Abstract
Aerobic exercise (AEx) exerts antidepressant effects, although the neurobiological mechanisms underlying such effects are not well understood. Reduced brain-derived neurotrophic factor (BDNF) and elevated cortisol have been implicated in the pathophysiology of depression and appear to normalize with antidepressant treatment. Thus BDNF and cortisol may serve as biological targets for developing AEx as an antidepressant treatment.
Purpose.
This study examined the effects of AEx, of different intensities, on serum BDNF and cortisol in individuals with and without depression.
Methods.
Thirteen participants with depression (10 female; age = 27.2 ± 6.9; Montgomery-Äsberg Depression Rating Scale (MADRS) = 21.7 ± 4.7) and thirteen control participants (10 female; age 27.2 ± 7.2; MADRS = 0.5 ± 0.9) participated. Experimental visits consisted of 15 minutes of low intensity cycling (LO) at 35% heart rate reserve (HRR), high intensity cycling (HI) at 70% HRR, or sitting (CON). During each visit, blood samples were obtained at baseline, immediately post-exercise (IP), and then every fifteen minutes post-exercise for one hour (15P, 30P, 45P, 60P). Group, condition, and time differences in BDNF and cortisol were assessed.
Results.
There were no group differences in cortisol and BDNF. Secondary analysis revealed that BDNF increased in an intensity-dependent nature at IP and cortisol was significantly elevated at 15P following HI. Changes in BDNF and cortisol showed significant linear relationships with changes in HR.
Conclusion.
HI AEx can elicit acute, transient increases in BDNF and cortisol in young, healthy, and physically active, non-depressed and mild-moderately depressed individuals. This work suggests AEx has potential to significantly impact central nervous system function and the magnitude of such impact may be directly driven by exercise intensity.
Keywords: depression, mental health, neuroplasticity, neurobiological, antidepressant, cortisol
Introduction
Depression has a lifetime prevalence of 20.6% in U.S. adults and generates an economic burden of over $200 billion annually in the U.S. (1). Treatment options rely heavily on pharmacotherapy, although approximately one-third of patients will not achieve remission with multiple pharmacotherapeutic trials (2). Furthermore, pharmacotherapy is often associated with significant side effects including nausea, sleep disturbances, sexual dysfunction, reduced seizure threshold, and electrocardiographic changes (3). Thus, alternative and adjunctive anti-depressant therapies, with limited side effects, are needed to improve remission rates.
Depression is a multifaceted condition and its causes are not fully understood; thus developing novel anti-depressant therapies presents a significant challenge. Psychosocial impairments, such as difficulties with relationships, and work and home responsibilities and neurobiological changes, including alterations in neurotransmitters, neurotrophins, and hormones are all believed to contribute to depression. Aerobic exercise (AEx) has documented efficacy as a treatment for depression and evidence suggests that AEx produces anti-depressant effects when employed singularly (4) or concomitant with pharmacological (5) or other non-pharmacological interventions (6). Aerobic exercise may modulate mood through several mechanisms including increased self-efficacy, increased motivation and energy, and improved psychosocial function. Additionally, AEx may elicit anti-depressant effects via positive neurobiological adaptations. Despite the documented benefits of AEx for improving mood and reducing depressive symptoms, our understanding of the neurobiological effects of AEx underlying these benefits remains limited.
The pathophysiology of depression involves a myriad of neurobiological alterations that manifest in reduced neuroplasticity of the brain. Neuroplasticity is the ability of the brain to adapt to internal and external stimuli through the restructuring and reorganization of neuronal connections (7). Plasticity of the brain is critical for learning and memory processes and appears to be a vital aspect of successful depression treatment (8). Evidence suggests that reduced neuroplasticity in depression may be influenced by brain-derived neurotrophic factor (BDNF) (9) and cortisol (10). BDNF is a demonstrated mediator of neuroprotective and neuroplastic processes (11) and current evidence indicates that peripheral BDNF concentrations are reduced in depressed patients (9). Preclinical evidence suggests that serum BDNF is representative of central concentrations (12) and can cross the blood-brain barrier (13). Cortisol, a widely studied glucocorticoid, can also have powerful effects on the central nervous system (10). Cortisol is released via the hypothalamo-pituitary-adrenal (HPA) axis in response to acute psychological and physiological stress. A dysfunctional HPA axis has been implicated in depression and is evidenced by elevated resting salivary cortisol levels (14, 15) and a dysregulated cortisol awakening response (15).
In addition to the cross-sectional evidence linking depression with reduced BDNF and elevated cortisol, intervention studies indicate a normalization of these pathophysiological markers with treatment (16-18). This suggests the possibility that circulating BDNF and cortisol might serve as biomarkers for treatment response and candidate targets for novel antidepressant treatments. The responses of BDNF and cortisol to aerobic exercise training interventions have been examined, yet limited and conflicting evidence exists demonstrating the efficacy of AEx to modulate circulating BDNF and cortisol in individuals with depression (19-21). While methodological differences, such as comparing assessments in plasma versus serum and storage and handling procedures, may have contributed to inconsistent results (16) these discrepancies may also result from variations in exercise prescription (i.e. frequency, intensity, duration and mode) in previous longitudinal studies. Thus, examining the acute response to AEx may serve as a useful model to begin to gain a better understanding of the capacity of AEx to modulate circulating BDNF and cortisol as well as differences in response of these biomarkers between those with and without and depression. Acute increases in plasma BDNF have been shown in depressed individuals in response to a single session of maximal AEx (22). Additionally, acute increases in serum BDNF have been shown in healthy and depressed individuals in response to submaximal exercise although there does not appear to be a linear relationship between exercise intensity and serum BDNF response (23-25). Further, whether inducing a stress response (measured by cortisol response) via exercise, impacts BDNF response in individuals with depression is unknown. To address these gaps in knowledge, the present study examined the neurobiological effects of different intensities of AEx in individuals with and without depression. We hypothesized that: 1) compared to non-depressed control participants, depressed participants would exhibit significantly reduced BDNF; and 2) compared to non-depressed control participants, depressed participants would exhibit significantly elevated cortisol. Furthermore, we expected a dose-response relationship such that BDNF and cortisol levels would increase with exercise intensity.
Methods
Participants
Twenty-eight individuals, fourteen with depression (DEP) and fourteen age and sex matched healthy controls (HC) participated in this study. After a brief phone screen for study eligibility, potential participants were scheduled for an in-person visit for further evaluation for eligibility and, if appropriate, obtain informed consent. All participants were assessed with the Mini International Neuropsychiatric Interview 5.0 (M.I.N.I.) (26), and the severity of depressive symptomology was assessed with the Montgomery-Äsberg Depression Rating Scale (MADRS) structured interview guide (27). The MADRS structured interview guide was selected to ensure that depression severity was assessed with a standardized battery of questions and to limit the potential of misinterpreting responses from a set of unstructured questions. Furthermore, the MADRS is widely used in depression literature allowing for comparison to other trials. Inclusion in the DEP group was restricted to individuals meeting M.I.N.I. criteria for a major depressive episode and/or who had a MADRS score > 10. To control for the effect of medication dosage titration on BDNF and cortisol participants taking anti-depressant medication(s) were permitted to participate if current medication dosage had been stable for a minimum of four weeks. Inclusion in the HC group was restricted to individuals with a MADRS scores ≤ 6, no self-reported prior history of depression or other neuropsychiatric conditions (confirmed with the M.I.N.I.), and no current, self-reported use of psychoactive drugs or medications. Further inclusion criteria were: 1) ages 18-50; and 2) physically able and safe to exercise (as assessed by Physical Activity Readiness Questionnaire). Exclusion criteria were: 1) self-reported primary diagnosis of another Axis 1 disorder; 2) self-reported secondary diagnosis of a psychotic disorder, cognitive disorder, substance-related disorder, or obsessive compulsive disorder; 3) current smoker; 4) history of seizures; 5) other diagnosed neurological or musculoskeletal disorder/injury, uncontrolled cardiovascular or metabolic disease, electronic or metal implants; 6) resting blood pressure ≥ 200 mmHg systolic or 100 mmHg diastolic. Participants were recruited via flyers, online and email advertisements, and through an anonymous, online research recruitment tool (www.researchmatch.org).
Procedures
All procedures were approved by the Medical University of South Carolina (MUSC) Institutional Review Board and were conducted in accordance with the Declaration of Helsinki. Prior to performing any experimental procedures, all participants provided written informed consent. Participants attended a total of three experimental sessions. All study visits were scheduled between the hours of 12:00 and 18:00, and each participant’s experimental visit time was scheduled such that baseline assessment occurred within a 90-minute window. The average time of baseline draw was 14:49 (SD: 0:56, Range: 13:23-16:47) and 14:25 (SD: 1:24, Range: 12:1 - 17:37) for the HC and DEP group, respectively. The purpose of conducting experimental visits in the afternoon and standardizing the baseline assessment time was to control for the potential impact of diurnal variations on BDNF and cortisol assessments. All participants were instructed to maintain their normal diet and activity habits while enrolled in the study, however participants were asked to refrain from alcohol use the night before and from exercise the morning of each visit. Time between experimental visits ranged from 1 to 35 days. On average, the HC and DEP groups completed experimental visits in 17 (Range: 3-55) and 19 (Range 3-67) days, respectively.
Aerobic Exercise
Participants completed the prescribed exercise bouts on a stationary cycle ergometer (Ergomedic 839E, Monark; Sweden). The three experimental conditions consisted of 15 minutes of low intensity cycling (LO), high intensity cycling (HI), or sitting on the cycle ergometer (CON). Prior to each session, each participant was fit with a heart rate monitor (Polar, USA) and resting blood pressure was assessed. During each session, heart rate was monitored continuously, and blood pressure and rate of perceived exertion (RPE) were assessed at baseline, every 5 minutes during exercise, immediately post-exercise, and then 5 minutes post-exercise. Exercise intensity was determined using the Karvonen equation. During low intensity exercise, participants cycled to achieve a heart rate of 35% of heart rate reserve (HRR) and during high intensity exercise participants cycled to achieve a heart rate of 70% HRR. Participants maintained pedaling at their preferred cadence as the cycle ergometer automatically adjusted the workload in order to maintain the target heart rate (THR) for the session. All participants were able to achieve their target heart within the first five minutes of the exercise bout.
Participants were not informed of the order of their experimental conditions and were told about their THR following baseline blood specimen collection and prior to beginning the exercise condition. The order of exercise conditions was randomized so that each permutation occurred only once for every six participants in each group.
Blood Specimen Collection and Analysis
After baseline assessments of HR, RPE, and blood pressure, a study nurse placed an intravenous catheter in a superficial forearm vein. The catheter tubing was maintained patent with an isotonic saline solution and was drained prior to obtaining blood specimen samples. Following placement of the intravenous catheter, participants remained seated quietly until 30 minutes had elapsed from the time of their arrival to the laboratory. During each experimental session (HI, LO and CON), blood samples were obtained at baseline (PRE), immediately post-exercise (IP), and then every fifteen minutes post-exercise for one hour (15P, 30P, 45P, 60P). Immediate post-exercise (IP) blood samples were taken within one minute of exercise cessation. Following exercise (or seated control), subjects remained seated on the cycle ergometer for the IP- blood sample. Subjects were then seated in a chair for the duration of the experimental session and all subsequent blood samples. Blood samples were collected into 5.0 mL serum-separating tubes (SST) containing gel and clot activator (Becton Dickinson Vacutainer SST, Franklin Lakes, NJ). The South Carolina Clinical and Translational Research Institute (SCTR) provided a mobile nurse to perform blood specimen collection and storage during each experimental session.
Blood samples were allowed to clot upright at room temperature (RT) for at least 30 minutes and were then centrifuged at RT for 15 min at 1500xg. The serum for each time point was then divided equally (~500 ul each) using a transfer pipet into four labeled 2.0 ml screw-cap microcentrifuge tubes (two per assay) and stored at −80°C until testing. On the day of testing, samples were removed from −80°C and allowed to come to RT for at least 1.5 hours on the bench top as per the assay protocol. After plating, the samples were then moved to a −20°C freezer until they were disposed of after 30 days. Enzyme-linked immunosorbent assays were performed to determine serum BDNF (Quantikine, R&D Systems, Minneapolis, MN) and serum cortisol (IBL International, Hamburg, Germany) concentrations according to manufacturer guidelines. All samples were run in duplicate and if the coefficient of variation exceeded 15% samples were assayed a second time. Each sample value represents the mean of the samples assayed.
Data Analysis
Statistical analyses
Data were assessed for normality, skewness, and kurtosis. Pearson product-moment and Spearman rank-order correlations were performed on baseline cortisol, BDNF, and MADRS scores for the DEP group to examine relationships between depression severity and baseline biomarker values. Group differences on demographic and baseline blood biomarker measures were assessed using Student t-tests or Wilcoxon rank-sum tests based on distributional assessment (SAS v9.4, Cary, NC). The effects of group, exercise condition, and time on serum BDNF and cortisol were examined using mixed linear models. Models were initially constructed with serum BDNF or cortisol as the dependent variable and time nested within condition as the independent variables. Time was nested within condition as the effect of time was expected to be primarily driven by exercise condition. Multiple random intercepts, slopes, and covariance structures were examined and the model the yielded the lowest Akaike information criterion (AIC) was selected for modeling with fixed effects. The purpose of this initial model construction was to account for the non-independence of repeated measures within and across multiple experimental conditions. Following initial model construction, the group variable (i.e. HC vs. DEP) was added as a covariate to examine the main effect of depression on serum BDNF and cortisol response. Finally, to control for demographic covariates, Body Mass Index (BMI), medication status, age, and gender were added to the model. Main effects and interactions of covariates were examined and Bonferroni adjustments were performed for pairwise comparisons to control for Type 1 error. Significance level was set a priori at α < .05 for all statistical tests.
Secondary Data Analysis
In the absence of significant between group differences, secondary within-subjects data analyses were performed. The purpose of this analysis was to determine if BDNF and cortisol response differences existed between exercise conditions; additionally, potential relationships between changes in HR, BDNF, and cortisol were assessed across groups. A one-way analysis of covariance (ANCOVA) was performed to examine the effect of condition on changes in BDNF and cortisol while controlling for group. To examine the extent to which exercise intensity explained changes in BDNF and cortisol, a simple linear regression analysis was conducted using mean change in HR as the predictor variable and changes in BDNF and cortisol as the outcome variables, respectively. Additionally, a correlational analysis was performed between changes in BDNF and cortisol to assess the relationship between HPA-axis activation and BDNF production.
Results
All fourteen participants in the HC group were able to provide samples for all conditions (n=14). During data inspection it was found that serum BDNF values from multiple samples of one HC participant exceeded outlier values that had been previously published in healthy controls (28). Additionally, one participant from the DEP group had samples that were incorrectly labeled during processing. Since these data were not representative of this sample or contained processing errors, data from these participants were removed prior to statistical analysis. Thus, the final sample analysis included a total of thirteen participants per group (Table 1). Due to difficulties with intravenous catheter placement, the DEP group was not able to provide samples all three conditions however each DEP participant was able to provide a blood sample for at least one experimental condition. All subjects in the HC group were able to provide blood sample for all conditions.
Table 1.
Sample demographic characteristics.
| Variable | Healthy Control (HC) n = 13 |
Depression (DEP) n = 13 |
p value |
|---|---|---|---|
| Sex (Female, n) | 10 | 10 | - |
| Age (years) | 27.2 ± 7.2 | 27.2 ± 6.9 | 0.83 |
| BMI (kg/m2) | 22.3 ± 1.9 | 25.3 ± 5.5 | < 0.05# |
| MADRS | 0.5 ± 0.9 | 21.7 ± 4.7 | < 0.001# |
| IPAQ (MET*min*week−1) | 6615 ± 5805 | 7353 ± 7504 | 0.56 |
| Mod/Vig. Activity (MET*min*week−1) | 1538 ± 1122 | 1118 ± 1529 | < 0.05# |
| Taking medication (n) | 0 | 9 | - |
| Resting HR (BPM) | 64.3 ± 10.3 | 73.0 ± 11.4 | < 0.001# |
| Resting Systolic BP (mmHg) | 106.8 ± 10.9 | 112.6 ± 11.1 | 0.23 |
| Resting Diastolic BP (mmHg) | 64.3 ± 6.5 | 70.1 ± 7.4 | 0.06 |
| Resting serum BDNF (ng/mL) | 29.2 ± 6.8 | 31.2 ± 7.2 | 0.23 |
| Resting serum cortisol (ng/mL) | 91.1 ± 60.4 | 91.8 ± 30.0 | 0.12 |
Values are presented as mean ± standard deviation
Significance determined by Wilcoxon rank-sum test
Mean MADRS scores were significantly different between the HC (0.5 ± 0.9) and DEP (21.7 ± 4.7) groups. The HC group had no reported history of depression or any other neuropsychiatric or neurological condition. All individuals in the HC group were non-smokers and medication free. Eleven of the DEP group reported a medical diagnosis of depression, with the remaining two meeting criteria for current major depressive episode on the M.I.N.I. The average self-reported length of current depressive episode for the DEP group was 8.2 ± 6.1 months with a range of 1 to 24 months. Nine DEP participants reported a stable dose (no changes within the previous four weeks) of at least one psychotropic medication and four DEP participants reported no current psychotropic medication usage. Psychotropic medications taken by participants included: fluoxetine (n=3), escitalopram (n=1), sertraline (n=1), vilazodone (n=1), duloxetine (n=3), alprazolam (n=1), memantine (n=1), quetiapine (n=1), mirtazapine (n=1), topiramate (n=1), rizatriptan (n=1), and trazadone (n=1).
Demographic and baseline biomarker results
Healthy control participants were sex and age (± 2 years) matched with DEP participants (Table 1). Between group differences (HC vs. DEP) at baseline included MADRS scores, BMI, weekly moderate-to-vigorous physical activity, and resting HR (Table 1). There were no other significant between group differences in demographic factors. Mean resting blood pressure was not significantly different between groups and no subjects reported having hypertension or taking antihypertensive medication. Mean baseline serum BDNF and cortisol values were not significantly different between groups.
Relationship of depression severity and baseline biomarkers
Spearman rank-order correlations indicated a significant moderate, positive correlation between MADRS and baseline cortisol (rs(32) = 0.65, p < .001) and no correlation between MADRS and baseline BDNF (rs(32) = −0.26, p = .15). There was no correlation between baseline cortisol and baseline BDNF (rs(32) = 0.09, p = .61).
Heart rate and RPE response to aerobic exercise
As a proof of concept check, between-condition differences in HR and RPE were examined. There was a main effect of condition on mean HR (F(2,70) = 538.27 p < .001) and mean RPE (F(2,70) = 156.34 p < .001). Post-hoc comparisons revealed all conditions were significantly different from one another for these outcomes (all p < .001; Table 2). There was a main effect of group on mean HR (F(1,70) = 13.12 p <. 001) but there was no main effect of group on mean RPE (F(1,70) = 0.28 p = 0.60).
Table 2.
Heart rate and rate of perceived exertion response to AEx
| Variable and Experimental Condition |
Healthy Control (HC) n = 13 |
Depression (DEP) n = 13 |
|---|---|---|
| Mean HR – CON (BPM)* | 69.5 ± 11.7 | 79.4 ± 10.6 |
| Mean RPE – CON^ | 6.3 ± 0.6 | 6.3 ± 0.7 |
| Mean HR – LO (BPM)* | 107.4 ± 8.2 | 115.4 ± 6.4 |
| Mean RPE – LO^ | 9.8 ± 2.0 | 10.4 ± 1.7 |
| Mean HR – HI (BPM)* | 153.8 ± 9.1 | 157.9 ± 4.3 |
| Mean RPE – HI^ | 14.2 ± 1.8 | 14.2 ± 2.0 |
Values are presented as mean ± standard deviation
Mean HR significantly different between conditions
Mean RPE significantly different between conditions
Effect of depression, exercise, and time on serum BDNF and cortisol
Effects on BDNF.
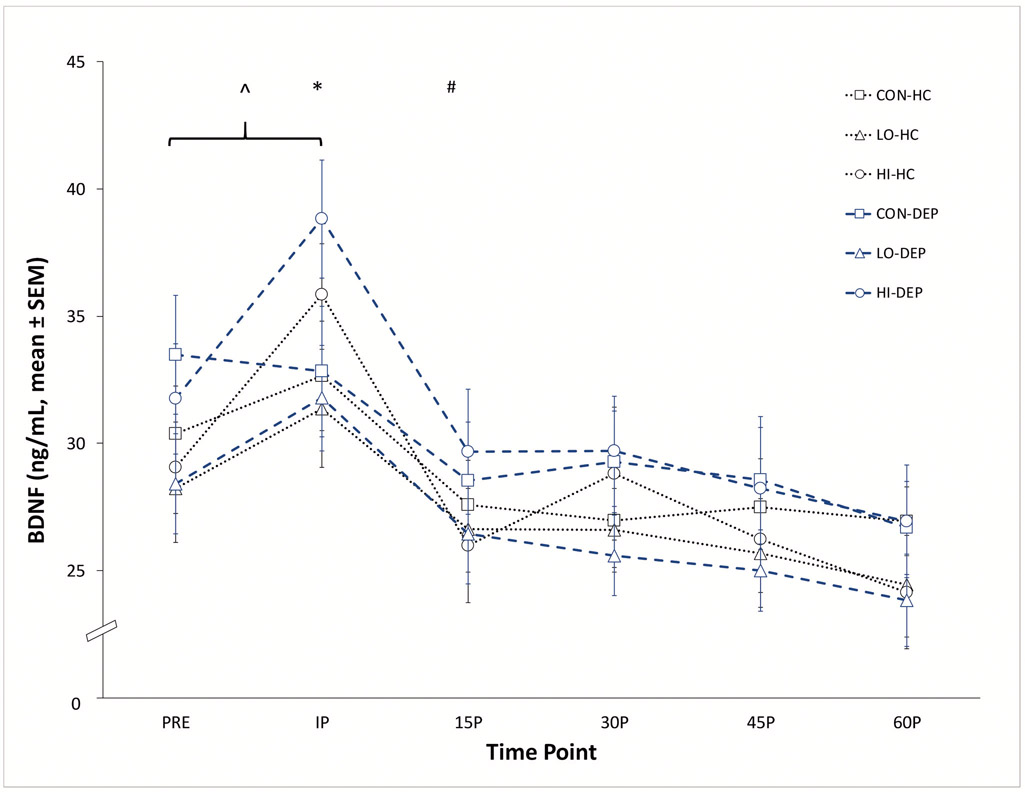
Serum BDNF data from all three experimental conditions were assessed to examine the effect of group, experimental condition and time on post-exercise BDNF response (Figure 1). Covariates BMI, age, medication status, and sex were included in the model. There was no main effect of group (F(1,20) = 0.12 p = 0.73) or any covariate on BDNF. There was a significant interaction between condition and time (F(17,377) = 18.77 p < .001). At PRE there was no significant difference in BDNF between conditions (F(2,377) = 1.87 p = 0.15). Bonferroni adjustments for multiple comparisons revealed that serum BDNF was significantly greater at IP in the HI compared to the LO (t(377) = −3.35, p = .003) and CON (t(377) = −2.59, p = .027) conditions, respectively. There was no significant difference in serum BDNF between the CON and LO conditions (t(377) = 0.41, p = 1.0) at the IP time point. BDNF at IP in the HI (t(377) = −8.04, p <.001) and LO (t(377) = −3.89, p = .019) conditions was significantly greater than the respective PRE values suggesting a significant increase from baseline in both exercise conditions. The effects of AEx were transient as BDNF at the 15P time point in the HI and LO conditions were not significantly different than PRE and there were no other between condition differences at any other time point after exercise (all adjusted p values > .05).
Figure 1.
Time course of absolute changes in serum BDNF in depressed (DEP) and non-depressed (HC) participants.
^ At IP, BDNF significantly greater than PRE in LO (p = .019) and HI (p < .001) conditions.
* BDNF significantly greater in HI condition compared to LO (p = .003) and CON (p = .027) conditions at IP.
# At 15P, BDNF not significantly different than PRE in LO and HI conditions (both p > .05).
Effects on cortisol.
Serum cortisol data from all three experimental conditions were assessed to examine the effect of group, experimental condition and time on post-exercise cortisol response (see Figure, Supplemental Digital Content 1, absolute changes in serum cortisol). Covariates BMI, age, medication status, and sex were included in the model. There was no main effect of group (F(1,20) = 1.42 p = 0.25) or any covariate on serum cortisol. There was a significant interaction between condition and time (F(17, 378) = 5.25 p < .001). At PRE there was no significant difference in cortisol between conditions (F(2,378) = 2.51 p = 0.08). Bonferroni adjustments for multiple comparisons revealed several significant interactions of time and condition on cortisol. At IP, 15P, 30P, 45P cortisol was significantly lower in the LO condition compared to the CON condition (t(378) = 2.91, p = .011; t(378) = 2.82, p = 0.02; t(378) = 3.08, p = .007; t(378) = 2.81, p = 0.02), respectively. In contrast, cortisol concentrations were higher in the HI condition compared to CON condition during the first 30 minutes of recovery (15P t(378) = −2.46, p = 0.04; 30P t(378) = −2.60, p = 0.03). In addition, cortisol was higher in the HI condition compared to the LO condition for the final 45 minutes of recovery (15P t(378) = −4.99, p < 0.001; 30P t(378) = −5.37, p < .001; 45P t(378) = −5.23, p < .001; 60P t(378) = −4.00, p = .012). In the HI condition, cortisol was not significantly different at 60P compared to PRE (t(378) = −2.60, p = 1.0) suggesting that cortisol returned to baseline levels within one hour post-exercise.
Secondary data analysis results.
Primary data analysis revealed no significant between group differences in BDNF and cortisol. Data were subsequently examined for differences between conditions amongst the entire sample. For this analysis, change scores were calculated from PRE to IP for BDNF and PRE to 15P for cortisol. Change scores at the IP and 15P were selected based on the significant between condition differences observed at these time points in the primary data analysis.
Effect of exercise condition on BDNF and cortisol.
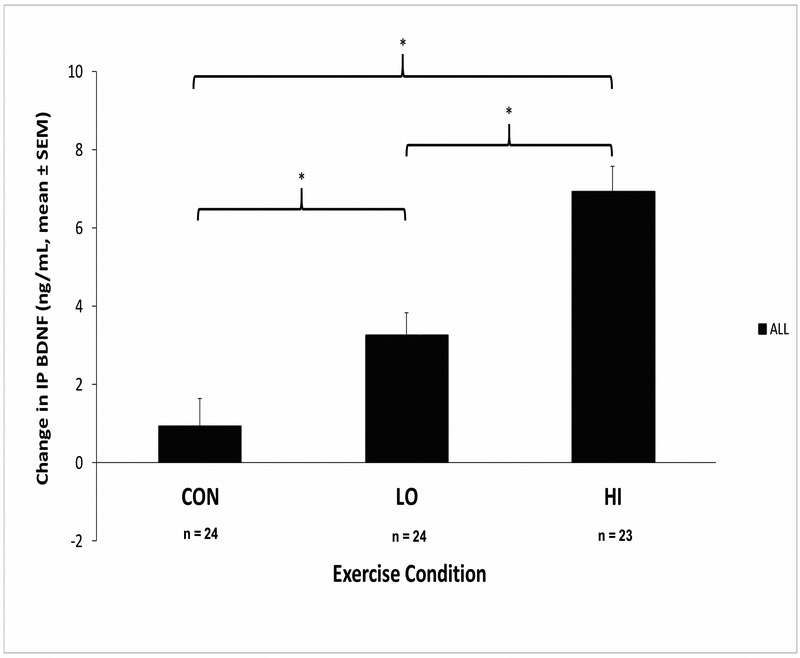
There was a significant effect of condition on the change in BDNF from PRE to IP (F(2,67) = 21.30 p < .001) while controlling for group (Figure 2). Mean BDNF changes and 95% confidence intervals in response to HI, LO, and CON conditions were 6.91 ng/mL (95%CI = 5.54 - 8.29), 3.24 ng/mL (95%CI = 2.04 - 4.44), and 0.94 ng/mL (95%CI = −0.50 – 2.38), respectively. Bonferroni adjustments revealed that change in BDNF in the HI condition was significantly greater compared to the LO (t(67) = −3.97, p < .001) and CON conditions (t(67) = −6.48, p < .001). Additionally, the LO condition elicited a significantly greater BDNF response than the CON condition (t(67) = −2.54, p = .04).
Figure 2.
Effect of condition on change in IP BDNF (HC and DEP groups combined)
* Significant between condition differences
There was also a significant effect of condition on the change in cortisol at 15P (F(2,67) = 17.01, p < .001) while controlling for group. Mean cortisol changes and 95% confidence intervals in response to HI, LO, and CON conditions were 48.0 ng/mL (95%CI = 25.4 – 70.5), −4.67 ng/mL (95%CI = −11.7 - 2.34), and −2.50 ng/mL (95%CI = −13.4 – 8.40), respectively. The HI condition elicited a significantly greater cortisol response compared to the LO (t(67) = −5.17, p < .001) and CON conditions (t(67) = −4.96, p < .001). There was no significant difference in cortisol between the LO and CON conditions (t(67) = .22, p = 1.0). Group had no significant effect on change in IP BDNF (F(1,67) = 1.58 p = .22) and 15P cortisol (F(1,67) = .04 p = .84).
Relationship of change in HR with change in BDNF and cortisol.
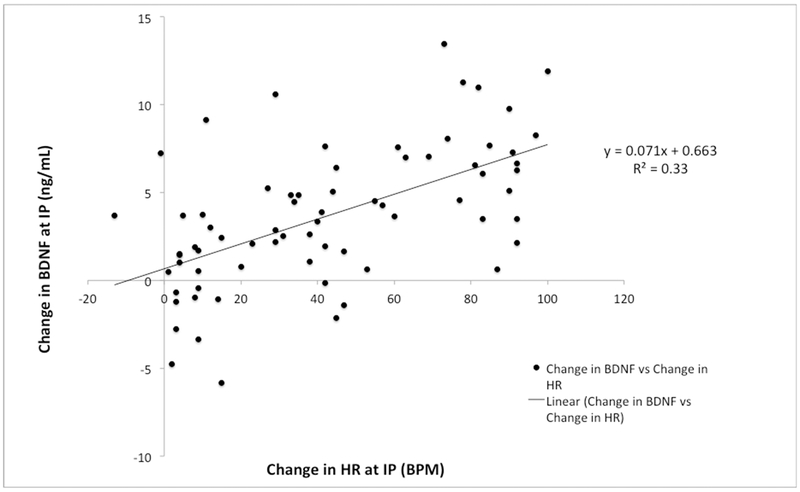
A simple linear regression was performed to examine change in post-exercise BDNF and change in HR. A significant relationship was found (F(1,69) = 34.1, p < .001, R2 = .33), with change in BDNF equal to .663 + .071 (BPM) ng/mL (Figure 3). Thus, at the IP time point, BDNF increased .71 ng/mL for every increase of 10BPM in HR from baseline. Similarly, we also examined change in cortisol based on HR response. A significant relationship was found (F(1,69) = 25.9, p < .001, R2 = .27) as change in cortisol was equal to −15.6 + .678 (BPM) ng/mL. Thus, at 15P cortisol increased 6.78 ng/mL for every increase of 10BPM in HR from baseline.
Figure 3.
Relationship of change in HR and BDNF.
Relationship of change in BDNF and change in cortisol.
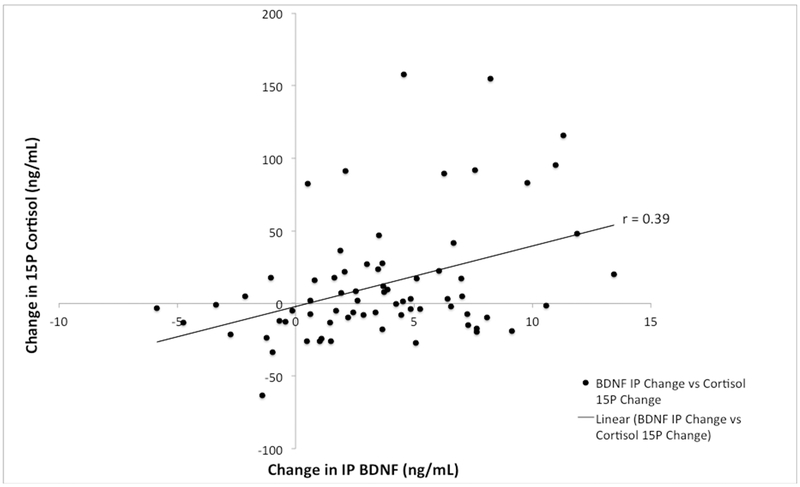
A Pearson product-moment correlation was performed to assess the association between the changes in BDNF and cortisol following exercise. There was a significant, small correlation between change in IP BDNF and change in IP cortisol (r = .29, n = 71, p = .02) and a significant, small, correlation between change in IP BDNF and change in 15P cortisol (r = .39, n = 71, p < .001, Figure 4).
Figure 4.
Relationship of change in BDNF and cortisol.
Discussion
The primary purpose of this study was to examine the acute neurobiological responses to aerobic exercise of different intensities in individuals with and without depression. To our knowledge this is the first investigation to examine the acute effects of different intensities of AEx on BDNF and cortisol responses in depressed vs. non-depressed (healthy controls) individuals. Contrary to our expectation there were no group differences in either serum BDNF or cortisol levels. Our finding that BDNF response did not differ between groups is consistent with previous work by Gustafsson and colleagues (22) who reported no significant effect of group in BDNF response to maximal aerobic exercise in 18 HC and 18 DEP subjects. Furthermore, the lack of between group differences in cortisol is consistent with Kiive et al. (29) who found no differences in cortisol response to maximal aerobic exercise in 22 HC and 24 DEP subjects. It should be noted that conflicting work by Krogh et al. (30) indicated a significant effect of group on cortisol response to maximal aerobic exercise in 44 HC and 137 DEP subjects. However, it is possible that the conflicting reports in the literature may be driven by the composition of samples as Kiive et al. (2004) studied an all male sample while Krogh et al. (2010) studied a sample that was 75.1% female. Females with depression have been shown to have greater resting cortisol concentrations compared to non-depressed females (31). Additionally, compared to males, females have demonstrated an exaggerated cortisol response to dexamethasone/corticotropin-releasing factor stimulation testing (32). Thus, sex differences in resting and exercise stimulated cortisol responses are a plausible explanation for the conflicting results in the literature although our results did not indicate that sex significantly impacted BDNF or cortisol.
Several aspects of this sample should be considered when interpreting the lack of between group differences in BDNF found in this trial. There was a small effect size (d=.29) when compared to healthy controls on resting BDNF, but we were insufficiently powered to detect this observed effect. A recent meta-analyses assessed the effect of depression on resting serum BDNF compared to non-depressed controls (33). In this study, Molendijk and colleagues reported a medium, negative effect of antidepressant-free depression (d=−.47) on resting BDNF concentrations when compared to healthy controls. However there was no difference in resting BDNF concentrations between those treated with antidepressants (d=.07) and healthy controls. Considering that ~70% of our DEP group were on a stable dose of anti-depressant medication, it is possible that BDNF levels were normalized by treatment, thereby explaining the lack of difference in resting and post-AEx BDNF. Anti-depressant treatment via selective serotonin reuptake inhibitor (SSRI), which was reported by all but one medicated participant (SSRI n=8; SNRI n=1), has been shown to increase serum BDNF in depressed patients to a level that is comparable to healthy controls (34). Pre-clinical evidence indicates that SSRI treatment stimulates endogenous BDNF production in cortical glial cells through increases in BDNF mRNA expression(35). Moreover, SSRI treatment increases activation of BDNF’s cellular receptor, tropomyosin receptor kinase B (trkB), which may enhance further neurotrophin signaling (36). To our surprise, on average, our DEP group was moderately-to-highly physically active. Lastly, while our DEP sample may have had less severe depressive symptoms and were relatively younger than in previous studies (22, 37) neither depressive symptom severity nor age was associated with resting BDNF(33). Relatedly, Meyer et al. reported that change in BDNF following a single session of AEx was not related to short-term changes in depressed mood (37), and previous work indicates that acute BDNF response to exercise can occur across the lifespan (38).
There was no effect of depression on resting cortisol in our sample. Meta-analytic research comparing adults with and without depression indicate a medium effect (d=.60) of depression on resting cortisol, although this effect size was reduced (d=.33) when stricter methodological standards were employed (39). Stetler and colleagues also reported significantly increased cortisol in older participants as well as inpatients and more severely depressed individuals (39). . Our younger, mild-to-moderately depressed cohort was likely a primary contributor to the lack of between group differences in resting cortisol. In response to AEx, both groups demonstrated similar cortisol responses in all three experimental conditions. In comparison to previous literature (40), the HC group seemingly demonstrated a normal HPA-axis response to AEx. Thus, it appears that our DEP group did not exhibit an abnormal stress response to AEx. Interestingly, females with depression have previously been shown to have a blunted cortisol response to psychosocial stressors compared to healthy controls while males demonstrate an exaggerated response (41). Although our limited sample prevents us from making comparisons across sex, our sample of primarily females did not appear to demonstrate such a response. In fact, during the most stressful condition (HI), the change in cortisol concentration of DEP females at 15P (60.5 ng/mL ± 43.6) was comparable to that of the HC females (45.5 ng/mL ± 66.0). This may suggest that HPA-axis dysfunction may not have been present in our DEP sample or that the mild-to-moderate nature of depression in this group did not significantly impact HPA-axis function. Another possibility is that depressed individuals who perform regular moderate-to-vigorous activity may maintain HPA-axis function and stress reactivity (42). Therefore, the physical activity status of our DEP group may serve as a protective mechanisms contributing to improved cortisol response to AEx.
Secondary analyses revealed that the sample, as a whole, demonstrated an intensity dependent change in BDNF immediately post-AEx. Cortisol was not affected in an intensity-dependent manner, however the HI condition elicited a significant increase at the 15P time point. Heart rate change was a significant predictor of both changes in IP BDNF and 15P cortisol, explaining 33% and 27% of the variance in each biomarker, respectively. Additionally, changes in BDNF and cortisol were positively associated with one another, although this relationship is likely driven by heart rate change or exercise intensity.
Effects of exercise intensity on BDNF
Our results indicated that exercise intensity appears to be an important feature in the ability of AEx to augment circulating BDNF. These results are previously corroborated by several studies examining acute BDNF responses to AEx in young, non-depressed cohorts (25, 40, 43). For example, Hotting and colleagues (25) examined the effect of exercise intensity on BDNF in young, healthy adults who cycled for a total of thirty minutes at an intensity similar to that used in our study. Consistent with our findings, their results indicated the high intensity (145BPM; ~80%HRmax) cycling group demonstrated a significantly greater BDNF response compared to the low intensity (99BPM; <57%HRmax) and control groups, immediately following exercise. Interestingly, their low intensity group did not demonstrate a significant change in BDNF compared to the non-exercise control group. Rojas Vega et al. (40) also reported similar results, finding no significant changes in BDNF following a 10-minute moderate intensity warm-up (~128BPM) but significant increases following a maximal capacity (~189BPM) cycling test. Ferris and colleagues (43) also reported an intensity dependent change in BDNF following maximal exercise, and 30 minutes of exercise at +10%, and −20% of ventilatory threshold (VT). Maximal (~175BPM) and +10% VT (~150BPM) cycling both elicited significant increases in BDNF while the −20% VT (~122BPM) failed to induce a significant change in BDNF. Our results combined with previous findings suggest that a single session of AEx can increases peripheral BDNF and that this increase occurs in an intensity-dependent manner, resulting in linear relationship between heart rate and BDNF.
Additionally, our project, along with others, has demonstrated that a single session of AEx can increase peripheral BDNF for a brief time before it returns to pre-exercise concentrations. The transient nature of this response is not fully understood but is potentially important to understanding the long-term effects of AEx on BDNF response. The ability of exercise to modulate BDNF expression may be stimulated by the contraction of skeletal muscle (44). Matthews and colleagues (2009) examined the effects of two hours of stationary cycling at 60%VO2max on serum BDNF and skeletal muscle BDNF mRNA in eight young, healthy, untrained men. Serum BDNF and muscle BDNF mrRNA via muscle biopsies were assessed at baseline, immediately after the exercise bout, and then at 3, 5, 8, 24, 48, and 72 hours after exercise. Results indicated that serum BDNF was significantly higher immediately after exercise and then returned to below baseline values for all time points afterwards. In contrast, BDNF mRNA expression peaked at five hours post-exercise and remained above baseline levels up to 72 hours post-exercise. Furthermore, muscle biopsies revealed a 50% increase in BDNF protein levels relative to baseline at 24 hours post-exercise. Thus, it appears that although circulating serum BDNF returns to baseline levels in a short period of time, the expression of BDNF in target tissues may remain elevated for 24-72 hours following high-intensity exercise. This evidence suggests that although circulating BDNF undergoes rapid up- and down-regulation in response to AEx the overall effects of a single session of aerobic exercise on BDNF expression may last several hours to several days post-exercise.
Demonstrating the ability of AEx to positively modulate BDNF is an important step in the continued pursuit of AEx as a viable option for anti-depressant treatment. Previous work examining the effects of exercise on BDNF has established the potential neurobiological underpinnings of the antidepressant response to exercise as elevated BDNF has been shown to be a strong predictor of remission after aerobic exercise treatment and reduced BDNF a strong predictor of non-response to aerobic exercise treatment for depression (45). Considering that BDNF has significant influence on neuroplasticity, AEx could have the potential to be utilized clinically for depression as well as other neuropsychiatric or neurological conditions in which neuroplasticity may be compromised. Based on the existing evidence, it appears that high intensity exercise is the most likely candidate to modulate BDNF expression.
Effects of exercise intensity on cortisol
A physical stressor, in this study high intensity AEx, demonstrated a predictable cortisol response in our sample. Our results indicated that high intensity AEx elicited a significant increase in cortisol at 15P, which is consistent with previous work examining the acute glucocorticoid responses to exercise (40). The delay in cortisol response to high AEx is a result of the activation of the HPA-axis and the adrenal glands and cortisol, which are working downstream from the pituitary gland and adrenocorticotropin hormone (ACTH). Seminal work by Luger and colleagues (46) showed that ACTH peaked during AEx at intensities of 70% and 90% of VO2max in young, healthy control subjects. Cortisol peaks occurred during the recovery period, which coincided with a reduction in ACTH. Additionally, the magnitude of change in ACTH and cortisol were intensity-dependent with near maximal AEx eliciting the greatest ACTH and subsequent cortisol responses. The temporal relationship of ACTH and cortisol in these conditions also suggests that the negative feedback mechanisms by which the HPA-axis is regulated were normal functioning as ACTH production reduced as cortisol concentrations increased.
The high intensity condition in the current study resulted in the greatest cortisol response and ultimately served as a test of HPA-axis response to stress. The time course of the cortisol response appeared to be similar to previously published work, although we did not assess corticotropin-releasing hormone (CRH) or ACTH to get a full picture of HPA-axis function. Acute bouts of AEx, specifically of high intensity, may serve as stress challenges to examine differences in HPA-axis function between healthy controls and depressed individuals, and perhaps even various subtypes of depression. Assessing the temporal relationships of CRH, ACTH, and cortisol are important in elucidating the function of HPA-axis in response to acute exercise in depressed cohorts and the potential adaptations that may occur with chronic exercise. It is possible that AEx may benefit those with depression through improved HPA-axis regulation that has been shown in non-depressed individuals with higher aerobic fitness (47). Thus, understanding the acute and chronic responses of the HPA-axis may provide valuable information regarding the neurobiological benefits of AEx in depression.
Relationship of cortisol and BDNF
Pre-clinical evidence suggests that glucocorticoids (i.e. cortisol) regulate BDNF mRNA expression (48) and chronic exposure to glucocorticoids reduces BDNF through disruption of the BDNF signaling pathway (49). In humans, Ambrus and colleagues reported an inverse relationship between serum cortisol and plasma BDNF in female suicide attempters (50). Additionally, they reported a positive relationship between concentration difficulties and serum cortisol and lower plasma BDNF. Further, Issa and colleagues reported inverse relationships between prefrontal cortex cortisol and BDNF and cerebrospinal fluid cortisol and BDNF in post-mortem schizophrenia subjects (51). The evidence can not directly indicate that reduced BDNF and elevated cortisol are the sole cause of depression or other neuropsychiatric conditions, however it does appear, at least from preclinical models, that cortisol can impact BDNF regulation and both may serve as potential therapeutic treatment targets.
Interestingly, BDNF appears to increase with remission of depressive symptoms and/or pharmacological antidepressant treatment (33). Also, following a three-month yoga-only program subjects demonstrated a significant inverse relationship between change in serum cortisol and change in serum BDNF, which would suggest that chronic exercise may reduce cortisol and increase BDNF (17) However, the relationship of these markers in response to either chronic or acute exercise is not well studied. In this current study, changes in BDNF and cortisol demonstrated weak, although significant, relationships with another. Additionally, change in IP BDNF demonstrated a stronger relationship with 15P cortisol (r = .39) than with IP Cortisol (r = .29) suggesting that peak changes in BDNF may precede peak changes in cortisol. Based on these results it would appear that a stimulus potent enough to evoke a cortisol response would be needed to acutely stimulate BDNF, however we are unable to conclude on whether cortisol regulation would improve over the course of an exercise training program.
Both BDNF and cortisol are of particular importance to neuropsychiatric and neurologically compromised populations due to their potential impact on neuroplasticity (52, 53). Given the sizeable amount of literature examining these biomarkers, the coupling of changes in BDNF and cortisol warrant further investigation. Examining the relationship of these biomarkers in response to acute and chronic treatment of depression may help in the advancement of treatment paradigms as well as identifying potential predictors of treatment response.
Limitations
Our study contained several limitations that should be considered when interpreting the results. First, our participants with depression were community-dwelling individuals with mild-moderate depression. Thus, the current results cannot be extrapolated to inpatient or more severely depressed patients. Additionally, our recruitment materials specifically stated that exercise was involved in this project. As a result, our study likely is at risk of selection bias of individuals that were more physically active or fit. We did not match our samples for activity level, which resulted in the HC group reporting more moderate-vigorous physical activity than the DEP group, although overall activity was not significantly different between groups. Our sample also contained a mix of males and females and, although we were sex-matched, there are likely sex-specific neurobiological differences for which we were unable to decipher in this sample. Next, although all participants were either taking a stable dose or were not currently using anti-depressant medication, it is possible that medications may have normalized biomarker responses in those taking medication, thus influencing group responses. Additionally, we did not assess our sample for the presence of the BDNF Val66Met polymorphism. While we are unable to account for the potential influence of this genetic variation on the BDNF response to exercise, recent work suggests that the presence of this polymorphism does not attenuate BDNF response to high intensity exercise (54). In humans, peripheral measures of BDNF may not be representative of central concentrations and caution should be taken when interpreting blood-based measures of BDNF as such. We did not correct BDNF and cortisol for potential plasma volume changes in response to exercise and may have overestimated their concentrations. Although previous work shows that when correcting for plasma volume changes BDNF and cortisol remain significantly elevated following exercise (55, 56). Since all sessions took place in the afternoon, participants were permitted to attend experimental sessions in a non-fasted state. As a result, we cannot exclude that a pre-experimental meal may have influenced either BDNF or cortisol results. Lastly, our sample was young and otherwise without comorbid conditions, thus our results cannot be generalized to older or elderly populations or those with multiple neuropsychiatric or other health conditions.
Future Directions
While this work shows that a single session of AEx can acutely stimulate BDNF production in young, healthy, and physically active, non-depressed and mild-moderately depressed individuals, more work is needed in this area. Future work should be aimed at studying the ability of AEx to stimulate BDNF in sedentary, more severely depressed, and medication-free individuals as well as other neuropsychiatric and neurologic cohorts. Whether long-term exercise training can enhance resting levels of BDNF in depressed cohorts and how this may correspond with antidepressant response remains unclear. Examining the relationship between treatment response and BDNF will be useful in identifying characteristics of responders to aerobic exercise treatment. Lastly, identifying exercise training parameters (i.e. mode, frequency, intensity, time) that elicit optimal psychobiological responses will be important in further establishing exercise as a clinically viable treatment for depression.
Conclusions
In light of the findings of this study, it appears that high-intensity AEx can elicit acute, transient increases in BDNF and cortisol in young, healthy, and physically active, non-depressed and mild-moderately depressed individuals. However, given the small sample size and the unique characteristics of the depression group, caution must be taken in interpreting these results to a larger population. Through the assessment of neurobiological markers, this work suggests AEx has the potential to significantly impact central nervous system function and that the magnitude of such impact may be directly driven by intensity of exercise. Establishing exercise as a neuromodulatory treatment option can be useful to the fields of neuropsychiatry and neurorehabilitation and further work should focus on the mechanistic underpinnings and the overall magnitude of effect in treatment studies. Questions remain regarding the ability of aerobic exercise to predictably modulate BDNF and cortisol in moderate to severely depressed, and treatment-resistant patients. Additionally, the benefit of adjunctive aerobic exercise to pharmacological anti-depressant treatment is established, however questions remain regarding its role in combination with less commonly prescribed treatments such as rTMS and ECT.
Supplementary Material
Supplement Figure. Time course of absolute changes in serum cortisol in depressed (DEP) and non-depressed (HC) participants.
* Cortisol significantly greater in HI condition compared to both LO (15P; p < 0.001, 30P; p < .001) and CON (15P; p = .04, 30P; p = .03) conditions
# Cortisol significantly greater in HI condition compared to LO condition (45P; p < .001, 60P; p = .012)
Acknowledgments
We would like to thank a dedicated group of study participants for their time and effort that made this study possible. A special thank you to Campbell Long and Kristen Buchanan, BSN, RN for their assistance with data acquisition and processing. This project was supported by funding from the National Institute of General Medical Sciences under grant number P20-GM109040 and by MUSC’s Clinical and Translational Science Award grant UL1 TR001450 from the National Center for Advancing Translational Sciences (NCATS).
Footnotes
Publisher's Disclaimer: Medicine & Science in Sports & Exercise® Published ahead of Print contains articles in unedited manuscript form that have been peer reviewed and accepted for publication. This manuscript will undergo copyediting, page composition, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered that could affect the content.
Conflict of Interest
The authors have no conflicts to report. The results of this study do not constitute endorsement by the American College of Sports Medicine. The results are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015. February;76(2):155–62. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006. November;163(11):1905–17. [DOI] [PubMed] [Google Scholar]
- 3.Bauer M, Pfennig A, Severus E, Whybrow PC, Angst J, Moller HJ, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J Biol Psychiatry. 2013. July;14(5):334–85. [DOI] [PubMed] [Google Scholar]
- 4.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med 2005. January;28(1):1–8. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007. Sep-Oct;69(7):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacquart SD, Marshak HH, Dos Santos H, Luu SM, Berk LS, McMahon PT, et al. The effects of simultaneous exercise and psychotherapy on depressive symptoms in inpatient, psychiatric older adults. Adv Mind Body Med 2014. Fall;28(4):8–17. [PubMed] [Google Scholar]
- 7.Classen J. Plasticity. Handb Clin Neurol 2013;116:525–34. [DOI] [PubMed] [Google Scholar]
- 8.Arnone D, McKie S, Elliott R, Juhasz G, Thomas EJ, Downey D, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2013. December;18(12):1265–72. [DOI] [PubMed] [Google Scholar]
- 9.Bus BA, Molendijk ML, Tendolkar I, Penninx BW, Prickaerts J, Elzinga BM, et al. Chronic depression is associated with a pronounced decrease in serum brain-derived neurotrophic factor over time. Mol Psychiatry. 2014. August 26. [DOI] [PubMed] [Google Scholar]
- 10.Travis SG, Coupland NJ, Hegadoren K, Silverstone PH, Huang Y, Carter R, et al. Effects of cortisol on hippocampal subfields volumes and memory performance in healthy control subjects and patients with major depressive disorder. J Affect Disord 2016. September 1;201:34–41. [DOI] [PubMed] [Google Scholar]
- 11.Zagrebelsky M, Korte M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology. 2014. January;76 Pt C:628–38. [DOI] [PubMed] [Google Scholar]
- 12.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 2002. August 16;328(3):261–4. [DOI] [PubMed] [Google Scholar]
- 13.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998. December;37(12):1553–61. [DOI] [PubMed] [Google Scholar]
- 14.Rhebergen D, Korten NC, Penninx BW, Stek ML, van der Mast RC, Oude Voshaar R, et al. Hypothalamic-pituitary-adrenal axis activity in older persons with and without a depressive disorder. Psychoneuroendocrinology. 2015. January;51:341–50. [DOI] [PubMed] [Google Scholar]
- 15.Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009. June;66(6):617–26. [DOI] [PubMed] [Google Scholar]
- 16.Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord 2015. March 15;174:432–40. [DOI] [PubMed] [Google Scholar]
- 17.Naveen GH, Varambally S, Thirthalli J, Rao M, Christopher R, Gangadhar BN. Serum cortisol and BDNF in patients with major depression-effect of yoga. Int Rev Psychiatry. 2016. June;28(3):273–8. [DOI] [PubMed] [Google Scholar]
- 18.Schule C, Baghai TC, Eser D, Zwanzger P, Jordan M, Buechs R, et al. Time course of hypothalamic-pituitary-adrenocortical axis activity during treatment with reboxetine and mirtazapine in depressed patients. Psychopharmacology (Berl). 2006. July;186(4):601–11. [DOI] [PubMed] [Google Scholar]
- 19.Lamego MK, Souza Moura AM, Paes F, Ferreira Rocha NB, de Sa Filho AS, Lattari E, et al. Aerobic Exercise Does Not Predict Brain Derived Neurotrophic Factor And Cortisol Alterations in Depressed Patients. CNS Neurol Disord Drug Targets. 2015;14(9):1116–28. [DOI] [PubMed] [Google Scholar]
- 20.Dinoff A, Herrmann N, Swardfager W, Gallagher D, Lanctot KL. The effect of exercise on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF) in major depressive disorder: A meta-analysis. J Psychiatr Res 2018. October;105:123–31. [DOI] [PubMed] [Google Scholar]
- 21.Beserra AHN, Kameda P, Deslandes AC, Schuch FB, Laks J, Moraes HS. Can physical exercise modulate cortisol level in subjects with depression? A systematic review and meta-analysis. Trends Psychiatry Psychother 2018. Oct-Dec;40(4):360–8. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson G, Lira CM, Johansson J, Wisen A, Wohlfart B, Ekman R, et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Res 2009. October 30;169(3):244–8. [DOI] [PubMed] [Google Scholar]
- 23.Meyer JD, Koltyn KF, Stegner AJ, Kim JS, Cook DB. Relationships between serum BDNF and the antidepressant effect of acute exercise in depressed women. Psychoneuroendocrinology. 2016. December;74:286–94. [DOI] [PubMed] [Google Scholar]
- 24.Meyer JD, Ellingson LD, Koltyn KF, Stegner AJ, Kim JS, Cook DB. Psychobiological Responses to Preferred and Prescribed Intensity Exercise in Major Depressive Disorder. Med Sci Sports Exerc 2016. November;48(11):2207–15. [DOI] [PubMed] [Google Scholar]
- 25.Hotting K, Schickert N, Kaiser J, Roder B, Schmidt-Kassow M. The Effects of Acute Physical Exercise on Memory, Peripheral BDNF, and Cortisol in Young Adults. Neural Plast 2016;2016:6860573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22,33;quiz 34-57. [PubMed] [Google Scholar]
- 27.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008. January;192(1):52–8. [DOI] [PubMed] [Google Scholar]
- 28.Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, et al. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep 2015. December 10;5:17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiive E, Maaroos J, Shlik J, Toru I, Harro J. Growth hormone, cortisol and prolactin responses to physical exercise: higher prolactin response in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2004. September;28(6):1007–13. [DOI] [PubMed] [Google Scholar]
- 30.Krogh J, Nordentoft M, Mohammad-Nezhad M, Westrin A. Growth hormone, prolactin and cortisol response to exercise in patients with depression. J Affect Disord 2010. September;125(1-3):189–97. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaka H, Maeshima H, Kida S, Kurita H, Shimano T, Nakano Y, et al. Gender differences in serum testosterone and cortisol in patients with major depressive disorder compared with controls. Int J Psychiatry Med 2013;46(2):203–21. [DOI] [PubMed] [Google Scholar]
- 32.Anthenelli RM, Heffner JL, Blom TJ, Daniel BE, McKenna BS, Wand GS. Sex differences in the ACTH and cortisol response to pharmacological probes are stressor-specific and occur regardless of alcohol dependence history. Psychoneuroendocrinology. 2018. August;94:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. 2014. July;19(7):791–800. [DOI] [PubMed] [Google Scholar]
- 34.Wolkowitz OM, Wolf J, Shelly W, Rosser R, Burke HM, Lerner GK, et al. Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011. August 15;35(7):1623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology (Berl). 2011. July;216(1):75–84. [DOI] [PubMed] [Google Scholar]
- 36.Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 2003. January 1;23(1):349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer JD, Koltyn KF, Stegner AJ, Kim JS, Cook DB. Relationships between serum BDNF and the antidepressant effect of acute exercise in depressed women. Psychoneuroendocrinology. 2016. September 28;74:286–94. [DOI] [PubMed] [Google Scholar]
- 38.Hakansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, et al. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J Alzheimers Dis 2017;55(2):645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011. Feb-Mar;73(2):114–26. [DOI] [PubMed] [Google Scholar]
- 40.Rojas Vega S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res 2006. November 22;1121(1):59–65. [DOI] [PubMed] [Google Scholar]
- 41.Zorn JV, Schur RR, Boks MP, Kahn RS, Joels M, Vinkers CH. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology. 2017. March;77:25–36. [DOI] [PubMed] [Google Scholar]
- 42.Gerber M, Ludyga S, Mücke M, Colledge F, Brand S, Pühse U. Low vigorous physical activity is associated with increased adrenocortical reactivity to psychosocial stress in students with high stress perceptions. Psychoneuroendocrinology. 2017. June/01;80:104–13. [DOI] [PubMed] [Google Scholar]
- 43.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 2007. April;39(4):728–34. [DOI] [PubMed] [Google Scholar]
- 44.Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009. July;52(7):1409–18. [DOI] [PubMed] [Google Scholar]
- 45.Rethorst CD, South CC, Rush AJ, Greer TL, Trivedi MH. Prediction of treatment outcomes to exercise in patients with nonremitted major depressive disorder. Depress Anxiety. 2017. July 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luger A, Deuster PA, Kyle SB, Gallucci WT, Montgomery LC, Gold PW, et al. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med 1987. May 21;316(21):1309–15. [DOI] [PubMed] [Google Scholar]
- 47.Traustadottir T, Bosch PR, Cantu T, Matt KS. Hypothalamic-pituitary-adrenal axis response and recovery from high-intensity exercise in women: effects of aging and fitness. J Clin Endocrinol Metab. 2004. July;89(7):3248–54. [DOI] [PubMed] [Google Scholar]
- 48.Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013. June 3;239:196–213. [DOI] [PubMed] [Google Scholar]
- 49.Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, et al. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience. 2010. February 17;165(4):1301–11. [DOI] [PubMed] [Google Scholar]
- 50.Ambrus L, Lindqvist D, Traskman-Bendz L, Westrin A. Hypothalamic-pituitary-adrenal axis hyperactivity is associated with decreased brain-derived neurotrophic factor in female suicide attempters. Nord J Psychiatry. 2016. November;70(8):575–81. [DOI] [PubMed] [Google Scholar]
- 51.Issa G, Wilson C, Terry AV Jr, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis 2010. September;39(3):327–33. [DOI] [PubMed] [Google Scholar]
- 52.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med 2010. September 1;40(9):765–801. [DOI] [PubMed] [Google Scholar]
- 53.Sale MV, Ridding MC, Nordstrom MA. Cortisol inhibits neuroplasticity induction in human motor cortex. J Neurosci 2008. August 13;28(33):8285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helm EE, Matt KS, Kirschner KF, Pohlig RT, Kohl D, Reisman DS. The influence of high intensity exercise and the Val66Met polymorphism on circulating BDNF and locomotor learning. Neurobiol Learn Mem 2017. October;144:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kallies G, Rapp MA, Fydrich T, Fehm L, Tschorn M, Teran C, et al. Serum brain-derived neurotrophic factor (BDNF) at rest and after acute aerobic exercise in major depressive disorder. Psychoneuroendocrinology. 2018. December 14;102:212–5. [DOI] [PubMed] [Google Scholar]
- 56.Kargotich S, Goodman C, Keast D, Fry RW, Garcia-Webb P, Crawford PM, et al. Influence of exercise-induced plasma volume changes on the interpretation of biochemical data following high-intensity exercise. Clin J Sport Med 1997. July;7(3):185–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure. Time course of absolute changes in serum cortisol in depressed (DEP) and non-depressed (HC) participants.
* Cortisol significantly greater in HI condition compared to both LO (15P; p < 0.001, 30P; p < .001) and CON (15P; p = .04, 30P; p = .03) conditions
# Cortisol significantly greater in HI condition compared to LO condition (45P; p < .001, 60P; p = .012)