Abstract
Neurons are highly polarized cells with extraordinary energy demands, mainly fulfilled by mitochondria. In response to altered neuronal energy state, mitochondria adapt to enable energy homeostasis and nervous system function. This adaptation, also called mitochondrial plasticity, can be observed at the level of form, function and position alterations. The main energy expenditure in neurons is localized at the synapse. In this review, we will discuss molecular mechanisms regulating mitochondrial plasticity at the synapse and how they contribute to information processing within neurons.
Introduction
The physical basis of the mind is the nervous system, which is composed of an intricate collection of specialized and highly polarized cells called neurons. Neurons extend their processes, axons, and dendrites over extremely long distances (more than a meter long for some peripheral neurons) and communicate with each other through specialized contacts called synapses. Although all neurons use energy, metabolic requirements vary among individual neurons and neuronal sub-compartments. Mitochondria provide 95% of this energy precisely when and where it is needed in the nervous system. As long as energy metabolism remains tightly coupled to neuronal function, the nervous system operates adequately. However, dysregulation of energy homeostasis underlies many chronic neurodegenerative diseases and neurological disorders. This review focuses on how energy homeostasis in neurons is achieved through positional, ultrastructural and functional plasticity of mitochondria.
The energy utilization puzzle in neurons
The brain constitutes only 2% of the body mass, yet consumes ~20% of the resting metabolic energy produced throughout the body. Within the brain, neurons utilize 75–80% of this energy, while the remainder energy is used by the neighboring glial cells. Housekeeping tasks, such as cytoskeletal dynamics, macromolecule synthesis, and organelle trafficking, use 25% of the total neuronal energy. Maintaining resting membrane potential (~15%), firing action potentials (~16%), and synaptic transmission (~44%, both on pre- and postsynaptic side) compose the energetically costly processes [1,2]. Thus, the majority of energy used by neurons is locally consumed at the synapse.
In neurons, energy demand increases by several fold during synaptic transmission and synaptic plasticity. Both the extended geometry of a neuron and its highly compartmentalized energy needs impose an additional burden on neuronal metabolism during periods of high energy demand. Cellular energy, in the form of adenosine triphosphate (ATP), is generated from assimilated nutrients via connected biochemical reactions that take place in the cytoplasm and mitochondria. For metabolic reactions to be properly coupled to spatiotemporal energy need, both feedforward and feedback regulatory mechanisms must be in place to sustain energy homeostasis in the neurons.
Glucose, the brain’s primary fuel source, is supplied by coordinated changes in neuronal activity and cerebral blood flow. The ability of neurons to rapidly uptake and utilize glucose has been a source of great debate in the brain metabolism field; hence, the task of glucose metabolism has been assigned to neighboring glial cells (see recent reviews discussing the astrocyte-to-neuron lactate shuttle hypothesis in depth [3–6]). However, recent studies strongly suggest that neurons, particularly nerve terminals, can rapidly metabolize glucose [4,7,8].
The first step of the breakdown of glucose occurs in the cytoplasm via glycolysis. Glycolytic production of pyruvate yields a net gain of two ATP molecules. Further metabolism of pyruvate in the mitochondria through oxidative phosphorylation yields approximately 36 ATP molecules. Metabolic flexibility allows the cells to utilize glycolysis for a rapid energy supply and oxidative phosphorylation for more efficient ATP production. This raises a key basic metabolic question: how do glycolytic and mitochondrial oxidative phosphorylation-based energy utilization differ during neurotransmission in neurons? Although there is conflicting evidence, overall, it seems that mitochondrial energy metabolism is preferred during basal activity, while both glycolysis and oxidative phosphorylation are important for maintaining ATP levels during the energy-demanding processes of synaptic transmission or synaptic plasticity [8–11].
Mitochondrial occupancy is generally much higher in neurons relative to other cell types, reflecting their reliance upon oxidative phosphorylation for ATP production. Mitochondria display an extensively connected network in the somatodendritic area, while forming more punctate units in axons (Figure 1). These observed differences in mitochondrial volume and composition further suggest subcompartment-specific energy needs in neurons. Synaptic activity-dependent regulation of mitochondrial position, form, and function is necessary for sustaining energy homeostasis. Glycolytic activity at the synapse has been recently reviewed elsewhere [4,6]. Here, we will address the complex role of mitochondria, specifically in energy-demanding presynaptic terminals and postsynaptic dendrites.
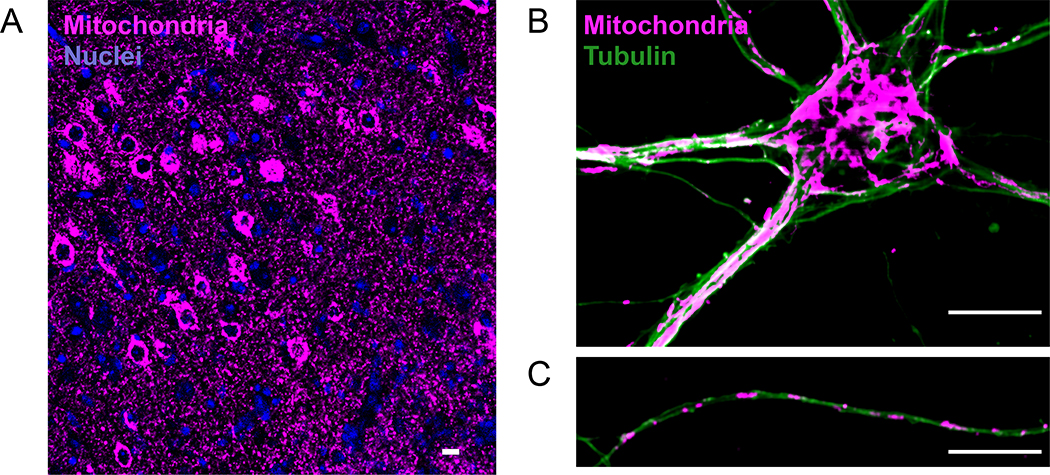
Figure 1.
Mitochondrial morphology and distribution in neurons. (a) Neuronal mitochondria at motor cortex, displaying high mitochondrial content in the brain. Cre-dependent expression of mitochondrial-SNAP-tag allowed neuron-specific labeling. (b) Mitochondrial morphology in somatodendritic, and (c) axonal compartment of cultured hippocampal neurons (14 days in vitro). Mitochondria (Anti-Tom20 staining), and microtubule network (Anti-tubulin staining) display unique properties in axons and dendrites. Mitochondria form a long and voluminous network in dendrites. In contrast, axonal mitochondria are homogeneous in size (1–2μm) and occupy a smaller cytoplasmic fraction (Scale bar=10μm).
Mitochondrial plasticity at the presynaptic terminal
In axons, discrete units of mitochondria (Figure 1C) are either in motion or in a stationary state. The fraction of motile mitochondria (ranging from 10–30% in primary neuron cultures and in vivo), as well as mitochondrial positioning along the axon, evolve as the neuron matures [12,13]. Newly generated neurons predominantly rely on glycolysis for differentiation. Coupled with increased mitochondrial biogenesis, neuronal metabolism shifts from glycolysis to oxidative phosphorylation as the neuron grows [14]. The maintenance and growth of axonal branches require selective immobilization of mitochondria [15]. In fully developed cortical neurons in vivo, axonal mitochondria undergo less movement while simultaneously localizing preferentially to presynaptic boutons [12,13]. Approximately 50% of all axonal mitochondria are located at the presynaptic boutons in early postnatal days (P15), followed by a drastic increase to 80% in adult rat hippocampal neurons [16]. Boutons with a resident mitochondrion are shown to have more stable synapses than those without mitochondria. Stimulation of synaptic plasticity – either via theta burst stimulation-mediated long-term potentiation (LTP) or retinal-lesion-induced cortical plasticity – induces no change in mitochondrial localization to presynaptic boutons [12,16]. Taken together, these findings suggest that, as neurons mature, the majority of axonal mitochondria reach their final destination, and this is not altered rapidly by synaptic plasticity mechanisms.
Although mitochondrial recruitment is not linked to presynaptic plasticity, these mitochondria still play a functional role when present at the boutons. Recent 3D electron micrographic reconstruction studies reveal that presynaptic boutons with at least one mitochondrion contain more synaptic vesicles (SVs) (Figure 2A) and have larger synapses than boutons lacking mitochondria [16]. Augmented SV release occurs more often in mitochondria-containing synaptic boutons after theta burst stimulation-induced LTP. Non-synaptic mitochondria, found in inter-bouton regions along the axon, participate in sustaining synaptic transmission at synapses without mitochondria [16,17]. Perturbation of the stationary mitochondria pool in axons, induced by knocking out the mitochondrial docking protein Syntaphilin (Figure 2D), increases synaptic transmission variability. While presynaptic boutons with resident mitochondria maintain vesicle release during repeated stimulation, mitochondrion-free boutons or boutons with motile mitochondria display more pulse-to-pulse variability [18]. Considering the rapid utilization yet limited diffusion rate of ATP in neurons (~0.3μm per millisecond), the distribution of mitochondria delimits the cytoplasmic energy landscape in mature neurons. Hence, prolonged changes in neuronal activity mainly shape mitochondrial localization at presynaptic sites – a critical element for maintaining homeostatic plasticity [12,19–21].
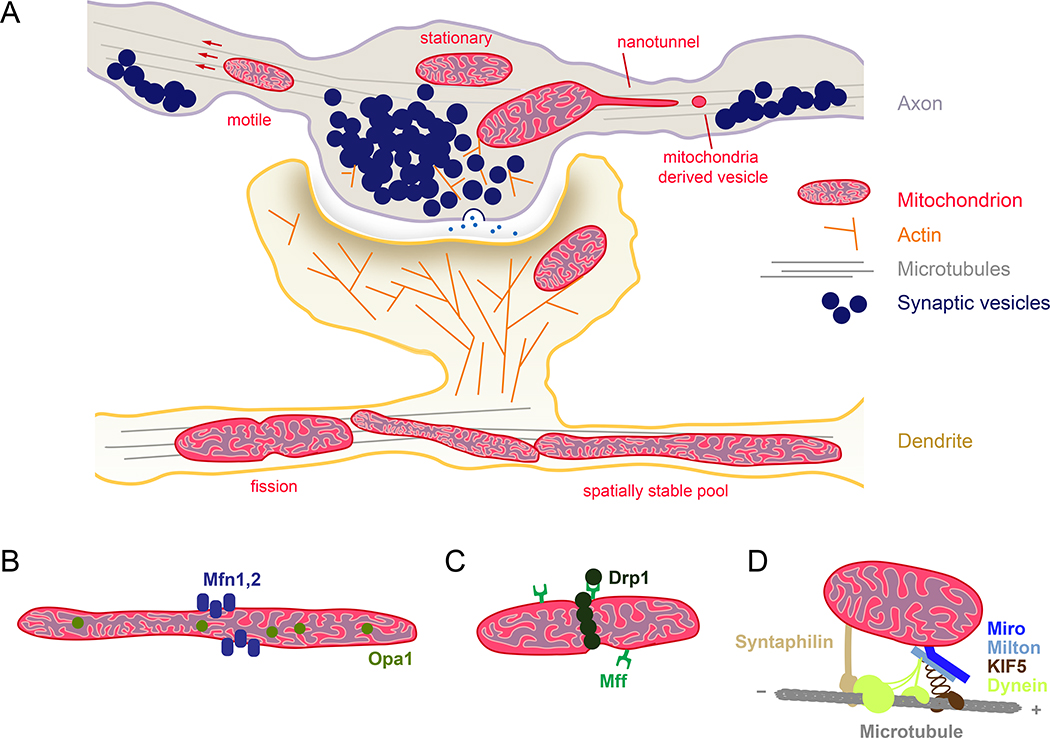
Figure 2.
Mitochondrial plasticity at the synapse. (a) Schematic representation of a synapse. Presynaptic terminals shown here as en passant boutons along the axon, and dendritic spines meet at the synapse. Regulation of long distance transport along microtubules and local actin cytoskeleton are critical for mitochondrial recruitment to synapse. ATP generated by mitochondria fuel synaptic activity, SV recycling, local protein synthesis and actin treadmilling. In response to enhanced energy demand and calcium buffering need, mitochondria alter their motility, size, cristae shape, and outer membrane dynamics at the synapse. Proteins displayed in (b-d) facilitate the ultrastructural plasticity of mitochondria. (b) Mitochondrial fusion machinery is composed of GTPase proteins, Mitofusin 1/2 and Opa1. Mitofusin isoforms display selectivity in different brain regions, axons, and dendrites [46]. In addition to inner mitochondrial membrane fusion, Opa1 also regulates cristae remodeling based on metabolic need, which directly influences oxidative phosphorylation level [59]. (c) Mitochondrial fission is mediated by the dynamin-related protein Drp1 and fission factor Mff. Compartmentalized activity of Drp1 and Mff is coupled to synaptic activity [20,54,60] and also regulated by the cellular metabolism [59]. (d) Mitochondrial motor adaptor complex, composed of mitochondrial protein Miro 1/2 (RHOT1/2), motor adaptor protein Milton 1/2 (TRAK1/2), links kinesin and dynein motor proteins to mitochondria and controls microtubule-based long-distance transport. Miro and Milton isoforms might be selectively utilized for axonal and dendritic mitochondrial transport [51,61]. Many signaling pathways (e.g., calcium, glucose, ROS, damage) converge on this complex, which allow mitochondrial positioning to be tailored to local environment. Neuron-specific mitochondrial protein Syntaphilin anchors mitochondria to microtubules. Synaphilin-based anchoring could also alter motor adaptor complex components according to the “engine-switch and break” model in response to neuronal activity [62].
How do mitochondria become stationary at the presynaptic terminal or retain their motility? Molecular interactions between mitochondria and axonal cytoskeletal tracks, such as actin filaments and microtubules, regulate mitochondrial distribution and dynamics. Mitochondrial transport along microtubules is facilitated via the motor adaptor complex, composed of Miro and Milton (Figure 2D). Multiple signaling pathways converge on this complex to tailor mitochondrial positioning. Enhanced intracellular glucose levels arrest mitochondria via Milton, which may enrich mitochondria at glycolytically active presynaptic terminals. Miro1 mediates activity-dependent presynaptic repositioning of mitochondria, which alters the strength of presynaptic calcium signals and facilitates rescaling of synaptic signals during homeostatic plasticity [19,22–24]. Syntaphilin (Figure 2D) is also required for axonal mitochondrial docking [25]. Considering presynaptic boutons and axon branching points are enriched with actin cytoskeleton, and the stationary mitochondria pool in axons is juxtaposed to filamentous actin, actin-based mechanisms might also function to immobilize mitochondria [26]. Further supporting the role of actin in mitochondrial anchoring, actin depolarization was found to mobilize axonal mitochondria [27]. The crucial synchronization of microtubule and actin-based mitochondrial transport in neurons may be directed by the interactions between Miro and Myosin XIX, an actin motor [28,29].
Mitochondria support activity-driven SV recycling at the presynaptic terminal. A plethora of neuronal functions, including pain perception, cognition, plasticity and metabolic regulation, also require presynaptic neuropeptides and neurotrophins, which are packed in dense core vesicles (DCVs). In contrast to SVs, which are made at the presynaptic release sites, DCVs must be newly generated in the cell body. Trafficking of DCVs to release sites is fueled by a localized glycolytic enzyme [30], but also requires axonal mitochondria [31]. In hypothalamic neurons expressing agouti-related peptide and proopiomelanocortin neuropeptides, presynaptic boutons with mitochondria contain more SVs, which follows from the previously mentioned high energy burden of the SV cycle. However, at neuromodulatory boutons, mitochondria number and DCV pool size display no correlation, which might suggest distinct energy or calcium buffering needs in these specialized release sites [32].
Synaptic activity drives ATP synthesis, which is likely mediated by calcium influx stimulating both glycolysis and oxidative phosphorylation. Although other calcium-driven processes aside from the SV cycle certainly contribute to acute ATP consumption, the SV cycle – specifically endocytosis – presents the largest ATP burden at nerve terminals [9,17]. To meet these activity-driven increases in energy demands during synaptic transmission, presynaptic boutons undergo two remarkable metabolic adaptations: firstly, the glucose transporter GLUT4 is translocated to the axonal plasma membrane. This metabolic adaptation allows glucose to more efficiently enter energetically demanding presynapses and promotes compensatory glycolysis [7]. Secondly, glycolytic enzymes redistribute and colocalize to form a metabolic compartment adjacent to synapses to meet these increased local energy demands [33]. Enhanced glucose flux can also induce mitochondrial motility arrest in axons [22]. Co-compartmentalization of glycolytic machinery together with mitochondria might allow metabolic flexibility and efficiency at active synapses [34].
Alterations in mitochondrial architecture, such as size (via fusion-fission) [20,35], inner membrane cristae packaging [36,37], outer membrane budding [38], and nanotunnel formation [39,40], contribute to, and are indicative of, mitochondrial function (Figure 2). Coordinated regulation of mitochondrial fusion and fission (Figure 2) defines axonal mitochondria size. Elongated presynaptic mitochondria, upon downregulation of mitochondrial fission factor (Mff), cause a reduction in neurotransmitter release. The presynaptic release deficits observed in this case are caused by enhanced mitochondrial calcium buffering capacity, despite having no detectable change in ATP level [20].
In the other direction, increased immediate energy needs at the synapse can reshape mitochondria, suggesting plastic metabolic mechanisms to increase ATP production capacity at presynaptic boutons. Presynaptic mitochondria in both glutamatergic neurons and fast-spiking parvalbumin-positive interneurons exhibit higher cristae density and a morphology conducive to high energy production, compared to mitochondria in slow-firing cannabinoid receptor-positive interneurons [36]. For example, when cristae morphology is disrupted via impairment of the mitochondrial contact site and cristae organizing system MICOS, mitochondrial motility arrest and abnormal morphology in presynaptic active zones are observed, which functionally may restrict neurotransmitter release [41]. These characteristics are indicative of activity-dependent mitochondrial plasticity, which can rapidly enhance fuel efficiency at the synapse.
Numerous enzymes, including kinases and metabolic sensor enzymes, customize mitochondrial properties in axons (see recent reviews [42,43]). The magnitude of signals that determine mitochondrial dynamics and function provide opportunities for mitochondrial plasticity based on the local axonal micro-environment. Further studies will reveal how the spatiotemporal integration of distinct signaling pathways coordinate mitochondrial bioenergetics and distribution throughout the axon.
Postsynaptic mitochondrial plasticity in dendrites
Mitochondria form an elongated network and occupy larger areas in dendrites compared to axons (Figure 1). This unique distribution of dendritic mitochondria starts before synapse formation and reaches a stable state in mature neurons. Similar to axons, dendritic mitochondria selectively localize to synapses [44–46]. However, mitochondria take up twice as much space in inhibitory interneuron dendrites compared to excitatory neuron dendrites, which may reflect the increased energy demands of interneurons due to higher activity levels [47]. In a recent electron microscopic reconstruction of mouse neocortex, mitochondria were rarely found inside dendritic spines – furthermore, of the few found, most were continuations of mitochondria in the parent dendrite [47,48]. These data contrast with an older study, which identified a sizable fraction of hippocampal dendritic spines (~8%) containing mitochondria [49]. Intriguingly, 87% of the dendritic spines in olfactory bulb granule cells contain mitochondria [50]. Altogether, these differences indicate synapse-specific regulation of mitochondrial positioning in postsynaptic regions and stress the need for further studies on the regulatory molecular mechanisms.
As previously described with regards to axonal mitochondria, dendritic mitochondrial distribution and function is also maintained by coordinated mitochondrial trafficking, fusion and fission. When Miro1 (Figure 2D) is impaired, mitochondria largely fail to localize to distal dendrites, dendritic complexity recedes, and, in mature neurons, neurons are affected in ways that mimic neurodegeneration [51]. Artificially depleting mitochondrial fission protein Drp1 (Figure 2C) decreases spine and synapse density, while enriching dendritic mitochondria by overexpressing Drp1 increases spine and synapse density [49]. Similarly, perturbations in mitochondrial fusion pathways, induced by Mfn2 (but not Mfn1) and Opa1 depletion (Figure 2B), cause defects in dendritic growth, spine formation and maintenance [46,52]. These studies imply that the mitochondrial network is coupled to dendritic postsynaptic morphology and function.
Several recent studies have probed the potential function of dendritic mitochondria. Mitochondria form spatially stable (~30μm) compartments in dendrites via local tethering to actin and microtubule cytoskeleton [45]. Synaptic activity modulates this unique mitochondrial architecture, which is essential for synaptic plasticity. LTP induction, a form of activity-dependent synaptic plasticity, triggers Drp1-dependent mitochondrial fission events in dendrites. Notably, prevention of mitochondrial fission impairs calcium elevations in the mitochondrial matrix, structural LTP in cultured neurons, and electrophysiological LTP in hippocampal slices [53]. LTP-induced mitochondrial fission can also have strong behavioral implications. Enhanced mitochondrial fission, via Drp1 phosphorylation, leads to behavioral adaptations in an addiction model, including increased cocaine-seeking after long-term abstinence from cocaine [54]. This activity-dependent synaptic plasticity is energetically costly, consuming ~4 ATP molecules per peptide bond formation in dendrites, and is supplied by spatially confined dendritic mitochondria [45]. While these studies reinforce the importance of mitochondrial oxidative phosphorylation, the precise role of glycolysis in dendrites is still unknown. Further analysis of activity-dependent metabolic pathways is necessary to clarify the molecular mechanisms underlying mitochondrial plasticity in dendrites.
Mitochondria are also able to modulate LTP in non-bioenergetic ways. For instance, endoplasmic reticulum (ER)-mitochondria tethering is essential for calcium homeostasis in dendrites. In the absence of successful ER-mitochondria tethering, activity-induced calcium release from the ER remains in the cytoplasm rather than in the mitochondrial matrix, which may alter dendritic branch-specific synaptic integration and plasticity [55]. Additionally, mitochondrial flashes (mitoflashes), which consist of transient increases in reactive oxygen species (ROS) production, mitochondrial depolarization, and alkalization inside the mitochondrial matrix, can act as signals to convert short-term synaptic changes into LTP [56]. Thus, together with ATP production, mitochondria can carry out multiple signaling functions in dendrites by releasing cytosolic ROS transients and modulating calcium levels.
Conclusions
In this review, we explored the crucial role of mitochondrial plasticity at the synapse through energy generation, calcium buffering, neurotransmitters synthesis, lipid transfer, ROS-dependent signaling pathways and cell death initiation. Each of these tasks requires synapse-, axon-, and dendritic compartment-specific tailoring within a neuron. Further studies are required to elucidate how neurons maintain a healthy mitochondria pool to accomplish these functions and avoid energy crises. Additionally, although we still have a limited understanding of how the mitochondrial proteome is maintained in neurons, heterogeneous energy utilization and mitochondrial activity among different brain regions and neuron classes likely necessitate custom-made proteomes (such as in excitatory vs inhibitory neurons - unpublished results). The fact that mitochondria are hotspots for local protein synthesis [45,57], combined with the possibility of mitochondrial material exchange between neuron-glia, further complicates matters. Despite much progress in our understanding of neuronal metabolism, many questions remain in order to fully understand the metabolic regulation of mitochondrial function in the nervous system.
Highlights.
Mitochondria fuel nervous system function.
Neuronal activity alters mitochondrial form and function.
Mitochondrial plasticity enables rapid energy supply in neurons.
Resident pre- and postsynaptic mitochondria support active synapses.
Acknowledgements
We thank Pekkurnaz Lab members for their insightful comments, Daniela Cassataro and Seungyoon B. Yu for their contribution to graphical design. Research in the laboratory of G.P. is funded by the University of California San Diego institutional funds, Parkinson’s Foundation (PF-JFA-1888), and National Institutes of Health (R35GM128823). M.J.R. is funded by NIH Training Grant T32NS061847. The authors apologize to colleagues whose work could not be cited in this review due to space limitations.
Footnotes
Conflict of interest
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Harris JJ, Jolivet R, Attwell D: Synaptic energy use and supply. Neuron 2012, 75:762–777. [DOI] [PubMed] [Google Scholar]
- 2.Howarth C, Gleeson P, Attwell D: Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab 2012, 32:1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yellen G: Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol 2018, 217:2235–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz-Garcia CM, Yellen G: Neurons rely on glucose rather than astrocytic lactate during stimulation. J Neurosci Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dienel GA: Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 2012, 32:1107–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashrafi G, Ryan TA: Glucose metabolism in nerve terminals. Curr Opin Neurobiol 2017, 45:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashrafi G, Wu Z, Farrell RJ, Ryan TA: GLUT4 Mobilization Supports Energetic Demands of Active Synapses. Neuron 2017, 93:606–615 e603.• This study demonstrates that synaptic activity triggers insertion of glucose transporters (GLUT4) into the presynaptic plasma membrane. Enhanced glucose uptake, via GLUT4, and glycolysis is likely to fuel synaptic function during sustained activity.
- 8.Diaz-Garcia CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G: Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metab 2017, 26:361–374 e364.• This study shows that stimulated neurons metabolize glucose, not lactate, by using a genetically encoded fluorescent NADH/NAD+ sensor in vivo.
- 9.Rangaraju V, Calloway N, Ryan TA: Activity-driven local ATP synthesis is required for synaptic function. Cell 2014, 156:825–835.•• Using a genetically encoded presynaptic ATP reporter, the authors showed that synaptic activity enhances ATP synthesis rate, which requires both glycolysis and oxidative phosphorylation.
- 10.Sobieski C, Fitzpatrick MJ, Mennerick SJ: Differential Presynaptic ATP Supply for Basal and High-Demand Transmission. J Neurosci 2017, 37:1888–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lujan B, Kushmerick C, Banerjee TD, Dagda RK, Renden R: Glycolysis selectively shapes the presynaptic action potential waveform. J Neurophysiol 2016, 116:2523–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smit-Rigter L, Rajendran R, Silva CA, Spierenburg L, Groeneweg F, Ruimschotel EM, van Versendaal D, van der Togt C, Eysel UT, Heimel JA, et al. : Mitochondrial Dynamics in Visual Cortex Are Limited In Vivo and Not Affected by Axonal Structural Plasticity. Curr Biol 2016, 26:2609–2616. [DOI] [PubMed] [Google Scholar]
- 13.Lewis TL Jr., Turi GF, Kwon SK, Losonczy A, Polleux F: Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Curr Biol 2016, 26:2602–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agostini M, Romeo F, Inoue S, Niklison-Chirou MV, Elia AJ, Dinsdale D, Morone N, Knight RA, Mak TW, Melino G: Metabolic reprogramming during neuronal differentiation. Cell Death Differ 2016, 23:1502–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courchet J, Lewis TL Jr., Lee S, Courchet V, Liou DY, Aizawa S, Polleux F: Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell 2013, 153:1510–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith HL, Bourne JN, Cao G, Chirillo MA, Ostroff LE, Watson DJ, Harris KM: Mitochondrial support of persistent presynaptic vesicle mobilization with age-dependent synaptic growth after LTP. Elife 2016, 5.•• The authors analyzed presynaptic mitochondrial distribution and ultrastructure by using 3D electron microscopy in hippocampal neurons. Their results indicate that mitochondria are selectively enriched at large boutons and supports activity-driven presynaptic vesicle mobilization.
- 17.Pathak D, Shields LY, Mendelsohn BA, Haddad D, Lin W, Gerencser AA, Kim H, Brand MD, Edwards RH, Nakamura K: The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem 2015, 290:22325–22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH: Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep 2013, 4:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccaro V, Devine MJ, Higgs NF, Kittler JT: Miro1-dependent mitochondrial positioning drives the rescaling of presynaptic Ca2+ signals during homeostatic plasticity. EMBO Rep 2017, 18:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis TL Jr., Kwon SK, Lee A, Shaw R, Polleux F: MFF-dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nat Commun 2018, 9:5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badal KKA K; Lamoureux P; Liu X; Reich A; Fallahi-Sichani M; Swarnkar S; Miller KE; Puthanveettil SV: Synapse Formation Activates a Transcriptional Program for Persistent Enhancement in the Bi-directional Transport of Mitochondria. Cell Reports 2019, 26:P507–517.E503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL: Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell 2014, 158:54–68. T•• This study identifies a novel molecular mechanism that allows mitochondria to sense glucose availability and to concentrate in glucose enriched areas in neurons.
- 23.Wang X, Schwarz TL: The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 2009, 136:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT: Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 2009, 61:541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH: Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 2008, 132:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganguly A, Tang Y, Wang L, Ladt K, Loi J, Dargent B, Leterrier C, Roy S: A dynamic formin-dependent deep F-actin network in axons. J Cell Biol 2015, 210:401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sood P, Murthy K, Kumar V, Nonet ML, Menon GI, Koushika SP: Cargo crowding at actin-rich regions along axons causes local traffic jams. Traffic 2018, 19:166–181. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Domenech G, Covill-Cooke C, Ivankovic D, Halff EF, Sheehan DF, Norkett R, Birsa N, Kittler JT: Miro proteins coordinate microtubule- and actin-dependent mitochondrial transport and distribution. EMBO J 2018, 37:321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oeding SJ, Majstrowicz K, Hu XP, Schwarz V, Freitag A, Honnert U, Nikolaus P, Bahler M: Identification of Miro1 and Miro2 as mitochondrial receptors for myosin XIX. J Cell Sci 2018, 131. [DOI] [PubMed] [Google Scholar]
- 30.Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F: Vesicular glycolysis provides on-board energy for fast axonal transport. Cell 2013, 152:479–491. [DOI] [PubMed] [Google Scholar]
- 31.Zhao T, Hao Y, Kaplan JM: Axonal Mitochondria Modulate Neuropeptide Secretion Through the Hypoxic Stress Response in Caenorhabditis elegans. Genetics 2018, 210:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atasoy D, Betley JN, Li WP, Su HH, Sertel SM, Scheffer LK, Simpson JH, Fetter RD, Sternson SM: A genetically specified connectomics approach applied to long-range feeding regulatory circuits. Nat Neurosci 2014, 17:1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang S, Nelson JC, Bend EG, Rodriguez-Laureano L, Tueros FG, Cartagenova L, Underwood K, Jorgensen EM, Colon-Ramos DA: Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function. Neuron 2016, 90:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal A, Pekkurnaz G, Koslover EF: Spatial control of neuronal metabolism through glucose-mediated mitochondrial transport regulation. Elife 2018, 7.•• This study describes a quantitative model for the glucose-dependent spatial organization of mitochondria and provides mathematical evidence that metabolic efficiency can be enhanced through regulated mitochondrial transport.
- 35.Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko TS, Ahmad J, Edwards RH, Sesaki H, Huang EJ, et al. : Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci 2014, 34:14304–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cserep C, Posfai B, Schwarcz AD, Denes A: Mitochondrial Ultrastructure Is Coupled to Synaptic Performance at Axonal Release Sites. eNeuro 2018, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins GA, Tjong J, Brown JM, Poquiz PH, Scott RT, Kolson DR, Ellisman MH, Spirou GA: The micro-architecture of mitochondria at active zones: electron tomography reveals novel anchoring scaffolds and cristae structured for high-rate metabolism. J Neurosci 2010, 30:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin MY, Cheng XT, Tammineni P, Xie Y, Zhou B, Cai Q, Sheng ZH: Releasing Syntaphilin Removes Stressed Mitochondria from Axons Independent of Mitophagy under Pathophysiological Conditions. Neuron 2017, 94:595–610 e596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent AE, Turnbull DM, Eisner V, Hajnoczky G, Picard M: Mitochondrial Nanotunnels. Trends Cell Biol 2017, 27:787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer TD, Dash PK, Liu J, Waxham MN: Morphology of mitochondria in spatially restricted axons revealed by cryo-electron tomography. PLoS Biol 2018, 16:e2006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai PI, Papakyrikos AM, Hsieh CH, Wang X: Drosophila MIC60/mitofilin conducts dual roles in mitochondrial motility and crista structure. Mol Biol Cell 2017, 28:3471–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misgeld T, Schwarz TL: Mitostasis in Neurons: Maintaining Mitochondria in an Extended Cellular Architecture. Neuron 2017, 96:651–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devine MJ, Kittler JT: Mitochondria at the neuronal presynapse in health and disease. Nat Rev Neurosci 2018, 19:63–80. [DOI] [PubMed] [Google Scholar]
- 44.Faits MC, Zhang C, Soto F, Kerschensteiner D: Dendritic mitochondria reach stable positions during circuit development. Elife 2016, 5:e11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangaraju V, Lauterbach M, Schuman EM: Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell 2019, 176:73–84 e15.•• This study characterizes spatially restricted mitochondrial pools in dendrites and shows their role in synaptic plasticity-coupled local protein synthesis.
- 46.Chen H, McCaffery JM, Chan DC: Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 2007, 130:548–562. [DOI] [PubMed] [Google Scholar]
- 47.Kasthuri N, Hayworth KJ, Berger DR, Schalek RL, Conchello JA, Knowles-Barley S, Lee D, Vazquez-Reina A, Kaynig V, Jones TR, et al. : Saturated Reconstruction of a Volume of Neocortex. Cell 2015, 162:648–661. [DOI] [PubMed] [Google Scholar]
- 48.Santuy A, Turegano-Lopez M, Rodriguez JR, Alonso-Nanclares L, DeFelipe J, Merchan-Perez A: A Quantitative Study on the Distribution of Mitochondria in the Neuropil of the Juvenile Rat Somatosensory Cortex. Cereb Cortex 2018, 28:3673–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Okamoto K, Hayashi Y, Sheng M: The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119:873–887. [DOI] [PubMed] [Google Scholar]
- 50.Cameron HA, Kaliszewski CK, Greer CA: Organization of mitochondria in olfactory bulb granule cell dendritic spines. Synapse 1991, 8:107–118. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Domenech G, Higgs NF, Vaccaro V, Ros H, Arancibia-Carcamo IL, MacAskill AF, Kittler JT: Loss of Dendritic Complexity Precedes Neurodegeneration in a Mouse Model with Disrupted Mitochondrial Distribution in Mature Dendrites. Cell Rep 2016, 17:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams PA, Morgan JE, Votruba M: Opa1 deficiency in a mouse model of dominant optic atrophy leads to retinal ganglion cell dendropathy. Brain 2010, 133:2942–2951. [DOI] [PubMed] [Google Scholar]
- 53.Divakaruni SS, Van Dyke AM, Chandra R, LeGates TA, Contreras M, Dharmasri PA, Higgs HN, Lobo MK, Thompson SM, Blanpied TA: Long-Term Potentiation Requires a Rapid Burst of Dendritic Mitochondrial Fission during Induction. Neuron 2018, 100:860–875 e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandra R, Engeln M, Schiefer C, Patton MH, Martin JA, Werner CT, Riggs LM, Francis TC, McGlincy M, Evans B, et al. : Drp1 Mitochondrial Fission in D1 Neurons Mediates Behavioral and Cellular Plasticity during Early Cocaine Abstinence. Neuron 2017, 96:1327–1341 e1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirabayashi Y, Kwon SK, Paek H, Pernice WM, Paul MA, Lee J, Erfani P, Raczkowski A, Petrey DS, Pon LA, et al. : ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science 2017, 358:623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu ZX, Tan X, Fang H, Lau PM, Wang X, Cheng H, Bi GQ: Dendritic mitoflash as a putative signal for stabilizing long-term synaptic plasticity. Nat Commun 2017, 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cioni JM, Lin JQ, Holtermann AV, Koppers M, Jakobs MAH, Azizi A, Turner-Bridger B, Shigeoka T, Franze K, Harris WA, et al. : Late Endosomes Act as mRNA Translation Platforms and Sustain Mitochondria in Axons. Cell 2019, 176:56–72 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong-Riley M: Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 1979, 171:11–28. [DOI] [PubMed] [Google Scholar]
- 59.Yu SB, Pekkurnaz G: Mechanisms Orchestrating Mitochondrial Dynamics for Energy Homeostasis. J Mol Biol 2018, 430:3922–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li H, Alavian KN, Lazrove E, Mehta N, Jones A, Zhang P, Licznerski P, Graham M, Uo T, Guo J, et al. : A Bcl-xL-Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat Cell Biol 2013, 15:773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, et al. : TRAK/Milton motor-adaptor proteins steer mitochondrial trafficking to axons and dendrites. Neuron 2013, 77:485–502. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Sheng ZH: Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol 2013, 202:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]