Abstract
Objective:
A high plasma level of inflammasome mediator interleukin 18 (IL-18) was associated with mortality in observational ARDS cohorts. Statin exposure increases both inflammasome activation and lung injury in mouse models. We tested whether randomization to statin therapy correlated with increased IL-18 in the ARDSNet SAILS trial.
Design:
Retrospective analysis of randomized controlled clinical trial
Setting:
Multicenter North American clinical trial, the ARDSNet Statins for Acutely Injured Lungs (SAILS)
Patients:
Six hundred eighty-three subjects with infection-related ARDS, representing 92% of the original trial population.
Interventions:
Random assignment of rosuvastatin or placebo for up to 28 days or 3 days after ICU discharge.
Measurements and Main results:
We measured plasma IL-18 levels in all SAILS patients with sample available at Day 0 (baseline, N=683) and Day 3 (after randomization, N=588). We tested the association between IL-18 level at baseline, rising IL-18, and the impact of statin therapy on 60-day mortality, adjusting for severity of illness.
Baseline plasma IL-18 level ≥800 pg/ml was highly associated with 60-day mortality, with a hazard of death of 2.3 (95% CI, 1.7–3.1). Rising plasma IL-18 was also associated with increased mortality. For each unit increase in log2(IL-18) at day 3 compared to baseline, the hazard of death increased by 2.3 (95% CI, 1.5–3.5). Subjects randomized to statin were significantly more likely to experience a rise in plasma IL-18 levels. Subjects with AKI, shock, low baseline IL-18, and those not receiving systemic corticosteroids were more likely to experience rising IL-18. Randomization to statin therapy was associated with rising in IL-18 in all of those subsets, however.
Conclusions:
Elevated baseline plasma IL-18 was associated with higher mortality in sepsis-induced ARDS. A rise in plasma IL-18 was also associated with increased mortality and was more common in subjects randomized to statin therapy in this clinical trial.
Keywords: Inflammasome, sepsis, IL-18, statin, ICU outcomes, ARDS
INTRODUCTION
Multiple sources of evidence suggest that 3-hydroxy-3 methyglutaryl coenzyme A (HMG-CoA) reductase inhibitors (commonly known as statins) might be beneficial in sepsis and sepsis-induced ARDS.(1, 2) Though mainly used clinically to lower cholesterol, statins are also recognized to have other potential benefits, including anti-inflammatory properties, immunomodulatory, antioxidant, and antithrombotic effects (3, 4), supporting their potential use in both sepsis and ARDS. While a number of trials demonstrated that subjects who were prescribed statins had better outcomes from both sepsis and ARDS,(5, 6) other trials failed to show a benefit of statin administration(7–10), including the recently completed NIH/ARDS Network-funded trial of rosuvastatin in sepsis-induced ARDS (Statins for Acutely Injured Lungs from Sepsis, known as the SAILS trial)(11).
While it is well-appreciated that statins can have side effects that include myositis, myalgias, and liver function test elevation, whether statins can paradoxically promote a pro-inflammatory state is less well-studied.(12) Our group previously showed that statins enhanced bleomycin-induced lung inflammation and fibrosis in mice via enhanced activation of the NLRP3 inflammasome.(13) The inflammasome is part of an innate immune complex response that is involved in pathogen recognition, leading to Caspase-1-mediated maturation and secretion of the pro-inflammatory cytokines Interleukin 1 beta (IL-1β) and IL-18.(14) Furthermore, our group reported that the inflammasome plays a critical role in mediating the pro-inflammatory response in murine sepsis and that this response is dependent upon mitochondrial integrity and an intact autophagy response to injury.(15) We additionally showed that the inflammasome-related cytokines IL-18 and IL-1β and caspase-1 protein levels were elevated in murine lung injury and in human infection-related ARDS.(15–17) Specifically, we found that in critically ill patients, elevated IL-18 plasma levels correlated with disease severity and predicted ICU mortality,(17) and similarly, circulating mitochondrial DNA (mtDNA) levels (likely upstream of inflammasome activation) were associated with ICU mortality.(15) (16)
Plasma samples obtained from subjects in the aforementioned SAILS trial at baseline and follow-up levels after randomization to statin therapy offer a unique opportunity to evaluate the association between statin exposure and inflammasome activation, as measured by IL-18 change over time. In addition, we assessed whether statin exposure modified the association between IL-18 and mortality.
METHODS
Study Design
Our study utilizes the patient population from the SAILS trial. The SAILS trial protocol has been published previously (11). Briefly, SAILS was a North American multi-center randomized controlled trial of rosuvastatin vs. placebo for patients with sepsis-induced ARDS of <48h duration. ARDS was defined as a P:F<300 using the American-European Consensus Conference definition of ARDS (18). 745 patients were randomized at the 44 participating sites, from March 2010 through September 2013. Patients were randomized to receive a 40 mg loading dose at enrollment followed by 20 mg per day until 3 days after discharge from ICU, day 28, or death, whichever came first. The primary outcome of SAILS was death prior to discharge home or hospital day 60. The clinical trial was approved by the Institutional Review Board at each of the 44 enrolling hospitals in the NHLBI ARDS Clinical trial network, and assent for participation was collected from patients and their families as part of SAILS trial enrollment at all centers, including consent to use biospecimens for future research for subjects included in this analysis. The ARDS Network provided approval for this project. Plasma samples were obtained from all subjects enrolled in the trial for time points of day 0, day 3, and day 6 of randomization (Supplemental Figure 1). Our study includes those subjects from the SAILS trial who have at least a day 0 measurement of plasma IL-18. Because of non-random missingness of 212 day 6 samples (particularly deaths and discharges), day 6 IL-18 values were not included in these analyses.
Plasma IL-18 protein Measurement
Plasma samples were measured in duplicate for total IL-18 levels by ELISA (RayBiotech, Inc., Norcross, GA, Cat# ELH-IL-18–001) using the manufacturer’s protocol, with levels normalized to the standard curve of known concentrations of IL-18 provided by the manufacturer. If duplicate values differed by >1 log and >400, the level was measured a third time, with the 2 closest values averaged for analysis. Average values of the duplicates were log2-transformed and used for analyses.
Statistical Methods
A subset of SAILS patients had plasma available for IL-18 measurement (Supplemental Figure 1). Baseline characteristics of patients in this subset, randomized to placebo or statin, as well as those of excluded subjects were described. To examine the association of elevated baseline levels of plasma IL-18 with mortality within 60 days, we employed Cox proportional hazards regression techniques, where IL-18 was dichotomized at ≥800 pg/ml, a cut-off pre-specified based on association with mortality in the Brigham and Women’s Hospital (BWH) Registry of Critical Illness (RoCI) population. (17) Models were adjusted for age, race, baseline glomerular filtration rate (GFR), APACHE III, and presence of shock. Acute kidney injury (AKI) was defined using the KDIGO criteria (with AKI defined by any rise in creatinine ≥ 0.3 within a rolling 48h of enrollment)(19) or creatinine at enrollment ≥1.5 the age, gender, and race-adjusted creatinine of a GFR of 75, as described previously.(20) The association of dichotomized IL-18 with secondary trial outcomes including ventilator free days, ICU-free days, renal and hepatic failure free days were also described.
In the subset of subjects in whom IL-18 values for both baseline and day 3 were available, a Cox proportional hazard model was utilized to determine whether change in IL-18 from baseline to day 3 was associated with 60-day mortality. Change in IL-18 level was also categorized into increasing, decreasing and stable trajectories. Increasing trajectory was defined as day 3 IL-18 value greater than 110% of baseline; decreasing trajectory was defined as day 3 IL-18 value less than 90% of baseline value, and stable trajectory was defined as day 3 IL-18 value within 10% of baseline IL-18 value. These cut-offs were selected to obtain 3 reasonably-sized groups with a change outside the range of error.
Finally, we assessed whether baseline patient characteristics or randomization to statin therapy were individually associated with rising IL-18 using Fisher’s exact test. A Cox proportional hazards model was used to characterize whether the association between rising IL-18 values and mortality varied by statin therapy or any other baseline characteristics found to be associated with rising IL-18 levels through use of an interaction term.
All tests were two-sided and conducted at the 0.05 level of significance. All analyses were performed using R v 3.0.1.
RESULTS
Of the 745 participants in the SAILS trial, 699 had at least a day 0 plasma sample available for testing and were thus eligible for inclusion in this analysis. However, day 0 (baseline) IL-18 could be measured in only 683 patients (91% of the entire clinical trial population) and the current study is restricted to this cohort. Baseline characteristics of patients are shown in Table 1 by randomization status. Notably, baseline characteristics do not differ qualitatively between those excluded and included in this study.
Table 1.
Baseline Demographics of SAILS subjects
| Clinical Characteristic | Placebo (343 subjects) | Statin (340 subjects) | Excluded (62 subjects) |
|---|---|---|---|
| Age (years) | 56 (43, 65) | 54 (41, 67) | 53 (43, 65) |
| Gender (% female) | 173 (50%) | 175 (52%) | 32 (52%) |
| White Race | 272 (79%) | 267 (79%) | 51 (82%) |
| APACHE III Score | 92 (76, 115) | 91 (71, 109) | 86 (74, 110) |
| Shock at baseline | 154 (50%) | 155 (51%) | 30 (55%) |
| P:F<200 mmHg | 229 (73%) | 240 (76%) | 41 (71%) |
| Malignancy1 | 45 (13%) | 38 (11%) | 6 (10%) |
| IV or po steroids≥ 20mg | 86 (28%) | 76 (25%) | 18 (32%) |
| AKI | 166 (51%) | 162 (51%) | 27 (48%) |
| Chronic Dialysis | 9 (3%) | 4 (1%) | 2 (3%) |
| ESLD | 17 (5%) | 17 (5%) | 4 (7%) |
| Baseline IL-18 Level (pg/ml) | 557 (412, 736) | 541 (374, 782) | N/A |
Values as N(%) or Median (IQR).
Malignancy= lymphoma, leukemia, or metastatic cancer
Baseline plasma IL-18 elevation is associated with increased mortality and morbidity
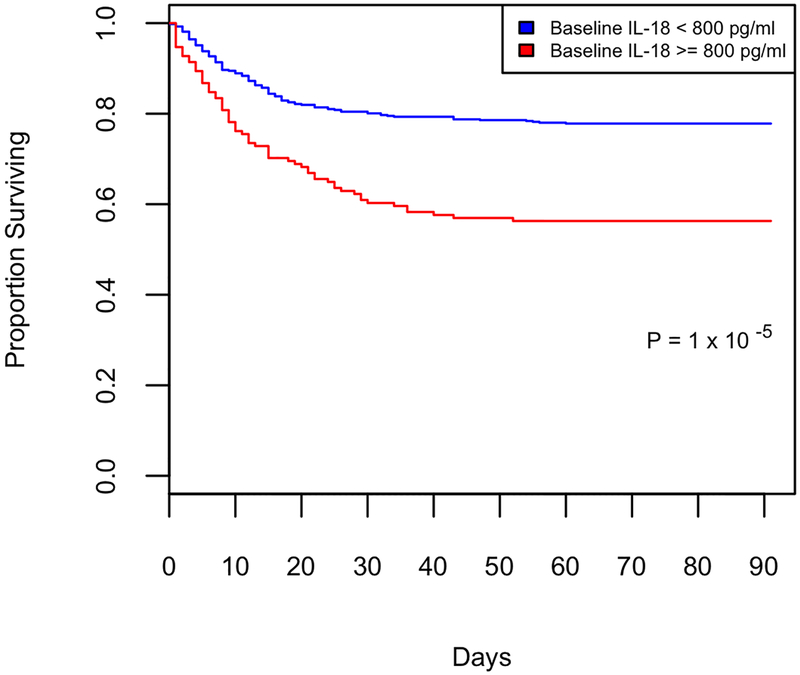
High plasma IL-18 early in the course of critical illness was associated with mortality in our observational ICU cohort (BWH RoCI).(17) The association between elevated baseline IL-18 level and risk of death was confirmed in SAILS (Figure 1). Specifically, the hazard ratio associated with a baseline IL-18 level ≥ 800 pg/ml was 2.2 (95% CI, 1.5–3.1) after adjusting for age, race, APACHE III, GFR, randomization to statin and shock (Cox proportional hazards p= <.001, Table 2). 60-day mortality rate (the primary outcome of SAILS) was 22% in subjects with baseline IL-18 < 800 pg/ml versus 43% in those with IL-18 ≥ 800 pg/ml (OR 2.7; 95% CI, 1.8–4.1, Fisher’s exact p=1 × 10−6).
Figure 1:
Survival dichotomized by baseline plasma IL-18 level. Kaplan-Meier curve of survival from enrollment (day 0) to day 90. Subjects with low baseline IL-18 level (<800 pg/ml, blue) vs. those with high baseline IL-18 level (≥ 800 pg/ml, red), p value 1 × 10−5 by Cox proportional hazards.
Table 2.
Association of plasma IL-18 levels with mortality at 60 days
| Variable | Hazard for Death | CI | P val | Adjusted Hazard | Adjusted CI | Pval | |
|---|---|---|---|---|---|---|---|
| Baseline IL18 ≥800 | 2.3 | 1.7–3.1 | <.001 | 2.21 | 1.5–3.1 | <.001 | |
| Log2 Rise in
IL-18 by Day 3 |
2.32 | 1.5–3.5 | <.001 | 2.31−3 | 1.3–4.1 | .004 | |
P values for Cox Proportional Hazards association with 60-day mortality.
Adjustment for covariates including age, gender, race, APACHE III, GFR, and presence of shock.
Adjusted for baseline IL18 level.
Additional adjustment for corticosteroid therapy and AKI
Primary results are dichotomized IL-18 at ≥ 800 pg/ml because this threshold performed well in a previous cohort; however, the association is robust to various analytic strategies. When log2 IL-18 was evaluated as a continuous measure, the association remained strongly significant, even after adjustment (Supplemental Table 1). The association with mortality is non-linear, with association driven by the highest quartile (Supplemental Figure 2, highest quartile cut-off is 763 pg/ml).
Approximately 15% (N=102) of SAILS subjects had previously taken statins. Baseline IL-18 levels were similar, with a median IL-18 of 569 pg/ml in those who had previously taken statins, vs. 543 pg/mL in those who were statin naïve (p Wilcoxon >.2). Baseline IL-18 ≥ 800 pg/ml was significantly associated with an increased risk of death by day 60 in both previously treated groups (OR 4.4; 95% CI, 1.5–12.8) and the statin naïve (OR 2.5; 95% CI, 1.6–4.0).
In addition to its association with mortality, baseline IL-18 level ≥ 800 pg/ml was associated with key secondary outcomes of the SAILS trial, including fewer ventilator-free days, ICU-free days, and fewer renal and hepatic failure free days (Supplemental Table 2, all p<.001).
Rising IL-18 is associated with increased mortality
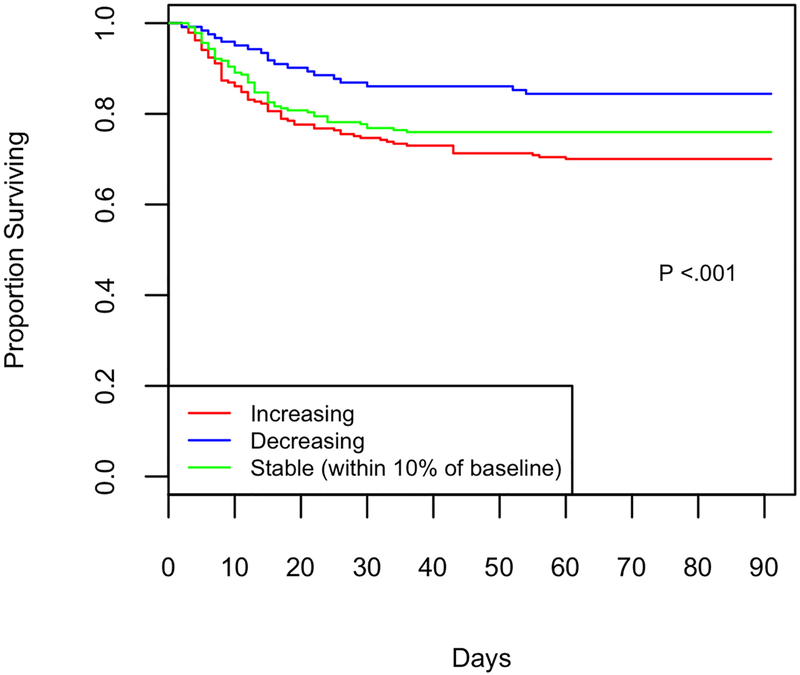
In the subset of 588 patients with day 3 IL-18 levels available, change in IL-18 was significantly associated with 60-day mortality even after adjusting for baseline IL-18 (P= <0.001). For each unit increase in log2(IL-18) at day 3 compared to baseline, the hazard rate increased by 2.3 (95% CI, 1.5–3.5, Table 2). This association remains significant when adjusted for additional covariates including baseline IL-18 level, age, gender, race, APACHE III, GFR, presence of shock, AKI, and systemic corticosteroid therapy (OR 2.3 (95% CI, 1.3–4.1). When change in IL-18 was categorized as stable, decreasing, or increasing as above, stable trajectory of IL-18 was observed in 229 (39%) of subjects, rising trajectory in 237 (40%), and falling in 122 (21%) of subjects. 71 (30%) of subjects with increasing IL-18 died by day 60 as compared to 19 (16%) of those who had decreasing IL-18 and 55 (24%) of those who had stable IL-18 (Chi square p value <.001, and Figure 2 Kaplan-Meier curve shows mortality to day 90). Rising IL-18 is associated with death in both statin naïve and previously treated patients.
Figure 2:
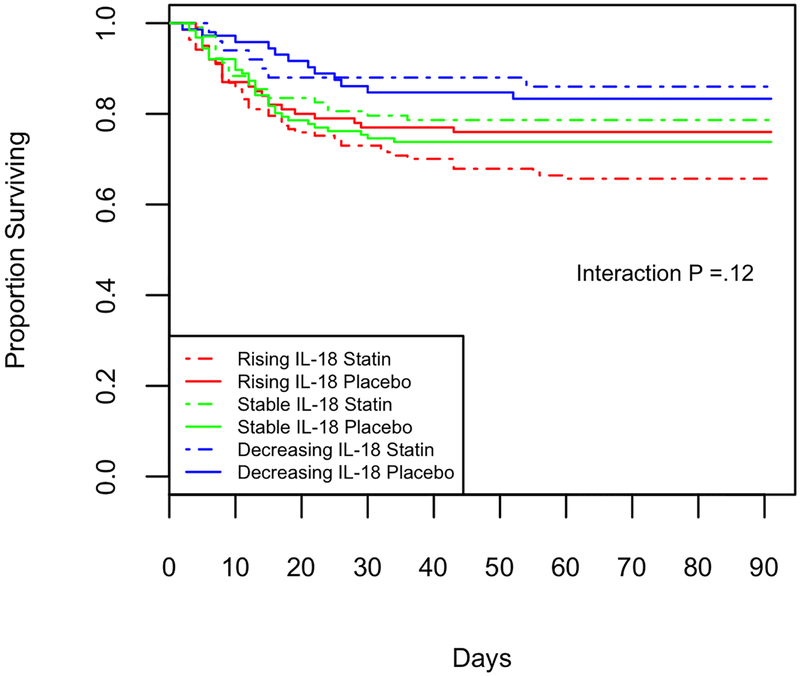
Survival grouped by day 3 IL-18 trajectory, Kaplan-Meier curve of survival from enrollment (day 0) to Day 90. Change characterized as increasing IL-18 (red, N=237), stable (green, within 10% of baseline, N = 229), falling (blue, N= 122). 2A: Mortality is highly associated with change in IL-18, (Chi squared p <.001). 2B: Subjects randomized to statin are shown with a dashed line, those randomized to placebo with a solid line. Mortality is highly associated with change in IL-18, and there is no detectable interaction by treatment group (p=.12).
Randomization to statin is associated with greater rise in plasma IL-18 level
Subjects randomized to statin therapy were more likely to have rising IL-18 levels by day 3. Day 3 Plasma IL-18 level rose a median of 47 pg/ml (IQR −22, 126) in subjects on statins, and 13 pg/ml in those randomized to placebo (P Wilcoxon = .001). When change in IL-18 was categorized as stable, decreasing, or increasing, 47% of subjects randomized to statin had rising IL-18 levels while 17% had decreasing IL-18; in contrast 34% of subjects randomized to placebo had rising IL-18 levels vs. 24% had decreasing IL-18 (Chi square p=0.002). Randomization to statin therapy was associated with more IL-18 rise regardless of prior statin exposure (p interaction = .5).
Approximately 50% of subjects randomized to statin had plasma rosuvastatin level measured at day 3; of these, only 37% (N= 64) were in the therapeutic range of >10 ng/ml. The rise in IL-18 from day 0 to day 3 was similar in those who achieved therapeutic levels (42% increasing, 23% decreasing) vs. those assigned to statin with subtherapeutic levels (48% increasing, 17% decreasing). This suggests a class effect of rosuvastatin on inflammasome activation rather than a dose response.
Although the magnitude of association between mortality and change in IL-18 was higher in patients on statins compared to those on placebo, there is not sufficient evidence to conclude that they are statistically different (p interaction=0.10) For each unit increase in log2(IL-18), the hazard ratio increased by 1.7 (95% CI, 0.85–3.3) in patients who received placebo. Similarly, for patients who received statin therapy, for each unit increase in log2(IL-18) the hazard rate increased by 2.6 (95% CI, 1.5–3.5). The Kaplan-Meier curves in Figure 2B show the interplay between statin/placebo and the different trajectories of IL-18. Subjects with an increasing trajectory of IL-18 have worse survival irrespective of statin therapy.
Baseline characteristics of subjects with rising IL-18
We next assessed whether baseline patient characteristics were more prevalent in patients with rising IL-18, to potentially identify a high and low risk patient population for future statin trials. As shown in Table 3, in addition to randomization to statins, baseline AKI, shock, and lower baseline IL-18 value were associated with increasing IL-18 and systemic steroid therapy was associated with a decreased rise in IL-18. Multivariable models assessing effect of IL-18 rise on mortality are adjusted for these variables. There was no significant interaction between any baseline clinical variable and randomization to statin therapy (all p interaction >.2). When data was stratified for shock, AKI, and corticosteroid use, randomization to statin therapy was associated with a higher odds of IL-18 rise in every case. Other baseline clinical variables associated with severity of illness, including APACHE III score, ARDS severity by Berlin criteria, or C-reactive protein (CRP) level, were not significantly associated with increasing IL-18.
Table 3.
Clinical Characteristics associated with rising IL-18 levels
| Clinical Characteristic | Rising IL-18 (237 subjects) |
Stable or falling IL-18 (351 subjects) | P value | |
|---|---|---|---|---|
| Age (years) | 55 (41, 68) | 56 (44, 65) | >.2 | |
| Female gender | 116 (49%) | 180 (51%) | >.2 | |
| White Race | 191 (81%) | 274 (78%) | >.2 | |
| APACHE III Score | 91 (71, 114) | 91 (75, 110) | >.2 | |
| Shock at baseline | 117 (54%) | 145 (46%) | .04 | |
| P:F<200 | 164 (74%) | 234 (72%) | >.2 | |
| Baseline CRP (mg/dL) | 23 (13, 31) | 22 (12, 31) | >.2 | |
| Randomization to statin | 137 (58%) | 153 (44%) | <.001 | |
| IV or po steroids ≥ 20 mg on Day 1 | 42 (20%) | 95 (31%) | .01 | |
| AKI | 132 (56%) | 159 (45%) | .01 | |
| ESLD | 13 (5%) | 15 (4%) | >.2 | |
| Malignancy | 33 (14%) | 40 (11%) | >.2 | |
| Baseline IL-18 (pg/ml) | 468 (348, 637) | 621 (448, 840) | <.001 | |
Values as N(%) or Median (IQR).
Malignancy= lymphoma, leukemia, or metastatic cancer. “Rising” defined by increase of IL18 by 10% at Day 3, compared with the 353 subjects with stable or falling values at Day 3. P values are for Wilcoxon’s rank sum for continuous variables, Fisher’s exact test for categorical variables. Clinical characteristics significantly associated with rising IL18 highlighted in BOLD.
DISCUSSION
Our prior preclinical data suggested the possibility that statins might exacerbate activation of the inflammasome pathway in the setting of lung injury (13), and our prior clinical data pointed toward activation of the inflammasome pathway in human ARDS as correlating with worse outcomes indicated, in part, by elevated plasma IL-18 levels (17). Thus, we investigated whether statin administration might increase plasma IL-18 levels during sepsis-induced ARDS and whether predictors of a favorable response to statins during critical illness could be identified. We found that a baseline plasma IL-18 level ≥ 800 pg/ml was associated with a markedly increased hazard of death of 2.2, similar to the hazard of death of 1.8 conferred by baseline shock, defined by need for vasopressors, in this population. Rising IL-18 level at day 3 was similarly associated with increased risk of death, and subjects randomized to statins were more likely to experience increasing IL-18, supporting prior work that revealed that statin therapy might lead to inflammasome activation.
It is important to acknowledge that the association between elevated and rising IL-18 plasma levels and increased mortality does not prove causation. In this study we examined plasma IL-18 levels as a surrogate of inflammasome activation, given correlation of this plasma marker in our prior work with other markers of inflammasome activation in correlative pre-clinical studies and human plasma.(17) Free IL-18, the active moiety, has indeed been shown to potentiate tissue damage in experimental models through a variety of mechanisms, including promoting inflammatory cell tissue infiltration via downstream pro-inflammatory effectors, such as inducible nitric oxide synthase, tumor necrosis factor-alpha, and chemokine production.(21, 22) Thus, IL-18 could serve as a causative agent in contributing to multisystem organ failure. Whether poor outcomes in SAILS relate to direct injury by IL-18, or inflammasome activation per se, or whether IL-18 is simply a surrogate for systemic inflammation cannot be determined with this study.
One potential limitation of our IL-18 plasma measurements is that the ELISA kit we used might detect both bound and unbound IL-18, and it is known that the IL-18 binding protein (ILBP) can bind and neutralize IL-18, thus altering levels of free IL-18 that is most likely the active moiety of interest.(23) However, ILBP has rarely been measured in IL-18 biomarker studies, and in the limited series in which it has been investigated, including one study in sepsis, the overall conclusions are that total IL-18 measured levels are generally reflective of free IL-18 levels, as IL-18 and ILBP levels likely rise in parallel during pro-inflammatory states, such that there is still a relative excess of circulating free IL-18 in disease.(24) Similarly, we did not find a significant influence of ILBP levels on relative IL-18 levels in a small cohort that we analyzed from this study (data not shown). Thus, in aggregate, while we believe that our IL-18 measurements correlate with inflammasome activation and likely downstream activation of pro-inflammatory cascades, we were not able to formally measure inflammasome activation in this cohort. However, our prior work and the literature(17) support the association of measured plasma IL-18 levels with the observed clinical outcomes as analyzed in this paper.
This study adds to our understanding of sepsis-induced ARDS in several important ways. First, we confirm the importance of the plasma IL-18 levels and potential inflammasome activation in a multi-center randomized controlled trial as a predictor of outcome in sepsis-induced ARDS, while prior evidence had relied on observational cohorts(17). These findings lend further support to the importance of the inflammasome in sepsis-induced ARDS.(25) Our data supports the possibility that the observed pro-inflammatory response to statins was mediated by inflammasome activation, given the disparate increase in plasma IL-18 levels in response to statins vs. placebo. Preclinical data demonstrated that statins can enhance inflammasome activation and bleomycin-induced lung injury in mice via upregulation of mitochondrial reactive oxygen species in macrophages(13). Interestingly, we previously found that the level of elevated circulating mitochondrial DNA correlates with ICU mortality in humans.(16) Furthermore, prior data had suggested that hydrostatic statins such as rosuvastatin, could be particularly prone to NLRP3 inflammasome activation in the presence of lung injury.(13) Plasma levels of rosuvastatin in SAILS level were substantially lower than anticipated(11), which makes the finding of increased inflammasome in statin-treated subjects in this cohort even more striking.
Given that patients randomized to statin had higher risk of rising IL-18, and those subjects with rising IL-18 were at markedly increased risk of death, one would predict that rosuvastatin therapy might have caused harm, rather than resulting in a null result (the primary outcome of the SAILS trial, which was stopped early for futility). Identifying baseline factors that predict IL-18 rise and/or inflammasome activation in response to statin therapy would be a major advance, as future statin trials might be targeted toward certain patient groups; unfortunately, as shown in Table 3, no such “inflammasome activation risk factors” could be identified with our available biomarkers or clinical baseline characteristics. Interestingly, subjects who received at least 20 mg of systemic steroids were less likely to experience IL-18 rise, but rising IL-18 in the steroid group was still associated with death, and randomization to statin was associated with increased rise in IL-18 in all subgroups.
Subjects with rising IL-18 experienced a higher hazard of death, regardless of treatment assignment (hazard 3.3 in statin group vs 1.8 in placebo). An interaction term for this was not statistically significant, though our power to appreciate such an interaction was limited, even in a trial of over 500 patients.
The question of whether a select subset may benefit (or be harmed) by statins remains unanswered. After the publication of the SAILS trial, the Phase 2b HARP2 randomized controlled trial of simvastatin for ARDS trial was published and was similarly negative. Recent re-analyses of both the SAILS and HARP2 datasets using latent class modeling showed differing efficacy signals in a hyper-inflammatory subset characterized by high IL-8, IL-6, and low bicarbonate. In HARP2, this hyperinflammatory subset benefitted from simvastatin therapy (32% mortality in the simvastatin group, vs. 45% in placebo).(26) In contrast, there was no benefit in the hyperinflammatory subset in SAILS (39.7% 60 day-mortality in the rosuvastatin group vs. 33.6% in placebo).(27) Interestingly, in the COPD Gene population-based study of smokers with over 2000 CT scans, statin therapy was associated with increased risk of interstitial abnormalities; in that study, the risk increased with the degree of statin hydrophilicity, with lowest risk for simvastatin, and highest risk for pravastatin and rosuvastatin.(13) This could be one explanation for apparent benefit of simvastatin in hyperinflammatory subset in HARP2 but a trend toward harm from rosuvastatin in the hyperinflammatory subset of SAILS. The interplay between inflammasome activation, plasma IL-18, and the inflammatory subphenotype identified by latent class modeling is an important area of future study. These data reveal the value of incorporating biomarker and functional studies in ARDS clinical trials, with the hope of identifying predictors of a favorable response to selected interventions.
In summary, in this study, we report that inflammasome activation, as indicated in part by plasma IL-18 levels both at baseline and over the first 3 days in ICU, correlates with higher mortality in a large prospective trial of patients with sepsis-induced ARDS. Also, some patients randomized to statin therapy experienced rises in plasma IL-18 levels that might correlate with inflammasome activation. These results highlight the heterogeneous nature of sepsis and ARDS and the importance of ongoing biology studies in human trials to better predict individualized responses to targeted interventions.
Supplementary Material
Supplementary Figure 1: Plasma availability by study date and randomization
Supplementary Figure 2: 60-day mortality is higher in subjects with high baseline IL-18. Mortality (in blue) is twice as high in the highest quartile, defined in this cohort by baseline IL-18 levels >763 pg/ml (OR of death 2.39, p<.001 by Fisher’s exact test).
Acknowledgements
Dr. Art Wheeler is included as a co-author posthumously, as he was involved in conception and design of this work.
Funding
NIH/NHLBI R01 HL112747, HL111024, HL51856, HL55330, Global Research Laboratory grant # 2016K1A1A2910779, K23 HL125663, NIH/NCATS KL2-TR-002385, and ARDSNET Investigators
Copyright form disclosure: Drs. Rogers, Hunninghake, Kozikowski, DeSouza, Liu, Matthay, Steingrub, Nakahira, Choi, and Baron received support for article research from National Institutes of Health (NIH). Dr. Rogers received funding from NIH/ National Heart, Lung, and Blood Institute (NHLBI) R01 HL112747, NIH/NHLBI K23 HL125663, NIH/NHLBI HL51856, NIH/NHLBI HL55330, Global Research Laboratory grant, and NIH/National Center for Advancing Translational Sciences (NCATS) KL2-TR-002385. Dr. Hunninghake received funding from consulting for Genentech, Boehringer-Ingelheim, the Gerson Lehrman Group, and Mistubishi Chemical for work unrelated to this submission. Dr. Kozikowski’s institution received funding from Brigham and Womens Hospital. Dr. DeSouza disclosed work for hire. Dr. Liu’s institution received funding from NHLBI, National Institute of Diabetes and Digestive and Kidney Disease, and she received funding from National Policy Forum on Critical Care and Acute Renal Failure, Achaogen (consultant), Durect (consultant), Theravance (consultant), Quark (consultant), Potrero Med (consultant), Amgen (stockholder), and Baxter (presenter at sponsored meeting). Dr. Matthay’s institution received funding from Bayer Pharmaceuticals, Department of Defense, GlaxoSmithKline, and he received other support from CS Berhling, Roche-Genentec, Quark Pharmaceuticals, Boerhinger-Ingelheim, Cerus Therapeutics, and NHLBI. Dr. Choi’s institution received funding from NIH; she received funding from Teva Pharmaceuticals; and she disclosed that she is a cofounder, stock holder and serves on the Scientific Advisory Board for Proterris, which develops therapeutic uses for carbon monoxide, and she has a use patent on CO. Dr. Baron’s institution received funding from the NIH, and she disclosed off-label product use of statin administration for infection-related ARDS. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Chalmers JD, Singanayagam A, Murray MP, et al. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med 2008;121(11):1002–1007 e1001. [DOI] [PubMed] [Google Scholar]
- 2.Thomsen RW, Riis A, Kornum JB, et al. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Archives of internal medicine 2008;168(19):2081–2087. [DOI] [PubMed] [Google Scholar]
- 3.Terblanche M, Almog Y, Rosenson RS, et al. Statins: panacea for sepsis? The Lancet Infectious diseases 2006;6(4):242–248. [DOI] [PubMed] [Google Scholar]
- 4.Liao JK, Laufs U. Pleiotropic effects of statins. Annual review of pharmacology and toxicology 2005;45:89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med 2011;183(5):620–626. [DOI] [PubMed] [Google Scholar]
- 6.Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med 2013;187(7):743–750. [DOI] [PubMed] [Google Scholar]
- 7.Kruger PS, Harward ML, Jones MA, et al. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Respir Crit Care Med 2011;183(6):774–781. [DOI] [PubMed] [Google Scholar]
- 8.Papazian L, Roch A, Charles PE, et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial. JAMA 2013;310(16):1692–1700. [DOI] [PubMed] [Google Scholar]
- 9.Patel JM, Snaith C, Thickett DR, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Critical care 2012;16(6):R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAuley DF, Laffey JG, O’Kane CM, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014;371(18):1695–1703. [DOI] [PubMed] [Google Scholar]
- 11.National Heart L, Blood Institute ACTN Truwit JD, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014;370(23):2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brealey DA, Singer M, Terblanche M. Potential metabolic consequences of statins in sepsis. Crit Care Med 2011;39(6):1514–1520. [DOI] [PubMed] [Google Scholar]
- 13.Xu JF, Washko GR, Nakahira K, et al. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med 2012;185(5):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nature reviews Immunology 2016;16(7):407–420. [DOI] [PubMed] [Google Scholar]
- 15.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology 2011;12(3):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahira K, Kyung SY, Rogers AJ, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS medicine 2013;10(12):e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolinay T, Kim YS, Howrylak J, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med 2012;185(11):1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149(3 Pt 1):818–824. [DOI] [PubMed] [Google Scholar]
- 19.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120(4):c179–184. [DOI] [PubMed] [Google Scholar]
- 20.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care 2004;8(4):R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Craft ML, Wang P, et al. IL-18 contributes to renal damage after ischemia-reperfusion. Journal of the American Society of Nephrology : JASN 2008;19(12):2331–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netea MG, Fantuzzi G, Kullberg BJ, et al. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J Immunol 2000;164(5):2644–2649. [DOI] [PubMed] [Google Scholar]
- 23.Novick D, Schwartsburd B, Pinkus R, et al. A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine 2001;14(6):334–342. [DOI] [PubMed] [Google Scholar]
- 24.Dinarello CA, Novick D, Kim S, et al. Interleukin-18 and IL-18 binding protein. Front Immunol 2013;4:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenck EJ, Ma KC, Murthy SB, et al. Danger Signals in the ICU. Crit Care Med 2018;46(5):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. The Lancet Respiratory medicine 2018;6(9):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha P, Delucchi KL, Thompson BT, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med 2018;44(11):1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Plasma availability by study date and randomization
Supplementary Figure 2: 60-day mortality is higher in subjects with high baseline IL-18. Mortality (in blue) is twice as high in the highest quartile, defined in this cohort by baseline IL-18 levels >763 pg/ml (OR of death 2.39, p<.001 by Fisher’s exact test).