Abstract
Mesenchymal stem cells (MSCs) have considerable translational potential in a wide variety of clinical disciplines and are the cellular foundation of individualized treatments of auto-immune, cardiac, neurologic and musculoskeletal diseases and disorders. While the cellular mechanisms by which MSCs exert their biological effects remain to be ascertained, it has been hypothesized that MSCs are supportive of local tissue repair through secretion of essential growth factors. Therapeutic applications of MSCs in peripheral nerve repair have recently been reported. This review focuses on how MSCs can promote nerve regeneration by conversion into Schwann-like cells, and discusses differentiation methods including delivery and dosing of naive or differentiated MSCs, as well as in vitro and in vivo outcomes. While MSC-based therapies for nerve repair are still in early stages of development, current progress in the field provides encouragement that MSCs may have utility in the treatment of patients with peripheral nerve injury.
Keywords: stromal cells, differentiation, Schwann cells, delivery method, allografts
INTRODUCTION
To achieve successful repair of peripheral nerve segmental defects, nerve autografts still supersede the results of all commercially available nerve graft substitutes (bioabsorbable conduits, vessels or processed allografts).(1) Nerve autografts are limited in availability and their harvest from patients automatically generates donor side morbidity. The application of MSCs has been actively considered for in vitro and in vivo studies seeking to improve outcomes of peripheral nerve reconstruction. MSCs potentially provide the necessary biological support for nerve substitutes to equate results obtained by autografts.(2, 3) Prior studies have also evaluated the application of Schwann cells to nerve graft substitutes and have demonstrated active expression of neurotrophic factors with encouraging outcomes. (4) However, clinical application of this technology is impractical, as it would require harvest of autologous nerve tissue to obtain autologous Schwann cells and extensive time to culture and grow the requisite number of Schwann cells for adequate seeding of the nerve graft substitutes. Alternatives to autologous Schwann cells would be the differentiation of autologous MSCs from the patient into Schwann-like cells. In vitro targeted stimulation of autologous MSCs has resulted in differentiation into Schwann-like cells without having to sacrifice autologous nerves.(4, 5) Hence, autologous MSCs can be harvested from the patient, differentiated into Schwann- like cells and be delivered to the site of nerve repair or seeded onto nerve graft substitutes to improve the regenerative environment. Important topics addressed in this review include methods for how to differentiate MSCs into Schwann-like cells, how to deliver naive or differentiated MSCs and the regenerative potential of differentiated MSCs compared to undifferentiated MSCs.
MESENCHYMAL STEM CELLS
MSCs can be isolated from a variety of tissues from the stromal vascular fraction that are extrinsic to blood vessels. They are most frequently obtained from either bone marrow or adipose tissue. Multiple studies have compared bone marrow and adipose MSCs and both sources yield viable MSCs that comply with minimal criteria for MSCs as defined in 2006 by the International Society for Cellular Therapy.(6) Key properties of MSCs include that they are plastic adherent, multi-potent and express canonical mesenchymal stem cell markers (CD44 and CD90), while other markers are absent (CD34 and CD45).(6, 7) In contrast to bone marrow, adipose tissue is more easily accessible and requires only minimally invasive methods (liposuction vs bone marrow harvest) to obtain adequate quantities of MSCs, while having a similar effect on nerve regeneration.(8, 9) MSCs that are derived from the stromal vascular fraction of adipose tissue are easily expanded and differentiated.(10, 11) These properties render adipose derived MSCs of particular interest for clinical applications compared to less accessible bone marrow derived MSCs.
A well-established method to derive MSCs from adipose tissue consists of mechanically disrupted and enzymatically digested tissues. The fat tissue obtained by liposuction is minced and enzymatically digested using collagenase type I. The undigested tissue is removed by filtration and the filtered solution is suspended in standard culture media containing a-MEM. For clinical applications, this media is supplemented with platelet lysate (PL) to obtain zoonotic free clinical grade MSCs. We note that although Fetal Bovine Serum (FBS) suffices for research applications, the cell populations that emerge upon proliferative expansion in PL versus FBS may differ in their molecular properties and these differences may result in functional differences in cell therapy applications. Upon centrifugation, low density adipocytes emerge at the top of the tube, while stromal cells from the vasculature of fat tissue are collected as a pellet. The pellet can be re-suspended in MEM containing growth supplements (e.g., PL or FBS) and antibiotics (e.g., penicillin/streptomycin solution) for subsequent culture as adherent MSCs.(7, 11) Overall, deriving MSCs from adipose tissue is well-described and technically simple to perform making it advantageous for clinical applications.
MECHANISM OF ACTION
There are two major hypotheses on how MSCs establish tissue regeneration. The first proposes that the exogenously administered MSCs have a structural function in tissue injury and thus differentiate in vivo into tissue that requires repair. Growth factors and other paracrine molecules produced by the surrounding tissue stimulate the MSCs to differentiate into the requisite cell type. Supportive studies at best only infer this mechanism. Orbay and colleagues labeled undifferentiated MSCs and reported that they were still detected after 3 months and expressed Schwann cell proteins in a rat-model.(12) Tomita and colleagues reported in a rat-model that a small fraction of their GFP-labeled MSCs were still present after 8 weeks and expressed myelin protein, suggesting that some trans-differentiation into Schwann cells occurred.(4) In this model, MSCs may be able to both repair and replace injured tissue. However, to date this model for MSC function remains largely untested. While there is no question that cellular differentiation is required for neuronal development, it is not clear whether therapies relying on MSCs replicate the normal differentiation of Schwann cells.
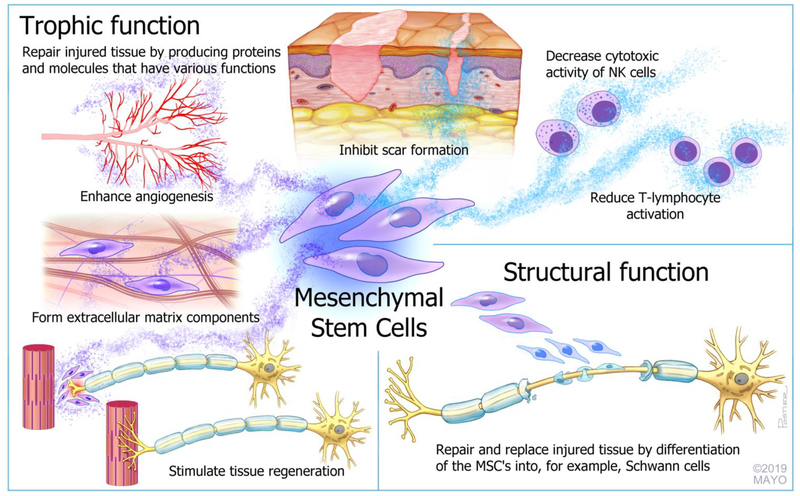
The second hypothesis for MSCs has more recently emerged and this concept poses that MSCs have trophic functions that are important for extracellular matrix remodeling and tissue regeneration.(13) At least a subset of MSCs are derived from pericytes, which are released upon tissue damage or disease. The proteins and molecules produced by the MSCs can enhance angiogenesis, inhibit scar formation and stimulate tissue regeneration.(14) In addition to maximizing the intrinsic regenerative capacity of the tissue, MSCs have key immunomodulatory roles. After the initial immunologic response to injury, pro-inflammatory cytokines produced by NK cells and T lymphocytes ‘activate’ the MSCs. MSCs subsequently prevent the inappropriate and overaggressive activation of T lymphocytes and decrease the cytotoxic activity of NK cells through feedback loops.(15, 16) This ‘trophic’ concept has been corroborated by findings in multiple in vitro and in vivo studies of enhanced gene expression and growth factor production after the introduction of MSCs to damaged tissue.(17, 18) Overall, MSCs most likely have a trophic function and their role in enhancing nerve regeneration is to maximize the intrinsic regenerative capacity of the nerve and minimize the inappropriate inflammatory response after nerve-injury. A dual function, in which a small fraction of the MSCs has a structural function by replacing injured tissue-cells, while the remaining part of the MSCs maximizes the intrinsic regenerative capacity of the injured tissue by producing growth factors and cytokines, is not inconceivable. The described different functions of MSCs are shown in Figure 1.
Figure 1.
Schematic overview of the (hypothesized) subset of functions of mesenchymal stromal cells.
In light of the hypotheses for the mechanisms of action of MSCs, several key questions need to be addressed prior to clinical implementation. These include the role of differentiation of MSCs prior to administration, the optimal dosing and time frame of application of differentiated versus undifferentiated MSCs and how MSCs need to be administered regardless of differentiation status.
APPLICATION OF MSCS
An important aspect for the clinical application of MSCs is that outside factors like local anesthetics or contrast medium can influence the viability of MSCs and should be taken into account in studies on the potential of MSCs for clinical applications. (19, 20) Although the outcomes of preclinical and clinical research on the use of MSCs have been promising in a wide variety of clinical disciplines, further research to determine the optimal doses and time points of implantation, the long-term risks and the long-term efficacy are needed to optimize outcomes of MSC- supported tissue regeneration.(21, 22)
MSCS IN PERIPHERAL NERVE REPAIR
Differentiation of MSCs into Schwann-like cells
The neural induction of MSCs by chemicals combined with growth factors is the most established method to obtain Schwann cell-like differentiation. The induction protocol by Kingham and colleagues is widely used and includes two preparation steps with ß-mercaptoethanol (for 24 hours) and all-trans-retinoic acid (for 72 hours). Subsequently the cells are placed in growth medium enriched with a differentiation cocktail containing Platelet-derived growth factor (PDGF-AA), basis fibroblast growth factor (bFGF), Forskolin and a member of the Neuregulin family (Neuregulin-1 ß1, Glial growth factor-2 or Heregulin- ß1). After 2 weeks in differentiation medium, the morphology of MSCs is altered into an elongated spindle shape, characteristic of Schwann cells. Immunohistochemistry and Western blot analysis after differentiation has revealed expression of several phenotype-specific surface markers, including GFAP, S100 and p75-NTR. (2, 4, 5, 11, 17, 23–25) Studies have demonstrated this protocol is also suitable for human MSCs and that the function of those differentiated cells is analogous to Schwann cells. (4, 26) Regardless of the fact that the effect of differentiated MSCs needs to be further examined and confirmed, these findings imply that research on targeted stimulation of MSCs could be applied in humans in the future and therefore has a serious clinical relevancy. The additional timing and cost of differentiation need to be justified prior to human trials.
The components of Kingham’s induction protocol each have specific biological functions. Forskolin activates adenylyl cyclase which increases the level of intracellular cyclic adenosine monophosphate (cAMP). cAMP causes an increase in the mitogenic responses of Schwann cells(27), in response to the mitogenic actions of the growth factors PDGF and bFGF/FGF2.(28) The neuregulin-1 family plays a crucial role in the actual development and evolution of Schwann cells. Their presence activates cascades promoting Schwann cell differentiation and expansion. The level of Neuregulin-1 (NRG1) determines differentiation of Schwann cells into myelinating or non-myelinating cells that are responsible for the development of group C nerve fibers. NRG1 levels determines axon size, which enables the myelinating Schwann cells to optimize myelin sheath thickness.(29, 30) Kingham’s protocol is currently the preferred method to obtain Schwann-like cells from adipose derived MSCs. The effect of altering the dosages of the different components of the differentiation protocol on the ultimate function of the Schwann-like cells is an interesting prospect for future research.
As targeted neurogenic stimulation to induce differentiation of MSCs is an expensive, time-consuming and inefficient process requiring several weeks of laboratory-based preparation time, efforts have been made to find alternative approaches. Safford and colleagues used a chemical induction medium with butylated hydroxyanisole, potassium chloride, valproic acid, Forskolin, hydrocortisone and insulin to differentiate murine and human MSCs. Within 5–6 hours following neuronal induction, they observed dramatic cell morphological changes in cytoskeletal organization and membrane appearance in MSC cultures which persisted up to 5 days. Beyond 5 days however, the differentiated MSCs lost viability and perished within 14 days of culture.(10) The induction protocol of Anghileri involves the culturing of MSCs for 72 hours in growth medium with exogenous bFGF (FGF2) and human epithelial growth factor as mitogenic factors to facilitate formation of cellular spheres. The spheres were induced to differentiate in media containing BDNF and all-trans-retinoic acid. After four weeks of culture, only half of the MSCs demonstrated the characteristic neuronal morphology, which expressed nestin and neuronal markers MAP-2 and NeuN, but lacked the expression of glial markers S100 and GFAP.(31) Ahmadi and colleagues compared the method of Anghileri to the chemical induction protocol of Woodburry and colleagues. Woodburry included an optimization step in which MSCs are initially induced by addition of ß-mercaptoethanol (BME) for 24 hours followed by induction of neural differentiation using dimethylsulfoxide (DMSO), BME and butylated hydroxyanisole for 1 to 5 days. Ahmadi noted the differentiation protocol of Anghileri significantly improved MSC survival and increased MSC viability, indicating the use of potentially toxic substances (e.g., DMSO and BME) may not be necessary and could be avoided for MSC differentiation.(32, 33) Thaler and colleagues confirmed the toxic effect of DMSO by demonstrating DMSO can initiate epigenetic changes which increased cell apoptosis.(34) Despite the attempts to equal the efficiency of Kingham’s differentiation protocol by alternative chemical induction methods, none have resulted in high percentages of viable Schwann-like cells. In an ideal scenario, MSCs can be differentiated by natural, non-toxic compounds that are largely available, cost-effective and which do not influence the viability of MSCs.
In an effort to find a method meeting the requirements listed above, studies have been performed on the effect of nerve tissue/nerve leachate to cell cultures, co-culture of MSCs with Schwann cells and the electrical stimulation of MSCs. The induction culture medium of Liu and colleagues consisted of 1cm fragments of rat sciatic nerves soaked in normal growth medium (i.e., DMEM and 10% FBS). After 2 days, nerve fragments were removed and adipose tissue derived MSCs were further cultured in the sciatic nerve leachate for another 3 days. Cells adopted a spindle-shape within 48 hours and reflected by expression of S100 and GFAP proteins as was confirmed by immunohistochemistry and western blot analysis, but the nerve autografts required for this protocol would not create a clinically viable therapeutic solution.(18) Liao compared three methods to induce adipose tissue derived MSCs, including (I) neural induction with chemicals only (i.e., media with 2% DMSO for 5 hours), (II) neural induction by chemicals combined with growth factors (i.e., NGF, bFGF/FGF2 and BDNF, as well as the cAMP-related drug Forskolin) for 2 weeks, and (III) neural induction by co-culture of MSCs with Schwann cells. Immunohistochemistry and gene expression analysis showed higher mRNA levels for S100, nestin and GFAP in method II and III compared to method I. Similar to Liu and others, autologous Schwann cells would pose a practical problem for the clinical implementation of method III.(35) Das and colleagues differentiated MSC into Schwann-like cells by electrical stimulation to alter cellular membrane potential. The majority of electrically induced MSCs (>80%) showed Schwann cell markers S100 and p75 and enhanced secretion of NGF compared to chemically induced MSCs or undifferentiated MSCs.(36) Although electrical differentiation is promising and may mimic aspects of normal neuronal cell differentiation, physical methods for differentiation have remained largely unexplored and it remains unclear whether electrical stimulation will have practical benefits compared to differentiation with growth factors.
Methods of administration and cell dosage
The desired method of cell delivery depends on the intended mechanism of action of MSCs. MSCs need to be delivered within the ultrastructure of nerves to fulfill a structural function or need to be able to migrate to the site of injury. Micro-injection of the MSCs has been described, but the consequences of injection to cell viability and the resulting ultrastructural trauma to the nerve are potential concerns. Jesuraj and colleagues reported the pressure build-up in the syringe and needle during injection reduces viability of cells after needle passage. (46) In contrast, Onishi and colleagues reported that adipose derived MSCs were fairly robust within a range of fluid pressures within the syringe upon expulsion. (37, 38) Studies that examined the viability of bone marrow derived MSCs post-injection have various conclusions ranging from no viability changes to a temporarily affected viability, to a reduced viability. (39–41) Increasing the needle gauge may intuitively reduce cell damage, but inserting a larger needle in a processed nerve graft is practically almost impossible and can easily cause tearing of the epineurium. In addition, uncontrolled micro-injection leads to a non-uniform distribution of cells and may result in local accumulation of clusters of MSCs that potentially block the ingrowth of the regenerating nerve rather than enhancing it.(38) The calibers of myelinated axon fibers (2 to 22μm) in proportion to the average diameter of MSCs (17.9 − 30.4μm) also may be problematic when MSCs are injected in the nerve allograft.(42–44) In case of using hollow nerve conduits, injection of MSCs will not harm the conduit itself, but it can still cause decreased viability of the cells and might obstruct axonal ingrowth. Furthermore, leakage of cells out of the nerve substitute is a recognized problem; the study by Jesuraj and colleagues showed only 10% of cells were successfully transferred after one million cells were injected.(38) The injection of MSCs in nerve substitutes is not clinically applicable due to low and uncontrollable delivery efficiencies and the potential damage to the cells and the nerves. Hence, future studies may consider alternative delivery methods for both differentiated and undifferentiated MSCs.
Intravenous injection of MSCs has been investigated as an alternative to MSC-injection that prevents nerve-damage and cell-leakage and focuses on the more likely trophic function of MSCs. Although the vasculature potentially delivers a subset of MSCs to the area of injury, the cells may not accumulate to a critical mass to enhance nerve regeneration. In addition, the relatively large size of MSCs causes entrapment in capillaries.(45) MSCs can also be administered by intramuscular injection which delivers cells locally with preservation of the nerve. Intramuscular injection of MSCs in the gastrocnemius muscle resulted in a significantly improved functional recovery and neuro- conduction velocity compared to intravenous injection of MSCs or sham injection.(46) It has been reported that intramuscular injection of MSCs leads to enhanced nerve regeneration.(47) Even though these findings are promising, the described techniques still require injection of cells which potentially decreases the viability of the cells. The enhanced outcomes after intravenous and intramuscular injection of MSCs, do confirm the previously suggested trophic function of MSCs.
Soaking nerve grafts in MSC-solutions is another described method of cell delivery. Thompson and colleagues compared the injection of cells to a soaking technique in which the nerve samples were pretreated with a micro- needle roller. Injection led to a higher number of cells in the inner and middle zones of the nerves, while soaking delivered a higher number of cells in the outer zone.(48) Dynamic seeding has been successful in vascular tissue engineering and resulted in a more efficient and uniform distribution of cells compared to static seeding with seeding efficiencies ranging from 38% to 90%.(49) This strategy was applied to a nerve-model by Rbia and colleagues. They non-traumatically seeded MSCs on the surface of a processed nerve allograft with the use of a bioreactor. This resulted in a uniform distribution of MSCs that were adhered to the nerve graft.(50) The cells did not migrate into the nerve allograft and the interaction between the MSCs and the nerve surface resulted in an upregulation of neurotrophic factors that potentially enhance nerve regeneration within the nerve graft.(50) Overall, dynamic seeding results in a uniform distribution of MSCs on nerve allografts that enables the cells to interact with the nerve ultrastructure with a high efficiency without harming the cells nor the nerve ultrastructure. To date, this is the most promising delivery method of MSCs to allograft nerves and might form the bridge towards individualized peripheral nerve repair in clinical practice.
Jesuraj and colleagues used a concentration of 1 ×10^5 cells/5uL to inject and compared it to a concentration of 10^6 cells/5uL. Their analysis revealed an injection efficiency of 10% for the 1×10^6 cells (100.000 cells) and 40% for the 1×10^5 cells (40.000 cells) of which only the larger dose was trackable by in vivo fluorescence. (38) Thompson and colleagues also soaked or injected their 10mm allograft segments with 1×10^6 cells/5uL, but did not report a total efficiency. (48) Rbia and colleagues used 1×10^6 cells to dynamically seed their nerve segments and reported a seeding efficiency of 89.2%, suggesting that almost 900.000 cells were attached to the surface of the 10mm nerve segment before in vivo implementation. (50) Wang and colleagues also used 1×10^6 MSCs, diluted in 1mL fluid, to inject in the gastrocnemius muscle.(46) Despite the wide variety of delivery efficiencies, there seems to be consensus that at least 1×10^6 MSCs need to be presented to the nerve graft to generate noticeable biological effects. However, no studies on the optimal dosing of MSCs have been reported. Dynamic seeding of MSCs appears to be a reliable, effective and well-studied delivery method (see table 1).
Table 1.
Overview of the pros and cons of the described delivery methods of MSCs.
| Delivery method | Efficiency | Pros | Cons |
|---|---|---|---|
| Injection into nerve grafts | 10–40% | - Delivers a high number of MSCs in the inner and middle nerve zones | - Reduced viability of MSCs - Damage to the ultrastructure of the nerve - Leakage of cells (conduits) -Local accumulation of MSCs |
| Intravenous injection | 100% | - No damage to the ultrastructure of the nerve - No cell leakage |
- Reduced viability of cells - Entrapment of MSCs in capillaries - Low number of MSCs at regeneration site |
| Intramuscular injection | 100% | - Locally delivers MSCs - No damage to the ultrastructure of the nerve - No cell leakage |
- Reduced viability of cells - Low number of MSCs at regeneration site |
| Soaking | Unknown | - Delivers MSCs in the outer nerve zones -Preserved viability of MSCs |
- Damage to the nerve (micro-needle roller) |
| Seeding | 89.2% | - Uniform distribution of MSCs -Preserved viability of MSCs - No damage to the ultrastructure of the nerve - No cell leakage |
- Interaction between MSCs and extracellular matrix is required |
None of the methods of administration reported to date have been specifically tested on differentiated MSCs, so direct comparisons between undifferentiated and differentiated MSCs with respect to their delivery efficiency are lacking. This could be a potential decisive factor as it has been emphasized that differentiation of MSC may decrease their potential to attach to surfaces.(51) It is essential that delivery methods are tested on differentiated MSCs as well as undifferentiated MSCs as the impact on clinical application is significant with respect to cost and time.
Differentiated MSCs versus undifferentiated MSCs In Vitro
The in vitro capabilities of differentiated MSCs in peripheral nerve repair have been extensively evaluated. Kingham and colleagues found that differentiated MSCs significantly extended the number and the length of formed neurites by motor neuron-like cells compared to undifferentiated MSCs.(11) In another study of Kingham, enhanced expression was observed for nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), vascular endothelial growth factor-A (VEGF-A) and angiopoietin-1 in differentiated MSC compared to undifferentiated MSCs.(17) ELISA analysis demonstrated enhanced secretion of BDNF, GDNF, angiopoietin-1 and VEGF-A upon differentiation of MSCs. These increased levels of growth factors resulted in higher total neurite outgrowth, longer neurites and a better angiogenic potency after removal of the factors that stimulate differentiation from the growth medium.(17) Tomita found similar results and showed differentiated human MSCs produced higher levels of neurotrophic factors like BDNF, NGF and GDNF compared to undifferentiated MSCs. The secretion of these neurotrophic factors resulted in a significantly increased percentage of neuron-bearing neurites, and a significant increase in both neurite length and number of neurons.(4) Ladak also demonstrated that co-culture of differentiated MSCs with dorsal root ganglion neurons led to longer and more arborous neurite outgrowth than undifferentiated MSCs.(23) As described previously, the same result was found with differentiated human MSCs.(26) In vitro studies that examined the interaction between undifferentiated and differentiated MSCs with a processed nerve allograft showed persistent enhanced expression of neurotrophic genes that subsequently led to the secretion of neurotrophic growth factors.(52, 53) In general, differentiated MSCs enhance the expression of neurotrophic genes and the secretion of neurotrophic proteins, resulting in increased neurite outgrowth in vitro. These in vitro results are promising and support the hypothesis that differentiated MSCs have a trophic function in nerve regeneration. The remark needs to be made that any agent or growth factor added to the growth medium may become embedded in the extracellular matrix (ECM) and might not be completely washed out after removal of the differentiation media. Thus, the enhanced gene-expressions and the increased neurite outgrowth could still be the effect of direct stimulation by the added growth factors instead of being positively influenced by the differentiated MSCs. In vivo research could eliminate this discrepancy.
Differentiated MSCs versus undifferentiated MSCs in vivo
When seeded on a conduit and transplanted in a rat-model, differentiated MSCs characterized by Kingham and colleagues increased the distance of axon regeneration and enhanced vascularity in nerve conduits compared to undifferentiated MSCs. These findings show that neurotrophic and angiogenic factors produced by differentiated MSCs interact with regenerative mechanisms that support repair of injured nerves by enhancing vascularization and improved nerve regeneration.(17) Ladak and colleagues found in vivo that differentiated MSCs seeded in a nerve conduit resulted in an equal number of regenerating axons across the nerve gap compared to seeded Schwann cells. However, the improved axon regeneration did not translate into improved electrodiagnostic parameters or increased muscle weight.(23) Keilhoff and colleagues compared the outcomes of Schwann cells, undifferentiated MSCs and differentiated MSCs injected in a devitalized muscle. The authors found both Schwann cells and differentiated MSCs contribute to appropriate regeneration while undifferentiated MSCs did not exhibit the ability to improve nerve repair.(47) Kappos showed in a rat sciatic nerve gap model that the addition of differentiated human MSCs to a nerve conduit led to functional outcomes (sciatic functional index and gastrocneumius muscle mass) that exceeded the results of undifferentiated human MSCs and Schwann cells.(54) In contrast, other studies showed low potential of the Schwann-like cells. Fox and colleagues demonstrated in a rat model that primary Schwann cells did not have a beneficial effect on nerve regeneration after 4 weeks when injected into nerve grafts.(55) Orbay and colleagues evaluated the effects of differentiated and undifferentiated MSCs when seeded in silicone tubes and compared the outcomes to empty silicone tubes and nerve grafts. Although the functional outcomes of both MSC- groups were significantly better than those of the other groups, there were no significant differences between differentiated or control MSCs.(24) Watanabe compared undifferentiated MSCs, differentiated MSCs and Schwann cells in a rat facial nerve gap model and came to similar conclusions in that all groups had a comparable amount of nerve regeneration and all cell based strategies gave functional results close to that of autografts.(56)
The advantages and disadvantages of differentiated versus undifferentiated MSCs in vitro and in vivo are presented in table 2. Although the majority of in vitro studies demonstrated a larger trophic potential of differentiated MSCs compared to undifferentiated MSCs, the in vivo outcomes were less unanimous. These conflicting results may be due to the embedded growth agents in the ECM that is generated in cell culture. Differences in differentiation methods, dosing and efficiency of cell delivery methods, and the composition of the nerve substitutes could affect the persistence of differentiation in vivo in the absence of the differentiation medium and could account for different outcomes. Further careful studies are required to confirm differentiated MSCs preserve their described enhanced trophic function in vivo.
Table 2.
Overview of the pros and cons of the use of undifferentiated versus differentiated MSCs in vitro and in vivo for peripheral nerve repair.
| Cell type | In vitro | In vivo | ||
|---|---|---|---|---|
| Pros | Cons | Pros | Cons | |
| Undifferentiated MSCs | - No extended preparation time - No extra preparation costs |
- Less expression of neurotrophic genes - Less production of neurotrophic growth factors |
- No extended preparation time - No extra preparation costs - Functional outcomes comparable to differentiated MSCs |
- Histologically less axon regeneration |
| Differentiated MSCs | - Enhanced expression of neurotrophic genes - Enhanced production of neurotrophic growth factors - Extended number and length of neurites - Suitable for human MSCs |
- Extended preparation time - Extra preparation costs |
- Increased axon regeneration distance - Enhanced vascularity |
- Inconsistency about functional outcomes - Extended preparation time - Extra preparation costs |
CONCLUSION
Adipose derived MSCs are easy to access, derive and expand. Furthermore, these cells can be successfully differentiated into Schwann-like cells. Therefore, adipose derived MSCs, and in particular adipose derived MSCs differentiated into Schwann-like cells have been broadly studied in the effort to improve the outcomes of peripheral nerve repair/reconstruction. The neural induction of MSCs by chemicals combined with growth factors (PDFG-AA, bFGF/FGF2, Forskolin, neuregulin-1/NRG1) remains the preferred method to obtain Schwann cell-like differentiation and has been validated for human MSCs differentiation. To obtain the putative trophic effect of MSCs, they should be delivered in a timely and non-traumatic method. Dynamic seeding of MSCs on nerve grafts meets these criteria. Despite the wide interest in the use of both differentiated and undifferentiated MSCs in peripheral nerve repair, the optimal delivery and dosing of differentiated MSCs is a rather under-explored research topic. The advantages of using undifferentiated versus differentiated MSCs remain to be further defined. In vitro studies have shown that differentiated MSCs permit enhanced expression of neurotrophic genes and the secretion of neurotrophic proteins, resulting in increased neurite outgrowth when compared to undifferentiated MSCs. The beneficial effect of differentiated MSCs has not yet been convincingly confirmed in in vivo studies. Future studies are needed to determine the ideal method of delivery and optimal dosages of differentiated and undifferentiated MSCs to nerve allografts to ascertain their regenerative potential for peripheral nerve reconstruction. These studies should consider the ultimate goal of clinical applications, as well as be cognizant of the time and cost issues of peripheral nerve reconstruction/repair.
Highlights.
- To obtain Schwann cell differentiation, MSCs need to be exposed to growth factors.
- Differentiated MSCs express enhanced neurotrophic genes and proteins in vitro.
- Dynamic seeding is an efficient and non-traumatic delivery method for MSCs.
- Advantages of undifferentiated vs differentiated MSCs need to be further defined.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations:
- μl
Microliter
- a-MEM
Minimum Essential Medium Eagle - Alpha Modification
- BDNF
Brain-Derived Neurotrophic Factor
- bFGF
Basis Fibroblast Growth Factor
- BME
ß-Mercaptoethanol
- cAMP
Cyclic Adenosine Monophosphate
- CF
Cystic Fibrosis
- DMEM
Dulbeccòs Modified Eagle Media
- DMSO
Dimethylsulfoxide
- ECM
Extracellular Matrix
- FBS
Fetal Bovine Serum
- GDNF
Glial Cell-Derived Neurotrophic Factor
- GFP
Green Fluorescent Protein
- MAP-2
Microtubule-Associated Protein 2
- MSC
Mesenchymal Stem Cells
- NeuN
Neuronal Nuclei
- NK
Natural Killer Cells
- NRG1
Neuregulin-1
- PDGF-AA
Platelet-Derived Growth Factor
- PL
Platelet Lysate
- VEGF-A
Vascular Endothelial Growth Factor A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests to declare.
REFERENCES
- 1.Rbia N, Shin AY. The Role of Nerve Graft Substitutes in Motor and Mixed Motor/Sensory Peripheral Nerve Injuries. J Hand Surg Am. 2017;42(5):367–77. [DOI] [PubMed] [Google Scholar]
- 2.Georgiou M, Golding JP, Loughlin AJ, Kingham PJ, Phillips JB. Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials. 2015;37:242–51. [DOI] [PubMed] [Google Scholar]
- 3.Hundepool CA, Nijhuis TH, Mohseny B, Selles RW, Hovius SE. The effect of stem cells in bridging peripheral nerve defects: a meta-analysis. Journal of neurosurgery. 2014;121(1):195–209. [DOI] [PubMed] [Google Scholar]
- 4.Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. [DOI] [PubMed] [Google Scholar]
- 5.Caddick J, Kingham PJ, Gardiner NJ, Wiberg M, Terenghi G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54(8):840–9. [DOI] [PubMed] [Google Scholar]
- 6.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. [DOI] [PubMed] [Google Scholar]
- 7.di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2010;63(9):1544–52. [DOI] [PubMed] [Google Scholar]
- 8.Mahmoudifar N, Doran PM. Mesenchymal Stem Cells Derived from Human Adipose Tissue. Methods in molecular biology (Clifton, NJ). 2015;1340:53–64. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and tissue research. 2007;327(3):449–62. [DOI] [PubMed] [Google Scholar]
- 10.Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochemical and biophysical research communications. 2002;294(2):371–9. [DOI] [PubMed] [Google Scholar]
- 11.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Experimental neurology. 2007;207(2):267–74. [DOI] [PubMed] [Google Scholar]
- 12.Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2011. [DOI] [PubMed] [Google Scholar]
- 13.Caplan AI, Hariri R. Body Management: Mesenchymal Stem Cells Control the Internal Regenerator. Stem cells translational medicine. 2015;4(7):695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan AI. Adult Mesenchymal Stem Cells: When, Where, and How. Stem cells international. 2015;2015:628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. Journal of immunology research. 2015;2015:394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell death and differentiation. 2014;21(2):216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem cells and development. 2014;23(7):741–54. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Zhang Z, Qin Y, Wu H, Lv Q, Chen X, et al. A new method for Schwann-like cell differentiation of adipose derived stem cells. Neuroscience letters. 2013;551:79–83. [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Smith J, Nie H, Wang Z, Erwin PJ, van Wijnen AJ, et al. Cytotoxicity of Local Anesthetics in Mesenchymal Stem Cells. American journal of physical medicine & rehabilitation. 2018;97(1):50–5. [DOI] [PubMed] [Google Scholar]
- 20.Wu T, Nie H, Dietz AB, Salek DR, Smith J, van Wijnen AJ, et al. Cytotoxic Effects of Nonionic Iodinated Contrast Agent on Human Adipose-Derived Mesenchymal Stem Cells. PM & R : the journal of injury, function, and rehabilitation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karussis D, Petrou P, Kassis I. Clinical experience with stem cells and other cell therapies in neurological diseases. Journal of the neurological sciences. 2013;324(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 22.Gogel S, Gubernator M, Minger SL. Progress and prospects: stem cells and neurological diseases. Gene therapy. 2011;18(1):1–6. [DOI] [PubMed] [Google Scholar]
- 23.Ladak A, Olson J, Tredget EE, Gordon T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Experimental neurology. 2011;228(2):242–52. [DOI] [PubMed] [Google Scholar]
- 24.Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012;65(5):657–64. [DOI] [PubMed] [Google Scholar]
- 25.di Summa PG, Kalbermatten DF, Raffoul W, Terenghi G, Kingham PJ. Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell-like differentiated adipose-derived stem cells and increase cell survival under stress conditions. Tissue engineering Part A. 2013;19(3–4):368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brohlin M, Mahay D, Novikov LN, Terenghi G, Wiberg M, Shawcross SG, et al. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neuroscience research. 2009;64(1):41–9. [DOI] [PubMed] [Google Scholar]
- 27.Kim HA, Ratner N, Roberts TM, Stiles CD. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(4):1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis JB, Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. The Journal of cell biology. 1990;110(4):1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Current opinion in neurobiology. 2006;16(5):492–500. [DOI] [PubMed] [Google Scholar]
- 30.Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. BioEssays : news and reviews in molecular, cellular and developmental biology. 2000;22(11):987–96. [DOI] [PubMed] [Google Scholar]
- 31.Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galie M, Sbarbati A, et al. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem cells and development. 2008;17(5):909–16. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadi N, Razavi S, Kazemi M, Oryan S. Stability of neural differentiation in human adipose derived stem cells by two induction protocols. Tissue & cell. 2012;44(2):87–94. [DOI] [PubMed] [Google Scholar]
- 33.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. Journal of neuroscience research. 2000;61(4):364–70. [DOI] [PubMed] [Google Scholar]
- 34.Thaler R, Spitzer S, Karlic H, Klaushofer K, Varga F. DMSO is a strong inducer of DNA hydroxymethylation in pre-osteoblastic MC3T3-E1 cells. Epigenetics. 2012;7(6):635–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao D, Gong P, Li X, Tan Z, Yuan Q. Co-culture with Schwann cells is an effective way for adipose- derived stem cells neural transdifferentiation. Archives of medical science : AMS. 2010;6(2):145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das SR, Uz M, Ding S, Lentner MT, Hondred JA, Cargill AA, et al. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Advanced healthcare materials. 2017;6(7). [DOI] [PubMed] [Google Scholar]
- 37.Onishi K, Jones DL, Riester SM, Lewallen EA, Lewallen DG, Sellon JL, et al. Human Adipose-Derived Mesenchymal Stromal/Stem Cells Remain Viable and Metabolically Active Following Needle Passage. PM & R : the journal of injury, function, and rehabilitation. 2016;8(9):844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jesuraj NJ, Santosa KB, Newton P, Liu Z, Hunter DA, Mackinnon SE, et al. A systematic evaluation of Schwann cell injection into acellular cold-preserved nerve grafts. J Neurosci Methods. 2011;197(2):209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garvican ER, Cree S, Bull L, Smith RK, Dudhia J. Viability of equine mesenchymal stem cells during transport and implantation. Stem cell research & therapy. 2014;5(4):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agashi K, Chau DY, Shakesheff KM. The effect of delivery via narrow-bore needles on mesenchymal cells. Regenerative medicine. 2009;4(1):49–64. [DOI] [PubMed] [Google Scholar]
- 41.Mamidi MK, Singh G, Husin JM, Nathan KG, Sasidharan G, Zakaria Z, et al. Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. Journal of translational medicine. 2012;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunderland S, Lavarack JO, Ray LJ. The caliber of nerve fibers in human cutaneous nerves. J COMP NEUROL. 1949;91(1):87–101. [DOI] [PubMed] [Google Scholar]
- 43.Ryu YJ, Cho TJ, Lee DS, Choi JY, Cho J. Phenotypic characterization and in vivo localization of human adipose-derived mesenchymal stem cells. Mol Cells. 2013;35(6):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge J, Guo L, Wang S, Zhang Y, Cai T, Zhao RC, et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem cell reviews. 2014;10(2):295–303. [DOI] [PubMed] [Google Scholar]
- 45.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplantation proceedings. 2007;39(2):573–6. [DOI] [PubMed] [Google Scholar]
- 46.Wang P, Zhang Y, Zhao J, Jiang B. Intramuscular injection of bone marrow mesenchymal stem cells with small gap neurorrhaphy for peripheral nerve repair. Neuroscience letters. 2015;585:119–25. [DOI] [PubMed] [Google Scholar]
- 47.Keilhoff G, Goihl A, Stang F, Wolf G, Fansa H. Peripheral nerve tissue engineering: autologous Schwann cells vs. transdifferentiated mesenchymal stem cells. Tissue engineering. 2006;12(6):1451–65. [DOI] [PubMed] [Google Scholar]
- 48.Thompson MJ, Patel G, Isaacs J, McMurtry J, Richards N, Daner W. Introduction of neurosupportive cells into processed acellular nerve allografts results in greater number and more even distribution when injected compared to soaking techniques. Neurological research. 2017;39(3):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villalona GA, Udelsman B, Duncan DR, McGillicuddy E, Sawh-Martinez RF, Hibino N, et al. Cell- seeding techniques in vascular tissue engineering. Tissue engineering Part B, Reviews. 2010;16(3):341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rbia N, Bulstra LF, Bishop AT, van Wijnen AJ, Shin AY. A simple dynamic strategy to deliver stem cells to decellularized nerve allografts. Plastic and reconstructive surgery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fairbairn NG, Meppelink AM, Ng-Glazier J, Randolph MA, Winograd JM. Augmenting peripheral nerve regeneration using stem cells: A review of current opinion. World journal of stem cells. 2015;7(1):11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Z, Wang Y, Peng J, Ren Z, Zhan S, Liu Y, et al. Repair of nerve defect with acellular nerve graft supplemented by bone marrow stromal cells in mice. Microsurgery. 2011;31(5):388–94. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Luo H, Zhang Z, Lu Y, Huang X, Yang L, et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials. 2010;31(20):5312–24. [DOI] [PubMed] [Google Scholar]
- 54.Kappos EA, Engels PE, Tremp M, Meyer zu Schwabedissen M, di Summa P, Fischmann A, et al. Peripheral Nerve Repair: Multimodal Comparison of the Long-Term Regenerative Potential of Adipose Tissue-Derived Cells in a Biodegradable Conduit. Stem cells and development. 2015;24(18):2127–41. [DOI] [PubMed] [Google Scholar]
- 55.Fox IK, Schwetye KE, Keune JD, Brenner MJ, Yu JW, Hunter DA, et al. Schwann-cell injection of cold- preserved nerve allografts. Microsurgery. 2005;25(6):502–7. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe Y, Sasaki R, Matsumine H, Yamato M, Okano T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. Journal of tissue engineering and regenerative medicine. 2017;11(2):362–74. [DOI] [PubMed] [Google Scholar]