Abstract
Patients with inadequate anti-cancer T cell responses experience limited benefit from immune checkpoint inhibitors and other immunotherapies that require T cells. Therefore, treatments that induce de novo anti-cancer T cell immunity are needed. One strategy – referred to as in situ vaccination – is to deliver chemotherapeutic or immunostimulatory drugs into tumors to promote cancer cell death and provide a stimulatory environment for priming T cells against antigens already present in the tumor. However, achieving sufficient drug concentrations in tumors without causing dose-limiting toxicities remains a major challenge. To address this challenge, nanomedicines based on nano-sized carriers (‘nanocarriers’) of chemotherapeutics and immunostimulants are being developed to improve drug accumulation in tumors following systemic (intravenous) administration. Herein, we present the rationale for using systemically administrable nanomedicines to induce anti-cancer T cell immunity via in situ vaccination and provide an overview of synthetic nanomedicines currently used clinically. We also describe general strategies for improving nanomedicine design to increase tumor uptake, including use of micelle- and star polymer-based nanocarriers. We conclude with perspectives for how nanomedicine properties, host factors, and treatment combinations can be leveraged to maximize efficacy.
Keywords: Nanomedicine and Biomaterials, chemotherapeutic and immunostimulant, nanoparticle and microparticle, immunogenic cell death, pattern recognition receptor
1. Introduction: role of anti-cancer T cells in durable tumor regression
Immunotherapies can be highly effective for prolonging the survival of patients with advanced cancers [1, 2]. Such strategies rely on T cells to mediate tumor-specific killing through T cell receptor (TCR) recognition of antigens presented as peptide-MHC complexes on cancer cell surfaces [3]. Analysis of the TCR specificity of tumor-infiltrating lymphocytes used in adoptive cell therapies (ACT) have revealed that both CD4 and CD8 T cells recognizing a variety of tumor antigens – including self-antigens and tumor-specific antigens (“neoantigens”) – are capable of mediating complete and durable tumor regression [4, 5]. Moreover, the ability of checkpoint inhibitors (CPIs), such as anti-PD1 and anti-CTLA4, to mediate durable tumor regression has been shown to correlate with tumor mutational burden (i.e. number of neoantigens) and T cell infiltration into tumors [6, 7]. A current challenge is that patients who lack pre-existing anticancer T cells experience limited to no benefit from ACT and CPIs [8]. Therefore, strategies for inducing de novo anti-cancer T cell immunity are needed.
One promising approach is to prime anti-cancer T cell responses using vaccines delivering tumor antigens. Conventional vaccination approaches include peptide-based tumor antigens combined with immuno-stimulants (adjuvants) [9]; nucleic acids [10], bacterial vectors [11] and viral vectors [12] expressing tumor antigens; and, antigen-presenting cells (APCs) pulsed with tumor antigens [13] (Fig. 1). Conventional vaccines are attractive because T cell immunity can be focused against specific antigens that are unique to cancer cells, thereby minimizing potential off-target toxicity. However, conventional vaccines require the identification and selection of suitable antigens, which can be a labor-intensive and challenging process given that tumor antigen composition and expression varies between patients and can even vary between different metastatic lesions within the same patient [14-16]. An alternative approach, sometimes referred to as in situ vaccination, is to use certain chemotherapies, immunostimulants, or even radiation therapy, to mediate tumor killing and convert tumors from an immuno-suppressive to immuno-supportive environment that makes existing tumor antigens accessible for T cell priming [17, 18] (Fig. 1). By relying on the tumor as the source of antigens, in situ vaccination provides the advantages that antigen identification is not required and that the same therapy can be applied to any patient.
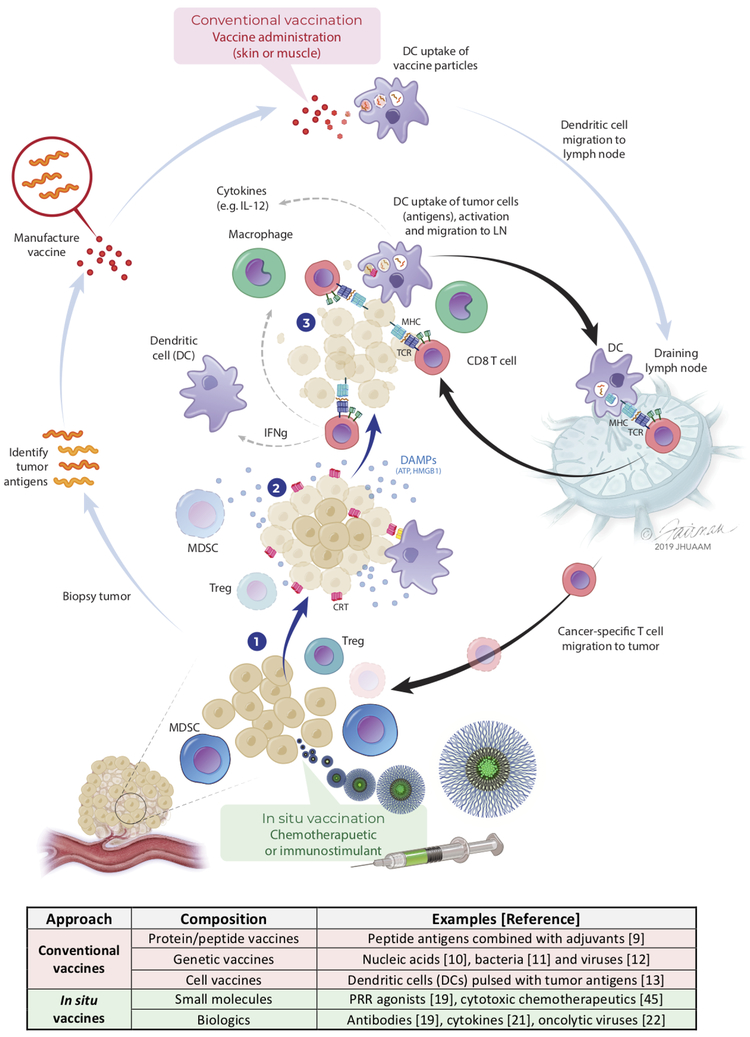
Figure 1: Conventional and in situ vaccination can be used to induce anti-cancer T cell immunity.
Conventional vaccines often use tumor antigens identified from biopsies, which are manufactured in one of several common formats (e.g., peptide/protein, nucleic acid, viral vector or dendritic cell-based) and then administered in the skin or muscle to prime anti-cancer T cells in lymph nodes. In contrast, in situ vaccination involves (1) delivery of immunostimulants or cytotoxic chemotherapeutics into tumors to (2) kill cancer cells, as well as deplete suppressor cells (MDSCs, Tregs) and/or promote immunogenic cancer cell death. Immunogenic cancer cell death is characterized by both the translocation of calreticulin (CRT) to cancer cell surfaces, which promotes their uptake by dendritic cells (DCs), as well as cancer cell release of danger associated molecular patterns (DAMPs) that recruit and activate immune cells. Activated DCs loaded with tumor antigens migrate to draining lymph nodes and prime anti-cancer T cells (3) that hone to tumors and kill cancer cells. The accompanying table summarizes different types of conventional and in situ vaccines that have been used for inducing T cell immunity.
In this review, we summarize data supporting the use of immunostimulants and chemotherapeutics for inducing anti-cancer T cell immunity via in situ vaccination and describe how formulations of such drugs in nanocarriers can be used as systemically administrable nanomedicines for enhanced safety and efficacy. We then highlight emerging nanocarrier technologies that overcome limitations of conventional approaches in the clinic. Throughout, we offer a first-hand perspective on the topics addressed based on our own experience developing and evaluating nanomedicines for inducing T cell immunity.
2. Immunostimulants and chemotherapeutics can induce T cell immunity via in situ vaccination
2.1. Agonists of pattern recognition receptors as immunostimulants for inducing anti-cancer T cell immunity
A variety of immunostimulants with diverse physical properties and mechanisms of action have shown efficacy for mediating tumor regression by in situ vaccination. These include agonists of pattern recognition receptors (PRRs) [19], antibodies (e.g., anti-CD40) [20], cytokines [21], oncolytic viruses [22] and even micron-sized bacteria (e.g., Bacillus Calmette-Guerin or BCG) [23] However, the following sections will limit discussion of immunostimulants to molecularly-defined agonists of PRRs, which are some of the most studied and – by virtue of their relatively small size – have the greatest need for formulations, e.g., nanocarriers, that can improve their uptake and retention in tumors.
PRRs are germ-line encoded receptors that function in the innate sensing of pathogens by a variety of cell types, particularly APCs [24]. Agonists of PRRs include a broad range of molecules that resemble or are derived from conserved structures of pathogens but are uncommon in humans. Among the various types of PRR agonists that have been identified, agonists of Toll-like receptor (TLR)-3 (dsRNA), TLR-4 (lipopolysaccharide and its synthetic analogs), TLR-7 (ssRNA and synthetic analogs of nucleotide bases), TLR-9 (CpG) and stimulator of interferon genes (STING) (e.g., cyclic dinucleotides (CDNs)) have been most extensively studied in the clinical setting [19] because these agonists induce type-I interferons (IFNs) needed for promoting Th1 CD4 and CD8 T cell immunity [25].
The most clinically advanced class of TLR agonists are imidazoquinoline-based TLR-7 agonists, which includes formulations of Imiquimod as a topical cream (Aldara®) used for treating cutaneous neoplasms and warts [26]. While several mechanisms have now been attributed to the anti-cancer activity of Imiquimod, the recognition that tumor regression is, in part, mediated by de novo priming of T cells against tumor antigens has provided a powerful proof-of-concept that in situ vaccination with immunostimulants is clinically relevant [27]. Agonists of TLR-3 and TLR-7 agonists have also been administered intravenously for treating disseminated cancers [28-30]; however, toxicities (i.e. flu-like symptoms) related to systemic immune activation were found to be dose-limiting. Therefore, many groups have opted to administer agonists of TLR-3 [19], TLR-7 [31, 32] and TLR-9 [33-36] by the intratumoral route to increase drug concentrations in the tumor while reducing systemic exposure.
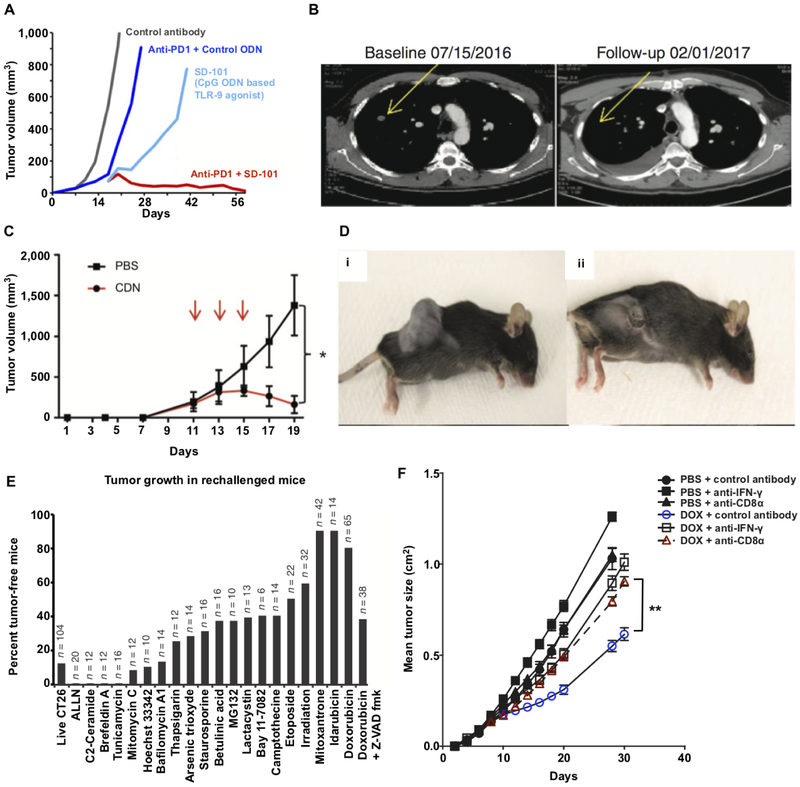
Intratumoral administration of the TLR-9 agonist, CpG, in mice [34] and humans [37] has been shown to lead to the induction of anticancer T cell immunity that is synergistic with CPIs and leads to regression of distal, non-treated tumor nodules (Fig. 2A,B). Similarly, intra-tumoral administration of the TLR-4 agonist, G100, a synthetic analog of LPS, in a stable emulsion was shown in mice and humans to promote systemic T cell immunity that correlated with regression of treated and distal nodules [38, 39].
Figure 2: In situ vaccination with immunostimulants and chemotherapeutics can promote durable tumor regression through immunological mechanisms.
(A) Mice with established CT26 tumors received either intratumoral (i.t.) injections with a CpG oligodeoxynucleotide (ODN)-based TLR-9 agonist, SD-101, i.t. SD-101 + systemic anti-PD1, i.t. control (inactive) ODN + systemic anti-PD1 or i.t. control ODN alone. Tumor growth kinetics are shown and indicate that i.t. treatment with the TLR-9 agonist (SD-101) combined with anti-PD1 leads to optimal tumor regression. (B) CT images taken from a patient with metastatic melanoma that was treated with SD-101 and anti-PD1 show regression of a non-injected tumor, suggesting that the treatment induced systemic anti-cancer T cell immunity. (C, D) Intratumoral injection of a CDN-based STING agonist leads to acute rejection of B16F10 melanoma tumors. Mice were implanted with 5×105 B16F10 cells on day 0 and then treated with either CDN or PBS for a total of three treatments (red arrows). Tumor growth kinetic (C) and representative images (D) of mice treated with PBS (i) or CDN (ii) are shown. (E) CT26 tumor cells were cultured with the indicated chemotherapeutic agents (x-axis) for 24-48 hours and then injected into the left flank of mice. Live tumor cells were injected into the right flank of the same mice 8 days later and the percentage of tumor-free mice was assessed 120 days later (note: n is the total number of mice used across multiple studies). Tumor rejection indicates that anti-cancer T cell immunity was primed by the treated cells, which suggests that the treatment promoted immunogenic cell death. (F) Mice with established MCA205 fibrosarcoma cells were treated with intratumoral doxorubicin or PBS and a subset of these mice were treated with either anti-IFN-γ or anti-CD8 antibodies to evaluate the impact of IFN-γ and CD8 T cell depletion on tumor regression by doxorubicin. Panel (A) adapted from ref. [34]; (B) adapted from ref. [37]; (C,D) adapted from ref. [41]; (E) adapted from ref. 48; and, (F) adapted from ref. 47.
STING agonists are another promising class of immunostimulants for in situ vaccination [40]. Intratumoral administration of CDN-based STING agonists has been shown to ablate large, established tumors through TNF-alpha mediated necrosis as well as T cell dependent mechanisms in mice [41] (Fig. 2C,D). These compelling data have motivated efforts to evaluate intratumoral administration of CDN-based STING agonists in the clinical setting as well as motivated a broader search for the identification of novel agonists of STING [42, 43].
2.2. Chemotherapeutics promote anti-cancer T cells by inducing immunogenic cancer cell death and/or depleting suppressor cells
Until recently, chemotherapies were believed to predominantly mediate tumor clearance through direct cytotoxic effects. However, it is increasingly recognized that tumor clearance with chemotherapies can involve multiple immune-mediated mechanisms that may act alone or in concert [44, 45].
For instance, it has been shown that chemotherapy-induced immunogenic cancer cell death is a major mechanism for driving CD8 T cell immunity associated with the efficacy of various chemotherapies [46], particularly anthracyclines [47, 48] (Fig. 2E,F). The mechanism involves translocation of calreticulin to tumor cell surfaces [48], which promotes their uptake by APCs, as well as tumor cell release of pro-inflammatory molecules (e.g., HMGB1 and ATP) that drive APC activation and migration to lymph nodes where T cell priming occurs [47] (Fig. 1). Chemotherapies can also promote tumor regression through the depletion or phenotypic conversion of different suppressor cell populations [44, 49]. Accordingly, cyclophosphamide as well as combination therapies using 5-fluorouracil have been shown to reduce the number and/or functionality of regulatory T cells (Tregs) [50, 51], while gemcitabine has been reported to deplete both Tregs and myeloid-derived suppressor cells (MDSCs) [52, 53].
2.3. Nanocarriers are needed to limit systemic drug exposure and maximize tumor accumulation
A major challenge to using immunostimulants and chemotherapeutics for in situ vaccination is that such drugs often exhibit broad biodistribution and rapid clearance from the blood following parenteral administration due to their small size and amphiphilic characteristics [54-56]. This means that high or frequent doses are needed to ensure that sufficient drug concentrations are maintained in tumors to mediate a therapeutic effect; however, drug accumulation in other tissues can quickly give rise to toxicities that are dose-limiting [30, 57].
To address this challenge, various particle-based formulations have been developed to physically restrict drug distribution as a means to limit systemic exposure (i.e. toxicity) and improve accumulation in tumors [58, 59]. While intratumoral administration of formulated drugs can be effective in certain settings [60], the intravenous route of administration may be needed for treating disseminated disease or inaccessible tumors. However, each route of administration has unique challenges and opportunities. An important consideration for the intravenous route is the size of particles comprising the drug formulation. Though micron-sized, or larger, particles may be suitable for use by local (e.g., intratumoral) routes of administration, such formulations given intravenously often have poor tumor penetration (see: section 4.2) and can form toxic emboli that occlude arteries [61]. Therefore, nano-sized macromolecular and particle carriers, referred to as nanocarriers, are needed for the delivery of immunostimulant and chemotherapeutic drugs as systemically administrable nanomedicines. This class of materials is the focus of section 3.
3. Nanocarriers for systemic administration of chemotherapeutics: status and challenges
3.1. Nanocarriers can improve drug safety and efficacy by modulating pharmacokinetics and distribution
Nanocarriers can be broadly defined as any nano-sized material, typically between 5-200 nm in diameter, that is used to transport drug molecules to a target tissue [62]. Common types of nanocarriers include vesicles based on lipids (liposomes) [63]; polymers of different architecture and hydrodynamic behavior (linear or branched random coils, polymer micelles, rigid polymer particles and polymersomes) [64]; dendrimers [65]; inorganic nanoparticles based on gold, iron oxide and silica [66]; carbon nanotubes [67]; and, recombinant proteins [68, 69] and virus-like particles [70].
Nanocarriers offer a variety of potential benefits for the delivery of chemotherapeutic and immunostimulant drugs, including increased solubility; slower degradation; reduced rates of blood clearance; and, lower systemic drug exposure [59]. However, the capacity of nanocarriers to passively accumulate in tumors largely on the basis of their size has provided the strongest impetus for their use. For instance, whereas low-molecular-weight drugs can be rapidly eliminated by the kidneys [71], nanocarriers (> 5 nm, diameter) evade renal elimination and can be designed for prolonged circulation. Longer circulation leads to greater exposure to tumor vasculature, which is characterized by slow blood flow [72] and irregular, “leaky” vessels [73, 74] that permit the extravasation of nanocarriers into tumors that lack functional lymphatic capillaries [75], thereby leading to passive nanocarrier accumulation into tumors by a process referred to as enhanced permeability and retention (EPR) [76, 77].
While a broad variety of nanocarriers have been developed for delivering drugs to tumors, only a limited number of these have been advanced to clinical trials and fewer still have been approved by regulatory authorities for human use [78]. The next section summarizes the status of the most clinically advanced synthetic nanocarriers, including liposomes, hydrophilic polymers and polymer micelles (Fig. 3), found in nanomedicines approved for clinical use. Readers are referred elsewhere (see: [78-80]) for reviews that provide a more complete summary of nanomedicines that have been approved by regulatory authorities and/or are undergoing clinical testing.
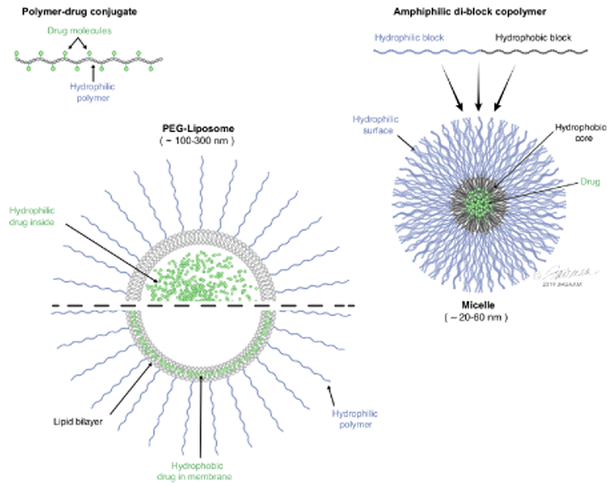
Figure 3: Nanomedicines based on polymer-drug conjugates, micelles and liposomes.
The polymer-drug conjugate represented in the cartoon schematic is a linear copolymer wherein multiple drug molecules are attached to the backbone of the polymer. The micelle particle represented in the schematic is comprised of multiple amphiphilic di-block polymers; the hydrophobic portion of the di-block polymer drives particle assembly and solubilizes hydrophobic drugs in the core of the particles, while the hydrophilic portion of the di-block polymer stabilizes the micelle. The liposome schematic shows a cross-section of a lipid bilayer vesicle coated with hydrophilic PEG chains; hydrophilic drugs can be encapsulated inside the liposomal particle, whereas hydrophobic drugs can insert into the bilayer membrane.
3.2. Nanomedicines based on liposomes, hydrophilic polymers and polymer micelles
3.2.1. Liposomes
Liposomes are lipid bilayer vesicles, which are typically hollow spheres of 100-300 nm diameter and often include a hydrophilic polymer surface coating, i.e. poly(ethylyene glycol) (PEG). The approval of a PEGylated liposomal formulation of doxorubicin (Doxil®) for the treatment of Kapsoi’s sarcoma by the FDA in 1995 was the first time a nanomedicine was approved for clinical use by a major regulatory authority [81, 82]. Since then, several liposomal formulations of chemotherapeutics have gained FDA approval, including doxorubicin (Doxil® and Myocet®), vincristine (Marqibo®), daunorubicin (DaunOxome®), cytarabine (Depocyt®), irinotecan (Onivyde®) and a co-formulation of cytaribine and irinotecan (Vyxeos®) [80, 83]. While liposomal formulations have demonstrated some benefit in altering the toxicity profile of certain chemotherapeutics, most have failed to demonstrate a significant improvement in efficacy [84, 85].
3.2.2. Hydrophilic polymers
An alternative nanocarrier approach is to covalently link multiple chemotherapeutic drug molecules to synthetic, hydrophilic polymers to yield macromolecular polymer-drug conjugates [86]. The most clinically advanced polymer-drug conjugate, referred to as paclitaxel poliglumex (Opaxio®), comprises multiple paclitaxel molecules covalently linked to poly(glutamic acid) (PGA) polymers and is approved by the FDA for treating several cancer types, including nonsmall lung cancer (NSCLC) [87, 88]. Other notable polymer-drug conjugates include platinum and anthracycline conjugates of linear N-(2-hydroxypropyl(methacrylamide)) (HPMA)-based copolymers [89, 90] as well as cyclodextrin-containing polymers conjugated to camptothecin, referred to as CRLX101[91]. Linear HPMA-based polymer-drug conjugates evaluated in the clinical setting have demonstrated lower toxicity and a higher maximum tolerated dose (MTD) compared with free drug but have failed to demonstrate an improvement in efficacy and are therefore no longer being developed [92]. Similarly, CRLX101 showed promise in preclinical animal models and early clinical testing but recently failed to demonstrate improved efficacy as compared with standard of care in renal cell cancer patients [93], though, several clinical studies evaluating the potential of CRLX101 for other indications are ongoing.
3.2.3. Polymer micelles
Another approach is to physically entrap hydrophobic chemotherapeutic drugs inside the core of micelles formed from amphiphilic polymers. The most clinically advanced micelle strategies use A-B type di-block copolymers consisting of hydrophilic PEG chains linked to hydrophobic polymers based on polyesters, e.g., poly(lactic acid) (PLA [94]), or substituted poly(amino acids), such as poly(L-glutamic acid) [95, 96]. PEG-PLA micelle formulations of paclitaxel (Genexol® PM) have been approved for several indications in South Korea and are currently undergoing clinical testing in other territories [80]. Clinical studies with current micelle approaches have demonstrated an improvement in the MTD with certain chemotherapies but have shown only modest survival benefit [94, 97].
3.2.4. Other nanocarriers used in nanomedicines
Other notable nanomedicines that are approved, or are at the late stages of clinical development, include chemotherapeutic drugs covalently attached to recombinant proteins (e.g., albumin [68] and antibodies [69]). Such strategies have demonstrated similar safety and efficacy as compared with synthetic nanomedicines but are based on recombinant technologies and are not covered herein. Finally, while a number of inorganic nanocarriers based on gold, iron oxide and silica have been evaluated extensively in the preclinical setting [66], few have entered late stages of clinical testing for chemotherapeutic and/or immunostimulant delivery. Though, the safe use of iron oxide nanoparticles in patients for other applications (i.e., treatment of anemia and as a contrast agent) suggests that further investigation of inorganic nanocarriers is warranted.
3.3. Current nanomedicines are limited by relatively low uptake into tumors
Only modest improvements in safety and efficacy have been observed with current nanomedicines compared with free, unformulated drugs, in the clinical setting [98, 99]. One observation to account for this shortcoming is that current nanomedicines typically provide no more than a 2-fold improvement in drug accumulation in tumors as compared with vital organs [100]. To characterize the full extent of this problem (i.e. low tumor accumulation), Wilhelm et al performed a meta-analysis of 117 preclinical studies and found that the median amount of nanocarrier accumulation in tumors is 0.7% of the injected dose [101]. While these observations have challenged assumptions that EPR can be relied upon to uniformly enable passive targeting of nanomedicines to tumors [98], they have also fueled efforts to better understand how nanocarrier properties impact tumor accumulation as a means to enable improved design of nanomedicines. The following sections highlight some of the results of these efforts.
4. Nanocarrier design parameters that impact nanomedicine accumulation in tumors
4.1. Clearance mechanisms and physical barriers reduce nanomedicine accumulation in tumors
Improving nanomedicines requires an understanding of the mechanisms that limit their accumulation in tumors, which include blood clearance mechanisms, physical barriers that impede extravasation and high interstitial pressures within the tumor [102-104]. Clearance mechanisms include passive elimination by the kidneys as well as active uptake of nanomedicines by cells of the reticuloendothelial system (RES) located in the liver and spleen [105-107]. Accumulation of nanomedicines into the tumor can also be blocked by physical barriers, including the occlusion of tumor blood vessels due to the physical stress of rapidly proliferating tumor cells [108]; the relatively low vascular volume [109]; and, the small porosity of basement membrane and extracellular matrix (ECM) coverage that dictate the size limit for particle extravasation into the tumor [110, 111]. Results of preclinical studies evaluating how different parameters (e.g., size, charge and shape) of nanomedicines can be modulated to reduce or slow clearance and maximize drug penetration into tumors are discussed below.
4.2. Sizes between 10-30 nm diameter enable prolonged circulation and deep tumor penetration
Hydrodynamic diameter is one of the principle factors dictating the kinetics of nanomedicine blood clearance as well as the extent of nanomedicine penetration into tumors [71, 112, 113]. Indeed, various studies have shown that particles less than ~ 5 nm diameter are rapidly eliminated from the circulation by the kidney but that tumor penetration is inversely proportional to size [114-117]. Accordingly, Popovic et al showed that ~ 10 nm particles can effectively extravasate and penetrate tumor tissue, whereas 30 nm particles showed much slower rates of extravasation and 125 nm particles were unable to extravasate [115]. These findings suggest that nanomedicines based on particles between 10-30 nm in diameter are optimal for avoiding renal clearance and maximizing tumor tissue penetration, though it should be emphasized that size is only one of several parameters that impact tumor uptake [103].
4.3. Neutral charge prevents non-specific interactions and permits deep tumor penetration
Nanomedicine surface charge is another critical parameter affecting blood half-life and tumor penetration. Nanomedicines comprising positively charged particles are rapidly cleared from the blood due to their non-specific interactions with negatively charged serum proteins, which can result in the formation of large aggregates that are efficiently phagocytized by RES cells [103]. On the other hand, positively charged nanomedicines show high accumulation in tumor vasculature, possibly due to electrostatic interactions with negatively charged proteoglycans of the basement membrane that may be exposed in tumor vessels [118, 119]. In contrast, particles with neutral and negative charge show higher blood circulation and overall less accumulation in tumors at a macroscopic level but improved extravasation as compared with cationic materials, with neutral materials limiting non-specific interactions and providing the best balance of tumor accumulation and penetration [118, 120-122]. Therefore, nanocarriers used in nanomedicines should be designed with neutral, or near-neutral charge for optimal accumulation in tumors. One means of modulating nanomedicine charge is to assemble particles through electrostatic interactions of oppositely charged materials. Indeed, several self-assembled technology platforms are being developed based on electrostatic interactions of immunostimulatory drugs [123-125], which provides a facile approach for modulating charge to achieve optimal tumor penetration.
4.4. Coating nanomedicines with hydrophilic polymers (e.g., PEG) reduces blood protein binding and clearance by RES cells
Another mechanism that can increase clearance of nanomedicines from the blood is their adsorption or binding of blood proteins (e.g., complement proteins and antibodies) that promote phagocytosis by RES cells. Approaches to modify the surfaces of nanomedicines with hydrophilic polymers, such as PEG, have been developed to reduce blood protein binding and increase circulation time [126, 127]. However, the density [128], functional group composition [129] and architecture [130] of the PEG surface coating have all been shown to have an impact on blood circulation time and remain areas of active investigation.
5. Optimizing nanomedicine design to maximize drug accumulation in tumors
5.1. Nanomedicines should be neutral and small, 10-30 nm, diameter, for optimal tumor uptake
The aforementioned studies suggest that nanomedicines should have a particle size between 10-30 nm, near neutral charge and surfaces coated with high densities of hydrophilic polymer chains to maximize drug accumulation in tumors through passive mechanisms [131] (Fig. 4). Two types of nanocarriers suitable for addressing these requirements are described below.
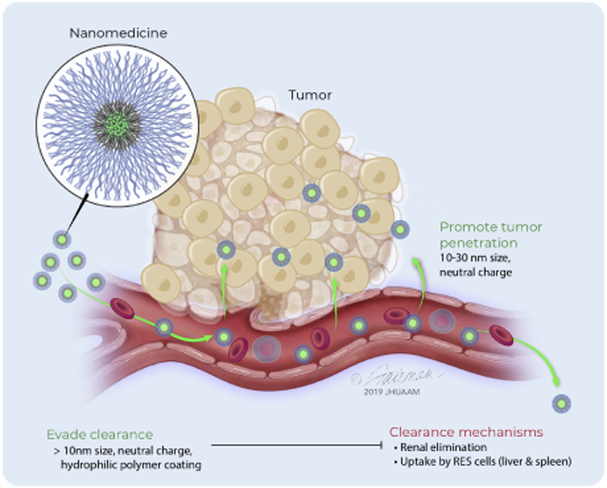
Figure 4: Nanomedicine properties that impact tumor accumulation.
Nanomedicines administered by the intravenous route must evade clearance by the kidneys and avoid capture by cells of the reticuloendothelial system (RES), principally located within the liver, spleen and lungs, to allow for prolonged circulation needed for tumor accumulation. Nanomedicines between 10-30 nm diameter with near neutral charge most efficiently extravasate and penetrate tumors.
5.2. Micelles offer high drug loading in small particles suitable for tumor targeting
In addition to micelle technologies used in nanomedicines approved for human use (see: section 3.2.3), various types of next generation systems are in preclinical and early stage clinical development. The potential advantages of micelles are that they permit high drug loading and are typically of a small, and tunable size range between 10-100 nm that is suitable for tumor accumulation [59]. Moreover, emerging technologies are systematically addressing historic challenges to using micelles. For instance, towards improving micelle stability, Lu et al recently showed that micelles with ultra-low critical micellar concentrations (10−6 mM) could be achieved using lipid amphiphiles comprising “superhydrophilic” zwitterionic polymers [132]. To further simplify micelle design and improve drug loading, Shamay et al recently showed that attachment of certain sulfated organic dyes directly to hydrophobic chemotherapeutics can result in an amphiphilic drug that self-assembles into micelles with ultra-high drug loading (up to 90%) [133]. Another way to improve drug molecule loading into the micelle is to covalently attach drug molecules directly to micelle-forming polymers through degradable linkers[134, 135]. This allows for greater control over the extent of drug loading as well as the timing and location of drug release, e.g., in response to low pH in the tumor [135]. Amphiphilic materials can also be prepared with different architectures, such as telodendrimers [136], or with stimuli-responsive properties to control the conditions under which micelle formation occurs [137]. Overall, the small size, high drug loading and chemical programmability of micelle systems makes them attractive for use in next-generation nanomedicines.
5.3. Dendrimers and star polymers can be synthesized with size and charge optimal for tumor targeting
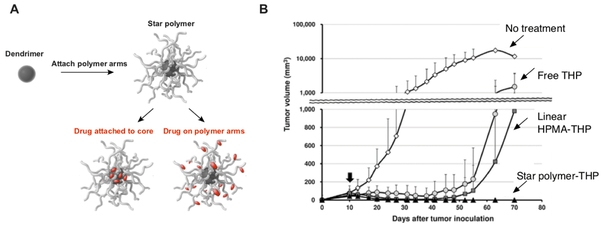
An alternative to using amphiphilic molecules that assemble into supramolecular structures is to chemically synthesize single macromolecules of a desirable size. Indeed, advances in coupling chemistry have made it possible to readily access chemically defined dendrimers with sizes > 10 nm diameter [65, 138]. While dendrimers alone have been used as nanocarriers for a variety of drug delivery applications [139, 140], further modification of dendrimers with the attachment of multiple hydrophilic polymer arms to produce “star polymers” can improve steric shielding and enable greater control over nanomedicine properties (e.g., size, drug loading, charge, etc.) that impact tumor accumulation and treatment efficacy [141] (Fig. 5A).
Figure 5: Nanomedicines based on star polymers mediate durable tumor regression.
(A) Star polymers can be prepared by the attachment of multiple hydrophilic polymer arms to dendrimer cores. Drug molecules are attached to the core and/or arms of the star polymer to produce star polymer-based nanomedicines. (B) Mice with established S-180 tumors were treated with 5 mg/kg equivalent dose of either the free anthracycline drug, THP (also referred to as Pirarubicin), THP conjugated to linear HPMA-based polymers (HPMA-THP) or THP conjugated to the arms of star polymers (star polymer-THP). Tumor volume was assessed at various timepoints thereafter. Panel (B) adapted from ref. [139].
The most clinically advanced nanomedicine based on star polymers – which is currently in phase II clinical testing – is a PEGylated poly(lysine)-based dendrimer with multiple docetaxel molecules linked to the dendrimer core [142, 143]. As an alternative to linking drug molecules to the dendrimer core, Etrych and colleagues have developed star polymers based on multivalent HPMA polymers linked to poly(amidoamine) (PAMAM) and 2,2-bis(hydroxymethyl)propionic acid (bis-MPA) dendrimers that enable attachment of multiple drug molecules to each of the polymer arms through pH-sensitive bonds designed to release drug in the acidic tumor microenvironment, thereby combining passive targeting with selectivity in terms of drug release [144, 145]. Anthracycline conjugates of the HPMA-based star polymers (~ 26 nm, diameter) showed higher tumor accumulation and improved tumor regression as compared with linear polymer-drug conjugates (~ 9 nm, diameter) (Fig. 5B) [144, 146], which suggests that star polymers may be helpful for overcoming limitations, i.e. low tumor accumulation, of earlier generation nanomedicines based on hydrophilic, linear polymer-drug conjugates [86, 92].
5.4. Nanomedicines based on micelle and star polymer carriers of anthracyclines induce durable anti-cancer T cell immunity
Importantly, many next generation nanomedicines leveraging the aforementioned advances in nanocarrier design, including HPMA-based star polymers [147], have been shown to be highly effective for inducing anti-cancer T cell immunity associated with tumor regression. For instance, Mastria et al recently showed that self-assembling nanoparticles based on peptide conjugates of doxorubicin administered intravenously improved tumor regression as compared with doxorubicin alone and that the enhanced efficacy was CD8 T cell dependent [148]. Moreover, while a variety of nanocarriers have been developed for delivering immunostimulants for use in vaccines to induce T cell immunity, many of these same systems can also be used in nanomedicines for cancer treatment via in situ vaccination [149-151]. Indeed, Kuai et al recently showed that lipid-based nanodiscs used for vaccination could also be used as chemotherapeutic nanomedicines for inducing T cell immunity that is synergistic with CPIs [152, 153]. These findings substantiate that nanomedicines optimized for tumor accumulation can be highly effective for mediating tumor clearance through the induction of durable anti-cancer T cell immunity.
6. Using host factors to improve nanomedicine accumulation into tumors and enhance T cell immunity
6.1. Leveraging the tumor microenvironment and host physiology to improve nanomedicine accumulation in tumors
A variety of strategies have been developed for improving nanocarrier accumulation into tumors through programmed responsiveness of the nanocarrier to properties that distinguish tumor tissue from normal tissue, such as pH [154], expression of certain enzymes [155] and hypoxia [156]. Targeting molecules, such as antibodies, aptamers or natural ligands of receptors that bind tumor-specific molecules (e.g., HER2) can also be used to trap materials in the tumor to improve accumulation and have shown some benefit for certain patients [157]. Nanomedicine accumulation into tumors can also be improved by altering host physiology [158]. Strategies include the reduction of nanocarrier blood clearance mechanisms (e.g., liver Kupffer cell depletion [107]) as well as the modulation of tumor blood flow and vessel leakiness [159-161]. Such strategies are orthogonal to - and therefore should be pursued in parallel with - approaches for improving the physical properties (e.g., size, charge, surface properties) of nanomedicines to improve drug accumulation in tumors.
6.2. Conditioning lymph nodes to enhance priming of memory T cells
Lymph nodes draining tumor tissue are sources of tumor antigens and can be targeted with immunostimulants to promote the priming and expansion of T cell immunity [162, 163]. As the conditions under which T cells are primed can impact the generation of memory T cells, which are provide durable anti-cancer immunity needed to prevent relapse [164, 165], it may also be beneficial to condition tumor-draining lymph nodes to modulate the quality of T cell responses primed [166]. In this regard, our lab recently showed that lymph node conditioning with microparticles carrying the immunomodulator rapamycin could alter the phenotypic quality of T cells generated against a melanoma antigen, and depending on the concentration of rapamycin, recently primed T cells could be directed towards a central memory, rather than effector, phenotype [167, 168]. These findings suggest that host conditioning of the tumor microenvironment and/or tumor draining lymph nodes [169] may be an effective strategy for eliciting high magnitude and quality (i.e. memory phenotype) anti-cancer T cell responses needed for clearing large tumors [170]. Though, additional studies will be needed to explore how best to combine systemically administered nanomedicines with host lymph node conditioning strategies.
7. Uptake of nanomedicines by phagocytic cells for the selective depletion and/or phenotypic conversion of suppressors cells
While many nanomedicines are designed to evade capture by phagocytic cells of myeloid lineage, immunotherapy is a setting where preferential targeting of myeloid lineage cells, such as MDSCs, may be beneficial. For instance, Jeanbart et al showed that pluronic-stabilized poly(propylene sulfide) (PPS) micelles carrying the cytotoxic drug 6-thioguanine could effectively deplete MDSCs in tumors as well as the periphery and that such depletion was associated with enhanced T cell mediated tumor clearance [171]. Rather than targeting their depletion, Rodell et al recently showed that a TLR-7 agonist formulated in cross-linked cyclodextrin-based nanoparticles could be used to convert tumor-associated macrophages from a suppressive M2 to an anti-tumorigenic, M1-like phenotype [172]. It is also worth noting that a major focus of the vaccine delivery field has been to develop nanocarriers capable of targeting different phagocytic populations in lymph nodes, spleen and liver, and thus lessons from this work may be useful in the design of nanomedicines for targeting phagocytic cells for cancer treatment [151, 173-178].
8. Future outlook and concluding remarks
While nanomedicines based on a broad range of chemotherapeutic and immunostimulant drugs have shown promise for mediating tumor regression through the induction of anti-cancer T cell immunity, low drug accumulation in tumors remains a significant challenge limiting the efficacy of such approaches. However, there is reason to be optimistic. Recent data suggests that doses of chemotherapeutic and immunostimulant drugs required for inducing anti-cancer T cells are lower than those required for tumor ablation [179, 180]. Indeed, Sivick et al recently showed that while high doses of a STING agonist immunostimulant ablated tumors, lower doses could be used to induce durable T cell immunity [180]. These findings–combined with the aforementioned improvements in nanomedicine design (see: section 5)–give reason to be optimistic that next generation nanomedicines will be able to reliably achieve sufficient drug concentrations in tumors needed for inducing anti-cancer T cell immunity while minimizing systemic drug exposure associated with toxicity. Optimizing the therapeutic potential of such next generation nanomedicines, though, will likely require combination with complementary therapies to unleash the full potential of T cells. Therefore, future studies should evaluate immunostimulant and/or chemotherapeutic nanomedicines alone and in combination with other therapies, particularly those that augment the action of T cells (e.g., CPIs, adenosine receptor antagonists, anti-tumor antibodies, cytokines, oncolytic viruses, etc.), to identify optimal combinations that maximize treatment efficacy. In conclusion, the tremendous promise of systemically administrable nanomedicines for improving the safety and efficacy of immunotherapies warrants vigorous development by industry and the scientific community as well as continued support of these activities by funding agencies.
Highlights.
In situ vaccination with chemotherapeutic and immunostimulant drugs can promote anticancer T cell immunity
Chemotherapeutic and immunostimulants formulated as nanomedicines can be used for systemic cancer treatment but are limited by low drug accumulation in tumors
Nanomedicines with small size, neutral charge, and hydrophilic polymer surface coatings evade blood clearance mechanisms and are optimal for tumor uptake
Next-generation micelles and star polymers offer improved capabilities for cancer therapy
Systemically administered nanomedicines offer potential to safely enhance T cell immunity
Acknowledgements:
This work was supported in part by the NIH (Award # R01EB027143), the Damon Runyon Cancer Research Foundation (Award # DRR3415), the United States Department of Veterans Affairs (Award # 1I01BX003690), the Czech Science Foundation (project 19-08176S) and the Ministry of Education, Youth and Sports of the Czech Republic within the National Sustainability Program II (project BIOCEV-FARLQ1604).
Footnotes
Competing financial interests:
GML is an employee and shareholder of Avidea Technologies, Inc., which is actively developing nanomedicines for cancer treatment. RL is a shareholder and advisor to Avidea Technologies, Inc. CMJ is an employee of the VA Maryland Health Care System. The views reported in this paper do not reflect the views of the Department of Veterans Affairs or the United States Government. CMJ has an equity position in Cellth Systems, LLC which is developing cancer diagnostic technologies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy, Science, 348 (2015) 69–74. [DOI] [PubMed] [Google Scholar]
- [2].Allison JP, Immune Checkpoint Blockade in Cancer Therapy: The 2015 Lasker-DeBakey Clinical Medical Research Award, JAMA, 314 (2015) 1113–1114. [DOI] [PubMed] [Google Scholar]
- [3].Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, Immunity, 39 (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [4].Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA, T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer, N Engl J Med, 375 (2016) 2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, Parkhurst MR, Yang JC, Rosenberg SA, Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer, Science, 344 (2014) 641–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R, Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial, Lancet, 387 (2016) 1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison T, Wang L, Ribas A, Wolchok JD, Chan TA, Genetic basis for clinical response to CTLA-4 blockade in melanoma, N Engl J Med, 371 (2014) 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM, Targeting neoantigens to augment antitumour immunity, Nat Rev Cancer, 17 (2017) 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Melief C, Peptide-Based Therapeutic Cancer Vaccines, 2018. [Google Scholar]
- [10].Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Husemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber C, Tureci O, Sahin U, Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy, Nature, 534 (2016) 396–401. [DOI] [PubMed] [Google Scholar]
- [11].Lin IY, Van TT, Smooker PM, Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery, Vaccines (Basel), 3 (2015) 940–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Draper SJ, Heeney JL, Viruses as vaccine vectors for infectious diseases and cancer, Nature reviews. Microbiology, 8 (2010) 62–73. [DOI] [PubMed] [Google Scholar]
- [13].Saxena M, Bhardwaj N, Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment, Trends Cancer, 4 (2018) 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal- Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB, Shafi S, Murugaesu N, Mitter R, Akarca AU, Linares J, Marafioti T, Henry JY, Van Allen EM, Miao D, Schilling B, Schadendorf D, Garraway LA, Makarov V, Rizvi NA, Snyder A, Hellmann MD, Merghoub T, Wolchok JD, Shukla SA, Wu CJ, Peggs KS, Chan TA, Hadrup SR, Quezada SA, Swanton C, Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade, Science, 351 (2016) 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, Gao J, Socci ND, Solit DB, Olshen AB, Schultz N, Taylor BS, Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity, Nat Biotechnol, 34 (2016) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, Gartner JJ, Zheng Z, Li YF, Ray S, Wunderlich JR, Somerville RP, Rosenberg SA, Immunogenicity of somatic mutations in human gastrointestinal cancers, Science, 350 (2015) 1387–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hammerich L, Binder A, Brody JD, In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf, Mol Oncol, 9 (2015) 1966–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S, In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment, Vaccine, 33 (2015) 7415–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Smith M, Garcia-Martinez E, Pitter MR, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L, Trial Watch: Toll-like receptor agonists in cancer immunotherapy, Oncoimmunology,7 (2018) e1526250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ, Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody, Clinical cancer research : an official journal of the American Association for Cancer Research, 17 (2011) 2270–2280. [DOI] [PubMed] [Google Scholar]
- [21].Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A, Weller M, Becher B, Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection, J Exp Med, 210 (2013) 2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Crammer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr., Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS, Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma, J Clin Oncol, 33 (2015) 2780–2788. [DOI] [PubMed] [Google Scholar]
- [23].Redelman-Sidi G, Glickman MS, Bochner BH, The mechanism of action of BCG therapy for bladder cancer--a current perspective, Nat Rev Urol, 11 (2014) 153–162. [DOI] [PubMed] [Google Scholar]
- [24].Iwasaki A, Medzhitov R, Toll-like receptor control of the adaptive immune responses, Nat Immunol, 5 (2004) 987–995. [DOI] [PubMed] [Google Scholar]
- [25].Coffman RL, Sher A, Seder RA, Vaccine adjuvants: putting innate immunity to work, Immunity, 33 (2010) 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].A.G. A, S.K. Tyring, Rosen T, Beyond a decade of 5% imiquimod topical therapy, Journal of drugs in dermatology : JDD, 8 (2009) 467–474. [PubMed] [Google Scholar]
- [27].Schon M, Schon MP, The antitumoral mode of action of imiquimod and other imidazoquinolines, Curr Med Chem, 14 (2007) 681–687. [DOI] [PubMed] [Google Scholar]
- [28].Lampkin BC, Levine AS, Levy H, Krivit W, Hammond D, Phase II trial of poly(I,C)-LC, an interferon inducer, in the treatment of children with acute leukemia and neuroblastoma: a report from the Children’s Cancer Study Group, Journal of biological response modifiers, 4 (1985) 531–537. [PubMed] [Google Scholar]
- [29].Witt PL, Ritch PS, Reding D, McAuliffe TL, Westrick L, Grossberg SE, Borden EC, Phase I trial of an oral immunomodulator and interferon inducer in cancer patients, Cancer Res, 53 (1993) 5176–5180. [PubMed] [Google Scholar]
- [30].Dummer R, Hauschild A, Becker JC, Grob JJ, Schadendorf D, Tebbs V, Skalsky J, Kaehler KC, Moosbauer S, Clark R, Meng TC, Urosevic M, An exploratory study of systemic administration of the toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma, Clinical cancer research : an official journal of the American Association for Cancer Research, 14 (2008) 856–864. [DOI] [PubMed] [Google Scholar]
- [31].Singh M, Khong H, Dai Z, Huang XF, Wargo JA, Cooper ZA, Vasilakos JP, Hwu P, Overwijk WW, Effective innate and adaptive antimelanoma immunity through localized TLR7/8 activation, J Immunol, 193 (2014) 4722–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fakhari A, Nugent S, Elvecrog J, Vasilakos J, Corcoran M, Tilahun A, Siebenaler K, Sun J, Subramony JA, Schwarz A, Thermosensitive Gel-Based Formulation for Intratumoral Delivery of Toll-Like Receptor 7/8 Dual Agonist, MEDI9197, J Pharm Sci, 106 (2017) 2037–2045. [DOI] [PubMed] [Google Scholar]
- [33].Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R, In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study, J Clin Oncol, 28 (2010) 4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, Coffman RL, Guiducci C, Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells, Proc Natl Acad Sci U S A, 113 (2016) E7240–E7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R, Eradication of spontaneous malignancy by local immunotherapy, Sci Transl Med, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kwong B, Liu H, Irvine DJ, Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy, Biomaterials, 32 (2011) 5134–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ, Barve M, Daniels GA, Wong DJ, Schmidt EV, Candia AF, Coffman RL, Leung ACF, Janssen RS, SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study, Cancer Discov, 8 (2018) 1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sagiv-Barfi I, Lu H, Hewitt J, Hsu FJ, Meulen J.t., Levy R, Intratumoral Injection of TLR4 Agonist (G100) Leads to Tumor Regression of A20 Lymphoma and Induces Abscopal Responses, Blood, 126 (2015) 820. [Google Scholar]
- [39].Bhatia S, Miller NJ, Lu H, Longino NV, Ibrani D, Shinohara MM, Byrd DR, Parvathaneni U, Kulikauskas R, Ter Meulen J, Hsu FJ, Koelle DM, Nghiem P, Intratumoral G100, a TLR4 Agonist, Induces Antitumor Immune Responses and Tumor Regression in Patients with Merkel Cell Carcinoma, Clinical cancer research : an official journal of the American Association for Cancer Research, 25 (2019) 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW Jr., Gajewski TF, Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity, Cell Rep, 11 (2015) 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Francica BJ, Ghasemzadeh A, Desbien AL, Theodros D, Sivick KE, Reiner GL, Hix Glickman L, Marciscano AE, Sharabi AB, Leong ML, McWhirter SM, Dubensky TW Jr., Pardoll DM, Drake CG, TNFalpha and Radioresistant Stromal Cells Are Essential for Therapeutic Efficacy of Cyclic Dinucleotide STING Agonists in Nonimmunogenic Tumors, Cancer Immunol Res, 6 (2018) 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, Tran JL, Moore P, Lehmann S, Eberl HC, Muelbaier M, Schneck JL, Clemens J, Adam M, Mehlmann J, Romano J, Morales A, Kang J, Leister L, Graybill TL, Charnley AK, Ye G, Nevins N, Behnia K, Wolf AI, Kasparcova V, Nurse K, Wang L, Li Y, Klein M, Hopson CB, Guss J, Bantscheff M, Bergamini G, Reilly MA, Lian Y, Duffy KJ, Adams J, Foley KP, Gough PJ, Marquis RW, Smothers J, Hoos A, Bertin J, Design of amidobenzimidazole STING receptor agonists with systemic activity, Nature, (2018). [DOI] [PubMed] [Google Scholar]
- [43].Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, Lea J, Frankel AE, Fu YX, Chen ZJ, Gao J, A STING-activating nanovaccine for cancer immunotherapy, Nat Nanotechnol, 12 (2017) 648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Menard C, Martin F, Apetoh L, Bouyer F, Ghiringhelli F, Cancer chemotherapy: not only a direct cytotoxic effect, but also an adjuvant for antitumor immunity, Cancer Immunol Immunother, 57 (2008) 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G, Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents, Cancer Cell, 28 (2015) 690–714. [DOI] [PubMed] [Google Scholar]
- [46].Wang YJ, Fletcher R, Yu J, Zhang L, Immunogenic effects of chemotherapy-induced tumor cell death, Genes Dis, 5 (2018) 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ, Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors, Cancer Res, 71 (2011) 4809–4820. [DOI] [PubMed] [Google Scholar]
- [48].Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G, Calreticulin exposure dictates the immunogenicity of cancer cell death, Nat Med, 13 (2007) 54–61. [DOI] [PubMed] [Google Scholar]
- [49].Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G, Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance, Immunity, 39 (2013) 74–88. [DOI] [PubMed] [Google Scholar]
- [50].Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H, Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide, Blood, 105 (2005) 2862–2868. [DOI] [PubMed] [Google Scholar]
- [51].Maeda K, Hazama S, Tokuno K, Kan S, Maeda Y, Watanabe Y, Kamei R, Shindo Y, Maeda N, Yoshimura K, Yoshino S, Oka M, Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity, Anticancer Res, 31 (2011) 4569–4574. [PubMed] [Google Scholar]
- [52].Eriksson E, Wenthe J, Irenaeus S, Loskog A, Ullenhag G, Gemcitabine reduces MDSCs, tregs and TGFbeta-1 while restoring the teff/treg ratio in patients with pancreatic cancer, J Transl Med, 14 (2016) 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, Utikal J, Umansky V, Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression, Front Immunol, 9 (2018) 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Newell DR, Pharmacokinetic determinants of the activity and toxicity of antitumour agents, Cancer Surv, 8 (1989) 557–603. [PubMed] [Google Scholar]
- [55].Kintzel PE, Dorr RT, Anticancer drug renal toxicity and elimination: dosing guidelines for altered renal function, Cancer Treat Rev, 21 (1995) 33–64. [DOI] [PubMed] [Google Scholar]
- [56].Harrison LI, Astry C, Kumar S, Yunis C, Pharmacokinetics of 852A, an imidazoquinoline Toll-like receptor 7-specific agonist, following intravenous, subcutaneous, and oral administrations in humans, J Clin Pharmacol, 47 (2007) 962–969. [DOI] [PubMed] [Google Scholar]
- [57].Schmitt A, Gladieff L, Laffont CM, Evrard A, Boyer JC, Lansiaux A, Bobin-Dubigeon C, Etienne-Grimaldi MC, Boisdron-Celle M, Mousseau M, Pinguet F, Floquet A, Billaud EM, Durdux C, Le Guellec C, Mazieres J, Lafont T, Ollivier F, Concordet D, Chatelut E, Factors for hematopoietic toxicity of carboplatin: refining the targeting of carboplatin systemic exposure, J Clin Oncol, 28 (2010) 4568–4574. [DOI] [PubMed] [Google Scholar]
- [58].Milling L, Zhang Y, Irvine DJ, Delivering safer immunotherapies for cancer, Adv Drug Deliv Rev, 114 (2017) 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shi J, Kantoff PW, Wooster R, Farokhzad OC, Cancer nanomedicine: progress, challenges and opportunities, Nat Rev Cancer, 17 (2017) 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Aznar MA, Tinari N, Rullan AJ, Sanchez-Paulete AR, Rodriguez-Ruiz ME, Melero I, Intratumoral Delivery of Immunotherapy-Act Locally, Think Globally, J Immunol, 198 (2017) 31–39. [DOI] [PubMed] [Google Scholar]
- [61].Poursaid A, Jensen MM, Huo E, Ghandehari H, Polymeric materials for embolic and chemoembolic applications, J Control Release, 240 (2016) 414–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nanocarriers as an emerging platform for cancer therapy, Nat Nanotechnol, 2 (2007) 751–760. [DOI] [PubMed] [Google Scholar]
- [63].Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K, Liposomes and polymersomes: a comparative review towards cell mimicking, Chem Soc Rev, 47 (2018) 8572–8610. [DOI] [PubMed] [Google Scholar]
- [64].Liechty WB, Kryscio DR, Slaughter BV, Peppas NA, Polymers for drug delivery systems, Annu Rev Chem Biomol Eng, 1 (2010) 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mignani S, Rodrigues J, Tomas H, Roy R, Shi X, Majoral JP, Bench-to-bedside translation of dendrimers: Reality or utopia? A concise analysis, Adv Drug Deliv Rev, 136–137 (2018) 73–81. [DOI] [PubMed] [Google Scholar]
- [66].Samer B, Mohamad H, Stefano P, Pietro R, Giuseppe C, Giuseppe T, Flavio R, Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic, Current Medicinal Chemistry, 25 (2018) 4269–4303. [DOI] [PubMed] [Google Scholar]
- [67].Bhattacharya K, Mukherjee SP, Gallud A, Burkert SC, Bistarelli S, Bellucci S, Bottini M, Star A, Fadeel B, Biological interactions of carbon-based nanomaterials: From coronation to degradation, Nanomedicine, 12 (2016) 333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kratz F, Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles, J Control Release, 132 (2008) 171–183. [DOI] [PubMed] [Google Scholar]
- [69].Beck A, Goetsch L, Dumontet C, Corvaia N, Strategies and challenges for the next generation of antibody-drug conjugates, Nat Rev Drug Discov, 16 (2017) 315–337. [DOI] [PubMed] [Google Scholar]
- [70].Rohovie MJ, Nagasawa M, Swartz JR, Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery, Bioeng Transl Med, 2 (2017) 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV, Renal clearance of quantum dots, Nat Biotechnol, 25 (2007) 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sevick EM, Jain RK, Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure, Cancer Res, 49 (1989) 3506–3512. [PubMed] [Google Scholar]
- [73].Folkman J, Angiogenesis in cancer, vascular, rheumatoid and other disease, Nat Med, 1 (1995) 27–31. [DOI] [PubMed] [Google Scholar]
- [74].Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK, McDonald DM, Openings between defective endothelial cells explain tumor vessel leakiness, Am J Pathol, 156 (2000) 1363–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK, Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation, Cancer Res, 60 (2000) 4324–4327. [PubMed] [Google Scholar]
- [76].Matsumura Y, Maeda H, A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs, Cancer Res, 46 (1986) 6387–6392. [PubMed] [Google Scholar]
- [77].Greish K, Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines, J Drug Target, 15 (2007) 457–464. [DOI] [PubMed] [Google Scholar]
- [78].Caster JM, Patel AN, Zhang T, Wang A, Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 9 (2017). [DOI] [PubMed] [Google Scholar]
- [79].Alphandery E, Grand-Dewyse P, Lefevre R, Mandawala C, Durand-Dubief M, Cancer therapy using nanoformulated substances: scientific, regulatory and financial aspects, Expert Rev Anticancer Ther, 15 (2015) 1233–1255. [DOI] [PubMed] [Google Scholar]
- [80].Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST, Challenges and strategies in anti-cancer nanomedicine development: An industry perspective, Adv Drug Deliv Rev, 108 (2017) 25–38. [DOI] [PubMed] [Google Scholar]
- [81].Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M, Amantea MA, Kaplan LD, Doxorubicin encapsulated in liposomes containing surface-bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS- related Kaposi’s sarcoma, J Clin Pharmacol, 36 (1996) 55–63. [DOI] [PubMed] [Google Scholar]
- [82].Barenholz Y, Doxil(R)--the first FDA-approved nano-drug: lessons learned, J Control Release, 160 (2012) 117–134. [DOI] [PubMed] [Google Scholar]
- [83].Talati C, Lancet JE, CPX-351: changing the landscape of treatment for patients with secondary acute myeloid leukemia, Future Oncol, 14 (2018) 1147–1154. [DOI] [PubMed] [Google Scholar]
- [84].O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C, C.B.C.S. Group, Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer, Ann Oncol, 15 (2004) 440–449. [DOI] [PubMed] [Google Scholar]
- [85].Seetharamu N, Kim E, Hochster H, Martin F, Muggia F, Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer, Anticancer Res, 30 (2010) 541–545. [PubMed] [Google Scholar]
- [86].Ekladious I, Colson YL, Grinstaff MW, Polymer-drug conjugate therapeutics: advances, insights and prospects, Nature Reviews Drug Discovery, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Langer CJ, O’Byrne KJ, Socinski MA, Mikhailov SM, Lesniewski-Kmak K, Smakal M, Ciuleanu TE, Orlov SV, Dediu M, Heigener D, Eisenfeld AJ, Sandalic L, Oldham FB, Singer JW, Ross HJ, Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer, J Thorac Oncol, 3 (2008) 623–630. [DOI] [PubMed] [Google Scholar]
- [88].Verschraegen CF, Skubitz K, Daud A, Kudelka AP, Rabinowitz I, Allievi C, Eisenfeld A, Singer JW, Oldham FB, A phase I and pharmacokinetic study of paclitaxel poliglumex and cisplatin in patients with advanced solid tumors, Cancer Chemother Pharmacol, 63 (2009) 903–910. [DOI] [PubMed] [Google Scholar]
- [89].Rademaker-Lakhai JM, Terret C, Howell SB, Baud CM, De Boer RF, Pluim D, Beijnen JH, Schellens JH, Droz JP, A Phase I and pharmacological study of the platinum polymer AP5280 given as an intravenous infusion once every 3 weeks in patients with solid tumors, Clinical cancer research : an official journal of the American Association for Cancer Research, 10 (2004) 3386–3395. [DOI] [PubMed] [Google Scholar]
- [90].Seymour LW, Ferry DR, Kerr DJ, Rea D, Whitlock M, Poyner R, Boivin C, Hesslewood S, Twelves C, Blackie R, Schatzlein A, Jodrell D, Bissett D, Calvert H, Lind M, Robbins A, Burtles S, Duncan R, Cassidy J, Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer, Int J Oncol, 34 (2009) 1629–1636. [DOI] [PubMed] [Google Scholar]
- [91].Svenson S, Wolfgang M, Hwang J, Ryan J, Eliasof S, Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101, J Control Release, 153 (2011) 49–55. [DOI] [PubMed] [Google Scholar]
- [92].Duncan R, Vicent MJ, Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities, Adv Drug Deliv Rev, 62 (2010) 272–282. [DOI] [PubMed] [Google Scholar]
- [93].Voss MH, Hussain A, Vogelzang N, Lee JL, Keam B, Rha SY, Vaishampayan U, Harris WB, Richey S, Randall JM, Shaffer D, Cohn A, Crowell T, Li J, Senderowicz A, Stone E, Figlin R, Motzer RJ, Haas NB, Hutson T, A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma, Ann Oncol, 28 (2017) 2754–2760. [DOI] [PubMed] [Google Scholar]
- [94].Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, Rha SY, Lee MY, Ro J, Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer, Breast Cancer Res Treat, 108 (2008) 241–250. [DOI] [PubMed] [Google Scholar]
- [95].Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, Nakatomi I, Yokoyama M, Kataoka K, Kakizoe T, NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel, Br J Cancer, 92 (2005) 1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cabral H, Nishiyama N, Kataoka K, Optimization of (1,2-diamino- cyclohexane)platinum(M)-loaded polymeric micelles directed to improved tumor targeting and enhanced antitumor activity, J Control Release, 121 (2007) 146–155. [DOI] [PubMed] [Google Scholar]
- [97].Ahn HK, Jung M, Sym SJ, Shin DB, Kang SM, Kyung SY, Park JW, Jeong SH, Cho EK, A phase II trial of Cremorphor EL-free paclitaxel (Genexol-PM) and gemcitabine in patients with advanced non-small cell lung cancer, Cancer Chemother Pharmacol, 74 (2014) 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Danhier F, To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?, J Control Release, 244 (2016) 108–121. [DOI] [PubMed] [Google Scholar]
- [99].Gammon JM, Dold NM, Jewell CM, Improving the clinical impact of biomaterials in cancer immunotherapy, Oncotarget, 7 (2016) 15421–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Nakamura Y, Mochida A, Choyke PL, Kobayashi H, Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer?, Bioconjug Chem, 27 (2016) 2225–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours, Nature Reviews Materials, 1 (2016) 16014. [Google Scholar]
- [102].Alexis F, Pridgen E, Molnar LK, Farokhzad OC, Factors affecting the clearance and biodistribution of polymeric nanoparticles, Mol Pharm, 5 (2008) 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ernsting MJ, Murakami M, Roy A, Li SD, Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles, J Control Release, 172 (2013) 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hua S, de Matos MBC, Metselaar JM, Storm G, Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization, Front Pharmacol, 9 (2018) 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tsoi KM, MacParland SA, Ma XZ, Spetzler VN, Echeverri J, Ouyang B, Fadel SM, Sykes EA, Goldaracena N, Kaths JM, Conneely JB, Alman BA, Selzner M, Ostrowski MA, Adeyi OA, Zilman A, McGilvray ID, Chan WC, Mechanism of hard-nanomaterial clearance by the liver, Nat Mater, 15 (2016) 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW, Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination, J Control Release, 240 (2016) 332–348. [DOI] [PubMed] [Google Scholar]
- [107].Tavares AJ, Poon W, Zhang YN, Dai Q, Besla R, Ding D, Ouyang B, Li A, Chen J, Zheng G, Robbins C, Chan WCW, Effect of removing Kupffer cells on nanoparticle tumor delivery, Proc Natl Acad Sci U S A, 114 (2017) E10871–E10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK, Pathology: cancer cells compress intratumour vessels, Nature, 427 (2004) 695. [DOI] [PubMed] [Google Scholar]
- [109].Harrington KJ, Rowlinson-Busza G, Syrigos KN, Abra RM, Uster PS, Peters AM, Stewart JS, Influence of tumour size on uptake of(111)ln-DTPA-labelled pegylated liposomes in a human tumour xenograft model, Br J Cancer, 83 (2000) 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Yokoi K, Kojic M, Milosevic M, Tanei T, Ferrari M, Ziemys A, Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment, Cancer Res, 74 (2014) 4239–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Miao L, Huang L, Exploring the tumor microenvironment with nanoparticles, Cancer Treat Res, 166 (2015) 193–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kobayashi H, Brechbiel MW, Nano-sized MRI contrast agents with dendrimer cores, Adv Drug Deliv Rev, 57 (2005) 2271–2286. [DOI] [PubMed] [Google Scholar]
- [113].Seymour LW, Duncan R, Strohalm J, Kopecek J, Effect of molecular weight (Mw) of N- (2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats, J Biomed Mater Res, 21 (1987) 1341–1358. [DOI] [PubMed] [Google Scholar]
- [114].Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A, Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers, J Natl Cancer Inst, 98 (2006) 335–344. [DOI] [PubMed] [Google Scholar]
- [115].Popovic Z, Liu W, Chauhan VP, Lee J, Wong C, Greytak AB, Insin N, Nocera DG, Fukumura D, Jain RK, Bawendi MG, A nanoparticle size series for in vivo fluorescence imaging, Angew Chem Int Ed Engl, 49 (2010) 8649–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K, Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size, Nat Nanotechnol, 6 (2011) 815–823. [DOI] [PubMed] [Google Scholar]
- [117].Mei L, Rao J, Liu Y, Li M, Zhang Z, He Q, Effective treatment of the primary tumor and lymph node metastasis by polymeric micelles with variable particle sizes, J Control Release, 292 (2018) 67–77. [DOI] [PubMed] [Google Scholar]
- [118].Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, Torchilin VP, Munn L, Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors, Cancer Res, 62 (2002) 6831–6836. [PubMed] [Google Scholar]
- [119].Krasnici S, Werner A, Eichhorn ME, Schmitt-Sody M, Pahernik SA, Sauer B, Schulze B, Teifel M, Michaelis U, Naujoks K, Dellian M, Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels, Int J Cancer, 105 (2003) 561–567. [DOI] [PubMed] [Google Scholar]
- [120].He C, Hu Y, Yin L, Tang C, Yin C, Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles, Biomaterials, 31 (2010) 3657–3666. [DOI] [PubMed] [Google Scholar]
- [121].Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P, Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles, PLoS One, 6 (2011) e24374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Wang H-X, Zuo Z-Q, Du J-Z, Wang Y-C, Sun R, Cao Z-T, Ye X-D, Wang J-L, Leong KW, Wang J, Surface charge critically affects tumor penetration and therapeutic efficacy of cancer nanomedicines, Nano Today, 11 (2016) 133–144. [Google Scholar]
- [123].Chiu YC, Gammon JM, Andorko JI, Tostanoski LH, Jewell CM, Modular Vaccine Design Using Carrier-Free Capsules Assembled from Polyionic Immune Signals, ACS Biomater Sci Eng, 1 (2015) 1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tostanoski LH, Jewell CM, Engineering self-assembled materials to study and direct immune function, Adv Drug Deliv Rev, 114 (2017) 60–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zeng Q, Zhang P, Zeng X, Tostanoski LH, Jewell CM, Advanced manufacturing of microdisk vaccines for uniform control of material properties and immune cell function, Biomater Sci, 6 (2017) 115–124. [DOI] [PubMed] [Google Scholar]
- [126].Akiyama Y, Mori T, Katayama Y, Niidome T, The effects of PEG grafting level and injection dose on gold nanorod biodistribution in the tumor-bearing mice, J Control Release, 139 (2009) 81–84. [DOI] [PubMed] [Google Scholar]
- [127].Ishihara T, Maeda T, Sakamoto H, Takasaki N, Shigyo M, Ishida T, Kiwada H, Mizushima Y, Mizushima T, Evasion of the accelerated blood clearance phenomenon by coating of nanoparticles with various hydrophilic polymers, Biomacromolecules, 11 (2010) 2700–2706. [DOI] [PubMed] [Google Scholar]
- [128].Walkey CD, Olsen JB, Guo H, Emili A, Chan WC, Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake, J Am Chem Soc, 134 (2012) 2139–2147. [DOI] [PubMed] [Google Scholar]
- [129].Shimizu T, Abu Lila AS, Fujita R, Awata M, Kawanishi M, Hashimoto Y, Okuhira K, Ishima Y, Ishida T, A hydroxyl PEG version of PEGylated liposomes and its impact on anti-PEG IgM induction and on the accelerated clearance of PEGylated liposomes, Eur J Pharm Biopharm, 127 (2018) 142–149. [DOI] [PubMed] [Google Scholar]
- [130].Hsu HJ, Han Y, Cheong M, Kral P, Hong S, Dendritic PEG outer shells enhance serum stability of polymeric micelles, Nanomedicine, 14 (2018) 1879–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Durymanov MO, Rosenkranz AA, Sobolev AS, Current Approaches for Improving Intratumoral Accumulation and Distribution of Nanomedicines, Theranostics, 5 (2015) 1007–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lu Y, Yue Z, Xie J, Wang W, Zhu H, Zhang E, Cao Z, Micelles with ultralow critical micelle concentration as carriers for drug delivery, Nature Biomedical Engineering, 2 (2018) 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Shamay Y, Shah J, Isik M, Mizrachi A, Leibold J, Tschaharganeh DF, Roxbury D, Budhathoki-Uprety J, Nawaly K, Sugarman JL, Baut E, Neiman MR, Dacek M, Ganesh KS, Johnson DC, Sridharan R, Chu KL, Rajasekhar VK, Lowe SW, Chodera JD, Heller DA, Quantitative self-assembly prediction yields targeted nanomedicines, Nat Mater, 17 (2018) 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Hu X, Jing X, Biodegradable amphiphilic polymer-drug conjugate micelles, Expert Opin Drug Deliv, 6 (2009) 1079–1090. [DOI] [PubMed] [Google Scholar]
- [135].Chytil P, Sirova M, Kudlacova J, Rihova B, Ulbrich K, Etrych T, Bloodstream Stability Predetermines the Antitumor Efficacy of Micellar Polymer-Doxorubicin Drug Conjugates with pH-Triggered Drug Release, Mol Pharm, 15 (2018) 3654–3663. [DOI] [PubMed] [Google Scholar]
- [136].Bharadwaj G, Nhan V, Yang S, Li X, Narayanan A, Macarenco AC, Shi Y, Yang D, Vieira LS, Xiao W, Li Y, Lam KS, Cholic acid-based novel micellar nanoplatform for delivering FDA-approved taxanes, Nanomedicine (Lond), 12 (2017) 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Laga R, Janouskova O, Ulbrich K, Pola R, Blazkova J, Filippov SK, Etrych T, Pechar M, Thermoresponsive Polymer Micelles as Potential Nanosized Cancerostatics, Biomacromolecules, 16 (2015) 2493–2505. [DOI] [PubMed] [Google Scholar]
- [138].Bugno J, Hsu HJ, Pearson RM, Noh H, Hong S, Size and Surface Charge of Engineered Poly(amidoamine) Dendrimers Modulate Tumor Accumulation and Penetration: A Model Study Using Multicellular Tumor Spheroids, Mol Pharm, 13 (2016) 2155–2163. [DOI] [PubMed] [Google Scholar]
- [139].Palmerston Mendes L, Pan J, Torchilin VP, Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy, Molecules, 22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zhang M, Zhu J, Zheng Y, Guo R, Wang S, Mignani S, Caminade AM, Majoral JP, Shi X, Doxorubicin-Conjugated PAMAM Dendrimers for pH-Responsive Drug Release and Folic Acid-Targeted Cancer Therapy, Pharmaceutics, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ren JM, McKenzie TG, Fu Q, Wong EH, Xu J, An Z, Shanmugam S, Davis TP, Boyer C, Qiao GG, Star Polymers, Chem Rev, 116 (2016) 6743–6836. [DOI] [PubMed] [Google Scholar]
- [142].Leong NJ, Mehta D, McLeod VM, Kelly BD, Pathak R, Owen DJ, Porter CJH, Kaminskas LM, Doxorubicin Conjugation and Drug Linker Chemistry Alter the Intravenous and Pulmonary Pharmacokinetics of a PEGylated Generation 4 Polylysine Dendrimer in Rats, J Pharm Sci, 107 (2018) 2509–2513. [DOI] [PubMed] [Google Scholar]
- [143].Mehta D, Leong N, McLeod VM, Kelly BD, Pathak R, Owen DJ, Porter CJH, Kaminskas LM, Reducing Dendrimer Generation and PEG Chain Length Increases Drug Release and Promotes Anticancer Activity of PEGylated Polylysine Dendrimers Conjugated with Doxorubicin via a Cathepsin-Cleavable Peptide Linker, Mol Pharm, 15 (2018) 4568–4576. [DOI] [PubMed] [Google Scholar]
- [144].Etrych T, Strohalm J, Chytil P, Rihova B, Ulbrich K, Novel star HPMA-based polymer conjugates for passive targeting to solid tumors, J Drug Target, 19 (2011) 874–889. [DOI] [PubMed] [Google Scholar]
- [145].Kostkova H, Schindler L, Kotrchova L, Kovar M, Sirova M, Kostka L, Etrych T, Star Polymer-Drug Conjugates with pH-Controlled Drug Release and Carrier Degradation, Journal of Nanomaterials, 2017 (2017) 10. [Google Scholar]
- [146].Nakamura H, Koziolova E, Etrych T, Chytil P, Fang J, Ulbrich K, Maeda H, Comparison between linear and star-like HPMA conjugated pirarubicin (THP) in pharmacokinetics and antitumor activity in tumor bearing mice, Eur J Pharm Biopharm, 90 (2015) 90–96. [DOI] [PubMed] [Google Scholar]
- [147].Rihova B, Kovar M, Immunogenicity and immunomodulatory properties of HPMA-based polymers, Adv Drug Deliv Rev, 62 (2010) 184–191. [DOI] [PubMed] [Google Scholar]
- [148].Mastria EM, Cai LY, Kan MJ, Li X, Schaal JL, Fiering S, Gunn MD, Dewhirst MW, Nair SK, Chilkoti A, Nanoparticle formulation improves doxorubicin efficacy by enhancing host antitumor immunity, J Control Release, 269 (2018) 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Yoo E, Salyer ACD, Brush MJH, Li Y, Trautman KL, Shukla NM, De Beuckelaer A, Lienenklaus S, Deswarte K, Lambrecht BN, De Geest BG, David SA, Hyaluronic Acid Conjugates of TLR7/8 Agonists for Targeted Delivery to Secondary Lymphoid Tissue, Bioconjug Chem, 29 (2018) 2741–2754. [DOI] [PubMed] [Google Scholar]
- [150].Sevimli S, Knight FC, Gilchuk P, Joyce S, Wilson JT, Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants, ACS Biomaterials Science & Engineering, 3 (2017) 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, Kumari S, Kelly RL, Kwan BH, Abraham W, Hu K, Mehta NK, Kauke MJ, Suh H, Cochran JR, Lauffenburger DA, Wittrup KD, Irvine DJ, Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses, Nat Med, 22 (2016) 1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kuai R, Yuan W, Son S, Nam J, Xu Y, Fan Y, Schwendeman A, Moon JJ, Elimination of established tumors with nanodisc-based combination chemoimmunotherapy, Sci Adv, 4 (2018) eaao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ, Designer vaccine nanodiscs for personalized cancer immunotherapy, Nat Mater, 16 (2017) 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Nakamura H, Etrych T, Chytil P, Ohkubo M, Fang J, Ulbrich K, Maeda H, Two step mechanisms of tumor selective delivery of N-(2-hydroxypropyl)methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage, J Control Release, 174 (2014) 81–87. [DOI] [PubMed] [Google Scholar]
- [155].Ruan S, Cao X, Cun X, Hu G, Zhou Y, Zhang Y, Lu L, He Q, Gao H, Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release, Biomaterials, 60 (2015) 100–110. [DOI] [PubMed] [Google Scholar]
- [156].Yang S, Wang Y, Ren Z, Chen M, Chen W, Zhang X, Stepwise pH/reduction-responsive polymeric conjugates for enhanced drug delivery to tumor, Mater Sci Eng C Mater Biol Appl, 82 (2018) 234–243. [DOI] [PubMed] [Google Scholar]
- [157].Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL, Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities, J Control Release, 277 (2018) 1–13. [DOI] [PubMed] [Google Scholar]
- [158].Stylianopoulos T, Munn LL, Jain RK, Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside, Trends Cancer, 4 (2018) 292–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Nagamitsu A, Greish K, Maeda H, Elevating blood pressure as a strategy to increase tumor-targeted delivery of macromolecular drug SMANCS: cases of advanced solid tumors, Jpn J Clin Oncol, 39 (2009) 756–766. [DOI] [PubMed] [Google Scholar]
- [160].Seki T, Fang J, Maeda H, Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application, Cancer Sci, 100 (2009) 2426–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Seki T, Carroll F, Illingworth S, Green N, Cawood R, Bachtarzi H, Subr V, Fisher KD, Seymour LW, Tumour necrosis factor-alpha increases extravasation of virus particles into tumour tissue by activating the Rho A/Rho kinase pathway, J Control Release, 156 (2011) 381–389. [DOI] [PubMed] [Google Scholar]
- [162].Fransen MF, Arens R, Melief CJ, Local targets for immune therapy to cancer: tumor draining lymph nodes and tumor microenvironment, Int J Cancer, 132 (2013) 1971–1976. [DOI] [PubMed] [Google Scholar]
- [163].Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA, Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response, Biomaterials, 35 (2014) 814–824. [DOI] [PubMed] [Google Scholar]
- [164].Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, Woo E, Friedmann PS, King EV, Thomas GJ, Sanchez-Elsner T, Vijayanand P, Ottensmeier CH, Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer, Nat Immunol, 18 (2017) 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Ribas A, Shin DS, Zaretsky J, Frederiksen J, Cornish A, Avramis E, Seja E, Kivork C, Siebert J, Kaplan-Lefko P, Wang X, Chmielowski B, Glaspy JA, Tumeh PC, Chodon T, Pe’er D, Comin-Anduix B, PD-1 Blockade Expands Intratumoral Memory T Cells, Cancer Immunol Res, 4 (2016) 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Gosselin EA, Eppler HB, Bromberg JS, Jewell CM, Designing natural and synthetic immune tissues, Nat Mater, 17 (2018) 484–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Gammon JM, Gosselin EA, Tostanoski LH, Chiu YC, Zeng X, Zeng Q, Jewell CM, Low- dose controlled release of mTOR inhibitors maintains T cell plasticity and promotes central memory T cells, J Control Release, 263 (2017) 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Andorko JI, Tostanoski LH, Solano E, Mukhamedova M, Jewell CM, Intra-lymph node injection of biodegradable polymer particles, J Vis Exp, (2014) e50984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].O’Neill NA, Eppler HB, Jewell CM, Bromberg JS, Harnessing the lymph node microenvironment, Curr Opin Organ Transplant, 23 (2018) 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, Borman ZA, Kerkar SP, Scott CD, Finkelstein SE, Rosenberg SA, Restifo NP, Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice, Clinical cancer research : an official journal of theAmerican Association for Cancer Research, 17 (2011) 5343–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Jeanbart L, Kourtis IC, van der Vlies AJ, Swartz MA, Hubbell JA, 6-Thioguanine-loaded polymeric micelles deplete myeloid-derived suppressor cells and enhance the efficacy of T cell immunotherapy in tumor-bearing mice, Cancer Immunol Immunother, 64 (2015) 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R, TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour- associated macrophages to enhance cancer immunotherapy, Nature Biomedical Engineering, 2 (2018) 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmuller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA, In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity, Nat Biotechnol, 33 (2015) 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, Ma Y, Zhang F, Tian R, Ni Q, Cheng S, Wang Z, Lu N, Yung BC, Wang Z, Lang L, Fu X, Jin A, Weiss ID, Vishwasrao H, Niu G, Shroff H, Klinman DM, Seder RA, Chen X, Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy, Nat Commun, 8 (2017) 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM, Improving Vaccine and Immunotherapy Design Using Biomaterials, Trends Immunol, 39 (2018) 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ, Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants, J Clin Invest, 125 (2015) 2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Structure-based programming of lymph-node targeting in molecular vaccines, Nature, 507 (2014) 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Lynn GM, Chytil P, Francica JR, Lagova A, Kueberuwa G, Ishizuka AS, Zaidi N, Ramirez-Valdez RA, Blobel NJ, Baharom F, Leal J, Wang AQ, Gerner MY, Etrych T, Ulbrich K, Seymour LW, Seder RA, Laga R, Impact of Polymer-TLR-7/8 Agonist (Adjuvant) Morphology on the Potency and Mechanism of CD8 T Cell Induction, Biomacromolecules, 20 (2019) 854–870. [DOI] [PubMed] [Google Scholar]
- [179].Landreneau JP, Shurin MR, Agassandian MV, Keskinov AA, Ma Y, Shurin GV, Immunological Mechanisms of Low and Ultra-Low Dose Cancer Chemotherapy, Cancer Microenviron, 8 (2015) 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Sivick KE, Desbien AL, Glickman LH, Reiner GL, Corrales L, Surh NH, Hudson TE, Vu UT, Francica BJ, Banda T, Katibah GE, Kanne DB, Leong JJ, Metchette K, Bruml JR, Ndubaku CO, McKenna JM, Feng Y, Zheng L, Bender SL, Cho CY, Leong ML, van Elsas A, Dubensky TW Jr., McWhirter SM, Magnitude of Therapeutic STING Activation Determines CD8(+) T Cell-Mediated Anti-tumor Immunity, Cell Rep, 25 (2018) 3074–3085 e3075. [DOI] [PubMed] [Google Scholar]