Abstract
Dopamine controls motor functions, motivation, and reward-related learning through G-protein coupled receptor signaling. The current working model is that upon release, dopamine diffuses to influence many target cells via wide-spread receptors. Recent studies, however, suggest that dopamine release is fast and generates small signaling hotspots. In this review, we summarize progress on the understanding of the dopamine release apparatus and evaluate how its properties may shape dopamine signaling during firing. We discuss how mechanisms of regulation may act through this machinery and propose that striatal architecture for dopamine signaling may have evolved to support rapid dopamine coding.
Introduction
The midbrain dopamine system is composed of two major pathways with distinct functions [1–3], The nigrostriatal pathway originates from dopamine neurons in the substantia nigra pars compacta (SNc), projects to the dorsal striatum, and is mostly associated with motor control. The mesolimbic pathway consists of dopamine neuron somata in the ventral tegmental area and projects to the ventral striatum, also known as the nucleus accumbens, for the control of reward processing. Functions of these pathways are affected in many brain disorders including Parkinson’s disease, schizophrenia and addiction. Most midbrain dopamine neurons have spontaneous pacemaker firing at 0.2-10 Hz and can switch to synchronous burst firing, which is important in each pathway for controlling respective behaviors. Detailed and specific insight into the molecular machinery for dopamine release is sparse, and mechanisms are often assumed from studies of fast synaptic vesicle exocytosis or of neuroendocrine secretion. We here review recent progress on the dopamine secretory apparatus and discuss how axonal mechanisms shape and regulate dopamine signaling with spatial and temporal precision. Dopamine is also released in the midbrain from somata and dendrites. While we focus on axonal dopamine release and compare its mechanisms to release of fast synaptic neurotransmitters, we include important recent advances in the understanding of somatodendritic release for comparison.
Exocytotic release of dopamine
At fast synapses, the force for fusion of synaptic vesicles with the presynaptic plasma membrane is provided by formation of the SNARE complex between the vesicular SNARE Synaptobrevin-2/VAMP-2 and the plasma membrane SNARE proteins Syntaxin-1 and SNAP-25 [4], Synaptic vesicle release is restricted to release sites called active zones, which precisely align neurotransmitter secretion with postsynaptic sensing mechanisms. This architecture warrants rapid and efficient exchange of information [5], The active zone consists of protein scaffolds that dock and prime a small portion of neurotransmitter-filled vesicles close to voltage-gated Ca2+ channels [6], When an action potential depolarizes the presynaptic plasma membrane, Ca2+ enters through these channels and rapidly triggers vesicle fusion via the Ca2+ sensors Synaptotagmin 1, 2 or 9 [4,7], Neurotransmitters are then released and diffuse across the synaptic cleft to activate postsynaptic receptors.
Compared to fast synapses, the secretory biology of dopamine neurons exhibits unique properties. Instead of forming classical point-to-point synapses, vesicle filled en-passant boutons of dopamine axons, called varicosities, are typically not associated with postsynaptic densities [8] (but see [9**]). Dopamine receptors, which are G-protein coupled receptors (GPCRs) [10], are normally encountered outside of synaptic areas [9**,11,12], In addition to axonal release, dopamine neurons also secrete dopamine from their cell bodies and dendrites, referred to as somatodendritic release [13–15], Together, these distinct morphological features indicate that dopamine transmission is different from classical synaptic transmission. A prominent model is that dopamine functions through volume transmission, in which neurotransmitters diffuse in tissue to activate relatively distant receptors for signaling [16,17], It has remained unclear whether dopamine release is mediated by the same protein machinery that is employed at fast synapses. Alternative hypotheses are that dopamine release is more similar to slower forms of secretion, for example those employed by neuroendocrine cells for hormone secretion or those for neuropeptide secretion. Although much less is known about the molecular architecture and functions of these secretory machines, they have at least partially distinct SNARE and active zone protein requirements [18–20], Finally, non-vesicular forms of release, for example through reversal of the dopamine transporter, may further contribute to dopamine transmission [21].
A convincing body of literature has established that most dopamine transmission is mediated by vesicular exocytosis. First, electron microscopy showed that axonal dopamine varicosities are densely packed with clusters of small, clear vesicles [9**,11,12], Somatodendritic dopamine release may also originate from specialized secretory organelles, as dopamine is mainly stored in tubulo-vesicular structures resembling the smooth endoplasmic reticulum in the soma or dendrites [22], Second, quantal events, reminiscent of individual vesicular packets of dopamine, can be recorded using amperometry or whole cell voltage clamp (see Box 1 for dopamine release measurements) from dopamine axons or cell bodies [14,23–25], Third, dopamine transmission is eliminated by blockade or knockout of the vesicular monoamine transporter type 2 (VMAT2) [26,27].
Box 1: Measurements of dopamine release.
Electrochemical detection
Fast-scan cyclic voltammetry (FSCV) is widely used for detecting dopamine release (see [34*] for an example). A small carbon fiber electrode is placed into the tissue in vivo or in vitro and a triangular wave (typically −0.6 to 1 V) is applied at a fast scan rate (>400 V/s). Different compounds generate oxidation and reduction currents at different voltages during the scan, with the peak current for dopamine appearing at ~0.6 V. The acquisition rate is usually set at 10 Hz but can be as high as 100 Hz. FSCV requires subtraction of a baseline current and is therefore only suitable to report changes in dopamine, and the scan rate is slow compared to the speed of exocytosis.
Amperometry is similar, but a constant potential (~0.6 V for dopamine) is applied to the electrode (see [25,30*] for examples). Its advantage is the high temporal resolution, which is limited only by the sampling rate. The specificity for dopamine is limited and amperometry can only be used when dopamine is the major electroactive substance. Oxidation consumes dopamine, which may contribute to the decay of the signal.
Microdialysis followed by high performance liquid chromatography is used to measure absolute dopamine levels in vivo (see [30*,48*] for examples). Its advantage is that it is sufficiently sensitive to determine basal dopamine levels the brain of a living animal, but the low temporal resolution makes it unsuited to assess fast dopamine transients, and tissue damage by the probe may influence the measurements.
Whole-cell recording
D2 IPSC recordings report the activation of dopamine D2 receptors. In midbrain dopamine neurons, D2 receptors are coupled to G-protein activated inwardly rectifying potassium channels (GIRK) to produce an inhibitory postsynaptic current (IPSC) upon activation by dopamine [13]. In the striatum, endogenous D2 coupled GIRKs are not present, but GIRKs can be virally expressed to report D2 activation [52,53*]. D2 IPSCs do not report all dopamine release, but only release that activates D2 receptors. The method relies on GPCR signal transduction, which produces a delay of ~50-100 ms between dopamine release and detection.
LGC-53 is a dopamine sensitive chloride channel expressed in C.elegans. When medium spiny neurons are transduced with viruses for striatal expression of LGC-53, a fast chloride current with good sensitivity and specificity for dopamine is measured [24]. It is unclear how the localization of virally expressed LGC-53 compares to that of dopamine receptors and how its activation relates to the activation of endogenous receptors.
Imaging
VMAT-pHluorin is a pH-sensitive fluorophore that is attached intraluminally to the vesicular monoamine transporter 2 (VMAT2). When VMAT-pHluorin labelled vesicles fuse with the plasma membrane, the fluorescent signal increases because the acidic vesicle lumen is neutralized. This method permits measurements of fusion of single vesicles from individual varicosities (see [40] for an example). Since dopamine neurons co-release other neurotransmitters, and transfected or transduced VMAT-pHluorin may not exactly reproduce the localization of endogenous VMAT2, this method may not always precisely report exocytosis of endogenous dopamine vesicles.
Fluorescent false neurotransmitters (FFNs) are VMAT2 substrates that are selectively loaded into monoamine-containing vesicles. When vesicles are released, FFN diffusion leads to a decrease in fluorescence detected in varicosities, which serves as a readout for release (see [43*] for an example). The method permits analyses of single varicosities, but the sensitivity is limited because only a small portion of vesicles from a terminal are released even under strong stimulation.
Genetically encoded dopamine sensors (dLight [61**] and GrabDA [62**]) are fluorescent indicators obtained by engineering of dopamine receptors tagged with circularly permutated GFP. These recently developed probes have great potential because they exhibit high sensitivity and spatiotemporal resolution in vitro and in vivo. It is currently uncertain whether the sensors are localized like the respective dopamine receptors.
Nanotube sensors are near-infrared fluorescent single-walled carbon nanotubes conjugated with single strand oligonucleotides that are directly placed into tissue in vivo or in vitro (see [60] for an example). Upon dopamine binding, a large increase in fluorescence is observed. These sensors respond rapidly and are highly sensitive, making them well suited to study spatial and temporal properties of dopamine release. Specificity is limited because they detect other catecholamines and ascorbic acid.
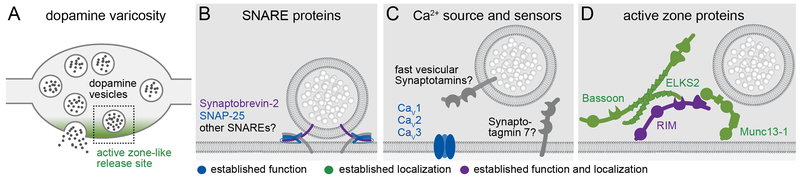
Vesicular release relies on SNARE proteins to mediate membrane fusion. Dopamine release is partially sensitive to botulinum toxins A and B, which cleave SNAP-25 or Synaptobrevin-2, respectively [28,29], and Synaptobrevin-2 is localized to dopamine varicosities [30*]. It appears, however, that release is resistant to tetanus toxin, which also cleaves Synaptobrevin-2 [29], These experiments may be confounded by indirect effects of the toxins on dopamine release via inhibiting the release of dopamine secretion modulators (for example acetylcholine [31,32]), or dopamine secretion could be resistant to toxins because dopamine neurons may lack machinery for toxin uptake. Hence, while these data generally support that dopamine release is vesicular (Figure 1), future studies will have to conclusively identify relevant SNAREs.
Figure 1. Molecular machinery for axonal dopamine secretion.
A) Schematic of a dopamine varicosity with an active zone-like release site in green. Dotted rectangle represents the area shown in B-D.
B) SNARE proteins have been implicated in dopamine release [28,29] and Synaptobrevin-2 has been localized to varicosities [30*], but their cleavage has only partial effects on release.
C) While Ca2+ sensors for axonal dopamine release are not known, Synaptotagmin 7 may partially mediate somatodendritic release [39]. Cav1-3 family Ca2+ channels all contribute to axonal dopamine secretion [34*].
D) The active zone proteins RIM, ELKS2, Munc13-1 and Bassoon are clustered in dopamine axons [9**,30*,41], and RIM is essential for action potential triggered dopamine release [30*]. Adapted in part from reference [4].
Dopamine release is triggered by Ca2+ and is steeply dependent on extracellular Ca2+, with potential differences between axonal release and somatodendritic release [33], Several early studies assessed the dependence of dopamine release on specific voltage-gated Ca2+ channels, but some of the effects may have been indirect because the manipulations may have impaired the triggering of axonal dopamine release via inhibiting cholinergic interneurons [31,32], A recent important study re-evaluated this point by either activating dopamine neurons optogenetically, or by blocking nicotinic acetylcholine receptors (nAChRs) during electrical stimulation, and found that Cav2 (N- and P/Q-type), Cav3 (T-type) and Cav1 (L-type) channels contribute to dopamine release [34*]. This is different from classical synapses, which nearly exclusively rely on Cav2 channels [4,35], The dependence of dopamine release on Cav1 channels is particularly interesting, because inhibiting these channels may help dopamine neuron survival [36].
The Ca2+ sensors for triggering axonal dopamine release remain unknown (Figure 1). At fast synapses, Synaptotagmin 1,2 and 9 trigger rapid exocytosis, and Synaptotagmin 7 and Doc2 mediate Ca2+ sensitivity of asynchronous and spontaneous release, and of facilitation [4,7,37], Conclusive studies of Ca2+ sensors in striatal dopamine axons have not been performed, and loss of function studies to test for necessity of specific Ca2+ sensor proteins will be necessary. Synaptotagmin 7 is necessary for efficient somatodendritic dopamine release [38], but it remains to be tested whether Synaptotagmin 7 functions as a Ca2+ sensing protein for dopamine release. Non-Ca2+-binding Synaptotagmins were also implicated in dopamine release. A recent study evaluated exo- and endocytotic roles of Synaptotagmin 11 in dopamine neurons [39], and Synaptotagmin 4 was shown to mediate somatodendritic release [38], Because Synaptotagmin 4 and 11 do not bind Ca2+ [7], their roles in dopamine release are not as Ca2+ sensors.
Active zone-like sites are essential for dopamine release
Hallmarks of synaptic transmission are its speed and its spatial precision: synaptic vesicles fuse within less than a millisecond upon arrival of an action potential and their exocytosis is restricted to release sites precisely opposed to postsynaptic receptors [5], This spatial and temporal precision is mediated by the active zone, a protein network that consists of the molecular scaffolds RIM, ELKS, Bassoon, Munc13, Liprin-α and RIM-BP. The active zone tethers primed, release ready vesicles to the presynaptic plasma membrane close to Ca2+ channels [6], Given the dogma of volume transmission, it remained unclear until recently whether dopamine release necessitates such machinery.
Work over the past two years has established that axonal dopamine release requires active zone-like release sites. First, dopamine release is rapid, it occurs within milliseconds of an action potential, and it has a high initial release probability when assessed in acute striatal brain slices [30*]. This indicates that release-ready vesicles must be tethered close to Ca2+ channels, which is a functional definition of an active zone [4,6], Notably, release probability is more variable in cultured dopamine neurons [40,41], and it is uncertain what the release probability is in an intact brain of a behaving animal. Second, dopamine axons contain active zone-like protein scaffolds with co-clustering of RIM, ELKS2, Bassoon, and likely Munc13-1 (Figure 1), fulfilling a morphological definition of an active zone-like site [30*]. This is supported by independent studies showing that Bassoon [41] and ELKS [9**] are present in striatal dopamine axons, and is further supported by a recent report that C.elegans dopaminergic neurons also contain clustered active zone proteins [42], Third, conditional knockout of RIM abolished action potential-triggered dopamine release nearly completely, and partially disrupted the active zone-like scaffold, establishing the necessity of these sites for release [30*]. Together, these findings establish that dopamine release is fast and is restricted to active zone-like sites.
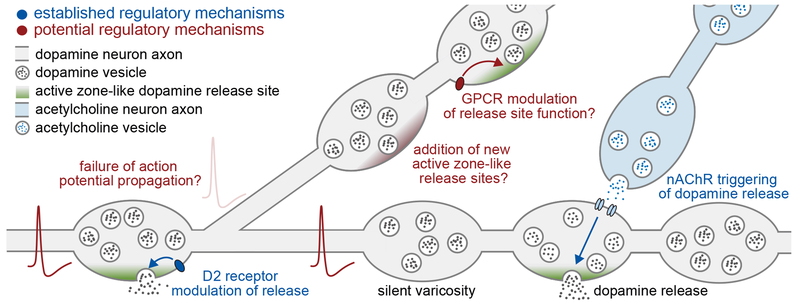
A recent study found that only ~20% of dopamine varicosities are active in response to depolarization [43*], as established with fluorescent false neurotransmitters (FFNs) that mimic dopamine uptake and release (Box 1). This finding indicates that many varicosities may be silent. The secretory architecture of dopamine axons supports this model (Figure 2), because only ~30% of dopamine varicosities contained active zone-like release sites with RIM, and RIM is essential for action potential triggered dopamine release [30*]. Given that 30% of dopamine varicosities contain postsynaptic densities [12], and some form GABAergic synaptic structures with the presence of presynaptic neurexin and postsynaptic neuroligin-2 [9**], one model that may arise is that dopamine is only released at synapses, which is further discussed in the section “Implications for dopamine signaling”.
Figure 2. Active zone-centric model for the regulation of dopamine release.
Established (blue) and potential (red) mechanisms that are involved in the control of dopamine release. Adapted in part from [30*].
These findings raise additional questions: Are dopamine neuron active zones identical to those at classical, fast synapses, or are there major differences? What is the precise molecular composition of dopamine release sites and how is Ca2+ source-sensor coupling organized? How does each molecular component of a dopamine release site participate in dopamine vesicle exocytosis? Does somatodendritic dopamine release rely on similar active zone machinery? We are only at the beginning of understanding the dopamine secretory machine. There are several hints, however, that dopamine axons and their release sites are not identical to “average” conventional axons and active zones. First, the release sites in dopamine axons are unusually sparse, different from most neurons [30*,43*]. Second, dopamine release has differential protein requirements at active zone-like sites, with RIM being more important and ELKS less important than at fast synapse [30*,44,45], Third, the active zone-like protein clusters have a different morphological appearance [30*]. Together, these observations indicate that the active zone-like structure in dopamine neurons is functionally specialized, and systematic experimentation will be necessary to provide detailed answers for these questions.
Regulation of dopamine release
Axonal dopamine release is not scaling linearly with Ca2+ signals or somatic action potential firing, two parameters that are often used as a proxy for dopamine signaling in behavioral experiments. Instead, the amount of extracellular dopamine that is sensed by receptors may be regulated at many stages including action potential propagation, dopamine production and vesicular loading, control of the number and efficiency of vesicular release sites, and regulation of dopamine reuptake. We will here focus on recent progress of key regulators of dopamine release (Figure 2): the dependence on action potential firing, striatal acetylcholine, and dopamine D2 autoreceptors. A common feature of these factors is that they strongly modulate efficiency of the dopamine vesicle fusion step, but the underlying molecular mechanisms have remained elusive.
Action potentials are a strong driving force for dopamine release. During microdialysis, reverse dialysis of tetrodotoxin into the striatum to block action potential firing reduces extracellular dopamine levels by ~70% [30*]. However, not every action potential releases the same amount of dopamine. An important example for this is short-term plasticity: due to the high initial release probability, release from dopamine neurons depresses strongly during a short action potential train, and the depression persists for tens of seconds after firing [30*,46], Given the tonic firing of dopamine neurons at 0.2-10 Hz, it is likely that dopamine neurons are in a state of depletion, and extended quiescence before action potential firing is likely positively correlated with the amount of dopamine released by an action potential.
In some cases, dopamine release is independent of somatic action potential firing. Dopamine release is powerfully triggered by activation of β2 containing nAChRs localized on dopamine axons, independent of ascending action potentials from midbrain dopamine neuron somata [31,32], Because the striatal cholinergic interneurons are firing tonically [47], they may strongly influence dopamine signaling. Another perhaps related example is reward expectation, during which striatal dopamine levels gradually increase without enhanced dopamine neuron firing [48*]. Hence, local striatal mechanisms lead to increases in extracellular dopamine independent of somatic dopamine neuron action potentials, and it will be important to identify these mechanisms and assess their roles in dopamine signaling.
It is also possible that not all somatic dopamine neuron action potentials reach the dopamine varicosities. Dopamine axons are unmyelinated and extensively arborized [49], Therefore, action potential propagation is costly as it requires activation of membrane conductances over large axonal surfaces, and modeling supports that propagation failures may be common in these complex axons [50], A recent study further showed that local opening of potassium conductances can inhibit action potential propagation through shunting [51].
Dopamine axons express Gi-coupled D2 autoreceptors that are sensitive to tonic and phasic dopamine [52,53*]. Activation of these autoreceptors inhibits dopamine synthesis, enhances dopamine uptake and regulates VMAT2 expression [3], At the soma, D2 receptors are coupled to GIRK potassium channels and their activation inhibits dopamine neuron firing [13], An interesting question is whether D2 receptor activation, and activation of other axonal GPCRs, can directly regulate components of the active zone-like dopamine release sites. Given that dopamine release requires these sites and several active zone proteins are regulated by GPCR signaling, for example via protein kinase A [6], these sites would be predestined as molecular substrates for regulation. Alternatively, or complementary to regulating individual sites, the overall number of axonal release sites could also be regulated. This may be a particularly powerful way of modulating dopamine release, because at baseline, only ~30% of dopamine vesicle clusters are associated with secretory sites [30*].
Implications for dopamine signaling
Dopamine has long been considered a volume transmitter that diffuses upon release to mediate effects in many cells over a large area, and on a slow time scale [8,16,17], Recent progress, however, has started to paint a more complex picture, with evidence for both fast and slow coding mechanisms for dopamine volume transmission [1,2], Recording and imaging studies found that dopamine codes events on the order of hundreds of milliseconds [54], and that dopamine neuron activity is regulated on similarly rapid time scales [55-58*]. These studies establish sub-second coding roles for dopamine, but it has remained unclear how the striatal organization of dopamine release and reception could account for such rapid coding functions. The firing of dopamine axons elicits dopamine release with very fast kinetics, and this release activates dopamine receptors with a signaling speed of tens of milliseconds [30*,52,53*]. An interesting working model is that action potential-driven dopamine release may preferably or exclusively happen at active zone-like sites, and most dopamine varicosities are silent because they do not contain such sites [30*,43*]. Hence, extracellular dopamine levels are highly spatially and temporally variable, with hotspots of short-lived peaks of dopamine. A better understanding of the spatial organization of dopamine receptors relative to these dopamine hot spots will be essential for understanding how the structural organization of the striatum supports the time scales of dopamine coding. Receptors could be evenly distributed on target cells, only “synaptically” clustered, or be organized in any way between these two extremes. Electron microscopic studies indeed found that ~30% of dopamine varicosities are associated with postsynaptic specializations [8,11], and it is possible that these specializations exactly align with the sparse active zone-like dopamine release sites. Dopamine neurons corelease GABA [27], and GABAergic receptors are precisely opposed to dopaminergic presynaptic structures containing ELKS2/CAST [9**]. The precise distribution of the two major dopamine receptor families (D1 Gs- and D2 Gi-GPCRs) relative to the secretory sites remains to be elucidated. Although dopamine receptors are often found extrasynaptically [9**,11,12], a recent study found that D2 receptors tagged with GFP by knockin are present in small clusters in the midbrain [59], In addition, dopamine is detected in hotspots upon release, diffusion is confined [60] and striatal D2-receptors are activated rapidly [52,53*]. It is thus possible that dopamine is only released at synaptic sites and sensed at nearby, possibly clustered receptors. A synapse-like organization with postsynaptic GABA receptors and nearby synaptic or perisynaptic dopamine receptor clusters could be one way to support rapid dopamine coding.
We have here summarized recent progress on the striatal secretory machine for dopamine and have hypothesized how this secretory machine could be embedded in local striatal release triggering and dopamine sensing mechanisms to support fast dopamine functions. Future work will need to assess these hypotheses and will rely on new tools. Recent progress in the development of microscopic methods, including fluorescent dopamine sensors [61**,62**] and superresolution microscopy will help to better assess the microarchitecture and function of striatal dopamine signaling.
Highlights.
Striatal dopamine release is mediated by vesicular exocytosis
Active zone-like sites are required for rapid and efficient dopamine secretion
Localized dopamine release generates signaling hotspots and is powerfully regulated
Some striatal dopamine release is independent of ascending action potentials
Future work should assess triggering and sensing mechanisms for fast dopamine coding
Acknowledgments
This work was supported by the National Institutes of Health (R01NS083898, R01NS103484 and R01MH113349, all to P.S.K.), by the Dean’s Initiative Award for Innovation (to P.S.K.), by a Harvard-MIT Joint Research Grant (to P.S.K.), and by a Gordon postdoctoral fellowship (to C.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References
- 1.Berke JD: What does dopamine mean? Nat. Neurosci. 2018, 21:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz W: Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci 2007, 30:259–288. [DOI] [PubMed] [Google Scholar]
- 3.Sulzer D, Cragg SJ, Rice ME: Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 2016, 6:123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaeser PS, Regehr WG: Molecular Mechanisms for Synchronous, Asynchronous, and Spontaneous Neurotransmitter Release. Annu. Rev. Physiol 2014, 76:333–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biederer T, Kaeser PS, Blanpied TA: Transcellular Nanoalignment of Synaptic Function. Neuron 2017, 96:680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Südhof TC: The Presynaptic Active Zone. Neuron 2012, 75:11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang ZP, Sudhof TC: Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol 2010, 22:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Descarries L, Watkins KC, Garcia S, Bosler O, Doucet G: Dual character, asynaptic and synaptic, of the dopamine innervation in adult rat neostriatum: A quantitative autoradiographic and immunocytochemical analysis. J. Comp. Neurol 1996, 375:167–186. [DOI] [PubMed] [Google Scholar]
- 9**.Uchigashima M, Ohtsuka T, Kobayashi K, Watanabe M: Dopamine synapse is a neuroligin-2-mediated contact between dopaminergic presynaptic and GABAergic postsynaptic structures. Proc. Natl. Acad. Sci. U. S. A 2016, 113:201514074. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examines the association of dopamine varicosities with postsynaptic specializations using immuno-electron microscopy and fluorescent imaging in striatal slices. It establishes that dopamine varicosities synapse onto GABA-ergic postsynaptic structures that contain neuroligin 2, and that ELKS2/CAST and neurexin are present in the presynapse.
- 10.Missale C, Nash SRS, Robinson SW, Jaber M, Caron MG: Dopamine receptors: from structure to function. Physiol. Rev 1998, 78:189–225. [DOI] [PubMed] [Google Scholar]
- 11.Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey Al: Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 1995, 65:709–30. [DOI] [PubMed] [Google Scholar]
- 12.Cailld I, Dumartin B, Bloch B: Ultrastructural localization of D 1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996, 730:17–31. [DOI] [PubMed] [Google Scholar]
- 13.Beckstead MJ, Grandy DK, Wickman K, Williams JT: Vesicular Dopamine Release Elicits an Inhibitory Postsynaptic Current in Midbrain Dopamine Neurons. Neuron 2004, 42:939–946. [DOI] [PubMed] [Google Scholar]
- 14.Gantz SC, Bunzow JR, Williams JT: Spontaneous Inhibitory Synaptic Currents Mediated by a G Protein-Coupled Receptor. Neuron 2013, 78:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH: Sorting of Vesicular Monoamine Transporter 2 to the Regulated Secretory Pathway Confers the Somatodendritic Exocytosis of Monoamines. Neuron 2005, 48:619–633. [DOI] [PubMed] [Google Scholar]
- 16.Agnati LF, Zoli M, Stromberg I, Fuxe K: Intercellular communication in the brain: Wiring versus volume transmission. Neuroscience 1995, 69:711–726. [DOI] [PubMed] [Google Scholar]
- 17.Rice ME, Cragg SJ: Dopamine spillover after quantal release: Rethinking dopamine transmission in the nigrostriatal pathway. Brain Res. Rev 2008, 58:303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borisovska M, Zhao Y, Tsytsyura Y, Glyvuk N, Takamori S, Matti U, Rettig J, Sudhof T, Bruns D: v-SNAREs control exocytosis of vesicles from priming to fusion. EMBO J.2004, 24:2114–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimojo M, Courchet J, Pieraut S, Torabi-Rander N, Sando R, Polleux F, Maximov A: SNAREs Controlling Vesicular Release of BDNF and Development of Callosal Axons. Cell Rep. 2015, doi: 10.1016/j.celrep.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Bospoort R, Farina M, Schmitz SK, de Jong A, de Wit H, Verhage M, Toonen RF: Munc13 controls the location and efficiency of dense-core vesicle release in neurons. J. Cell Biol 2012, 199:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkenburger BH, Barstow KL, Mintz IM: Dendrodendritic Inhibition Through Reversal of Dopamine Transport. Science. 2001, 293:2465–2470. [DOI] [PubMed] [Google Scholar]
- 22.Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM: Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J. Neurosci 1996, 16:4135–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borisovska M, Bensen AL, Chong G, Westbrook GL: Distinct Modes of Dopamine and GABA Release in a Dual Transmitter Neuron. J. Neurosci 2013, 33:1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kress GJ, Shu H-J, Yu A, Taylor A, Benz A, Harmon S, Mennerick S: Fast Phasic Release Properties of Dopamine Studied with a Channel Biosensor. J. Neurosci. 2014, 34:11792–11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staal RG, Mosharov EV, Sulzer D: Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci. 2004, 7:341–346. [DOI] [PubMed] [Google Scholar]
- 26.Fon EA, Pothos EN, Sun B-CC, Killeen N, Sulzer D, Edwards RH: Vesicular Transport Regulates Monoamine Storage and Release but Is Not Essential for Amphetamine Action. Neuron 1997, 19:1271–1283. [DOI] [PubMed] [Google Scholar]
- 27.Tritsch NX, Ding JB, Sabatini BL: Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 2012, 490:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortin GD, Desrosiers CC, Yamaguchi N, Trudeau LE: Basal somatodendritic dopamine release requires snare proteins. J. Neurochem 2006, 96:1740–1749. [DOI] [PubMed] [Google Scholar]
- 29.Bergquist F, Niazi HS, Nissbrandt H: Evidence for different exocytosis pathways in dendritic and terminal dopamine release in vivo. Brain Res. 2002, 950:245–253. [DOI] [PubMed] [Google Scholar]
- 30*.Liu C, Kershberg L, Wang J, Schneeberger S, Kaeser PS: Dopamine Secretion Is Mediated by Sparse Active Zone-like Release Sites. Cell 2018, 172:706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the molecular composition and function of active-zone like release sites in dopamine varicosities. It is demonstrated that action potential triggered dopamine release is rapid and requires the release site scaffold RIM, but not ELKS. Remarkably, active zone like sites are only present in approximately one third of the dopamine varicosities.
- 31.Zhou F-MM, Liang Y, Dani JA: Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 2001,4:1224–1229. [DOI] [PubMed] [Google Scholar]
- 32.Threlfell S, Lalic T, Platt NJ, Jennings K a., Deisseroth K, Cragg SJ: Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75:58–64. [DOI] [PubMed] [Google Scholar]
- 33.Chen BT, Moran KA, Avshalumov MV, Rice ME: Limited regulation of somatodendritic dopamine release by voltage-sensitive Ca2+ channels contrasted with strong regulation of axonal dopamine release. J. Neurochem. 2006, 96:645–655. [DOI] [PubMed] [Google Scholar]
- 34*.Brimblecombe KR, Grade CJ, Platt NJ, Cragg SJ: Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J. Physiol. 2015, 593:929–946. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examines the contribution of different voltage gated Ca2+ channels to dopamine release without the confound of cholinergic release triggering. It establishes that Cav1, 2 and 3 channels contribute to dopamine release with some regional differences between the dorsal striatum and nucleus accumbens. This is different from fast synapses, where Cav2 channels predominate.
- 35.Takahashi T, Momiyama A: Different types of calcium channels mediate central synaptic transmission. Nature 1993, 366:156–158. [DOI] [PubMed] [Google Scholar]
- 36.Kang S, Cooper G, Dunne SF, Dusel B, Luan C-H, Surmeier DJ, Silverman RB: CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat. Commun. 2012, 3:1146. [DOI] [PubMed] [Google Scholar]
- 37.Jackman SL, Turecek J, Belinsky JE, Regehr WG: The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 2016, 529:88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendez JA, Bourque MJ, Fasano C, Kortleven C, Trudeau LE: Somatodendritic dopamine release requires synaptotagmin 4 and 7 and the participation of voltage-gated calcium channels. J. Biol. Chem 2011, 286:23928–23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Kang X, Zhou L, Chai Z, Wu Q, Huang R, Xu H, Hu M, Sun X, Sun S, et al. : Synaptotagmin-11 is a critical mediator of parkin-linked neurotoxicity and Parkinson’s disease-like pathology. Nat. Commun 2018, 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan P-YY, Ryan TA: Calbindin controls release probability in ventral tegmental area dopamine neurons. Nat. Neurosci. 2012, 15:813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniel JA, Galbraith S, lacovitti L, Abdipranoto A, Vissel B: Functional Heterogeneity at Dopamine Release Sites. J. Neurosci. 2009, 29:14670–14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipton DM, Maeder Cl, Shen K: Rapid Assembly of Presynaptic Materials behind the Growth Cone in Dopaminergic Neurons Is Mediated by Precise Regulation of Axonal Transport. Cell Rep. 2018, 24:2709–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Pereira DB, Schmitz Y, Meszaros J, Merchant P, Hu G, Li S, Henke A, Lizardi-Ortiz JE, Karpowicz RJ, Morgenstern TJ, et al. : Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat. Neurosci. 2016, 19:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study develops and uses a new fluorescent false neurotransmitter, FFN200, which is taken up into vesicles via VMAT2. While most dopamine terminals exhibit depolarization induced Ca2+ entry, only ~20% of them release FFN200 in response to action potentials, indicating that many dopamine varicosities are silent.
- 44.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC: RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 2011, 144:282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Held RG, Liu C, Kaeser PS: ELKS controls the pool of readily releasable vesicles at excitatory synapses through its N-terminal coiled-coil domains. Elite 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Zhang X, Xu H, Zhou L, Jiao R, Liu W, Zhu F, Kang X, Liu B, Teng S, et al. : Temporal components of cholinergic terminal to dopaminergic terminal transmission in dorsal striatum slices of mice. J. Physiol. 2014, 592:3559–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H: Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron 2004, 43:133–43. [DOI] [PubMed] [Google Scholar]
- 48*.Mohebi A, Pettibone J, Hamid A, Wong J-M, Kennedy R, Berke J: Forebrain dopamine value signals arise independently from midbrain dopamine cell firing. bioRxiv 2018, doi: 10.1101/334060. [DOI] [Google Scholar]; This study examines dopamine levels in the nucleus accumbens and somatic dopamine neuron firing in the ventral tegmental area during reward-driven learning. Remarkably, striatal dopamine increases during motivation without an increase in somatic firing, indicating that extracellular dopamine is regulated independent of somatic firing.
- 49.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T: Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 2009, 29:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pissadaki EK, Bolam JP: The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson’s disease. Front. Comput. Neurosci. 2013, 7:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Condon M, Platt N, Roberts B, Tseu M-Y, Vietti-Michelina S, Threlfell S, Cragg SJ: Plasticity in striatal dopamine release is governed by release-independent depression and the dopamine transporter. bioRxiv 2018, doi: 10.1101/392753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marcott PF, Mamaligas AA, Ford CP: Phasic Dopamine Release Drives Rapid Activation of Striatal D2-Receptors. Neuron 2014, 84:164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Marcott PF, Gong S, Donthamsetti P, Grinnell SG, Nelson MN, Newman AH, Birnbaumer L, Martemyanov KA, Javitch JA, Ford CP: Regional Heterogeneity of D2-Receptor Signaling in the Dorsal Striatum and Nucleus Accumbens. Neuron 2018, 98:575–587. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this and a previous study [52], the authors virally express GIRK channels to characterize D2 receptor activation in medium spiny neurons. D2 receptor signaling is remarkably fast but has regional heterogeneity.
- 54.Yagishita S, Hayashi-Takagi A, Ellis-Davies GCR, Urakubo H, Ishii S, Kasai H: A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014, 345:1616–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ, Daw ND, Witten IB: Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat. Neurosci 2016, 19:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.da Silva JA, Tecuapetla F, Paixao V, Costa RM: Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 2018, 554:244–248. [DOI] [PubMed] [Google Scholar]
- 57.Howe MW, Dombeck DA: Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 2016, 535:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Menegas W, Akiti K, Amo R, Uchida N, Watabe-Uchida M: Dopamine neuronsprojecting to the posterior striatum reinforce avoidance of threatening stimuli. Nat. Neurosci 2018, 21:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigates the function of a subset of midbrain dopamine neurons that project to the tail of the striatum. The authors find that these neurons do not encode reward value, but instead are specifically involved in avoidance of threatening stimuli. Notably, information coding by these dopamine neurons exerts rapid, sub-second behavioral effects.
- 59.Robinson BG, Bunzow JR, Grimm JB, Lavis LD, Dudman JT, Brown J, Neve KA, Williams JT: Desensitized D2 autoreceptors are resistant to trafficking. Sci. Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beyene AG, Delevich K, ODonnell JTDB, Piekarski DJ, Lin WC, Thomas AW, Yang SJ, Kosillo P, Yang D, Wilbrecht L, et al. : Imaging Striatal Dopamine Release Using a Non-Genetically Encoded Near-Infrared Fluorescent Catecholamine Nanosensor. bioRxiv 2018, doi: 10.1101/356543. [DOI] [Google Scholar]
- 61**.Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong W-H, Folk RW, Broussard GJ, Liang R, Jang MJ, et al. : Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science. 2018, 360:eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, et al. : A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018, 174:481–496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]; References 61 and 62 present the generation of highly specific, genetically encoded optical sensors that detect dopamine release with high spatial and temporal resolution. It is expected that these new tools will be widely used to study dopamine secretion and function.