Abstract
Background
Fever is common in mechanically ventilated patients and may be uniquely detrimental in those with lung injury because of its injurious effects on pulmonary vascular permeability and alveolar epithelium. We evaluated the association of fever and antipyretic medication with mortality in mechanically ventilated emergency department (ED) patients.
Methods
This is a retrospective cohort study of 1264 patients requiring mechanical ventilation initiated in the ED with subsequent admission to an intensive care unit. Maximum body temperature was recorded for the first 24 hours after ED admission and categorized into four categories: <37°C, 37–38.2°C, 38.3–39.4°C, and ≥39.5°C. The primary outcome was 28-day mortality. We conducted a planned subgroup analysis of patients with sepsis at the time of intubation. Multivariable Cox proportional hazard ratios were used to assess the relationship between temperature, antipyretics, and mortality.
Results
Multivariable Cox proportional hazard ratios demonstrated that a maximum temperature ≥39.5°C was associated with increased mortality (aHR 1.59 [95% CI 1.05–2.39]). In the subgroup of patients with sepsis, a maximum temperature of 38.3–39.4°C was associated with survival (aHR 0.61 [95% CI 0.39–0.99]). There was no difference in 28-day mortality between patients who did and did not receive antipyretic medication in either the overall cohort or the septic subgroup.
Conclusion
High fever (≥39.5°C) was associated with increased risk for mortality in mechanically ventilated patients. However, in patients with sepsis, moderate fever (38.3–39.4°C) was protective. Antipyretic medication was not associated with changes in outcome. This suggests that fever may have different implications in septic versus non-septic mechanically ventilated patients.
Keywords: mechanical ventilation, sepsis, fever, mortality, antipyretics, acute respiratory distress syndrome
Introduction
Each year over 250,000 patients require intubation and mechanical ventilation in U.S. emergency departments (ED) (1). With a mortality of greater than 30% and annual costs over $27 billion, mechanical ventilation is disproportionately morbid and costly (2). Approximately 20% of mechanically ventilated patients develop acute respiratory distress syndrome (ARDS), an inflammatory condition with substantial morbidity and mortality that can be caused or worsened by mechanical ventilation (3–7). As such, there is a critical need to improve outcomes in this high risk population. The first 24 hours represent a key window where interventions may have maximum impact (8, 9).
Fever is present in up to 60% of mechanically ventilated patients, and treatment with antipyretic medications is common (10, 11). Fever may be especially detrimental to those at risk for ARDS because fever increases vascular pulmonary endothelial injury, pulmonary accumulation of neutrophils, and alveolar epithelial apoptosis, as well as raising overall metabolic burden and oxygen consumption (12–16). This theory is supported by animal models that suggest fever increases mortality after lung injury (12, 17, 18). However, fever also has beneficial immunologic effects, including enhanced immunity, (19) increased efficacy of antibiotics, (20) and inhibition of pathogen growth (21, 22). Several studies have found that fever is associated with improved survival in the setting of infection (9, 23, 24). Furthermore, antipyretic therapy has not been shown to improve long-term clinical outcomes (25, 26).
Currently it is unknown whether the harmful effects of fever outweigh the potential benefits in mechanically ventilated patients. Unfortunately, most studies of fever and antipyretic treatment have included mixed populations of critically ill patients without regard to ventilatory or infection status. Only two previous studies have assessed the relationship between fever and clinical outcomes specifically in mechanically ventilated patients (10, 27). These studies yielded mixed results, with poor reproducibility and low power. Therefore, the objective of our current study was to evaluate the impact of temperature and antipyretic therapy on mortality and clinical outcomes in patients with acute respiratory failure requiring mechanical ventilation. We hypothesized that fever would be associated with worse clinical outcomes in this population, especially in non-septic patients who would presumably benefit least from the immunomodulatory effects of fever.
Materials and Methods
Study population
This was a cohort study using a database of consecutively enrolled patients with mechanical ventilation initiated in the Emergency Department (ED) at a tertiary academic medical center between September 2009 and March 2016. The database was created as part of a clinical investigation that assessed outcomes associated with the implementation of ED lung-protective mechanical ventilation (8, 28). The study was approved by the institutional review board under waiver of informed consent.
Inclusion/exclusion criteria
All mechanically ventilated adult ED patients were screened for inclusion. Exclusion criteria were: (1) death or cessation of mechanical ventilation within 24 hours, (2) chronic mechanical ventilation, (3) presence of a tracheostomy, (4) transfer to a different hospital from the ED, (5) presence of ARDS in the ED, and (6) presence of neurologic injury (i.e. cardiac arrest and brain injury). Patients with neurologic injury were excluded based on the known deleterious effects of hyperthermia in this patient population (29–31).
Measurements
Baseline characteristics included demographics, comorbid conditions, illness severity (i.e. Sequential Organ Failure Assessment [SOFA]), reason for mechanical ventilation, ED length of stay, location of intubation, and ED requirement for blood products. For patients meeting sepsis criteria, suspected source, culture sites, and microbiology results were also collected. All temperature measurements and routes of measurement were collected from the ED and Intensive Care Unit (ICU) for the first 24 hours after presentation to the ED. Administration of antipyretic medication (ibuprofen and acetaminophen) given during this 24-hour period was recorded.
Patients were assigned to one of four temperature groups (<37°C, 37.0–38.2°C, 38.3–39.4°C, or ≥39.5°C) based on their maximum body temperature (Tmax) within 24 hours of ED admission These temperature groups were chosen a priori to be consistent with prior literature evaluating temperature in critically ill patients (9, 32, 33). Fever was defined as a core temperature (esophageal, bladder, rectal, or pulmonary catheter) of 38.3°C or greater as recommended by the American College of Critical Care Medicine and the Infectious Diseases Society of America guidelines for evaluation of fever in the ICU (34). Peripheral temperature measurements were adjusted to estimate core temperature by adding 0.3°C to axillary routes and 0.2°C to all other peripheral routes, based on the findings of a meta-analysis of the relationship between core and peripheral temperature measurements (35). Antipyretic medication received in the first 24 hours was assessed binarily based on whether either ibuprofen or acetaminophen was or was not administered during this timeframe. Both medications were given enterally, there were no instances of IV acetaminophen or ibuprofen use. Our cohort excluded patients that died within 24 hours of ED arrival; thus, we chose to assess temperature and antipyretic medication only within the first 24 hours so as to avoid survivor bias in our analysis.
The primary outcome was 28-day mortality. Secondary outcomes were progression to ARDS and ventilator-, hospital-, and ICU-free days. ARDS was defined according to the Berlin definition and was assessed through day seven (28, 36).
Statistical analysis
Baseline patient demographics were assessed with descriptive statistics, including mean (standard deviation) and median (interquartile range). Normality was assessed using histograms and the Kolmogorov-Smirnov test. Categorical variables were compared using the chi-squared test or Fisher’s exact. Continuous variables were compared using the independent samples t-test or Mann-Whitney U test.
Primary and secondary outcomes were assessed using univariable comparisons. Continuous outcomes were compared using the Kruskal-Wallis test after assessing normality. Kaplan-Meier survival curves for 28-day mortality were compared using the log-rank test.
To determine the independent effect of body temperature on 28-day mortality and progression to ARDS, a Cox Proportional Hazards model was used. Patients who are shown as censored were discharged alive who had additional follow-up before 28-days, but do not have additional records to confirm they were alive on day 28. These patients contribute to the analysis until their last day of follow-up. The 37.0–38.2°C temperature group was considered to be the reference group. A priori, we included age and SOFA score in these models, given the known association of these factors with worse clinical outcomes in critically ill patients.
All other baseline variables that varied with the outcome of interest with a p-value less than 0.05 on univariable comparisons were considered for inclusion in the regression model. These were evaluated with forward, backward, and stepwise regression based on Akaike Information Criterion (AIC) with a significance level of 0.10 for entry and removal. The proportional hazards assumption was tested using Schoenfeld residuals both quantitatively and graphically. Collinearity diagnostics were evaluated to ensure variable independence. Adjusted hazard ratios (aHR) and corresponding 95% confidence intervals and p-values are reported for each variable in the model. All tests were two-tailed and a p-value of 0.05 was used to establish significance.
Outcomes were assessed in the overall cohort and in the subset of patients intubated for sepsis. Sepsis was defined as the presence of two or more SIRS criteria in the setting of proven or suspected infection (37). Vital signs and laboratory data were evaluated to determine whether SIRS criteria were met. Proven infection was identified by positive culture data. Suspected infection was defined as the initiation of antibiotics by the care team. Patients with sepsis were analyzed separately because of evidence suggesting that the association between fever and mortality may vary based on infection status (23, 29, 38). Because ibuprofen and acetaminophen may be given to afebrile patients for analgesia, a sensitivity analysis was also performed to test the effect of antipyretics in the subgroup of patients who developed fever within 24 hours.
The presence of hypothermia is known to be a strong predictor of mortality in septic and non-septic patients (23, 39). Therefore, we also performed a sensitivity analysis excluding patients who had a minimum temperature < 36°C within the first 24 hours after ED admission. We presumed hypothermia would be most prevalent among patients with the lowest peak temperatures. Therefore, the goal of this sensitivity analysis was to remove the potential bias of hypothermia on mortality in these groups.
Results
Demographics
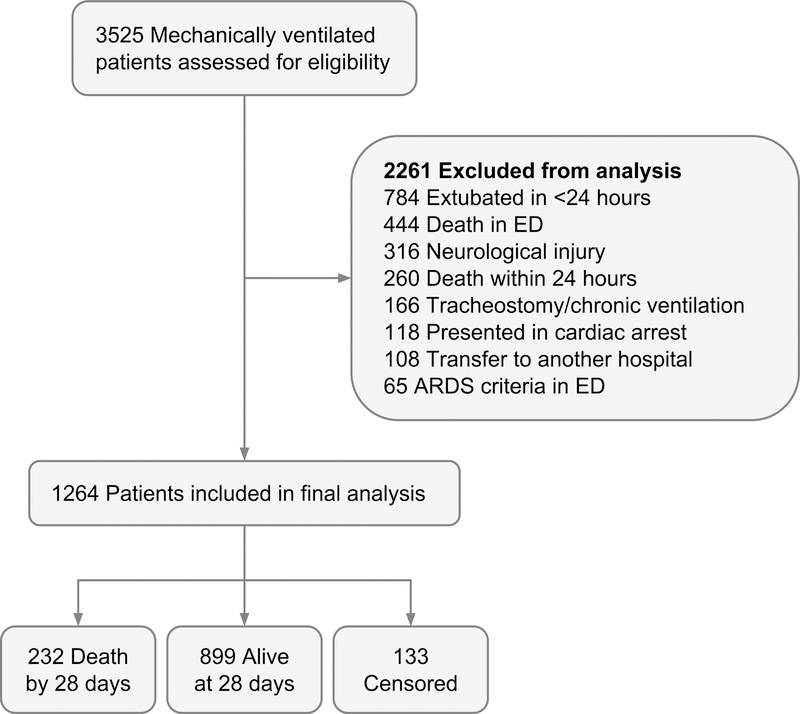
A total of 1264 patients were included in the final analysis (Figure 1). 28-day mortality was 22.5% for the overall group and 35.9% for the sepsis subgroup. ARDS developed in 11% of the overall group and in 23% of the septic subgroup.
Figure 1.
Patient inclusion flow diagram. ED, Emergency Department; ARDS, Acute Respiratory Distress Syndrome.
Table 1 presents baseline characteristics of the study population related to mortality. Maximum temperature did not vary among survivors and nonsurvivors (mean 38.4°C [0.8°C] vs 38.4°C [1.1°C]). Non-survivors averaged a lower minimum temperature compared to survivors (35.3°C [1.8°C] vs 35.8°C [1.8°C], p < .001). Nonsurvivors were also more likely than survivors to have core temperature, rather than peripheral temperature monitored, and had more frequent temperature measurements during the first 24 hours after ED admission. There was no difference between the two groups in the percentage of patients who received antipyretic therapy (13.7% in survivors vs 13.8% in nonsurvivors).
Table 1.
Baseline characteristics of survivors and nonsurvivors
| Survivors | Nonsurvivors | p | |
|---|---|---|---|
| n=1032 | n=232 | ||
| Age, years | 56.9 (16.8) | 65.3 (16.3) | <0.001 |
| Female, n (%) | 474 (45.9) | 109 (47.0) | 0.828 |
| BMI, n (%) | 0.022 | ||
| Underweight (<18.5) | 62 (6.0) | 14 (6.0) | |
| Normal weight (18.5–24.9) | 316 (30.6) | 93 (40.1) | |
| Overweight (25–29.9) | 267 (25.9) | 59 (25.4) | |
| Obese (≥ 30) | 387 (37.5) | 66 (28.4) | |
| Comorbidities, n (%) | |||
| Diabetes | 376 (36.4) | 83 (35.8) | 0.910 |
| COPD | 295 (28.6) | 57 (24.6) | 0.249 |
| CHF/Pulmonary edema | 261 (25.3) | 56 (24.1) | 0.778 |
| Cancer | 149 (14.4) | 80 (34.5) | <0.001 |
| Immunosuppression | 108 (10.5) | 33 (14.2) | 0.126 |
| Renal Failure | 93 (9.0) | 16 (6.9) | 0.364 |
| Cirrhosis | 74 (7.2) | 32 (13.8) | 0.002 |
| SOFAa | 4 (3–6) | 6 (3.75–8) | <0.001 |
| Reason for mechanical ventilation, n (%) | <0.001 | ||
| Sepsis | 326 (31.6) | 117 (49.0) | |
| Trauma | 143 (13.9) | 23 (9.6) | |
| COPD | 107 (10.4) | 14 (5.9) | |
| CHF/pulmonary edema | 86 (8.3) | 13 (5.4) | |
| Asthma | 39 (3.8) | 0 (0) | |
| Other | 331 (32.1) | 72 (30.1) | |
| ED length of stay, (h) | 5.9 (3.9–8.3) | 5.9 (4.0–7.8) | 0.838 |
| Received blood products in ED, n (%) | 102 (9.9) | 52 (22.4) | <0.001 |
| ICU type, n (%) | 0.032 | ||
| MICU | 700 (67.8) | 150 (64.6) | |
| SICU | 169 (16.4) | 28 (12.1) | |
| CCU | 75 (7.3) | 30 (12.9) | |
| NSICU | 74 (7.2) | 21 (9.0) | |
| CTICU | 14 (1.4) | 3 (1.3) | |
| Max temperature in 24 hours (°C) | 38.4 (0.8) | 38.4 (1.1) | 0.272 |
| Max temperature in 24 hours | 0.002 | ||
| <37°C | 57 (5.5) | 23 (9.9) | |
| 37 – 38.2°C | 606 (58.7) | 127 (54.7) | |
| 38.3 – 39.4°C | 290 (28.1) | 52 (22.4) | |
| ≥ 39.5°C | 79 (7.6) | 30 (12.9) | |
| Min temperature in 24 hours (°C) | 35.8 (1.8) | 35.3 (1.8) | <0.001 |
| Time between temperature measurements, hours | 2.0 (1.0–4.0) | 1.75 (1.0–2.6) | <0.001 |
| Core temperature measurement, n (%)b | 594 (57.5) | 158 (68.1) | 0.004 |
| Received antipyretics, n (%) | 141 (13.7) | 32 (13.8) | 1 |
Continuous variables are reported as mean (standard deviation) and median (interquartile range). SOFA, Sequential Organ Failure Assessment; ICU, Intensive Care Unit, MICU, Medicine Intensive Care Unit; SICU, Surgical Intensive Care Unit; CCU, Cardiac Care Unit; NSICU, Neurosurgical Intensive Care Unit; CTICU, Cardiothoracic Intensive Care Unit.
Modified score, which excludes Glasgow Coma Scale.
Core temperature defined as > 50% of measurements from a core thermometer, including bladder, rectal, esophageal, and pulmonary artery catheter.
Among patients with sepsis (Supplemental Digital Content, Table 1), survivors averaged a higher minimum temperature (36.2°C [1.3°C] vs 35.4°C [1.9°C], p < 0.001) and had less frequent temperature measurements. One-fourth (25.7%) of septic survivors received antipyretics versus 14.5% of septic nonsurvivors (p = 0.031).
Univariable Comparisons
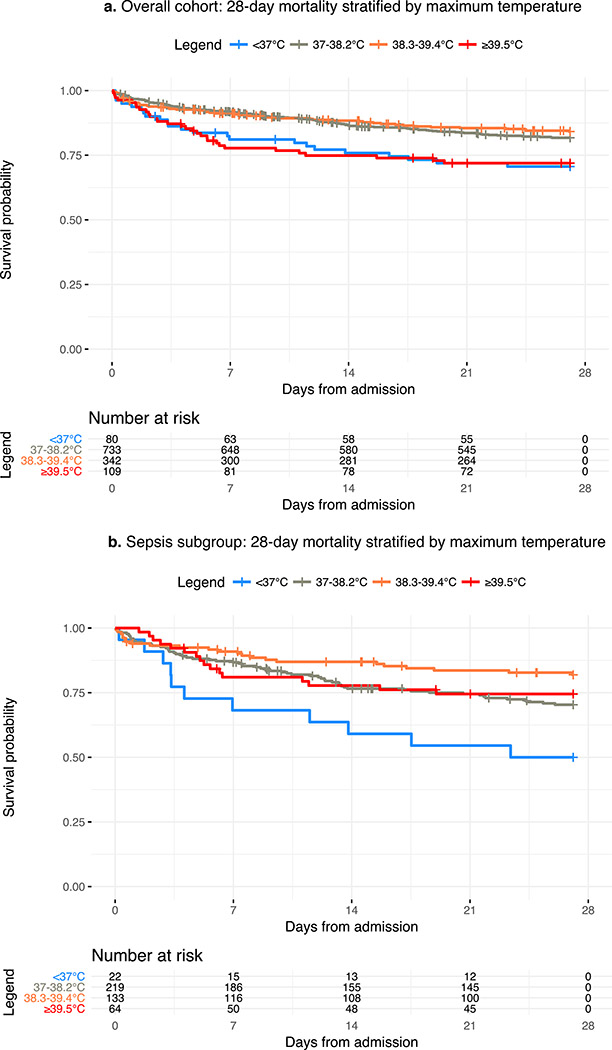
In the overall cohort, patients with a Tmax <37°C or ≥39.5°C had significantly higher 28-day mortality compared to the reference group (HR 1.8 [95% CI 1.1–2.7] and 1.7 [95% CI 1.1–2.5] respectively) (Figure 2a, Table 2). Patients with a Tmax ≥39.5°C also had a significantly higher progression to ARDS (HR 2.1 [95% CI 1.3–3.6]) (Table 2). These patients also had the lowest median ventilator-, hospital-, and ICU-free days (median 20.1 [IQR 0.0–25.2], 9.9 [IQR 0.0–18.1], and 18.5 [IQR 0.0–23.5] respectively) (Table 2).
Figure 2.
Survival curves for 28-day mortality stratified by maximum temperature within 24 hours. 2a: Overall cohort. 2b: Sepsis subgroup. Maximum temperature range: (1) <37°C, (2) 37–38.2°C, (3) 38.3–39.4°C and (4) ≥39.5°C. Category 2 is used as reference.
Table 2.
Clinical outcomes stratified by maximum temperature achieved within 24 hours
| Outcome | <37°C | 37–38.29°C | 38.3–39.5°C | >39.5°C | p |
|---|---|---|---|---|---|
| Overall | |||||
| 28-day mortalitya | 1.8 (1.1–2.7) | Ref. | 0.9 (0.6–1.2) | 1.7 (1.1–2.5) | 0.001 |
| ARDSa | 1.3 (0.7–2.7) | Ref. | 1.1 (0.7–1.7) | 2.1 (1.3–3.6) | 0.027 |
| Ventilator free daysb | 22.2 (0–26.1) | 24.6 (17.9–26.3) | 24.0(14.9–25.9) | 20.1 (0–25.2) | <0.001 |
| Hospital free daysb | 14.5 (0–21.6) | 16.9 (3.4–21.6) | 13.8 (0.5–20.9) | 9.9 (0–18.1) | <0.001 |
| ICU free daysb | 20.9 (0–24.9) | 23.0 (15.1–25.1) | 22.1 (12.9–24.6) | 18.5 (0–23.5) | <0.001 |
| Sepsis Subgroup | |||||
| 28-day mortalitya | 1.9 (1.0–3.8) | Ref. | 0.6 (0.4–0.9) | 0.8 (0.5–1.5) | 0.008 |
| ARDSa | 1.5 (0.6–3.8) | Ref. | 0.9 (0.6–1.6) | 1.3 (0.7–2.5) | 0.632 |
| Ventilator free daysb | 0 (0–21.5) | 21.5 (0–25.1) | 22.3 (12.5– 25.2) | 19.6 (0–24.5) | 0.016 |
| Hospital free daysb | 0 (0–5.9) | 11.1 (0–18.1) | 11.8 (0–19.2) | 10.4 (0–16.9) | 0.007 |
| ICU free daysb | 0 (0–17.2) | 19.5 (0–23.2) | 20.5 (10.5–23.9) | 18.1 (0–22.6) | 0.004 |
Survival data displaying Cox proportional hazard ratio, (95% confidence interval).
Continuous data displaying median, (interquartile range), p-value calculated with Kruskal-Wallis rank sum test for continuous outcomes, log-rank test for survival analysis. ARDS, acute respiratory distress syndrome; ICU, intensive care unit.
In the sepsis subgroup, patients with a Tmax <37°C had significantly higher 28-day mortality (HR 1.9 [95% CI 1.0–3.8]) as compared to the reference group (Figure 2b, Table 2). As opposed to the overall cohort, septic patients with Tmax ≥39.5°C had similar mortality compared to the reference group (HR 0.8 [95% CI 0.5–1.5]), and those with Tmax of 38.3–39.4°C had the lowest mortality (HR 0.6 [95% CI 0.4–0.9]) (Figure 2b, Table 2). There were no significant associations between Tmax category and progression to ARDS, even in the setting of fever ≥39.5 (HR 1.3 [95% CI 0.7–2.5]). The 38.3–39.4°C temperature range had the highest median ventilator-, hospital-, and ICU-free days (median 22.3 [IQR 12.5–25.2], 11.8 [IQR 0–19.2], and 20.5 [IQR 10.5–23.9] respectively) while the <37°C category had the fewest (median 0.0 [IQR 0.0–21.5], 0.0 [IQR 0.0–5.9]), and 0.0 [IQR 0.0–17.2] respectively) (Table 2).
Multivariable Comparisons
In the overall cohort, a Tmax ≥39.5°C was significantly associated with higher mortality (aHR 1.59 [95% CI 1.05–2.39]) after adjusting for identified confounders (Table 3). Conversely, in the sepsis subgroup, a Tmax ≥39.5°C was not a predictor of death (aHR 1.49 [95% CI 0.52–1.59]) and a Tmax of 38.3–39.4°C was associated with survival (aHR 0.61 [95% CI 0.39–0.99]) (Table 3). Multivariable Cox models for progression to ARDS did not yield any significant differences between Tmax groups in either the overall group or the sepsis subgroup (Supplemental Digital Content, Table 2).
Table 3.
Multivariable Cox proportional hazards model for 28-day mortality
| Variable | aHR | 95%CI | p |
|---|---|---|---|
| Overall | |||
| Agea | 1.03 | 1.02–1.04 | <0.001 |
| Comorbidity | |||
| Cancer | 2.18 | 1.66–2.87 | <0.001 |
| Cirrhosis | 1.65 | 1.12–2.45 | 0.012 |
| SOFAa | 1.07 | 1.02–1.13 | 0.006 |
| Received blood products in ED | 1.88 | 1.35–2.61 | <0.001 |
| Ventilated due to sepsis | 1.41 | 1.07–1.87 | 0.016 |
| Max temperature in 24 hoursb | |||
| <37°C | 1.43 | 0.91–2.26 | 0.119 |
| 38.3 – 39.5°C | 0.90 | 0.65–1.25 | 0.529 |
| >39.5°C | 1.59 | 1.05–2.39 | 0.027 |
| Sepsis Subgroup | |||
| Age | 1.02 | 1.01–1.04 | <0.001 |
| Comorbidity | |||
| Cancer | 2.16 | 1.46–3.19 | <0.001 |
| Cirrhosis | 2.03 | 1.15–3.59 | 0.014 |
| SOFAa | 1.08 | 1.00–1.15 | 0.040 |
| Received blood products in ED | 1.65 | 1.02–2.67 | 0.042 |
| Pulmonary source of infection | 0.67 | 0.46–0.98 | 0.042 |
| Max temperature in 24 hours b | |||
| <37°C | 1.46 | 0.75–2.83 | 0.265 |
| 38.3 – 39.5°C | 0.61 | 0.38–0.99 | 0.045 |
| >39.5°C | 1.49 | 0.52–1.59 | 0.732 |
For continuous varibles, hazard ratios reflect the increased hazard of 28-day mortality for a one-unit increase in the variable.
For categorical variables, the reference category 37–38.29°C, is removed. aHR, adjusted hazard ratio; CI confidence interval; SOFA, sequential organ failure assessment.
Antipyretic Medications
Baseline characteristics of antipyretic use are described in Supplemental Digital Content, Table 3. There was no significant difference in 28-day mortality or progression to ARDS among febrile patients who did and did not receive antipyretic therapy in the overall group and sepsis subgroup (Supplemental Digital Content, Table 4). Although univariable analysis of the sepsis subgroup showed that a significantly higher portion of survivors received antipyretic medications (Supplemental Digital Content, Table 1), this effect did not persist in the multivariable analysis (Table 3) or in the univariate analysis of the febrile subgroup of septic patients (Supplemental Digital Content, Table 4).
Sensitivity Analyses
Patients who developed fever in the first 24 hours had no significant difference in 28-day mortality or progression to ARDS in univariate analysis in both the overall group and the sepsis subgroup. Excluding patients who developed hypothermia in the first 24-hours of ED presentation did not substantially change our findings in both univariate and multivariate analysis of 28-day mortality in the overall group or sepsis subgroup (Supplemental Digital Content, Table 5 & 6, Figure 1).
Discussion
This cohort study was designed to evaluate the association between body temperature and clinical outcomes in mechanically ventilated patients. We found that high fever (≥39.5°C) within 24 hours of ED admission was an independent predictor of 28-day mortality. In patients with sepsis, fever in the 38.3–39.4°C range was associated with lower 28-day mortality compared to normothermia. Furthermore, treatment with antipyretic medication was not found to be associated with mortality in either the overall group or the sepsis subgroup, even when limited to patients with fever.
Our findings align with previous studies suggesting that elevated body temperatures are of contrasting prognostic significance in critically ill patients with and without sepsis (23, 38, 40) and add to a growing body of evidence demonstrating a potential benefit of hyperthermia in sepsis (41). There are several mechanisms by which fever may be protective in sepsis. Fever modulates the host response to infection, boosting both cellular and innate immunity (42). Fever also has been shown to decrease inflammatory cytokines and increase expression of cytoprotective heat shock proteins, which could limit the injurious effects of infection (43, 44). Additionally, fever may directly inhibit pathogen growth and increase antibiotic susceptibility (45). Our study supplements this literature through its focus on mechanically ventilated patients, a population at risk for ARDS, in whom it has been hypothesized that fever may worsen clinical outcomes by increasing inflammation and worsening epithelial injury (15, 17–22).
Few previous studies have assessed body temperature in mechanically ventilated patients. In a prospective cohort study of mechanically ventilated patients with ARDS by Netzer et al (10), one or more days of hypothermia or three or more days of fever both delayed mechanical ventilator liberation; however, only hypothermia was significantly associated with in-hospital mortality. The relationship between hypothermia and mortality was further supported in a study of patients with ARDS which found an inverse correlation between body temperature and death (27). However, fever was shown to be protective, and ventilator liberation was not assessed. Both studies had a high proportion of participants with sepsis, which may have biased or obscured results due to the potentially disparate results in patients with and without sepsis (23, 29, 38). Furthermore, the impact of antipyretic therapy was not assessed in either of these studies. We addressed these issues in our current study by conducting a subgroup analysis on patients with sepsis, including an analysis of antipyretic administration in all patients and those with fever, and using multivariable analysis to assess relationships between various temperature ranges and our outcomes.
On univariable analysis of the sepsis subgroup, we found a higher likelihood of survival among patients who received antipyretics. This effect did not persist in our multivariable analysis of antipyretics in septic patients with fever. Rather than a true benefit of antipyretic medication, this trend likely reflects the consistent survival benefit of fever we noted in our sepsis subgroup Thus, we did not find evidence of antipyretic benefit or harm, in agreement with high-quality randomized controlled trials and a meta-analysis suggesting antipyretic therapies have little effect on long-term mortality in critically ill septic patients (25, 26, 46).
Few prior studies explicitly address antipyresis in mechanically ventilated patients (47, 48). A large administrative data analysis in mechanically ventilated septic patients found antipyretic therapy (including both pharmacological and physical cooling) was associated with increased mortality (47). However, the proportion of patients receiving pharmacological therapy was equal in the survivor and nonsurvivor groups, suggesting that the overall benefit of antipyretic therapy was driven by the effect of external cooling. We did not evaluate external cooling in our patient population, so this may explain the difference in our results. Two studies have evaluated therapeutic hypothermia via external cooling in mechanically ventilated patients with ARDS (48, 49). Both demonstrated lower mortality in cooled patients, but these studies were limited by low sample size, lack of randomization, and control groups that contained historical controls.
The association we found between high fever and death in our overall group cannot be explained by an increased progression to ARDS, as we did not find a significant association between fever and ARDS in the overall group or sepsis subgroup.
This study has several limitations. It is a single-center, retrospective study, and as such is subject to the flaws inherent to this study design and cannot infer causality. We have attempted to address this through multivariable models accounting for statistically and clinically significant variables. Regardless, future prospective studies are needed to support and verify our conclusions. Our study includes peripheral temperature measurements which have a lower accuracy and when compared to central temperature monitoring (35). We have attempted to control for this by adjusting peripheral measurements as described in our methods. Because we evaluated body temperature only within the first 24 hours of presentation, it is unclear whether body temperature later in the course of critical illness affect outcomes or whether duration of fever is clinically important. Similarly, we cannot account for antipyretic therapy administered prior to ED presentation. Our analysis was based on maximum temperature rather than a continuum of measured temperatures, as is consistent with other studies (27, 50). Also, in terms of triaging resources to critically ill patients in the ED and ICU, the prognostic value of temperature during the first 24 hours is likely to be most clinically significant. We did not assess the degree to which lack of fever may have delayed recognition of sepsis and appropriate treatment. However prior work has demonstrated a deleterious effect of hypothermia and normothermia in septic patients even after adjusting for antibiotic omission and IV fluid in the ED (50).
Conclusion
In mechanically ventilated patients, high fever (≥39.5°C) is independently associated with death. In septic mechanically ventilated patients, however, fever (38.3–39.4°C) is associated with survival.
Supplementary Material
Acknowledgements
We thank Noor Al Hammadi, Statistical Data Analyst in the Department of Biostatistics at Washington University School of Medicine in St. Louis for assistance with statistical planning and analysis.
Conflicts of Interest and Sources of Funding:
Emily Evans was supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Number TL1 TR002344. Anne Drewry and Brian Fuller were supported by the Washington University Institute of Clinical and Translational Sciences grant KL2 TR000450. Emily Evans, Anne Drewry, Brian Fuller, and Brian Gage were supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Richard Hotchkiss reports he received study funding from RevImmune (Accentia Biopharmaceuticals, Inc., Tampa, FL) and grants from the National Institutes of Health. Emily Evans, Rebecca Doctor, Brian Gage, Brian Fuller, and Anne Drewry declare they have no conflicts of interest.
List of abbreviations
- aHR
Adjusted Hazard Ratio
- AIC
Akaike Information Criterion
- ARDS
Acute Respiratory Distress Syndrome
- CI
confidence interval
- ED
Emergency department
- ICU
Intensive Care Unit
- SOFA
Sequential Organ Failure Assessment
- Tmax
Maximum Temperature
References
- 1.Easter BD, Fischer C, Fisher J: The use of mechanical ventilation in the ED. Am J Emerg Med 30:1183–1188, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM: The epidemiology of mechanical ventilation use in the United States*. Crit Care Med 38:1947–1953, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh D, Boyd Bigelow D, Petty T, Levine B: ACUTE RESPIRATORY DISTRESS IN ADULTS. Lancet 290:319–323, 1967. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA: The Acute Respiratory Distress Syndrome. N Engl J Med 342:1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Ricard J-D, Dreyfuss D, Saumon G: Ventilator-induced lung injury. Curr Opin Crit Care 8:12–20, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Sutherasan Y, Vargas M, Pelosi P: Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Crit Care 18:211, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neto AS, Simonis FD, Barbas CS V., Biehl M, Determann RM, Elmer J, Friedman G, Gajic O, Goldstein JN, Linko R, et al. : Lung-Protective Ventilation With Low Tidal Volumes and the Occurrence of Pulmonary Complications in Patients Without Acute Respiratory Distress Syndrome. Crit Care Med 43:2155–2163, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Fuller BM, Ferguson IT, Mohr NM, Drewry AM, Palmer C, Wessman BT, Ablordeppey E, Keeperman J, Stephens RJ, Briscoe CC, et al. : Lung-Protective Ventilation Initiated in the Emergency Department (LOV-ED): A Quasi-Experimental, Before-After Trial. Ann Emerg Med 38:1573–1582, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundén-Cullberg J, Rylance R, Svefors J, Norrby-Teglund A, Björk J, Inghammar M: Fever in the Emergency Department Predicts Survival of Patients With Severe Sepsis and Septic Shock Admitted to the ICU*. Crit Care Med 45:591–599, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Netzer G, Dowdy DW, Harrington T, Chandolu S, Dinglas VD, Shah NG, Colantuoni E, Mendez-Tellez PA, Shanholtz C, Hasday JD, et al. : Fever is associated with delayed ventilator liberation in acute lung injury. Ann Am Thorac Soc 10:608–615, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niven DJ, Laupland KB, Tabah A, Vesin A, Rello J, Koulenti D, Dimopoulos G, de Waele J, Timsit J-F, EUROBACT Investigators: Diagnosis and management of temperature abnormality in ICUs: a EUROBACT investigators’ survey. Crit Care 17:R289, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice P, Martin E, He J-R, Frank M, DeTolla L, Hester L, O’Neill T, Manka C, Benjamin I, Nagarsekar A, et al. : Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol 174:3676–85, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, He J-R, Rice P, Frank M, Goldblum SE, et al. : Febrile-Range Hyperthermia Augments Pulmonary Neutrophil Recruitment and Amplifies Pulmonary Oxygen Toxicity. Am J Pathol 162:2005–2017, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagarsekar A, Tulapurkar ME, Singh IS, Atamas SP, Shah NG, Hasday JD: Hyperthermia promotes and prevents respiratory epithelial apoptosis through distinct mechanisms. Am J Respir Cell Mol Biol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipke AB, Matute-Bello G, Herrero R, Kurahashi K, Wong VA, Mongovin SM, Martin TR: Febrile-Range Hyperthermia Augments Lipopolysaccharide-Induced Lung Injury by a Mechanism of Enhanced Alveolar Epithelial Apoptosis. J Immunol 184:3801–3813, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manthous CA, Hall JB, Olson D, Singh M, Chatila W, Pohlman A, Kushner R, Schmidt GA, Wood LDH: Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med 1995. [DOI] [PubMed] [Google Scholar]
- 17.Akinci OI, Celik M, Mutlu GM, Martino JM, Tugrul S, Ozcan PE, Yilmazbayhan D, Yeldandi AV, Turkoz KH, Kiran B, et al. : Effects of body temperature on ventilator-induced lung injury. J Crit Care 20:66–73, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S, Hotchkiss JR, Takahashi T, Olson D, Adams AB, Marini JJ: Effect of core body temperature on ventilator-induced lung injury. Crit Care Med 32:144–9, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Berman JD, Dwyer DM, Wyler DJ: Multiplication of Leishmania in human macrophages in vitro. Infect Immun 26:375–379, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackowiak PA, Ruderman AE, Martin RM, Many WJ, Smith JW, Luby JP: Effects of physiologic variations in temperature on the rate of antibiotic-induced bacterial killing. Am J Clin Pathol 76:57–62, 1981. [DOI] [PubMed] [Google Scholar]
- 21.Small PM, Täuber MG, Hackbarth CJ, Sande MA: Influence of body temperature on bacterial growth rates in experimental pneumococcal meningitis in rabbits. Infect Immun 52:484–7, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu CM, Tian SF, Ren GF, Zhang YM, Zhang LX, Liu GQ: Occurrence of temperature-sensitive influenza A viruses in nature. J Virol 41:353–359, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young PJ, Saxena M, Beasley R, Bellomo R, Bailey M, Pilcher D, Finfer S, Harrison D, Myburgh J, Rowan K: Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med 38:437–444, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Drewry AM, Ablordeppey EA, Murray ET, Dalton CM, Fuller BM, Kollef MH, Hotchkiss RS: Monocyte Function and Clinical Outcomes in Febrile and Afebrile Patients with Severe Sepsis. SHOCK 1, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drewry AM, Ablordeppey EA, Murray ET, Stoll CRT, Izadi SR, Dalton CM, Hardi AC, Fowler SA, Fuller BM, Colditz GA: Antipyretic Therapy in Critically Ill Septic Patients: A Systematic Review and Meta-Analysis. Crit Care Med 45:806–813, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young P, Saxena M, Bellomo R, Freebairn R, Hammond N, van Haren F, Holliday M, Henderson S, Mackle D, McArthur C, et al. : Acetaminophen for Fever in Critically Ill Patients with Suspected Infection. N Engl J Med 373:2215–2224, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Schell-Chaple HM, Puntillo KA, Matthay MA, Liu KD, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network L and BIARDSN: Body temperature and mortality in patients with acute respiratory distress syndrome. Am J Crit Care 24:15–23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuller BM, Ferguson I, Mohr NM, Stephens RJ, Briscoe CC, Kolomiets AA, Hotchkiss RS, Kollef MH: Lung-protective ventilation initiated in the emergency department (LOV-ED): a study protocol for a quasi-experimental, before-after trial aimed at reducing pulmonary complications. BMJ Open 6:e010991, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena M, Young P, Pilcher D, Bailey M, Harrison D, Bellomo R, Finfer S, Beasley R, Hyam J, Menon D, et al. : Early temperature and mortality in critically ill patients with acute neurological diseases: trauma and stroke differ from infection. Intensive Care Med 41:823–832, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC: Impact of Fever on Outcome in Patients With Stroke and Neurologic Injury: A Comprehensive Meta-Analysis. Stroke 39:3029–3035, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Puccio AM, Fischer MR, Jankowitz BT, Yonas H, Darby JM, Okonkwo DO: Induced Normothermia Attenuates Intracranial Hypertension and Reduces Fever Burden after Severe Traumatic Brain Injury. Neurocrit Care 11:82–87, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laupland KB, Shahpori R, Kirkpatrick AW, Ross T, Gregson DB, Stelfox HT: Occurrence and outcome of fever in critically ill adults. Crit Care Med 36:1531–1535, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Laupland KB, Zahar J-R, Adrie C, Schwebel C, Goldgran-Toledano D, Azoulay E, Garrouste-Orgeas M, Cohen Y, Jamali S, Souweine B, et al. : Determinants of temperature abnormalities and influence on outcome of critical illness*. Crit Care Med 40:145–151, 2012. [DOI] [PubMed] [Google Scholar]
- 34.O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, et al. : Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med 36:1330–49, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Niven DJ, Gaudet JE, Laupland KB, Mrklas KJ, Roberts DJ, Stelfox HT: Accuracy of Peripheral Thermometers for Estimating Temperature. Ann Intern Med 163:768, 2015. [DOI] [PubMed] [Google Scholar]
- 36.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS: Acute Respiratory Distress Syndrome. JAMA 307:2526–33, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–55, 1992. [DOI] [PubMed] [Google Scholar]
- 38.Lee BH, Inui D, Suh GY, Kim JY, Kwon JY, Park J, Tada K, Tanaka K, Ietsugu K, Uehara K, et al. : Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care 16:R33, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushimoto S, Gando S, Saitoh D, Mayumi T, Ogura H, Fujishima S, Araki T, Ikeda H, Kotani J, Miki Y, et al. : The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: An analysis from a multicenter, prospective survey of severe sepsis. Crit Care 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peres Bota D, Lopes Ferreira F, Mélot C, Vincent JL: Body temperature alterations in the critically ill. Intensive Care Med 30:811–816, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Osborn TM, Phillips G, Lemeshow S, Townsend S, Schorr CA, Levy MM, Dellinger RP: Sepsis Severity Score. Crit Care Med 42:1969–1976, 2014. [DOI] [PubMed] [Google Scholar]
- 42.Evans SS, Repasky EA, Fisher DT: Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 15:335–349, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD: Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 68:1265–1270, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasday JD, Singh IS: Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones 5:471–80, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackowiak PA, Marling-Cason M, Cohen RL: Effects of temperature on antimicrobial susceptibility of bacteria. J Infect Dis 145:550–3, 1982. [DOI] [PubMed] [Google Scholar]
- 46.Bernard GR, Wheeler AP, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson WJ, Wright PE, Christman BW, Dupont WD, et al. : The Effects of Ibuprofen on the Physiology and Survival of Patients with Sepsis. N Engl J Med 336:912–918, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Ye S, Xu D, Zhang C, Li M, Zhang Y: Effect of Antipyretic Therapy on Mortality in Critically Ill Patients with Sepsis Receiving Mechanical Ventilation Treatment. Can Respir J 2017:3087505, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villar J, Slutsky AS: Effects of induced hypothermia in patients with septic adult respiratory distress syndrome. Resuscitation 26:183–192, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Slack DF, Corwin DS, Shah NG, Shanholtz CB, Verceles AC, Netzer G, Jones KM, Brown CH, Terrin ML, Hasday JD: Pilot Feasibility Study of Therapeutic Hypothermia for Moderate to Severe Acute Respiratory Distress Syndrome. Crit Care Med 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henning DJ, Carey JR, Oedorf K, Day DE, Redfield CS, Huguenel CJ, Roberts JC, Sanchez LD, Wolfe RE, Shapiro NI: The Absence of Fever Is Associated With Higher Mortality and Decreased Antibiotic and IV Fluid Administration in Emergency Department Patients With Suspected Septic Shock. Crit Care Med 45:e575–e582, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.