Abstract
The ionotropic GABA receptor (GABAAR) mediates fast inhibition in the brain. The GABAAR pore-forming (α, β, and non-α/β) subunits were isolated approximately 30 years ago and have since been the focus of extensive studies. As a result, many properties of GABAARs, including subunit assembly and channel and pharmacological properties, have been discovered. However, several of the underlying mechanisms, such as for the synaptic localization of GABAARs, remain unsolved. A reinvestigation of native GABAAR complexes in the brain and primary neurons identified two major molecular constituents, namely, the transmembrane GARLH/LHFPL protein family and the inhibitory synaptic protein neuroligin 2. This identification of the principal components of native receptor complexes may provide new mechanistic insight on receptor regulation.
Introduction
Fast synaptic inhibition in the vertebrate brain is mediated by receptors for GABA, the major inhibitory neurotransmitter. These receptors, GABAARs, are ionotropic; they are GABA-gated anion channels localized at synapses. Here, I review recent progress regarding the molecular composition, assembly, and localization of GABAARs.
Molecular composition of the native GABAAR complex
The GABAAR was first purified from bovine brains via SDS-PAGE as two bands at 53 and 56–59 kDa [1], which were identified as α and β subunits, respectively, by a combination of protein sequencing and molecular cloning [2]. So far, 19 genes have been identified as GABAAR ion channel pore-forming subunits (six encoding α subunits, three encoding β subunits, and ten encoding non-α/β subunits [including three γ subunits]) [3,4]. Because combinatorial expression of these cDNAs in heterologous cells resulted in channels exhibiting GABA-evoked activity [2], these pore-forming subunits have been a focus of GABAAR research. As a result, the subunit compositions of synaptic and extrasynaptic GABAARs and their distinct roles in phasic and tonic inhibition have been described [3–6].
Recently, the molecular constituents of several ionotropic neurotransmitter receptor complexes in vivo have been revealed. Some of the receptors form stable complexes with specific auxiliary subunits, which control receptor localization and channel properties [7,8]. These include TARP and cornichon-like molecules for AMPA-type glutamate receptors, Neto/Sol for mammalian kainate-type and invertebrate glutamate receptors, and MOLO-1 for invertebrate acetylcholine receptors [9–13]. The list of auxiliary subunits of cationic channels is growing and was reviewed recently in detail [14,15].
Blue native (BN) PAGE is a powerful approach that reveals stable complexes formed in vivo [16] and has been used to detect stable neurotransmitter receptor complexes [11,17,*18]. As there is no SDS in this procedure, the preservation of protein complexes is better than with SDS-PAGE, although the Coomassie brilliant blue dye or other ingredients may break some protein interactions. With BN-PAGE, protein complexes and the protein alone can be observed in a single sample. For example, silver staining of SDS-PAGE gels following the co-immunoprecipitation of TARP and AMPA receptors (AMPARs) from brain showed that AMPARs and TARPs are primary interactors, respectively [19,20]; however, the portions of TARP that were bound to and unbound from AMPARs (TARPed and TARPless AMPARs, respectively) could not be observed simultaneously in one lane. By contrast, both TARPed and TARPless AMPARs can be detected simultaneously via BN-PAGE, because their different molecular weights result in distinct migrations through the gel. BN-PAGE analyses showed that a majority of AMPARs form complexes with TARPs in crude cerebellar lysates solubilized with Triton X-100 [17,21].
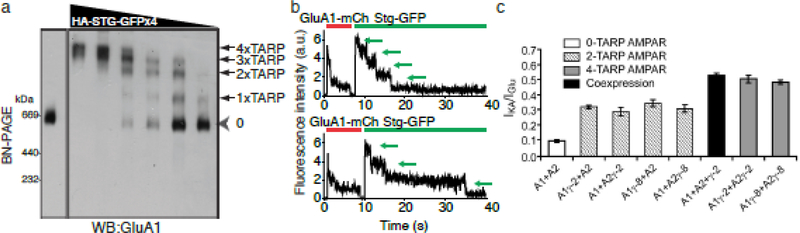
Furthermore, BN-PAGE can be used to reveal the stoichiometry of the constituents of the protein complex. Too high expression of transmembrane proteins, such as from the transient transfection of plasmids in HEK cells, induces protein aggregation, which can be detected on BN-PAGE at >1 MDa (Tomita laboratory, unpublished). Thus, an expression system that tightly regulates expression levels is ideal, such as with the injection of cRNAs into Xenopus laevis oocytes. In cRNA-injected oocytes, the AMPAR tetramer is detected as a single band of around 660 kDa, whereas four additional larger bands are detected when TARP is coexpressed (Figure 1a) [21]. This suggests that each AMPAR tetramer interacts with 1 to 4 TARPs at a stoichiometry that is dependent on the TARP expression level. Two other approaches using single-fluorescent molecule counting of TARPed AMPARs (Figure 1b) and distinct pharmacological properties of TARPed and TARPless AMPARs (Figure 1c) resulted in similar conclusions [22,23]. However, the TARP stoichiometry toward the AMPAR complex in vivo is proposed to be 1 or 2 according to BN-PAGE or electrophysiology, respectively [21,23].
Figure 1: Strategies for revealing the stoichiometry of molecular constituents.
Various approaches can reveal the stoichiometry of a component on a receptor. (a) An approach with BN-PAGE involves counting the number of distinct bands when expressing various ratios of a receptor and another constituent. Image modified from[21]. (b) An imaging approach involves measuring the number of distinct fluorescence signals in each receptor complex. Image modified from [22]. (c) An electrophysiological approach utilizes pharmacological features of a receptor/interactor complex. Image modified from [23].
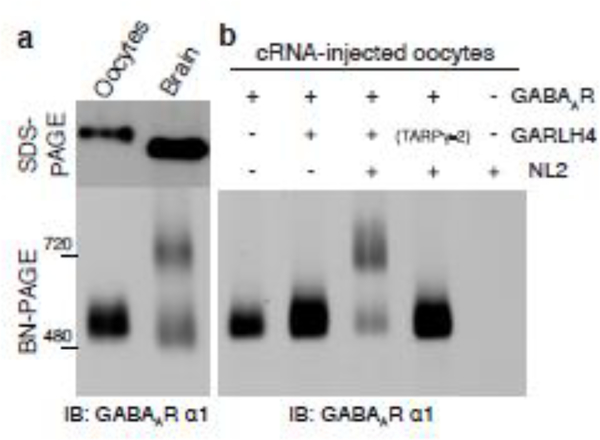
BN-PAGE with several anti-GABAAR antibodies revealed two discrete bands at 500 and 720 kDa for GABAAR complexes in the cerebellum (Figure 2a) and in primary cultured neurons [**24,*25]. By contrast, a single band at 520 kDa was detected by analyses of cRNA-injected Xenopus laevis oocytes coexpressing GABAAR α1, β2, and γ2 subunits or the β2-α1-β2-α1-γ2 concatenated pentamer [26]. This band is slightly higher than the lower band detected from cerebellum via SDS-PAGE, presumably because of a difference in the molecular weight of the α1 subunit (Figure 2a) [**24,*25]. The 720 kDa band detected from brain lysates but not from cRNA-injected oocytes indicates the presence of other proteins with GABAARs in the brain. The detergents necessary to solubilize membrane proteins from tissues and cells potentially break protein interactions. In the case of the GABAARs, membranes solubilized with Triton X-100 and lauryl maltose neopentyl glycol showed approximately 50% and 80% of the 720 kDa GABAAR complex, respectively [**24]. This suggests that at least 80% of GABAARs form a stable 720 kDa complex in the cerebellum and Triton X-100 partially breaks the 720 kDa complex. Purification and mass spectrometry analysis of this complex identified GABAAR subunits (α, β, and γ), a GABAAR regulatory lipoma HMGIC fusion partner like (LHFPL)-like molecule (GARLH), and neuroligin 2 as molecular constituents [**24]. GARLH family proteins are tetra-membrane-spanning proteins that share homology with LHFPLs. Among the six isoforms of LHFPLs, LHFPL3 and LHFPL4 are confirmed to interact with GABAARs and neuroligins and were named GARLH3 and GARLH4, respectively, to reflect their function [**24]. In addition, both neuroligin 2 and GARLH4/LHFPL4 are independently identified as inhibitory synaptic molecules [27,28].
Figure 2: Molecular constituents of a native GABAAR complex.
(a) Many protein complexes are preserved with BN-PAGE, but the molecular weight is inaccurate due to strong effects of the folding of each component. The α1 subunit is detected at 500 kDa in oocytes expressing α1, β2, and γ2 subunits but migrates at 500 and 720 kDa when isolated from the cerebellum. The difference in molecular weights of recombinant and native GABAARs suggests the existence of unidentified constituents in the native receptor complex. Image modified from [**24]. (b) In cRNA-injected oocytes coexpressing GABAAR (α1, β2, γ2), GARLH, and neuroligin 2, the receptor complex migrates at 720 kDa, similarly to native receptors. However, the native receptor complex is not reconstituted when either GARLH or neuroligin 2 is missing. Image modified from [**24].
Assembly of the native GABAAR complex
How do GARLHs and neuroligin 2 in the cerebellum interact and assemble as a GABAAR complex? GABAAR pore-forming subunits form a pentamer, with GABA-gated channels comprising a concatenation of five subunits ordered β2-α1-β2-α1-γ2 [26]. The structure of GABAARs at an atomic resolution was first revealed as a homopentamer of β3 subunits, revealing the pentameric assembly and its molecular architecture [29]. Recently, atomic structures of GABAARs containing the γ subunit and with various pharmaceutical reagents were solved using cryo-electron microscopy [**30–**32], revealing the stoichiometry of the GABAAR subunits and the residues responsible for intersubunit interactions as well as the pentameric arrangement.
The GABAAR in the brain forms a tripartite complex with GARLH and neuroligin 2, observed as the 720 kDa band via BN-PAGE (Figure 2a) [**24,*25]. The complex can be reconstituted from cRNA-injected oocytes coexpressing the GABAAR subunits (α1, β2, γ2), GARLH, and neuroligin 2 (Figure 2b) [**24]. In this complex, the GABAAR interacts with GARLH and GARLH interacts with neuroligin 2, but the GABAAR does not interact with neuroligin 2 [**24], indicating that GARLH partners GABAARs with neuroligin 2 (Figure 3). The γ subunit plays a critical role in the formation of this complex. When the γ2 subunit is not expressed in the cRNA-injected oocyte system, α1 and β2 subunits form pentamers of 520 kDa that do not complex with GARLH and neuroligin 2 [**24].
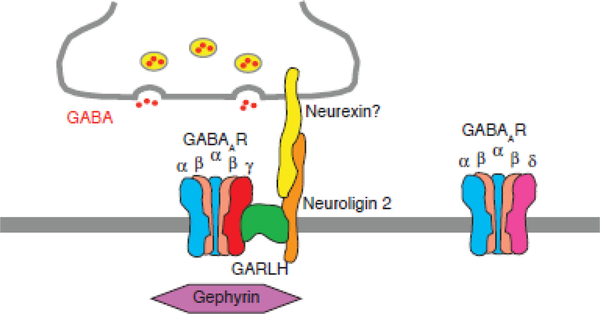
Figure 3: Synaptic localization of the GABAAR complex.
The γ2 subunit-containing GABAARs form a tripartite complex with GARLH and neuroligin at synapses, whereas δ subunit-containing GABAARs fail to form the complex and localize to extrasynaptic sites. The synaptic localization of gephyrin requires the synaptic localization of the tripartite complex. Neurexin might be a presynaptic recruiter of the complex.
Furthermore, both γ2 and α1 subunits from cerebellar granule cell lysates from wild-type mice migrate dominantly at 720 kDa on BN-PAGE gels [*25]. By contrast, the α1 subunit migrates at 500 kDa when γ2 knockout cells are used [*25]. These results suggest that the γ2 subunit interacts with GARLH to recruit neuroligin 2 to the 720 kDa complex (Figure 3).
Analyses of cerebellar GABAAR complexes solubilized with lauryl maltose neopentyl glycol showed that α6 and δ subunits migrate dominantly at 480 kDa on BN-PAGE gels, whereas α1 and γ2 subunits migrate dominantly at 720 kDa [*25]. Each GABAAR pentamer from cerebellar granule cells contains presumably only one non-α/β subunit [33,34], i.e., either δ or γ. Thus, the incorporation of the δ subunit blocks the incorporation of the γ subunit into the pentamer and its interaction with GARLH (Figure 3).
Unlike other auxiliary subunits [7,8], GARLH has not been shown to modulate the channel properties of GABAARs, such as the 50% effective concentration (EC50) of the full agonist GABA and the partial agonist THIP and the 50% inhibitory concentration (IC50) of the antagonist picrotoxin [**24], although GARLHs may modulate other channel properties that have not yet been tested. Future structure studies may reveal whether GARLH modulates the channel properties of GABAARs by identifying structures at both open and closed states. For example, early structure-function studies of TARPs suggested that the first extracellular domain of TARPs and a residue in the AMPAR ligand-binding domain are involved in the modulation of AMPAR channel properties [35,36]. Furthermore, the atomic structure of the TARP/AMPAR complex showed that the acid residues in the first extracellular loop of TARP interact with the ligand-binding domain of AMPARs while the channel pore is opening [37–40]. Interestingly, the same structure studies also showed that the TARP stoichiometry on AMPARs varies. Future analyses of the structures of the GABAAR/GARLH/neuroligin 2 complex with various pharmacological reagents may reveal whether GARLH modulates GABAAR channel properties. Thus far, the structures of neuroligin 1/2 and a GARLH homolog, LHFPL5, have been solved [41–45]. Despite the high amino acid sequence identity (61.8%) between GARLH4 and LHFPL5, LHFPL5 does not interact with GABAARs [**24]. A comparison of the amino acid sequences of GARLH4 and LHFPL5 may reveal potential residues involved in the interaction with GABAAR. A structure analysis of the GABAAR complex with GARLH and neuroligin will eventually reveal all responsible residues and the principal rules for their cooperation.
GABAAR complexes and their localization
GABAARs localize at both synaptic and extrasynaptic sites in the plasma membrane to mediate phasic and tonic inhibition, respectively [5]. In phasic inhibition, GABA released from presynaptic terminals binds to postsynaptic GABAARs for fast signaling, whereas tonic inhibition involves the binding of ambient extracellular GABA to extrasynaptic GABAARs to modulate resting membrane potentials and cell excitability. In principle, GABAARs with and without γ subunits can localize at synaptic and extrasynaptic sites, respectively [46–49]. Although GABAARs can function without the γ subunit in heterologous cells [2], GABAARs exhibit no synaptic activity in the γ2 knockout mice [*25,50]. The γ2 subunit enhances the conductance of GABAARs and thus the amplitude of synaptic GABAAR signals [47,51], though α1 subunit-containing receptors do not localize to synapses in γ2 knockout neurons [*25,50], supporting dual role of the γ2 subunit in channel properties and localization of GABAARs.
The knockdown of GARLH via shRNA and CRISPR substantially reduced the synaptic clustering of α1-containing GABAARs and the frequency of GABAAR-mediated miniature inhibitory postsynaptic currents [**24]. These effects were confirmed in a subsequent study of GARLH4/LHFPL4 knockout mice [**52]. Notably, surface GABAAR activity measured as GABA-evoked currents was unaltered [**24]. These papers establish GARLH as a critical determinant of inhibitory synaptic strength by controlling synaptic clustering, but not surface expression, of GABAARs. Importantly, the 720 kDa complex of GABAAR/GARLH/neuroligin was absent from GARLH knockdown neurons, with increased expression of the 500 kDa of GABAAR pentamer [**24]. The combined requirements for the γ2 subunit in inhibitory transmission and GARLH interactions suggest that GARLH controls synaptic clustering of GABAARs through γ2 subunit interactions.
The inhibitory molecule that recruits the tripartite complex to inhibitory synapses remains unknown. Both gephyrin and neuroligin 2 are inhibitory postsynaptic molecules, and gephyrin has been proposed as the scaffolding molecule that recruits GABAARs to inhibitory synapses [53]. The knockdown of GARLH was not expected to alter gephyrin localization if indeed gephyrin recruits the GABAAR/GARLH complex to inhibitory synapses. However, the synaptic clustering of gephyrin was substantially reduced in neurons with GARLH knockdown and knockout [**24,**52]. A simple interpretation of these results is that another molecule recruits the tripartite complex to inhibitory synapses. But perhaps GARLH may play roles in trafficking gephyrin to the plasmamembrane. In this case, GARLH disruption causes loss of gephyrin at synapses as a result of loss of gephyrin trafficking. This should be clarified in the future.
Although neuroligin 2 might be a molecule responsible for inhibitory localization of the complex, its synaptic localization is also reduced in GARLH knockdown and knockout neurons [**24,54]. Perhaps the interaction with GARLH stabilizes neuroligin 2, and the subsequent reduction in neuroligin 2 in knockdown/knockout neurons diminishes its synaptic abundance. This could be tested by examining the localization of neuroligin 2 in mutants of GARLH or neuroligin 2 that exhibit normal expression of the protein. Neuroligins interact with neurexin [55], of which the more than 1,000 splice variants and three orthologs play roles in both excitatory and inhibitory transmission [56–58]. The identification of an isoform that is expressed specifically at inhibitory synapses and interacts with neuroligin 2 in the tripartite GABAAR complex will advance the mechanistic understanding of inhibitory synaptic clustering. Alternatively, neurexin interactors that may be expressed specifically by either inhibitory or excitatory neurons may coordinate the localization of neurexins at inhibitory or excitatory synapses. Interestingly, no inhibitory transmission was observed in the olfactory bulbs of neurexin 3α/β knockout mice [59]. This may suggest that neurexin 3α/β interacts with the tripartite complex in the olfactory bulb. Investigations in the near future should examine such an interaction as well as determine the mechanism by which neurexins target excitatory and inhibitory synapses.
Conclusion
Extensive studies have focused on the pore-forming subunits of GABAARs since their molecular cloning 30 years ago [2]. These studies have provided remarkable findings regarding subunit assembly, channel properties, their interactors, and many clinically relevant pharmacological reagents. Meanwhile, several questions remain unsolved, such as those regarding the synaptic localization of GABAARs. The reinvestigation of the molecular constituents of native GABAAR complexes identified other constituents, including a novel GARLH protein family and an inhibitory synaptic protein, neuroligin 2. This identification of the principal components of native receptor complexes provides opportunities to obtain further mechanistic insight into the synaptic localization of GABAARs and to develop new pharmaceutical reagents with greater selectivity.
Highlights:
The molecular composition of native GABAAR complexes was recently identified.
Recent advances have been made regarding the assembly of this complex.
Surface expression of GABAARs is independent of GARLHs
GARLHs control synaptic clustering of the GABAAR complexes.
Acknowledgments
I thank the members of the Tomita lab for critical reading of the manuscript. S.T. is supported by NIH MH115705.
Footnotes
Conflict of interest
The author declares no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sigel E, Stephenson FA, Mamalaki C, Barnard EA: A gamma-aminobutyric acid/benzodiazepine receptor complex of bovine cerebral cortex. J Biol Chem (1983) 258(11):6965–6971. [PubMed] [Google Scholar]
- 2.Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, et al. : Sequence and functional expression of the gaba a receptor shows a ligand-gated receptor super-family. Nature (1987) 328(6127):221–227. [DOI] [PubMed] [Google Scholar]
- 3.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ: International union of pharmacology. Xv. Subtypes of gamma-aminobutyric acida receptors: Classification on the basis of subunit structure and receptor function. Pharmacol Rev (1998) 50(2):291–313. [PubMed] [Google Scholar]
- 4.Olsen RW, Sieghart W: International union of pharmacology. Lxx. Subtypes of gamma-aminobutyric acid(a) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev (2008) 60(3):243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrant M, Nusser Z: Variations on an inhibitory theme: Phasic and tonic activation of gaba(a) receptors. Nat Rev Neurosci (2005) 6(3):215–229. [DOI] [PubMed] [Google Scholar]
- 6.Luscher B, Fuchs T, Kilpatrick CL: Gabaa receptor trafficking-mediated plasticity of inhibitory synapses. Neuron (2011) 70(3):385–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan D, Tomita S: Defined criteria for auxiliary subunits of glutamate receptors. J Physiol (2012) 590(Pt 1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson AC, Nicoll RA: The expanding social network of ionotropic glutamate receptors: Tarps and other transmembrane auxiliary subunits. Neuron (2011) 70(2):178–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS: Functional studies and distribution define a family of transmembrane ampa receptor regulatory proteins. J Cell Biol (2003) 161(4):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, Tomita S: A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron (2009) 61(3):385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, Klocker N: Functional proteomics identify cornichon proteins as auxiliary subunits of ampa receptors. Science (2009) 323(5919):1313–1319. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Mellem JE, Brockie PJ, Madsen DM, Maricq AV: Sol-1 is a cub-domain protein required for glr-1 glutamate receptor function in c. Elegans. Nature (2004) 427(6973):451–457. [DOI] [PubMed] [Google Scholar]
- 13.Boulin T, Rapti G, Briseno-Roa L, Stigloher C, Richmond JE, Paoletti P, Bessereau JL: Positive modulation of a cys-loop acetylcholine receptor by an auxiliary transmembrane subunit. Nat Neurosci (2012) 15(10):1374–1381. [DOI] [PubMed] [Google Scholar]
- 14.Bowie D: Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins. J Biol Chem (2018) 293(48):18789–18802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greger IH, Watson JF, Cull-Candy SG: Structural and functional architecture of ampa-type glutamate receptors and their auxiliary proteins. Neuron (2017) 94(4):713–730. [DOI] [PubMed] [Google Scholar]
- 16.Schagger H, Cramer WA, von Jagow G: Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal Biochem (1994) 217(2):220–230. [DOI] [PubMed] [Google Scholar]
- 17.Vandenberghe W, Nicoll RA, Bredt DS: Stargazin is an ampa receptor auxiliary subunit. Proc Natl Acad Sci U S A (2005) 102(2):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank RA, Komiyama NH, Ryan TJ, Zhu F, O’Dell TJ, Grant SG: Nmda receptors are selectively partitioned into complexes and supercomplexes during synapse maturation. Nature communications (2016) 7(11264.* One of the figures analyzed receptor complexes systematically using BN-PAGE.
- 19.Tomita S, Fukata M, Nicoll RA, Bredt DS: Dynamic interaction of stargazing-like tarps with cycling ampa receptors at synapses. Science (2004) 303(5663):1508–1511. [DOI] [PubMed] [Google Scholar]
- 20.Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS: Molecular constituents of neuronal ampa receptors. J Cell Biol (2005) 169(3):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KS, Yan D, Tomita S: Assembly and stoichiometry of the ampa receptor and transmembrane ampa receptor regulatory protein complex. J Neurosci (2010) 30(3):1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hastie P, Ulbrich MH, Wang HL, Arant RJ, Lau AG, Zhang Z, Isacoff EY, Chen L: Ampa receptor/tarp stoichiometry visualized by single-molecule subunit counting. Proc Natl Acad Sci U S A (2013) 110(13):5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Lu W, Milstein AD, Nicoll RA: The stoichiometry of ampa receptors and tarps varies by neuronal cell type. Neuron (2009) 62(5):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki T, Hoyos-Ramirez E, Martenson JS, Morimoto-Tomita M, Tomita S: Garlh family proteins stabilize gabaa receptors at synapses. Neuron (2017) 93(1138–1152.** This study identified a tripartite complex of GABAAR, GARLH, and neuroligin as the native GABAAR complex in vivo and revealed that GARLH is required for synaptic clustering of GABAARs and inhibitory transmission.
- 25.Martenson JS, Yamasaki T, Chaudhury NH, Albrecht D, Tomita S: Assembly rules for gabaa receptor complexes in the brain. eLife (2017) 6(* This study provided a principal rule of subunit specificity for the interaction of GABAAR and GARLH and their function in synaptic localization.
- 26.Baur R, Minier F, Sigel E: A gaba(a) receptor of defined subunit composition and positioning: Concatenation of five subunits. FEBS letters (2006) 580(6):1616–1620. [DOI] [PubMed] [Google Scholar]
- 27.Varoqueaux F, Jamain S, Brose N: Neuroligin 2 is exclusively localized to inhibitory synapses. European journal of cell biology (2004) 83(9):449–456. [DOI] [PubMed] [Google Scholar]
- 28.Heller EA, Zhang W, Selimi F, Earnheart JC, Slimak MA, Santos-Torres J, Ibanez-Tallon I, Aoki C, Chait BT, Heintz N: The biochemical anatomy of cortical inhibitory synapses. PloS one (2012) 7(6):e39572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller PS, Aricescu AR: Crystal structure of a human gaba receptor. Nature (2014) 512(270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu S, Noviello CM, Teng J, Walsh RM, Jr., Kim JJ, Hibbs RE: Structure of a human synaptic gabaa receptor. Nature (2018) 559(7712):67–72.** Three studies (30–32) showed the structure of γ2 subunit-containing GABAARs expressed in heterologous cells. As the γ2 subunit is a major acting site of pharmacological reagents and a critical determinant of synaptic localization, this structure is significant.
- 31.Phulera S, Zhu H, Yu J, Claxton DP, Yoder N, Yoshioka C, Gouaux E: Cryo-em structure of the benzodiazepine-sensitive alpha1beta1gamma2s tri-heteromeric gabaa receptor in complex with gaba. eLife (2018) 7(** Three studies (30–32) showed the structure of γ2 subunit-containing GABAARs expressed in heterologous cells. As the γ2 subunit is a major acting site of pharmacological reagents and a critical determinant of synaptic localization, this structure is significant.
- 32.Aricescu R: Heteromeric gabaa receptor structures in positively-modulated active states. bioRxiv (2018).** Three studies (30–32) showed the structure of γ2 subunit-containing GABAARs expressed in heterologous cells. As the γ2 subunit is a major acting site of pharmacological reagents and a critical determinant of synaptic localization, this structure is significant.
- 33.Araujo F, Ruano D, Vitorica J: Absence of association between delta and gamma2 subunits in native gaba(a) receptors from rat brain. European journal of pharmacology (1998) 347(2–3):347–353. [DOI] [PubMed] [Google Scholar]
- 34.Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W: Subunit composition and quantitative importance of hetero-oligomeric receptors: Gabaa receptors containing alpha6 subunits. J Neurosci (1998) 18(7):2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS: Stargazin modulates ampa receptor gating and trafficking by distinct domains. Nature (2005) 435(7045):1052–1058. [DOI] [PubMed] [Google Scholar]
- 36.Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS: Stargazin interacts functionally with the ampa receptor glutamate-binding module. Neuropharmacology (2007) 52(1):87–91. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Zhao Y, Wang Y, Shekhar M, Tajkhorshid E, Gouaux E: Activation and desensitization mechanism of ampa receptor-tarp complex by cryo-em. Cell (2017) 170(6):1234–1246 e1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI: Elucidation of ampa receptor-stargazin complexes by cryo-electron microscopy. Science (2016) 353(6294):83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI: Channel opening and gating mechanism in ampa-subtype glutamate receptors. Nature (2017) 549(7670):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Chen S, Yoshioka C, Baconguis I, Gouaux E: Architecture of fully occupied glua2 ampa receptor-tarp complex elucidated by cryo-em. Nature (2016) 536(7614):108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arac D, Boucard AA, Ozkan E, Strop P, Newell E, Sudhof TC, Brunger AT: Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-ca2+ interactions. Neuron (2007) 56(6):992–1003. [DOI] [PubMed] [Google Scholar]
- 42.Gangwar SP, Zhong X, Seshadrinathan S, Chen H, Machius M, Rudenko G: Molecular mechanism of mdga1: Regulation of neuroligin 2:Neurexin transsynaptic bridges. Neuron (2017) 94(6):1132–1141 e1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ge J, Elferich J, Goehring A, Zhao H, Schuck P, Gouaux E: Structure of mouse protocadherin 15 of the stereocilia tip link in complex with lhfpl5. eLife (2018) 7( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JA, Kim D, Won SY, Han KA, Park D, Cho E, Yun N, An HJ, Um JW, Kim E, Lee JO et al. : Structural insights into modulation of neurexin-neuroligin trans-synaptic adhesion by mdga1/neuroligin-2 complex. Neuron (2017) 94(6):1121–1131 e1126. [DOI] [PubMed] [Google Scholar]
- 45.Elegheert J, Cvetkovska V, Clayton AJ, Heroven C, Vennekens KM, Smukowski SN, Regan MC, Jia W, Smith AC, Furukawa H, Savas JN et al. : Structural mechanism for modulation of synaptic neuroligin-neurexin signaling by mdga proteins. Neuron (2017) 95(4):896–913 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nusser Z, Sieghart W, Somogyi P: Segregation of different gabaa receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci (1998) 18(5):1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y, et al. : Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type a receptors. Proc Natl Acad Sci U S A (1995) 92(17):7749–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD et al. : Ligand-gated ion channel subunit partnerships: Gabaa receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J Neurosci (1997) 17(4):1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR et al. : Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type a receptor delta subunit knockout mice. Proc Natl Acad Sci U S A (1999) 96(22):12905–12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B: Postsynaptic clustering of major gabaa receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci (1998) 1(7):563–571. [DOI] [PubMed] [Google Scholar]
- 51.Fisher JL, Macdonald RL: Single channel properties of recombinant gabaa receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse l929 cells. J Physiol (1997) 505 (Pt 2)(283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davenport EC, Pendolino V, Kontou G, McGee TP, Sheehan DF, Lopez-Domenech G, Farrant M, Kittler JT: An essential role for the tetraspanin lhfpl4 in the cell-type-specific targeting and clustering of synaptic gabaa receptors. Cell reports (2017) 21(1):70–83.** This study established the GARLH/LHFPL4 knockout mouse and validated the role of GARLH/LHFPL4 in regulating inhibitory transmission in vivo.
- 53.Tyagarajan SK, Fritschy JM: Gephyrin: A master regulator of neuronal function? Nat Rev Neurosci (2014) 15(3):141–156. [DOI] [PubMed] [Google Scholar]
- 54.Wu M, Tian HL, Liu X, Lai JHC, Du S, Xia J: Impairment of inhibitory synapse formation and motor behavior in mice lacking the nl2 binding partner lhfpl4/garlh4. Cell reports (2018) 23(6):1691–1705. [DOI] [PubMed] [Google Scholar]
- 55.Sudhof TC: Synaptic neurexin complexes: A molecular code for the logic of neural circuits. Cell (2017) 171(4):745–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treutlein B, Gokce O, Quake SR, Sudhof TC: Cartography of neurexin alternative splicing mapped by single-molecule long-read mrna sequencing. Proc Natl Acad Sci U S A (2014) 111(13):E1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schreiner D, Nguyen TM, Russo G, Heber S, Patrignani A, Ahrne E, Scheiffele P: Targeted combinatorial alternative splicing generates brain region-specific repertoires of neurexins. Neuron (2014) 84(2):386–398. [DOI] [PubMed] [Google Scholar]
- 58.Ullrich B, Ushkaryov YA, Sudhof TC: Cartography of neurexins: More than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron (1995) 14(3):497–507. [DOI] [PubMed] [Google Scholar]
- 59.Aoto J, Foldy C, Ilcus SM, Tabuchi K, Sudhof TC: Distinct circuit-dependent functions of presynaptic neurexin-3 at gabaergic and glutamatergic synapses. Nat Neurosci (2015) 18(7):997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]