Abstract
OBJECTIVES:
To quantify non-enhancing tumor (NT) component in clear cell renal cell carcinoma (ccRCC) and assess its association with histologically defined tumor necrosis, stage, and survival outcomes.
METHODS:
Among 183 patients with ccRCC from multi-institutional changes in CT attenuation of tumor voxels were used to quantify percent of NT. Associations of NT with histologic tumor necrosis, and tumor stage/grade were tested using Wilcoxon signed-rank test and with survival outcomes using Kaplan-Meier’s curves/Cox regression analysis.
RESULTS:
NT was higher in ccRCC with tumor necrosis (11% versus 7%; p-value=0.0404) and higher pathological stage (p-value: 0.042 and <0.001, respectively). Patients with greater NT had higher incidence of cancer recurrence after resection (p-value <0.001) and cancer-specific mortality (p-value <0.001).
CONCLUSION:
NT on preoperative CT scans in patients with ccRCC correlates with tumor necrosis and stage and may serve as an independent imaging prognostic biomarker for cancer recurrence and cancer specific survival.
INTRODUCTION:
Kidney cancer is among the 10 most common cancers in the United States with a rising incidence over the past decade.1 More than 90% of kidney cancers are adenocarcinomas, and of those, clear cell renal cell carcinoma (ccRCC) is the predominant pathologic subtype, representing up to 85% of renal adenocarcinomas.2,3 The natural history of ccRCC is variable with some tumors exhibiting an indolent growth pattern, and others demonstrating aggressive behavior including local recurrence following resection and distant metastases.4,5 Histologically-defined tumor necrosis in ccRCC has been shown to be an independent negative prognostic factor for metastasis and overall survival.6,7 In fact, histologically-defined tumor necrosis is integrated into prognostic scoring systems such as the Stage, Size, Grade and Necrosis (SSIGN) score.8
Cross-sectional imaging with CT and MRI has been at the forefront in detecting renal cancers and providing pre-surgical staging which allows for appropriate management. Tumor necrosis is a histologic finding. The definition of necrosis on imaging is a controversial topic given the fact that cystic change, hemorrhage and fibrosis all show non or hypo enhancement on contrast enhanced cross sectional imaging, similar to necrosis. When necrosis is mentioned it is usually determined subjectively based on the non-enhancing tumor component (NT) and is not quantified.
The standardization of imaging protocols, especially CT scanning protocols, allows for consistency and reproducibility between different institutions. This consistency also allows for computer-based quantitative analysis. Thus the calculation of enhancing component (viable tumor) and NT (non-viable tumor) after semi-automated segmentation is feasible. Viable components of ccRCC enhance with intravenous contrast on abdominal cross-sectional imaging9,10, while necrotic, fibrotic, hemorrhagic and cystic components demonstrate no internal enhancement.11 Enhancement is defined as an increase in attenuation by ≥ 20 Hounsfield units between pre and post-contrast CT imaging.12 Any tumor component that does not increase in attenuation after the administration of intravenous contrast likely represent a non-viable component, possibly necrosis.13
We hypothesize that volumetric assessment of NT may serve as a quantitative measure of non-viable tumor and may correlate with tumor necrosis (given the low prevalence of cystic ccRCC in general and specifically among this cohort). This quantitative approach may allow for a more objective radiological assessment of necrosis, or at minimum an imaging based surrogate for necrosis, and lessen the inter-reader variability reported in the literature for qualitatively assessing for the presence of necrosis.11,14
Given the fact that larger ccRCC tend to have higher percentage of necrosis as a result of the tumor outgrowing its blood supply11, we aimed to generate a size-independent quantitative measure of ccRCC NT. Our primary objective was to explore the association between NT and histologically-defined tumor necrosis. Our secondary objective was to assess NT’s association with gene mutation status and tumor stage/grade and explore the prognostic association of NT with cancer recurrence and cancer-specific survival outcomes.
MATERIALS AND METHODS:
Patient Data and Imaging Studies:
This was a single-center retrospective analysis of a publicly available multi-center cohort utilizing The Cancer Genome Atlas (TCGA) data.15 Patients with ccRCC were recruited at multiple medical centers, where patients’ informed consents and institutional review boards’ approvals were obtained. All demographic and imaging data were de-identified to comply with the Health Insurance Portability and Accountability Act (HIPAA). The pathological specimens from surgical excision were submitted to the TCGA for pathological assessment and genomic analysis. The corresponding imaging studies were stored on The Cancer Imaging Archive (TCIA).16
183 patients with pre-surgical contrast enhanced abdominal CT scans, pathological data, and survival outcomes were retrieved from the TCGA and TCIA.
Tumor Segmentation:
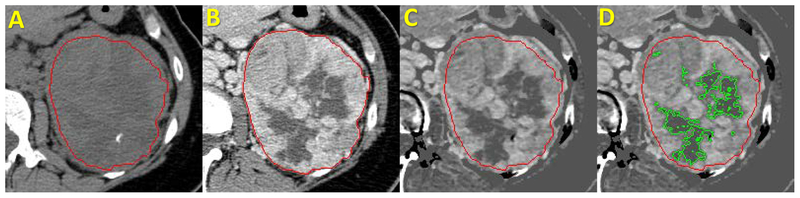
One fellowship trained abdominal radiologist with 9 years of experience performed the segmentations. The radiologist was blinded to the various study endpoints (pathology at surgery and patient outcomes). For image analysis we used a MatLab (MathWorks, Natick, Massachusetts)17 based dedicated software application to visualize and segment ccRCC from each patient’s CT scan. This semi-automated algorithm, combining the region-based active contours and a level set approach, was used in a slice-by-slice fashion (i.e. the entire ccRCC tumor was segmented). The initial step for tumor segmentation required the radiologist to manually select a region-of-interest (ROI) that roughly enclosed the tumor region on a single CT slice. Rough boundary localization of the tumor was then automatically generated by the software algorithm and propagated to consecutive slices, serving as an initial ROI for subsequent segmentations on the neighboring images. The final tumor segmentation boundaries were then verified and fine-tuned by the radiologist (Figure 1). Tumors were segmented on the pre- and post contrast series. The difference in attenuation in each voxel of the tumor volume in patients who had both pre-and post-contrast enhanced CT images (n=170) was used to define enhancing tumor and NT. Example of the segmentation steps is displayed in Figure 1. NT was defined as any tumor voxel that did not increase in CT attenuation values after intravenous contrast administration by 20 or more Hounsfield Units (HU) in comparison with the corresponding voxel on the pre-contrast CT12,18. In patients with only post-contrast enhanced CT (i.e. no pre-contrast imaging, n=13), we estimated solid tumor component using a radiologist-controlled semi-automated process embedded in another MatLab based application that localized regions within ccRCC that had low attenuation values (i.e. <20 HU) to represent the NT. In order to control for ccRCC tumor size (larger tumors are more likely to demonstrate larger NT and potentially necrosis19) we created a size-independent variable, which was defined as the percentage of NT, ((non-enhancing volume/whole ccRCC volume) × 100).
Figure 1. Steps of Tumor Segmentation and Quantification of Non-enhancing Tumor volume.
Steps of ccRCC Segmentations and Estimation of non-enhancing tumor component on CT Scan of The Abdomen before and after Intravenous Contrast. A. precontrast phase; B. Postcontrast phase; C. Postcontrast-precontrast subtraction; D. Postcontrast-precontrast subtraction with green line delineating the tumor necrosis. Red line delineates the ccRCC margins.
Clinical and Pathological Data:
Patient demographics and survival outcomes, as well as tumor pathology (stage, grade and presence or absence of necrosis) were retrieved from the TCGA web port and adjudicated based on TCGA consortium review. The presence of necrosis on pathology was defined as a categorical outcome (present versus absent).
Statistical Analysis:
Patient and tumor related data were summarized using frequency and percentage for categorical variables, and using median and range for continuous variables. Patients were censored at their last follow-up date if they were alive or deceased from non-ccRCC related illness (cancer-specific survival-CSS) and if alive without cancer recurrence (recurrence-free survival-RFS).
Given the non-normal distribution of NT, we chose the median value as an appropriate dividing point to discriminate between ccRCC tumors with high and low NT. Comparison of continuous variables between the two subsamples was performed with Wilcoxon rank-sum test. Comparison of categorical variables between the two subsamples was performed with Chi-Square or Fisher’s exact test. The Kaplan-Meier curves and the log-rank test were used to test the difference in cancer recurrence and cancer-specific survival between the two subsamples. Multivariate Cox regression models were built to assess the independent effect of NT on cancer recurrence and cancer-specific survival while controlling for age at diagnosis and pathological staging. A two-tailed p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS:
183 patients were included in this multi-institutional cohort of ccRCC. The median age at diagnosis was 59 years old (range 34 – 88 years). The majority of patients were male (66%) and most of them were white (96%). The median unidimensional tumor size was 5 cm (range 1 – 17). Median follow-up time was 3.4 years (interquartile range (IQR) = 1.7, 5.0) during which 46 patients (25%) had recurrence and 36 patients (20%) died of ccRCC. Larger tumors tended to have higher percentage of NT on imaging (b-estimate= 0.22, p-value<0.0001). The NT was not normally distributed with median of 8% and inter-quartile range between 3, and 17%. The median NT was higher in ccRCC with histologically defined tumor necrosis than in those without (11% versus 7%; p-value= 0.0404). There was no significant difference in NT between genders or race (Table 1). Patients with VHL mutation demonstrated less NT than their counterparts (6% versus 9%; p-value= 0.0115). No significant association was detected between other tested somatic gene mutation status and NT. The association between NT and pathological grading was in the expected direction and trended towards significance (p-value= 0.057); despite the lack of statistical significance, ccRCC with greater than median NT tended to be grade 4 more frequently than their counterparts (Table 1). NT was greater in patients with AJCC pathological stage II, III, and IV in comparison with stage I (11% versus 5%; p-value<0.001).
Table 1.
Patient Characteristics, Overall and Stratified by non-enhancing component.
| Characteristic | Overall n = 183 |
Patients with non-enhancing tumor component < median n = 91 |
Patients with non-enhancing tumor component ≥ median n =92 |
p-value** |
|---|---|---|---|---|
| Age at Diagnosis (years) | 59 (34, 88) | 59 (34, 88) | 59 (39, 79) | 0.8691 |
| Maximum Tumor Dimension (cm) | 5 (1, 17) | 4 (1, 14) | 7 (2, 17) | <0.0001 |
| Gender | ||||
| Female | 62 (34) | 31 (34) | 31 (34) | 0.958 |
| Male | 121 (66) | 60 (66) | 61 (66) | |
| Race | ||||
| white | 176 (96) | 87 (96) | 89 (97) | 0.897 |
| Others | 7 (4) | 4 (4) | 3 (3) | |
| Tumor Grade | ||||
| G1 | 1 (0.5) | 1 (1) | 0 (0) | 0.0589 |
| G2 | 72 (39) | 40 (44) | 32 (35) | |
| G3 | 79 (43) | 41 (45) | 38 (41) | |
| G4 | 31 (17) | 9 (10) | 22 (24) | |
| AJCC Stage | ||||
| Stage I | 96 (53) | 65 (71) | 31 (34) | <0.0001 |
| Stage II | 14 (8) | 2 (2) | 12 (13) | |
| Stage III | 48 (26) | 19 (21) | 29 (32) | |
| Stage IV | 25 (14) | 5 (5) | 20 (22) | |
| Distant Metastasis | ||||
| M0 | 160 (87) | 86 (95) | 74 (80) | 0.0041 |
| M1 | 23 (13) | 5 (5) | 18 (20) | |
| VHL | ||||
| 0 | 71 (39) | 30 (33) | 41 (45) | 0.0776 |
| 1 | 100 (55) | 56 (62) | 44 (48) | |
| NA | 12 (6) | 5 (5) | 7 (8) | |
| PBRM1 | 0.2970 | |||
| 0 | 119 (65) | 63 (69) | 63 (69) | |
| 1 | 52 (28) | 23 (25) | 29 (32) | |
| NA | 12 (7) | 5 (5) | 7 (8) | |
| SETD2 | 0.5960 | |||
| 0 | 157 (86) | 78 (86) | 79 (86) | |
| 1 | 14 (8) | 8 (9) | 6 (7) | |
| NA | 12 (7) | 5 (5) | 7 (8) | |
| BAP1 | 0.6198 | |||
| 0 | 155 (85) | 77 (85) | 78 (85) | |
| 1 | 16 (9) | 5 (5) | 7 (8) | |
| NA | 12 (7) | 9 (10) | 7 (8) | |
| KDM5C | 0.3014 | |||
| 0 | 163 (89) | 80 (88) | 83 (90) | |
| 1 | 8 (4) | 5 (5) | 7 (8) | |
| NA | 12 (7) | 6 (7) | 2 (2) |
N (%) for categorical variables; Median (range) for continuous variables
P-values are from Wilcoxon rank-sum test for continuous characteristics and Fisher’s exact test for categorical characteristics.
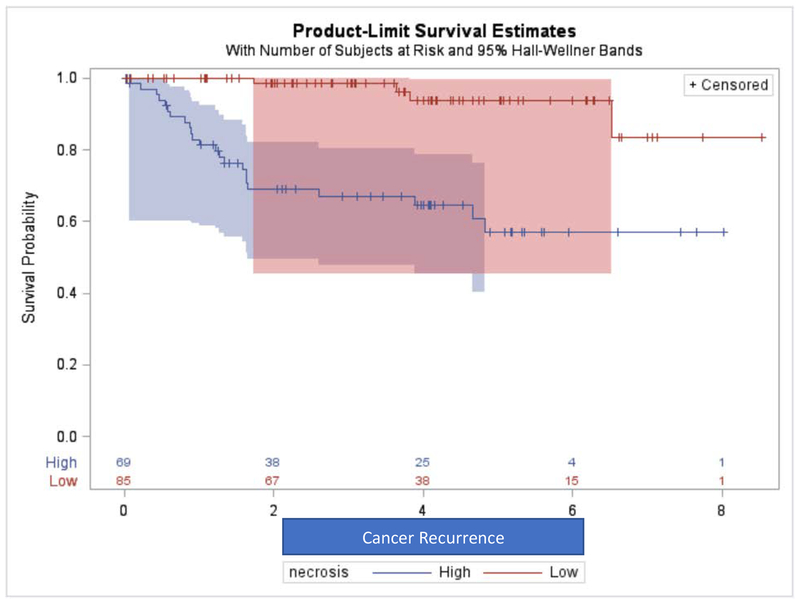
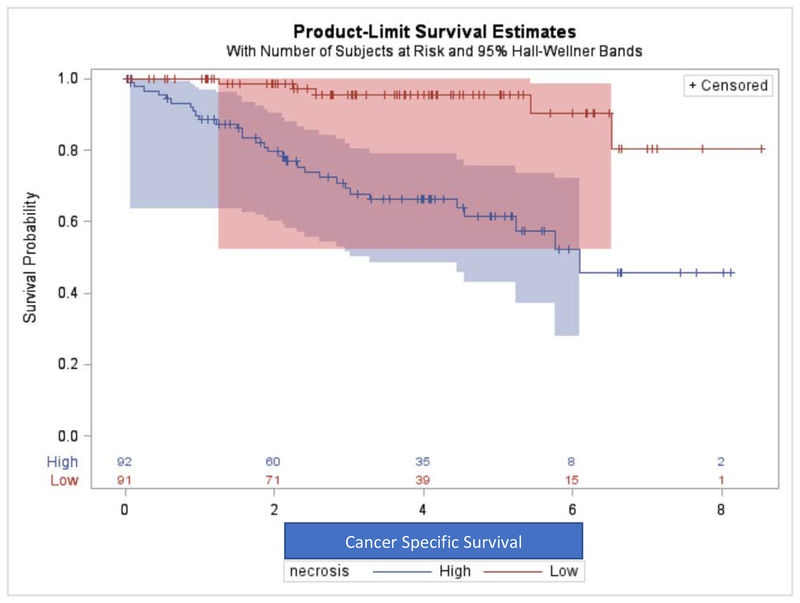
Patients with greater NT had higher incidence of cancer recurrence after resection (Log-rank test p-value<0.001) and higher incidence of cancer-specific mortality (Log-rank test p-value<0.001) as shown in Kaplan-Meier curves in Figures 2 and 3. Table 2 shows that in multivariate Cox-regression analysis controlling for patient’s age at diagnosis and pathological staging, patients with greater NT had higher hazards of cancer recurrence (Hazard Ratio HR= 6.14, p-value= 0.003) and higher hazard of cancer-specific death (HR= 3.00, p-value= 0.040).
Figure 2.
Association of Percent Non-enhancing Tumor with Cancer Recurrence Reflected on Kaplan-Meier Curves.
Figure 3.
Association of Percent Non-enhancing Tumor with Cancer-Specific Survival Reflected on Kaplan-Meier Curves.
Table 2.
Multivariate Cox-Regression Model Results Testing Association between Percent of Non-enhancing Tumor with Survival Outcomes
| Outcome | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Cancer Recurrence | 6.14 | 1.87 – 20.10 | 0.003 |
| Cancer-Specific Mortality | 3.00 | 1.05 – 8.58 | 0.040 |
DISCUSSION:
The results of this study suggest that NT in ccRCC, an objective and quantitative metric obtained from contrast enhanced abdominal CT imaging, correlates with the presence of necrosis on pathology. Furthermore, in addition to its association with VHL mutation status and pathological staging, it is an important independent prognostic factor for cancer recurrence and cancer specific survival.
The presence of necrosis at pathology is a well described negative prognostic factor which is used by clinicians to counsel and manage patients post-operatively.6,7 Evidence of this is manifested in the decision to include it as part of the SSIGN score.20,21 However, thus far its prognostic use has been limited to patients undergoing resection due to the fact that its definition is dependent on pathology review of resected tumors. NT, a potential biomarker which in this study was associated with the presence of necrosis on pathology, could theoretically provide useful prognostic information to the clinician in the preoperative setting, potentially supporting the decision to operate rather than manage conservatively and even risk stratify patients in terms of more or less invasive surgery (radical versus partial nephrectomy, lymph node dissection, ablation etc).
Beddy et. al. retrospectively studied 75 patients with ccRCC and preoperative MRIs.14 Two radiologists subjectively evaluated tumors for the presence of necrosis among other features. They found that necrosis was associated with disease progression. Their results agree with ours but importantly we used an objective, quantitative method of assessing nonviable tumor (necrosis) rather than qualitative assessment which is prone to inter-reader variability. Furthermore, we demonstrated an association with various clinically important patient outcomes including tumor stage at presentation, cancer recurrence and cancer-specific mortality accounting for time between surgical treatment and evidence of cancer recurrence and cancer specific mortality. Similarly, Hötker et al. conducted a retrospective study including a large cohort of ccRCC patients at single institutional focusing on the qualitative assessment of ccRCC necrosis.22 They found that ccRCC with extensive tumor necrosis as defined by radiologists assessment (more than 2/3rds) was associated with worse disease specific survival after controlling for pathologic stage. Our results are in agreement with these but extend it to use quantitative assessment and add more generalizability given the implementation of multi-institutional cohort in this study.
There were several limitations to this study. This was a retrospective analysis of a publicly available multi-site cohort. Therefore, there is the potential for selection bias similar to any retrospective research. A prospective study would be helpful to confirm our findings. Our approach of quantifying changes in attenuation values between pre and post contrast imaging was performed by one blinded radiologist. Although the analysis is meant to be quantitative and objective, we did not attempt to replicate the analysis by another radiologist. Therefore, we cannot comment on the reproducibility of the analysis. 13 patients did not have precontrast imaging and defining NT in these patients was done differently (<20 Hounsfield Units on postcontrast imaging). We chose to include these patients in the study in order to capture as many data points as possible and increase the statistical power. The TCGA and TCIA databases did not include the percentage of necrosis in each tumor on pathological assessment, rather simply the presence or absence. This represents an oversimplification as necrosis may be present in variable amounts and this likely has additional significance. An additional important limitation that warrants addressing is the definition of viable tumor using contrast enhancement. While it is true that the 20 HU cutoff is accepted in the literature, there have been reports of viable tumor enhancing less than 20 HU. Israel et. al defined an increase of 10–19 HU as indeterminate for enhancement.23 Although ccRCC is a typically avidly enhancing tumor, it is also true that it is uniquely heterogeneous. As such, there may be non-necrotic components that could have displayed minimal enhancement (between 10 and 19 HU) and could possibly be misclassified as necrosis based on our cutoff of 20 HU (i.e. overestimating tumor necrosis on imaging). Nevertheless, hypoenhancement of RCC typically occurs in papillary subtype renal cell carcinoma rather than in ccRCC24 and thus limits the effect of this potential overestimation of non-enhancing component in this cohort of ccRCC. Also important to note is the factor of phase of imaging following contrast administration; varying timing of imaging after intravenous contrast administration can potentially affect the extent of enhancement in ccRCC. Studies performed in an earlier corticomedullary phase of enhancement may exaggerate the hypoenhancing component of the tumor, leading to an overestimation of non-enhancing component/tumor necrosis. Conversely, studies performed later during the nephrographic phase of enhancement may potentially result in underestimation of NTV because of potential pseudo-enhancement. However, this limitation may extend across institutions and not necessarily result in disparate non-enhancing component results, given the standardization of CT renal imaging protocols and reproducibility of different post-contrast phases. Regardless, future prospective work should ensure the use of uniform imaging parameters including phase of imaging.
A final major caveat to point out is the lack of direct visual correlation between NT and pathology. We do not know what NT actually represents in tumors (cystic change, necrosis, hemorrhage, fibrosis or a combination). Therefore it would be incorrect to assume that NT represents necrosis. Rather, we have shown that NT correlates with the presence of necrosis and more importantly, has prognostic utility in terms of patient outcomes.25
At the same time, our study has several strengths including the multi-institutional cohort with reliably and consistently assessed pathological specimens, exploration of non-enhancing component association with genetic mutation, and robust clinical outcomes including follow up to assess cancer recurrence and cancer specific survival. The multi-institutional nature of this cohort expands the generalizability of its results despite the retrospective approach. Future studies are needed to confirm our findings and potentially set the ground for CT-based quantitative imaging biomarker.
CONCLUSION:
NT in ccRCC correlates with the presence of histologically-defined tumor necrosis and has potential to serve as an imaging-based biomarker predicting clinical outcome in preoperative setting when pathology is not yet available.
Funding:
Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748)
Abbreviations:
- ccRCC
clear cell Renal Cell Carcinoma
- TCGA
The Cancer Genome Atlas
- TCIA
The Cancer Imaging Archive
- CT
computed tomography
- VHL
von Hippel-Lindau
- AJCC
American Joint Committee on Cancer
- CSS
cancer-specific survival-CSS
- RFS
recurrence-free survival
- OS
odds Ratio
- HR
hazard ratio
- NT
non-enhancing tumor
Footnotes
No IRB was required (de-identified and publicly available data)
| Name | Disclosure |
|---|---|
| Firas S Ahmed | No conflict of interest |
| Oguz Akin | No conflict of interest |
| Hiram Shaish | No conflict of interest |
| Lyndon Luk | No conflict of interest |
| Xiaotao Guo | No conflict of interest |
| Hao Yang | No conflict of interest |
| Emily Zabor | No conflict of interest |
| Irina Ostrovnaya | No conflict of interest |
| A Ari Hakimi | No conflict of interest |
| Binsheng Zhao | No conflict of interest |
| Lawrence H Schwartz | No conflict of interest |
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nature reviews Urology 2010;7:245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storkel S, van den Berg E. Morphological classification of renal cancer. World journal of urology 1995;13:153–8. [DOI] [PubMed] [Google Scholar]
- 4.Gulati S, Martinez P, Joshi T, et al. Systematic evaluation of the prognostic impact and intratumour heterogeneity of clear cell renal cell carcinoma biomarkers. European urology 2014;66:936–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang PA, Vickers MM, Heng DY. Clinical and molecular prognostic factors in renal cell carcinoma: what we know so far. Hematology/oncology clinics of North America 2011;25:871–91. [DOI] [PubMed] [Google Scholar]
- 6.Pichler M, Hutterer GC, Chromecki TF, et al. Histologic tumor necrosis is an independent prognostic indicator for clear cell and papillary renal cell carcinoma. American journal of clinical pathology 2012;137:283–9. [DOI] [PubMed] [Google Scholar]
- 7.Sengupta S, Lohse CM, Leibovich BC, et al. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer 2005;104:511–20. [DOI] [PubMed] [Google Scholar]
- 8.Sekar RR, Patil D, Baum Y, et al. A novel preoperative inflammatory marker prognostic score in patients with localized and metastatic renal cell carcinoma. Asian journal of urology 2017;4:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JK, Kim TK, Ahn HJ, Kim CS, Kim KR, Cho KS. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR American journal of roentgenology 2002;178:1499–506. [DOI] [PubMed] [Google Scholar]
- 10.Young JR, Margolis D, Sauk S, Pantuck AJ, Sayre J, Raman SS. Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 2013;267:444–53. [DOI] [PubMed] [Google Scholar]
- 11.Vargas HA, Delaney HG, Delappe EM, et al. Multiphasic contrast-enhanced MRI: single-slice versus volumetric quantification of tumor enhancement for the assessment of renal clear-cell carcinoma fuhrman grade. Journal of magnetic resonance imaging : JMRI 2013;37:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Israel G, Bosniak M. How I Do It: Evaluating Renal Masses. Radiology 2005;236:441–50. [DOI] [PubMed] [Google Scholar]
- 13.Pedrosa I, Sun MR, Spencer M, et al. MR imaging of renal masses: correlation with findings at surgery and pathologic analysis. Radiographics : a review publication of the Radiological Society of North America, Inc 2008;28:985–1003. [DOI] [PubMed] [Google Scholar]
- 14.Beddy P, Genega EM, Ngo L, et al. Tumor necrosis on magnetic resonance imaging correlates with aggressive histology and disease progression in clear cell renal cell carcinoma. Clinical genitourinary cancer 2014;12:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CIP TCGA Radiology Initiative. 2015,
- 16.The Cancer Imaging Archive. (Accessed 1/16/2014, 2014, at http://www.cancerimagingarchive.net/.)
- 17.MATLAB The Language of Technical Computing at http://www.mathworks.com/products/matlab/.)
- 18.Bae K, Heiken J, Siegel C, Bennett H. Renal Cysts: Is Attenuation Artifactually Increased on Contrast-enhanced CT Images? Radiology 2000;216. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Ma X, Li H, et al. Influence of tumor size on oncological outcomes of pathological T3aN0M0 renal cell carcinoma treated by radical nephrectomy. PloS one 2017;12:e0173953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. The Journal of urology 2002;168:2395–400. [DOI] [PubMed] [Google Scholar]
- 21.Zigeuner R, Hutterer G, Chromecki T, et al. External validation of the Mayo Clinic stage, size, grade, and necrosis (SSIGN) score for clear-cell renal cell carcinoma in a single European centre applying routine pathology. European urology 2010;57:102–9. [DOI] [PubMed] [Google Scholar]
- 22.Hotker AM, Karlo CA, Zheng J, et al. Clear Cell Renal Cell Carcinoma: Associations Between CT Features and Patient Survival. AJR American journal of roentgenology 2016;206:1023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israel GM, Bosniak MA. How I do it: evaluating renal masses. Radiology 2005;236:441–50. [DOI] [PubMed] [Google Scholar]
- 24.Sasaguri K, Takahashi N. CT and MR imaging for solid renal mass characterization. European journal of radiology 2018;99:40–54. [DOI] [PubMed] [Google Scholar]
- 25.Winters BR, Gore JL, Holt SK, Harper JD, Lin DW, Wright JL. Cystic renal cell carcinoma carries an excellent prognosis regardless of tumor size. Urologic oncology 2015;33:505.e9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]