfortable injection. Many natural and synthetic polymer hydrogels have been explored, such as (hydroxypropyl) methylcellulose, hyaluronic acid/gellan gum, PHEMA, poly(glycerol methacrylate), siloxanes, PVA, and poly(vinyl pyrrolidone) (Kirchhof et al., 2015). However, the limitations to these materials include immune response induction, fast absorption or degradation, inability to form a complete tamponade, and difficult delivery if not in situ-forming (Su et al., 2015). Chang et al. recently developed a zwitterionic in situ forming a hydrogel by thiol–ene Michael addition reaction between poly(MPDSA-co-AC) and α-PEG-MA crosslinkers (Chang et al., 2015). The zwitterionic hydrogels at thiol-ene ratios of 2:1 showed appropriate swelling profiles, rheological properties, and gelation time for use as a vitreous substitute. Their in vivo rabbit studies demonstrated no postoperative inflammation, likely due to the zwitterionic component of the hydrogel, and remained optically transparent with no abnormal structural changes observed in the rabbit eyes up to 1 month after implantation. Hyashi et al. developed a fast-forming hydrogel with ultralow polymer content to improve the drawbacks of high swelling pressure (Hayashi et al., 2017). Clusters of highly branched tetra-armed PEG prepolymers could be crosslinked in 10 minutes, allowing for injection and reduced surgical time. In vivo studies in rabbits demonstrated this hydrogel formulation as an in-fill vitreous substitute for over 1 year without any adverse side effects. A biomimetic in situ-forming hydrogel composed of thiolated gellan (collagen analog) and poly(methacrylamide-co-methacrylate) (hyaluronic acid analog) was also studied for its tunable properties as a vitreous substitute (Santhanam et al., 2016). Crosslinks form via disulfide bonds to cause the liquid injectable formulation to gel at body temperature. Preliminary work in rabbits showed that a hydrogel formulation of 0.9 mg/mL of gellan and 12 mg/mL poly(MAM-co-MAA-co-BMAC) remained optically clear, did not raise IOP, and did not cause retinal detachment or retinal thickness atrophy.
Despite promising laboratory results, there are no clinically available hydrogel vitreous substitutes. Many formulations including the current gold standards offer only short-term treatment as vitreous substitutes, with more clinical studies required to approve hydrogels for commercial use. The following review article provides extensive detail of relevant clinal and experimental vitreous substitute research (Su et al., 2015).
4.5. Intravitreal drug delivery hydrogels
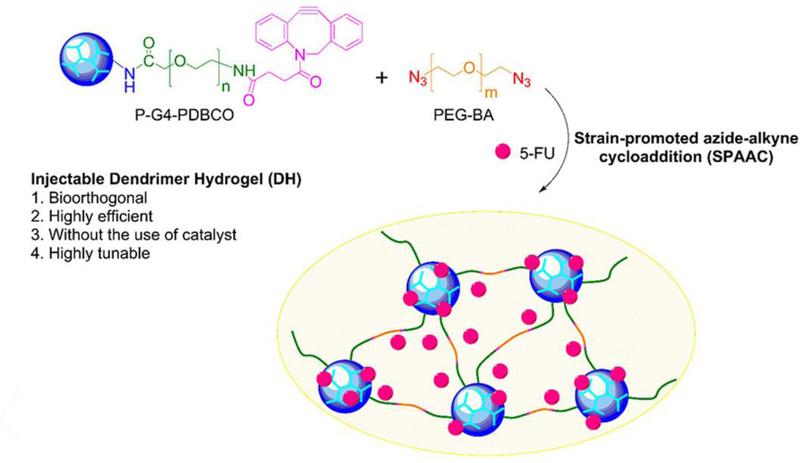
Unlike the challenges to vitreous substitute design, drug delivery hydrogels make up only a small volume of the vitreous space, do not necessarily need to be optically transparent, and generally are made to degrade over time. Indirect methods of delivery to the vitreous space, like systemic, suprachoroidal, and intrascleral suffer from low bioavailability in the target tissue and can have adverse side-effects. Intravitreal delivery, while invasive, can be safely performed by ophthalmologists to locally deposit the payload within the vitreous, with minimal occurrences of lens injury (0.006 %), retinal detachment (0.013 %), and endophthalmitis (0.018 %) (Meyer et al., 2016). However, many disease treatments, such as antiVEGF antibodies and bevacizumab for neovascularization suppression in diabetic retinopathy and AMD, require repeat injections that can increase the risk of complications. Hydrogels can be useful in taking on this challenge, by acting as a sustained release drug depot to minimize the need for multiple injections. Currently there are several implants on the market, such as Iluvien® to release fluocinolone acetonide (FA) for 36 months to treat diabetic macular edema, Retisert® to deliver FA to treat chronic noninfectious posterior uveitis, and Ozurdex® for dexamethasone delivery to treat macular edema (Syed, 2017, Kang-Mieler et al., 2014). While some of these implants can be made of biodegradable materials, some implants do require surgical removal or repositioning (Khurana et al., 2014). Like these polymeric implants, hydrogels can store higher dosages of the drug than otherwise tolerated in free-drug form, due to the sequestration into the hydrogel’s porous structures. Researchers have developed many sophisticated methods of loading therapeutics and promoting sustained and controlled release to reduce the number of injections. An essential factor for intraocular hydrogel delivery is the ability to inject it via small gauge syringes. Liquid hydrogels and in situ gelling hydrogels make this possible without requiring surgical implantation. Xu et al. developed a liquid hydrogel formulation using generation 4 poly(amidoamine) dendrimers modified with PEG and dibenzocyclooctyne groups to undergo crosslinking with PEG-bisazide chains for hydrogel formation via strain promoted azide-alkyne cycloaddition (Xu et al., 2017). The resulting gel was able to physically encapsulate the anticancer drug, camptothecin, and displayed an initial burst and sustained release profile in vitro. Although this formulation was tested in vivo in a mouse tumor model to show enhanced tumor reduction relative to free drug delivery, the liquid hydrogel formulation provides an attractive approach to delivering the formulation intravitreally. In situ forming gels, on the other hand, can be delivered before crosslinking has begun, with gelation occurring as the result of self-assembly, electrostatic interaction, and physiological stimuli. Temperature-sensitive hydrogels are among the most studied as the differences between conditions inside and outside the body is large enough to cause either crosslinking or drug release. Poly(N-isoporpylacrylamide) (PNIPAAm) is particularly attractive for it lower critical solution temperature of 32 °C, where at room temperature drugs can be loaded into tangled or crosslinked polymer chains and released as it increases to body temperature (37 °C) (Alexander et al., 2014, Hsiue et al., 2002). Egbu et al. developed two hydrogels composed of hyaluronic acid (HA) and PNIPAAm for the intraocular delivery of the antibody infliximab for the treatment of ocular inflammation (Egbu et al., 2018). One gel, called PEGDA-pNIPAAm-HA, was developed by chemically crosslinking NIPAAm in the presence of HA and infliximab with varying PEG-diacrylate (PEG-DA) weight percentages (1% and 3%). The other hydrogel forms by enzymatic crosslinking of HA functionalized with tyramine with infliximab with a 1% and 5% weight percent of HA-tyramine. All formulations were injectable, and in situ forming, however, the heat collapsible PEGDA-pNIPAAm-HA hydrogel displayed the most extended duration of infliximab release in a PK-Eye model – a two compartment in vitro model that mimics the anterior aqueous outflow and clearance of the human eye (Awwad et al., 2015).
Hydrogels can also be used to entrap and deliver stem cells to promote retinal and RPE tissue regeneration or provide neuroprotection by secretion of neurotrophic factors from cells encapsulated in hydrogels (Ballios et al., 2010, Gandhi et al., 2018, Zhang et al., 2011). Hydrogels can help overcome the challenge of integration of injected stem cells into the host retina, as well as provide long-term release of growth and neurotrophic factors to avoid high-frequency injections for anti-vascularization and protection of surviving cells in diseased retinas. Ballios et al. developed an injectable in situ forming hyaluronan and methylcellulose hydrogel for the delivery of retinal stem cells (RSCs) for retinal dysfunction and neural stem cells in stroke-injured brains (Ballios et al., 2015). Their results suggest higher RSC-derived rod survival and tissue integration, as well as cell distribution. The CD44 receptor on hyaluronan is implicated in the enhanced proliferation, migration, and distribution in RSC-derived photoreceptors and neural stem cells in their mouse animal models, relative to saline or artificial cerebrospinal fluid cell injections, respectively. This and other studies highlight the ability of ocular hydrogels to not only act as passive delivery vehicles of payloads but also to enhance the desired effect. For cell-based therapies, the advantages of natural monomer subunits outweigh those of synthetic origin for their intrinsic biocompatibility. Hunt et al. developed a 0.5% RGD-alginate hydrogel to encapsulate human embryonic stem cells/induced pluripotent stem cell-derived embryoid bodies, for the regeneration of the neural retina and RPE” (Hunt et al., 2017). Their in vitro work demonstrated that this hydrogel formulation could derive RPE and early neural retina, as an alternative to more frequently investigated hyaluronic acid scaffolds.
5. Conclusions
The field of hydrogels for ophthalmic therapy has dramatically increased since its early report in the 1960s. This trend has grown from topical vision correcting formulations (SCLs) and crystalline lens replacements due to cataracts to in situ “smart” drug delivery gels, vitreous substitutes, and cell scaffolds. While the innovations in drug encapsulation and release mechanisms, biocompatibility, and treatment duration have become highly sophisticated, the challenge of widespread application of hydrogel formulations in the clinic is still apparent. Issues concerning required sterilization, scale-up, shelf-life, and user compliance (healthcare professional and/or patient) must be overcome, before the advantages afforded by hydrogel therapeutic delivery can be realized. Globally, an estimated 246 million people are considered vision impaired and 39 million more considered blind, globally, with an associated financial cost of $3 trillion annually (Pascolini and Mariotti, 2012). Many of the diseases causing vision loss or blindness, especially in developing countries, are highly preventable and treatable.
Figure 7.
Schematic of injectable dendrimer hydrogel. Drug molecule is added during the gelation process between alkyne and azide groups. Reprinted with permission from (Xu et al., 2017). Copyright 2017 American Chemical Society.
Table 2.
Summary of covered hydrogel application examples in various regions of the eye.
| Segment of Eye | Hydrogel Application | Examples | |
|---|---|---|---|
| Anterior | |||
|
Soft Contact Lenses |
|||
|
Vision correcting |
• PHEMA |
||
| • PDMS | |||
| • silicone | |||
| Drug eluting | • PHEMA | ||
| • PAMAM dendrimer/PLGA nanoparticle | |||
| Wound Healing | |||
|
Corneal |
• ReSure® |
||
| • DuraSeal™ | |||
| Retinal | • Multi-arm modified PEG | ||
| Intraocular Lenses | |||
|
Lens |
• Surface-modified silicone |
||
| • Drug-eluting acrylate | |||
| Haptics | • Drug-eluting poly(L-lactide-co-ε-caprolactone) film | ||
| Posterior |
Vitreous Substitute |
• Zwitter-ionic poly(MPDSA-co-AC) |
|
| • tetra-armed PEG; thiolated gellan and poly(methacrylamide-co-methacrylate) | |||
| Intravitreal Hydrogels | |||
|
Drug eluting |
• Modified PAMAM dendrimer (clickable) |
||
| • temperature sensitive PNIPAAm | |||
| Cell scaffold | • hyaluronan and methylcellulose | ||
| • RGD-alginate | |||
Acknowledgements
This work was supported by the National Institutes of Health (R01EY024072).
Abbreviations
- AMD
Age-related macular degeneration
- BRB
Blood-retinal barrier
- DH
Dry heat
- EGF
Epithelial growth factors
- FA
Fluocinolone acetonide
- GAG
Glycosaminoglycan
- GI
Gamma irradiation
- IOL
Intraocular lens
- IOP
Intraocular pressure
- IVI
Intravitreal injection
- PAMAM
Poly(amidoamine) dendrimer
- PCO
Posterior capsule opacification
- PDMS
Poly(dimethyl siloxane)
- PEG
Poly(ethylene glycol)
- PHEMA
Poly(hydroxyethyl methacrylate)
- PMMA
Poly(methyl methacrylate)
- PNIPAAm
Poly(n-isoporpylacrylamide)
- PVA
Poly(vinyl alcohol)
- RPE
Retinal pigment epithelium
- RSC
Retinal stem cell
- SCL
Soft contact lens
- SH
Steam heat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ABELL RG, DARIAN-SMITH E, KAN JB, ALLEN PL, EWE SYP & VOTE BJ 2015. Femtosecond laser-assisted cataract surgery versus standard phacoemulsificati on cataract surgery: Outcomes and safety in more than 4000 cases at a single center. Journal of Cataract and Refractive Surgery, 41, 47–52. [DOI] [PubMed] [Google Scholar]
- AHMED EM 2015. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research, 6, 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER A, AJAZUDDIN KHAN,J & SARAF S 2014. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics, 88, 575–585. [DOI] [PubMed] [Google Scholar]
- ALOVISI C, PANICO C, DE SANCTIS U & EANDI CM 2017. Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery. Journal of Ophthalmology [DOI] [PMC free article] [PubMed]
- ANUMOLU SS, DESANTIS AS, MENJOGE AR, HAHN RA, BELONI JA, GORDON MK & SINKO PJ 2010. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials, 31, 964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AWWAD S, LOCKWOOD A, BROCCHINI S & KHAW PT 2015. The PK-Eye: A Novel In Vitro Ocular Flow Model for Use in Preclinical Drug Development. Journal of Pharmaceutical Sciences, 104, 3330–3342. [DOI] [PubMed] [Google Scholar]
- BALLIOS BG, COOKE MJ, DONALDSON L, COLES BLK, MORSHEAD CM, VAN DER KOOY D & SHOICHET MS 2015. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantatio n. Stem Cell Reports, 4, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLIOS BG, COOKE MJ, VAN DER KOOY D & SHOICHET MS 2010. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials, 31, 2555–2564. [DOI] [PubMed] [Google Scholar]
- BARICH DH, MUNSON EJ & ZELL MT 2005. PHYSICOCHEMICAL PROPERTIES, FORMULATION, AND DRUG DELIVERY. Drug Delivery: Principles and Applications, 57–71.
- BARLIYA T, SANDALON S, OFRI R, LIVNAT T & WEINBERGER D 2018. Transcleral approach for closing retinal tears using DuraSeal (TM) hydrogel sealant. Indian Journal of Ophthalmology, 66, 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZUKOVA D, PAGNOULLE C, DE PAUW-GILLET MC, DESBIEF S, LAZZARONI R, RUTH N, JEROME R & JEROME C 2007. Improved performances of intraocular lenses by poly(ethylene glycol) chemical coatings. Biomacromolecules, 8, 2379–2387. [DOI] [PubMed] [Google Scholar]
- CARREIRA AS, FERREIRA P, RIBEIRO MP, CORREIA TR, COUTINHO P, CORREIA IJ & GIL MH 2014. New drug-eluting lenses to be applied as bandages after keratoprosthesis implantation. International Journal of Pharmaceutics, 477, 218–226. [DOI] [PubMed] [Google Scholar]
- CASOLARO M, CASOLARO I & LAMPONI S 2012. Stimuli-responsive hydrogels for controlled pilocarpine ocular delivery. European Journal of Pharmaceutics and Biopharmaceutics, 80, 553–561. [DOI] [PubMed] [Google Scholar]
- CHANG J, TAO Y, WANG B, GUO BH, XU H, JIANG YR & HUANG YB 2015. An in situ-forming zwitterionic hydrogel as vitreous substitute. Journal of Materials Chemistry B, 3, 1097–1105. [DOI] [PubMed] [Google Scholar]
- CHUANG AT, MARGO CE & GREENBERG PB 2014. Retinal implants: a systematic review. British Journal of Ophthalmology, 98, 852–856. [DOI] [PubMed] [Google Scholar]
- COSTA JR, SILVA NC, SARMENTO B & PINTADO M 2015. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. European Journal of Clinical Microbiology & Infectious Diseases, 34, 1255–1262. [DOI] [PubMed] [Google Scholar]
- EGBU R, BROCCHINI S, KHAW PT & AWWAD S 2018. Antibody loaded collapsible hyaluronic acid hydrogels for intraocular delivery. European Journal of Pharmaceutics and Biopharmaceutics, 124, 95–103. [DOI] [PubMed] [Google Scholar]
- FANGUEIRO JF, ANDREANI T, EGEA MA, GARCIA ML, SOUTO SB, SILVA AM & SOUTO EB 2014. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. International Journal of Pharmaceutics, 461, 64–73. [DOI] [PubMed] [Google Scholar]
- FARKOUH A, FRIGO P & CZEJKA M 2016. Systemic side effects of eye drops: a pharmacokinetic perspective. Clinical Ophthalmology, 10, 2433–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-FERREIRO A, BARCIA MG, GIL-MARTINEZ M, VIEITES-PRADO A, LEMA I, ARGIBAY B, MENDEZ JB, LAMAS MJ & OTERO-ESPINAR FJ 2015. In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. European Journal of Pharmaceutics and Biopharmaceutics, 94, 342–351. [DOI] [PubMed] [Google Scholar]
- FERRAZ CC, VARCA GHC, LOPES PS, MATHOR MB & LUGAO AB 2014. Radio-synthesized polyacrylamide hydrogels for proteins release. Radiation Physics and Chemistry, 94, 186–189. [Google Scholar]
- GALANTE R, PARADISO P, MOUTINHO MG, FERNANDES AI, MATA JLG, MATOS APA, COLACO R, SARAMAGO B & SERRO AP 2015. About the effect of eye blinking on drug release from pHEMA-based hydrogels: an in vitro study. Journal of Biomaterials Science-Polymer Edition, 26, 235–251. [DOI] [PubMed] [Google Scholar]
- GALANTE R, PINTO TJA, COLACO R & SERRO AP 2018. Sterilization of hydrogels for biomedical applications: A review. Journal of Biomedical Materials Research Part B-Applied Biomaterials, 106, 2472–2492. [DOI] [PubMed] [Google Scholar]
- GANDHI JK, MANZAR Z, BACHMAN LA, ANDREWS-PFANNKOCH C, KNUDSEN T, HILL M, SCHMIDT H, IEZZI R, PULIDO JS & MARMORSTEIN AD 2018. Fibrin hydrogels as a xenofree and rapidly degradable support for transplantation of retinal pigment epithelium monolayers. Acta Biomaterialia, 67, 134–146. [DOI] [PubMed] [Google Scholar]
- GHOBRIL C & GRINSTAFF MW 2015. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chemical Society Reviews, 44, 1820–1835. [DOI] [PubMed] [Google Scholar]
- GONZALEZ-CHOMON C, CONCHEIRO A & ALVAREZ-LORENZO C 2011. Drug-Eluting Intraocular Lenses. Materials, 4, 1927–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMER N, BRANDL FP, KIRCHHOF S, MESSMANN V & GOEPFERICH AM 2015. Protein Compatibility of Selected Cross-linking Reactions for Hydrogels. Macromolecular Bioscience, 15, 405–413. [DOI] [PubMed] [Google Scholar]
- HAYASHI K, OKAMOTO F, HOSHI S, KATASHIMA T, ZUJUR DC, LI X, SHIBAYAMA M, GILBERT EP, CHUNG U, OHBA S, OSHIKA T & SAKAI T 2017. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nature Biomedical Engineering, 1. [Google Scholar]
- HOARE TR & KOHANE DS 2008. Hydrogels in drug delivery: Progress and challenges. Polymer, 49, 1993–2007. [Google Scholar]
- HOSHI S, OKAMOTO F, ARAI M, HIROSE T, SUGIURA Y, MURAKAMI T & OSHIKA T 2018. Patching retinal breaks with polyethylene glycol-based synthetic hydrogel sealant for retinal detachment in rabbits. Experimental Eye Research, 177, 117–121. [DOI] [PubMed] [Google Scholar]
- HSIUE GH, HSU SH, YANG CC, LEE SH & YANG IK 2002. Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials, 23, 457–462. [DOI] [PubMed] [Google Scholar]
- HUNT NC, HALLAM D, KARIMI A, MELLOUGH CB, CHEN JJ, STEEL DHW & LAKO M 2017. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomaterialia, 49, 329–343. [DOI] [PubMed] [Google Scholar]
- JI CD & SHI J 2012. Sterilization-free chitosan hydrogels for controlled drug release. Materials Letters, 72, 110–112. [Google Scholar]
- JIANG H, ZUO Y, ZHANG L, LI JD, ZHANG AM, LI YB & YANG XC 2014. Property-based design: optimization and characterization of polyvinyl alcohol (PVA) hydrogel and PVA-matrix composite for artificial cornea. Journal of Materials Science-Materials in Medicine, 25, 941–952. [DOI] [PubMed] [Google Scholar]
- KANG DERWENT JJ & MIELER WF 2008. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc, 106, 206–13; discussion 213–4. [PMC free article] [PubMed] [Google Scholar]
- KANG-MIELER JJ, OSSWALD CR & MIELER WF 2014. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opinion on Drug Delivery, 11, 1647–1660. [DOI] [PubMed] [Google Scholar]
- KHARKAR PM, KIICK KL & KLOXIN AM 2015. Design of thiol- and light-sensitive degradable hydrogels using Michael-type addition reactions. Polymer Chemistry, 6, 5565–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHURANA RN, APPA SN, MCCANNEL CA, ELMAN MJ, WITTENBERG SE, PARKS DJ, AHMAD S & YEH S 2014. Dexamethasone Implant Anterior Chamber Migration Risk Factors, Complications, and Management Strategies. Ophthalmology, 121, 67–71. [DOI] [PubMed] [Google Scholar]
- KIM YC, CHIANG B, WU XG & PRAUSNITZ MR 2014. Ocular delivery of macromolecules. Journal of Controlled Release, 190, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRCHHOF S, GOEPFERICH AM & BRANDL FP 2015. Hydrogels in ophthalmic applications. European Journal of Pharmaceutics and Biopharmaceutics, 95, 227–238. [DOI] [PubMed] [Google Scholar]
- KLEINMANN G, APPLE DJ, CHEW J, HUNTER B, STEVENS S, LARSON S, MAMALIS N & OLSON RJ 2006. Hydrophilic acrylic intraocular lens as a drug-delivery system for fourth-generation fluoroquinolones. Journal of Cataract and Refractive Surgery, 32, 1717–1721. [DOI] [PubMed] [Google Scholar]
- LIM HL, HWANG Y, KAR M & VARGHESE S 2014. Smart hydrogels as functional biomimetic systems. Biomaterials Science, 2, 603–618. [DOI] [PubMed] [Google Scholar]
- LIN CH, YEH YH, LIN WC & YANG MC 2014. Novel silicone hydrogel based on PDMS and PEGMA for contact lens application. Colloids and Surfaces B-Biointerfaces, 123, 986–994. [DOI] [PubMed] [Google Scholar]
- LIN QK, XU X, WANG BL, SHEN CH, TANG JM, HAN YM & CHEN H 2015. Hydrated polysaccharide multilayer as an intraocular lens surface coating for biocompatibility improvements. Journal of Materials Chemistry B, 3, 3695–3703. [DOI] [PubMed] [Google Scholar]
- LUO ZC, JIN L, XU L, ZHANG ZL, YU J, SHI S, LI XY & CHEN H 2016. Thermosensitive PEG-PCL-PEG (PECE) hydrogel as an in situ gelling system for ocular drug delivery of diclofenac sodium. Drug Delivery, 23, 63–68. [DOI] [PubMed] [Google Scholar]
- LV SX, TANG ZH, ZHANG DW, SONG WT, LI MQ, LIN J, LIU HY & CHEN XS 2014. Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. Journal of Controlled Release, 194, 220–227. [DOI] [PubMed] [Google Scholar]
- MATOSSIAN C, MAKARI S & POTVIN R 2015. Cataract surgery and methods of wound closure: a review. Clinical Ophthalmology, 9, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAULVI FA, LAKDAWALA DH, SHAIKH AA, DESAI AR, CHOKSI HH, VAIDYA RJ, RANCH KM, KOLI AR, VYAS BA & SHAH DO 2016. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. Journal of Controlled Release, 226, 47–56. [DOI] [PubMed] [Google Scholar]
- MEYER CH, KROHNE TU, ISSA PC, LIU ZP & HOLZ FG 2016. Routes for Drug Delivery to the Eye and Retina: Intravitreal Injections. Retinal Pharmacotherapeutics, 55, 63–70. [DOI] [PubMed] [Google Scholar]
- NALLASAMY N, GROVE KE, LEGAULT GL, DALUVOY MB & KIM T 2017. Hydrogel ocular sealant for clear corneal incisions in cataract surgery. Journal of Cataract and Refractive Surgery, 43, 1010–1014. [DOI] [PubMed] [Google Scholar]
- OELKER AM & GRINSTAFF MW 2008. Ophthalmic adhesives: a materials chemistry perspective. Journal of Materials Chemistry, 18, 2521–2536. [Google Scholar]
- PASCOLINI D & MARIOTTI SP 2012. Global estimates of visual impairment: 2010. British Journal of Ophthalmology, 96, 614–618. [DOI] [PubMed] [Google Scholar]
- ROBIN A & GROVER DS 2011. Compliance and adherence in glaucoma management. Indian Journal of Ophthalmology, 59, S93–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTHANAM S, LIANG J, STRUCKHOFF J, HAMILTON PD & RAVI N 2016. Biomimetic hydrogel with tunable mechanical properties for vitreous substitutes. Acta Biomaterialia, 43, 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ CL & MORCK DW 2010. Contact lenses as a drug delivery device for epidermal growth factor in the treatment of ocular wounds. Clinical and Experimental Optometry, 93, 61–65. [DOI] [PubMed] [Google Scholar]
- SHIKAMURA Y, YAMAZAKI Y, MATSUNAGA T, SATO T, OHTORI A & TOJO K 2016. Hydrogel Ring for Topical Drug Delivery to the Ocular Posterior Segment. Current Eye Research, 41, 653–661. [DOI] [PubMed] [Google Scholar]
- SIGFRIDSSON K & CARLSSON KE 2017. A preformulation evaluation of a photosensitive surface active compound, explaining concentration dependent degradation. European Journal of Pharmaceutical Sciences, 109, 650–656. [DOI] [PubMed] [Google Scholar]
- SU XY, TAN MJ, LI ZB, WONG MH, RAJAMANI L, LINGAM G & LOH XJ 2015. Recent Progress in Using Biomaterials as Vitreous Substitutes. Biomacromolecules, 16, 3093–3102. [DOI] [PubMed] [Google Scholar]
- SYED YY 2017. Fluocinolone Acetonide Intravitreal Implant 0.19 mg (ILUVIEN (R)): A Review in Diabetic Macular Edema. Drugs, 77, 575–583. [DOI] [PubMed] [Google Scholar]
- TAN DWN, LIM SG, WONG TT & VENKATRAMAN SS 2016. Sustained Antibiotic-Eluting Intra-Ocular Lenses: A New Approach. Plos One, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TETZ M & JORGENSEN MR 2015. New Hydrophobic IOL Materials and Understanding the Science of Glistenings. Current Eye Research, 40, 969–981. [DOI] [PubMed] [Google Scholar]
- THAM YC, LI X, WONG TY, QUIGLEY HA, AUNG T & CHENG CY 2014. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040 A Systematic Review and Meta-Analysis. Ophthalmology, 121, 2081–2090. [DOI] [PubMed] [Google Scholar]
- TING DSW, CHEUNG GCM & WONG TY 2016. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clinical and Experimental Ophthalmology, 44, 260–277. [DOI] [PubMed] [Google Scholar]
- TONG AY, GUPTA PK & KIM T 2018. Wound closure and tissue adhesives in clear corneal incision cataract surgery. Current Opinion in Ophthalmology, 29, 14–18. [DOI] [PubMed] [Google Scholar]
- TRUJILLO-DE SANTIAGO G, SHARIFI R, YUE K, SANI ES, KASHAF SS, ALVAREZ MM, LEIJTEN J, KHADEMHOSSEINI A, DANA R & ANNABI N 2019. Ocular adhesives: Design, chemistry, crosslinking mechanisms, and applications. Biomaterials, 197, 345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J, HE HL, COOPER RC & YANG H 2017a. In Situ-Forming Polyamidoamine Dendrimer Hydrogels with Tunable Properties Prepared via Aza-Michael Addition Reaction. Acs Applied Materials & Interfaces, 9, 10494–10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J, WILLIAMSON GS, LANCINA MG & YANG H 2017b. Mildly Cross-Linked Dendrimer Hydrogel Prepared via Aza-Michael Addition Reaction for Topical Brimonidine Delivery. Journal of Biomedical Nanotechnology, 13, 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG J, WILLIAMSON GS & YANG H 2018. Branched polyrotaxane hydrogels consisting of alpha-cyclodextrin and low-molecular-weight four-arm polyethylene glycol and the utility of their thixotropic property for controlled drug release. Colloids and Surfaces B-Biointerfaces, 165, 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG JJ & LIU F 2012. Imparting antifouling properties of silicone hydrogels by grafting poly(ethylene glycol) methyl ether acrylate initiated by UV light. Journal of Applied Polymer Science, 125, 548–554. [Google Scholar]
- WONG WL, SU XY, LI X, CHEUNG CMG, KLEIN R, CHENG CY & WONG TY 2014. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health, 2, E106–E116. [DOI] [PubMed] [Google Scholar]
- XING L, DORREPAAL SJ & GALE J 2014. Survey of intravitreal injection techniques and treatment protocols among retina specialists in Canada. Canadian Journal of Ophthalmology-Journal Canadien D Ophtalmologie, 49, 261–266. [DOI] [PubMed] [Google Scholar]
- XU LY, COOPER RC, WANG J, YEUDALL WA & YANG H 2017. Synthesis and Application of Injectable Bioorthogonal Dendrimer Hydrogels for Local Drug Delivery. Acs Biomaterials Science & Engineering, 3, 1641–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG H, TYAGI P, KADAM RS, HOLDEN CA & KOMPELLA UB 2012. Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. Acs Nano, 6, 7595–7606. [DOI] [PubMed] [Google Scholar]
- YASIN MN, SVIRSKIS D, SEYFODDIN A & RUPENTHAL ID 2014. Implants for drug delivery to the posterior segment of the eye: A focus on stimuli-responsive and tunable release systems. Journal of Controlled Release, 196, 208–221. [DOI] [PubMed] [Google Scholar]
- YU J, XU X, YAO FL, LUO ZC, JIN L, XIE BB, SHI S, MA HX, LI XY & CHEN H 2014. In situ covalently cross-linked PEG hydrogel for ocular drug delivery applications. International Journal of Pharmaceutics, 470, 151–157. [DOI] [PubMed] [Google Scholar]
- YU SH, ZHANG XY, TAN GX, TIAN L, LIU DD, LIU YX, YANG XG & PAN WS 2017. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydrate Polymers, 155, 208–217. [DOI] [PubMed] [Google Scholar]
- ZHANG K, HOPKINS JJ, HEIER JS, BIRCH DG, HALPERIN LS, ALBINI TA, BROWN DM, JAFFE GJ, TAO W & WILLIAMS GA 2011. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America, 108, 6241–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG YX, CHEN YF, SHEN XY, HU JJ & JAN JS 2016. Reduction- and pH-Sensitive lipoic acid-modified Poly(L-lysine) and polypeptide/silica hybrid hydrogels/nanogels. Polymer, 86, 32–41. [Google Scholar]