Abstract
OBJECTIVE:
Patients requiring admission to an intensive care unit (ICU) may subsequently experience cognitive decline. Our objective was to investigate longitudinal cognitive trajectories in older adults hospitalized in ICUs. We hypothesized that individuals hospitalized for critical illness develop greater cognitive decline compared to those who do not require ICU admission.
DESIGN:
A retrospective cohort study using prospectively collected cognitive scores of participants enrolled in the Mayo Clinic Study of Aging (MCSA) and ICU admissions retrospectively ascertained from electronic medical records. A covariate-adjusted linear mixed effects model with random intercepts and slopes assessed the relationship between ICU admissions and the slope of global cognitive z-scores and domains scores (memory, attention/executive, visuospatial and language).
SETTING:
ICU admissions and cognitive scores in the MCSA from October 1, 2004 through September 11, 2017.
PATIENTS:
Non-demented participants aged 50 through 91 at enrollment in the MCSA with an initial cognitive assessment and at least 1 follow-up visit.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Of 3,673 participants, 372 had at least one ICU admission with median [25th, 75th percentile] follow-up after first ICU admission of 2.5 [1.2, 4.4] years. For global cognitive z-score, admission to an ICU was associated with greater decline in scores over time compared to participants not requiring ICU admission (difference in annual slope = −0.028, 95%CI −0.044 to −0.012, P<0.001). ICU admission was associated with greater declines in memory (−0.029, 95%CI −0.047 to −0.011; P=0.002); attention/executive (−0.020, 95%CI −0.037 to −0.004; P=0.016); and visuospatial (−0.013, 95%CI −0.026 to −0.001; P=0.041) domains. ICU admissions with delirium were associated with greater declines in memory (interaction P=0.006) and language (interaction P=0.002) domains than ICU admissions without delirium.
CONCLUSIONS:
In older adults, ICU admission was associated with greater long-term cognitive decline compared to patients without ICU admission. These findings were more pronounced in those who develop delirium while in the ICU.
Keywords: cognitive aging, cognitive z-scores, cognitive domains: memory, attention/executive function, language, visuospatial skills, critical illness, intensive care unit, surgery, medical illness, critical care illness, Mayo Clinic Study of Aging, older adults
INTRODUCTION
Cognitive function in older adults may be impaired during and after hospitalization [1–4]. Several factors may contribute to cognitive decline [5], including illnesses necessitating hospitalization [6, 7], exposure to surgery and anesthesia [8, 9], and acute changes in cognitive function during hospitalization, including delirium [4, 10]. Given that studies examining cognition after hospital discharge are observational, it is difficult to account for confounding effects of comorbidities that may contribute to cognitive decline [1, 11]. There is some evidence that the severity of acute illness, rather than particular diagnosis, is associated with post-discharge cognitive impairment [12]. Although the construct of “disease severity” may be difficult to assess across heterogeneous illnesses, the need for admission to an intensive care unit (ICU) is one objective method of defining more severe disease or need for a major medical intervention. The presence of mild cognitive impairment (MCI) and dementia are independently associated with increased likelihood for admission to ICU [3, 13–15], highlighting the importance of baseline cognitive function when examining the effect of critical illness on subsequent cognitive trajectory. Additionally, acute complications during the hospitalization, such as delirium, can influence the degree of cognitive impairment in years following discharge [10].
The goal of the present study was to determine the association between ICU admission and the longitudinal trajectory of cognitive function. We hypothesized that patients admitted to the ICU would experience greater cognitive decline compared with patients not requiring ICU admission. To evaluate this hypothesis, we used data from the Mayo Clinic Study of Aging (MCSA), a population-based study adults aged 50 and older residing in Olmsted County, Minnesota.
MATERIALS AND METHODS
This study conforms to the requirements of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement [16] and was approved by the institutional review boards of Mayo Clinic and Olmsted Medical Center, Rochester, Minnesota. At MCSA enrollment, all participants provided written informed consent.
Selection of Participants
Study participants were enrolled in the MCSA, an epidemiologic study of the prevalence, incidence, and risk factors for mild cognitive impairment (MCI) among Olmsted County residents [17]. The present study included non-demented participants (cognitively unimpaired (CU) or with MCI) aged 50 through 91 at enrollment who were assessed from October 1, 2004 through September 11, 2017, including an initial assessment and at least 1 follow-up visit. Subjects who declined research authorization for the use of their medical records in research (Minnesota Statute 144.335) were excluded.
Exposure Definitions
The primary exposure was defined as ICU admission for any indication occurring after MCSA enrollment. Admissions were classified according to the type of ICU admission, including surgical, trauma (not requiring surgery), or medical. A surgical admission was defined as those with a surgical procedure performed within 12 hours before or 8 hours after admission; including unplanned and planned admissions after major procedures, e.g., cardiac surgery. Patients who did not have surgical procedures within 8 hours following admission, but were admitted through the emergency department to the Trauma, Critical Care, and General Surgery (TCGS) service were calssified as a separate ‘trauma ICU admission’ type. Many of these patients were admitted for acute medical illnesses (acute abdomen, acute chlecystectomy) ultimately requiring surgical procedures, but more than 8 hours after admission to the ICU; none had indications of traumatic brain injury in medical records. All other ICU admissions were classified as medical. Delirium during ICU admission was assessed using the Confusion Assessment Method for the ICU (CAM-ICU) score [18–20], per hospital protocol 3 times daily. Delirium was defined by the presence of at least one positive CAM-ICU evaluation during ICU admission, as described previously [21].
Neurological and Cognitive assessments
Details of the MCSA study, including clinical evaluation at baseline and at each 15-monthly follow-up involving 3 evaluators, have been described previously [17]. Briefly, the initial evaluation included assessment of participant’s demographics, medical history, memory (self-report) and family history of dementia. A study partner (informant) was interviewed in order to complete a Clinical Dementia Rating Scale [22] and the Functional Activities Questionnaire [23]. APOE genotyping was performed using standard methods.
Neurologic evaluation by a physician included the Short Test of Mental Status,[24] a modified Hachinski Ischemic Scale [25, 26], a modified Unified Parkinson’s Disease Rating Scale [27], and a neurologic examination. The neuropsychological evaluation included assessment of 4 cognitive domains using 9 tests: 1) attention/executive (Trail Making Test B and Digit Symbol Substitution Test) [28, 29]; 2) language (Boston Naming Test [30] and Category Fluency) [31]; 3) memory (Wechsler Memory Scale-Revised Logical Memory II [delayed recall], Wechsler Memory Scale-Revised Visual Reproduction- II [delayed recall] [32], and Auditory Verbal Learning Test (delayed recall) [33]; and 4) visuospatial skills (Wechsler Adult Intelligence Scale-Revised Picture Completion test and Wechsler Adult Intelligence Scale-Revised Block Design test) [28]. We calculated sample-specific z-scores for all cognitive tests where the MCSA 2004 enrollment cohort served as the reference distribution as described previously such that test scores have a standard deviation of 1 in the reference cohort [17, 34]. Domain scores for each of the four cognitive domains are the average of z-scores from individual tests within each domain, rescaled to have a standard deviation of 1 in the reference cohort. Global cognitive score was calculated by averaging the 4 domain scores and re-scaling to form a global z-score.
Clinical diagnoses were determined by a consensus committee including the neurologist, neuropsychologist, and nurse who evaluated each participant. Performance in a cognitive domain was compared with age-adjusted scores of cognitively unimpaired (CU) individuals previously obtained using Mayo’s Older American Normative Studies [33]. This approach relied on prior normative work in an independent sample from the same population. Participants with scores around 1.0 SD below the age-specific mean in the general population were considered for possible cognitive impairment. The operational definition of MCI was based on clinical judgment including a history from the patient and informant. Published criteria were used for the diagnosis: cognitive complaint, cognitive function not normal for age, essentially normal functional activities, no dementia [35]. A final decision about impairment in a cognitive domain was made after considering education, occupation, visual or hearing deficits, and reviewing all other participant information. The diagnosis of dementia was based on published criteria [36]. Participants who performed in the normal range and did not meet criteria for MCI or dementia were deemed CU.
Data Resources
Two separate data resources were used, the MCSA and Mayo Clinic institutional electronic medical records obtained through the Rochester Epidemiology Project (REP) medical records-linkage system [37]. The MCSA provided demographics and cognitive status. Comorbidities were ascertained via a detailed nurse abstraction of medical records. Because all ICUs in Olmsted County are on the Mayo Clinic campus, details regarding all ICU admissions were available through the Mayo Clinic electronic medical records. For each individual, details of all ICU admissions that occurred after the index date from participant entry into MCSA through most recent MCSA visit up to September 11, 2017 were abstracted.
Statistical Analyses
Baseline demographics and comorbidities were summarized using median [25th, 75th percentiles] for continuous variables and percentage for categorical variables according to the type of first ICU admission following MCSA enrollment. Characteristics of each ICU stay were described similarly.
The primary hypothesis concerned the relationship between ICU admission after MCSA study enrollment and subsequent cognitive trajectory. ICU admissions prior to MCSA enrollment were not included because complete data regarding ICU admissions, including CAM-ICU scores, were not available electronically before 2004. A linear mixed effects model with random intercepts and slopes assessed the relationship between ICU admissions and the average slope of global cognitive z-scores over time. Fixed effects included time (in years) following first available z-score to estimate a slope among unexposed and time (in years) following ICU admission(s). Since patients may have more than one ICU admission, we hypothesized that repeated admissions may have an additive effect. Five functional relationships for multiple ICU admissions were evaluated; details are given in the Supplemental Content: Statistical Analyses. Under the selected approach, model results describe the additive change in annual slope associated with each critical care admission. A negative change indicates greater cognitive decline over time following ICU admission.
Adjustment covariates were age at enrollment, smoking status, mid-life diagnosis of diabetes, hypertension, and dyslipidemia, history of atrial fibrillation, congestive heart failure, stroke, coronary artery disease, and alcohol problems, marital status, sex, APOE ε4 allele, education, Charlson comorbidity index, and cognitive status at enrollment. Models also adjusted for the interaction between these covariates and time since first z-score (slope). Secondary outcomes included z-scores for each cognitive domain, analyzed similarly.
A pre-specified secondary analysis assessed whether type of ICU admission (surgical, medical, or trauma) modified the relationship between ICU admission and slope of cognitive scores over time. In these models, type of ICU admission was defined based on the first admission following enrollment (surgical, medical, or trauma) and censored follow-up when subsequently admitted to the ICU for a different indication (e.g., medical ICU admission after previous surgical admission). An interaction P-value was calculated, and if significant, estimates of the change in slope were provided for each type. Similarly, whether delirium during the ICU stay modified the association between ICU admission and cognitive scores was assessed using a similar approach. In post-hoc analyses, MCI at enrollment and age at enrollment were assessed for moderating effects using interaction terms.
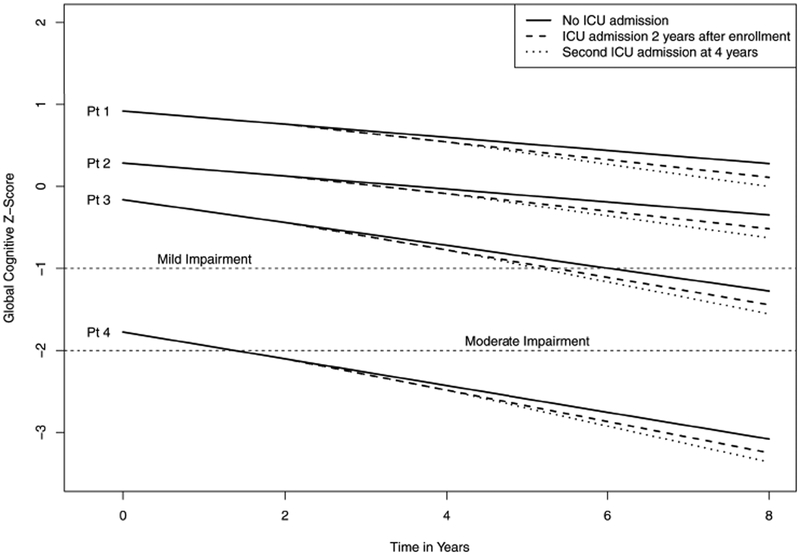
Data are described graphically in two ways. First, box plots describe the distribution of cognitive z-scores over time. Study visits were grouped into intervals (first z-score/enrollment, 8–22, 23–37, 38–52, and 53–67 months) based on the expected time between visits in the MCSA. Second, trajectories were simulated for four hypothetical patients to describe variation in cognitive z-scores at enrollment and trajectories over time. The four hypothetical patients have covariates selected to represent a range of healthy to unhealthy status (see respective Figure footnote for more details). Trajectories were simulated using linear combinations of model results with random terms set to zero to reflect an average subject with the given covariate values. For each hypothetical patient, ICU admission status was manipulated to simulate paths for (i) no ICU admission during the study, (ii) a single ICU admission 2 years after enrollment, and (iii) two ICU admissions with the first at 2 years and the second at 4 years after enrollment.
A P-value <0.05 was considered statistically significant. Analyses were performed using SAS statistical software (Version 9.4, SAS Institute, Inc, Cary, NC).
RESULTS
Of 5,576 MCSA participants enrolled during the study period, 132 were excluded due to inability to review patient medical history for possible ICU admissions, 87 were diagnosed with dementia at enrollment, and 1,684 did not have a follow-up global z-score during the study and were excluded (Figure 1). Among 3,673 included participants with cognitive z-scores at baseline and at least one follow-up study visit, the median [25th, 75th percentile] follow-up time after the index cognitive assessment was 4.0 [2.6, 6.4] years (mean (sd) = 4.7 (2.7)).Among 3,673 total participants, 3,301 participants did not have an ICU admission during followup and 372 (10.1%) had at least one ICU admission during follow-up. Of the 372 with at least one admission 299 (80%) were admitted once, 50 (13%) had 2 admissions, 16 (4%) had 3, and 7 (2%) had 4 or more admissions. For these 372 participants the median follow-up time and number of MCSA visits after their first ICU admission was 2.5 [1.2, 4.4] years (mean (sd) = 3.1 (2.4)) and 2 [1, 4] visits, respectively.
Figure 1.
Flow diagram of the Mayo Clinic Study of Aging (MCSA) participants in the present study.
Characteristics of study participants at the time of baseline MCSA assessment are presented in Table 1. Participants who required ICU admissions were older and had higher burden of preexisting comorbidities at the time of study entry. However, baseline cognitive z-scores, overall and by age groups, and MCI status were similar. Among the 372 participants who had ICU admissions during follow-up there were a total of 477 ICU admissions; details are summarized in Supplemental Table 1.The median duration of an ICU admission was 27 (21, 72) hours and mechanical ventilation was used in 127 (27%) of the admissions. Forty-seven unique patients experienced ICU delirium during 55 (12%) admissions.
Table 1:
Patient characteristics at enrollment summarized overall and by ICU admission*
| Characteristics | Overall (N=3673) |
No ICU Admissions (N=3301) |
1 or more ICU admissions (N=372) |
|---|---|---|---|
| Age at enrollment, years | 73 [66, 80] | 73 [65, 79] | 77 [73, 82] |
| Age ≥ 65 years at enrollment | 2859 (78%) | 2502 (76%) | 357 (96%) |
| Sex | |||
| Male | 1897 (52%) | 1666 (50%) | 231 (62%) |
| Female | 1776 (48%) | 1635 (50%) | 141 (38%) |
| BMI, kg/m2, n=3632 | 27.7 [24.9, 31.2] | 27.7 [24.8, 31.1] | 28.3 [25.0, 32.2] |
| BMI ≥ 30, n=3632 | 1178 (32%) | 1045 (32%) | 133 (36%) |
| Ever diagnosed alcohol problem, n=3646 | 164 (4%) | 145 (4%) | 19 (5%) |
| APOE E4 genotype, n=3632 | 1024 (28%) | 924 (28%) | 100 (27%) |
| Midlife diabetes, n=3672 | 307 (8%) | 281 (9%) | 26 (7%) |
| Midlife hypertension, n=3672 | 1478 (40%) | 1316 (40%) | 162 (44%) |
| Midlife dyslipidemia, n=3672 | 1831 (50%) | 1645 (50%) | 186 (50%) |
| Charlson comorbidity index | |||
| 1–2 | 2049 (56%) | 1905 (58%) | 144 (39%) |
| 3+ | 1624 (44%) | 1396 (42%) | 228 (61%) |
| Education, years | |||
| Less than 12 years | 189 (5%) | 161 (5%) | 28 (8%) |
| 12 years | 1025 (28%) | 903 (27%) | 122 (33%) |
| 13–15 years | 1016 (28%) | 915 (28%) | 101 (27%) |
| 16 years and above | 1443 (39%) | 1322 (40%) | 121 (33%) |
| Marital status, n=3668 | |||
| Single | 447 (12%) | 422 (13%) | 25 (7%) |
| Married | 2630 (72%) | 2356 (71%) | 274 (74%) |
| Widowed | 591 (16%) | 518 (16%) | 73 (20%) |
| Smoking status, n=3672 | |||
| Never | 1961 (53%) | 1801 (55%) | 160 (43%) |
| Former | 1545 (42%) | 1346 (41%) | 199 (53%) |
| Current | 166 (5%) | 153 (5%) | 13 (3%) |
| Baseline Cognitive status | |||
| Cognitively unimpaired | 3304 (90%) | 2973 (90%) | 331 (89%) |
| Mild cognitive impairment | 369 (10%) | 328 (10%) | 41 (11%) |
| Baseline Cognitive z-score, overall | 0.7 [−0.0, 1.4] | 0.7 [−0.0, 1.4] | 0.4 [−0.2, 1.0] |
| among Age ≤ 69 | 1.4 [0.9, 1.9] | 1.4 [0.9, 1.9] | 1.1 [0.5, 1.6] |
| among 70 ≤ Age ≤ 79 | 0.5 [−0.0, 1.1] | 0.5 [−0.0, 1.1] | 0.5 [−0.0, 1.1] |
| among 80 ≤ Age | −0.1 [−0.8, 0.5] | −0.1 [−0.9, 0.5] | 0.0 [−0.6, 0.7] |
Midlife is defined as age < 65 years. For patients who enrolled prior to age 65, midlife values reflect status at baseline. For variables with missing information, total number with complete data is summarized. *Baseline cognitive z-score is obtained on the first MCSA visit. Values are median [25th, 75th percentile] or n (percentage).
Primary analysis
At enrollment, the median [25th, 75th] percentiles of global cognitive z-scores were 0.69 [−0.04, 1.35] and declined over time (Figure 2A, Table 2). The slope of global cognitive z-score was significantly more negative in patients following ICU admission compared with those not requiring ICU admission (difference in slope = −0.028 per year, 95%CI −0.044 to −0.012, P<0.001, Table 2). Older age, female sex, higher Charlson comorbidity index, APOE ε4 allele presence, and MCI on enrollment (vs CU) were each associated with a more negative slope (interaction P<0.05 for all, Supplemental Table 2). Similar results were also observed for memory (difference in slope = −0.029, 95% CI −0.047 to −0.011; P=0.002); attention/executive (−0.020, 95%CI −0.037 to −0.004; P=0.016); and visuospatial domains (−0.013, 95%CI −0.026 to −0.001; P=0.041, Table 2).
Figure 2 A.
Box plots for global cognitive z-scores presented according to time since enrollment and exposure status at the time of Mayo Clinic Study of Aging (MCSA) visit. Time since enrollment was grouped into intervals based on planned time between MCSA visits (first z-score at enrollment, 8–22, 23–37, 38–52, and 53–67 months). A z-score measures how far a score is (in standard deviations) from the mean.
Table 2:
Cognitive scores and time following admission to the ICU*
| Scale Exposure count |
Slope est. |
Change in slope for each exposure est. (95% CI) |
P |
|---|---|---|---|
| Global z score | |||
| No admissions | −0.028 | ||
| Following 1 admission | −0.057 | −0.028 (−0.044, −0.012) | <0.001 |
| Following 2 admissions | −0.085 | ||
| Memory | |||
| No admissions | 0.018 | ||
| Following 1 admission | −0.011 | −0.029 (−0.047, −0.011) | 0.002 |
| Following 2 admissions | −0.040 | ||
| Language | |||
| No admissions | −0.038 | ||
| Following 1 admission | −0.05 | −0.012 (−0.028, 0.004) | 0.130 |
| Following 2 admissions | −0.062 | ||
| Visuospatial | |||
| No admissions | −0.007 | ||
| Following 1 admission | −0.020 | −0.013 (−0.026, −0.001) | 0.043 |
| Following 2 admissions | −0.034 | ||
| Attention/executive | |||
| No admissions | −0.056 | ||
| Following 1 admission | −0.077 | −0.021 (−0.037, −0.004) | 0.016 |
| Following 2 admissions | −0.097 |
Results are from multivariable linear mixed effects models. Slope estimate presented is for the average slope among all patients following the given number of admissions. As a result of the model specification, change in slope following ICU admission is the same after each subsequent ICU admission and therefore only presented once in the table. P-values test whether the change in slope due to ICU admission is significantly different from 0.
Figure 2B shows simulated paths of four hypothetical patients with escalating risks for cognitive decline (see legend) under no post-enrollment ICU admission and with one or two post-enrollment ICU admissions at 2 (and 4) years. These plots demonstrate substantial variability in global z-scores at enrollment and show that subsequent population average changes associated with ICU admissions are small relative to this overall variation.
Figure 2 B.
Simulated trajectories for four hypothetical patients under three scenarios: no critical care admission during follow-up; a single ICU admission at 2 years post-enrollment; and a second ICU admission 4 years post-enrollment. Follow-up is described from enrollment through 8 years. The four hypothetical patients were chosen to represent varying degrees of health at enrollment. Patient 1 is an 80-year-old female, never a smoker, married, with ≥16 years of education, with no comorbidities, APOE ε4 negative, and CU at enrollment. Patient 2 is an 80-year old male, never a smoker, married, 13–15 years of education, with prior history of coronary artery disease and a Charlson Comorbidity Index score of 2, APOE ε4 negative, and CU at enrollment. Patient 3 is an 80-year-old female, current smoker, single-partner status, 12 years of education, with prior history of stroke and a Charlson Comorbidity Index score of 2, APOE ε4 positive, and CU at enrollment. Patient 4 is an 80-year-old male, former smoker, single-partner status, <12 years of education, prior history of coronary artery disease and a Charlson Comorbidity Index score of 4, with midlife dyslipidemia, APOE ε4 positive, and mild cognitive impairment at enrollment. The plot demonstrates that changes over time attributable to ICU admission post-enrollment represent a subtle, although statistically significant, change in the average trajectory of cognitive z-scores relative to the variability in z-scores inherent in the population at enrollment. Diagnosis of MCI or dementia requires comprehensive clinical evaluation, but for illustration purposes we indicate z-score thresholds for mild and moderate impairment. Mild impairment is < −1, or more than 1 standard deviation below population norms; moderate impairment is typically < −2, more than 2 standard deviations below population norms. Abbreviations: MCI, mild cognitive impairment
Secondary analyses
The association between ICU admissions and global cognitive z-score slope did not differ between those who had MCI vs. CU at enrollment (index visit) (interaction P=0.705). Similarly, there was no evidence of a significant interaction between ICU admissions and age at enrollment (P=0.853) suggesting the association between ICU admission and global cognitive decline is similar regardless of patient age. Further, there was no evidence of an interaction with admission type (medical, surgical, or trauma, P=0.906). The same interactions (MCI vs CU, age, and admission type) were assessed for domain-specific cognitive scores and suggested no significant evidence of interaction (data not shown).
In a model including presence or absence of delirium as a modifier, decline was greater after delirium for domains of memory (interaction term P=0.006) and language (interaction term P=0.002) (Table 3). For global z-score, visuospatial, and attention/executive domain-specific z-scores the interaction was not statistically significant (interaction P=0.051, 0.270, 0.236 for global, visuospatial, and attention/executive domains, respectively, Table 3), but point estimates suggest worse outcomes following admission with presence of delirium.
Table 3:
Estimates according to presence of positive delirium assessment during critical care episode*
| No Delirium | Delirium | ||||
|---|---|---|---|---|---|
| Outcome | Change in slope est. (95% CI) |
P | Change in slope est. (95% CI) |
P | Interaction P |
| Global z score | −0.020 (−0.037, −0.003) | 0.025 | −0.066 (−0.111,−0.022) | 0.003 | 0.051 |
| Memory | −0.015 (−0.035, 0.005) | 0.136 | −0.090 (−0.140,−0.040) | <0.001 | 0.006 |
| Language | 0.000 (−0.017, 0.016) | 0.968 | −0.067 (−0.107,−0.027) | <0.001 | 0.002 |
| Visuospatial | −0.008 (−0.022, 0.006) | 0.259 | −0.028 (−0.061, 0.005) | 0.099 | 0.270 |
| Attention/executive | −0.015 (−0.033, 0.003) | 0.114 | .0.044 (−0.090, 0.002) | 0.059 | 0.236 |
Results are from multivariable linear mixed effects models. P-values test whether the change in slope due to ICU admission is significantly different from 0. Interaction p-values test whether the effect of ICU admission on slope differs according to whether or not a positive delirium score was recorded during the ICU admission.
DISCUSSION
The main finding of this study is that ICU admissions during hospitalization are associated with subsequent cognitive decline beyond the average decline observed in patients without critical care admissions. Greater cognitive decline was evident for memory, visuospatial, and attention/executive domains, the same domains affected by exposure to surgery and anesthesia [9] and Alzheimer’s disease dementia [38]. Among patients admitted to the ICU, the postadmission change in memory and language domains was significantly more pronounced in those who developed delirium.
A causal relationship between chronic diseases, critical illness and cognitive decline is difficult to decipher, and it remains unclear whether cognitive decline is related to severity of comorbid conditions, adverse events during ICU stay, or the hospitalization per se [1, 4, 39]. Cardiovascular and cerebrovascular diseases increase the risk for cognitive decline [40–42]. In a prior study, exposure to vascular surgery, but not other types of surgery, after age 60 was associated with MCI [8], suggesting that the need for vascular surgery was a marker of morbidity associated with MCI. Our study showed no evidence of an interaction with admission type; estimates and confidence intervals further suggested similar outcomes for surgical, including cardiac surgical patients, medical and trauma patients. Interpretation of causality is further complicated by reports that showed that MCI/dementia independently increase the risk for ICU admission [3, 14, 15]. We found no interaction between pre-existing MCI and cognitive decline following ICU admission.
Two studies suggest that at least part of long-term cognitive impairment is related to the ICU admission per se [1, 39]. Ehlenbach et al.[1] using the Cognitive Ability Screening Instrument score (0–100) on older adults reported 2.14 points lower scores (6.1 years mean follow-up) for those with critical care hospitalization compared to those who did not have critical care hospitalization. Iwashyna et al.[39] reported association between hospital admission for severe sepsis and persistent cognitive impairment in years following discharge: patients were followed up for up to 7.8 years prior to sepsis, and up to 8.3 years after surviving sepsis. In our study, we assessed study participants with a total follow up of median 4 years (among those with an ICU admission, with median [25th, 75th percentiles] 2.5 [1.2, 4.4] years of follow up following ICU admission) and found that the slope over time for global cognitive z-scores was significantly worse in those admitted to ICU compared with those not requiring hopitalziation with ICU admission. Furthermore, we confirmed the association between ICU delirium and long-term cognitive impairment [10, 43]. In a model including presence or absence of delirium as a modifier, decline was greater after delirium for domains of memory and language. However, it is unclear whether delirium is a marker for preexisting cognitive decline or whether delirium could produce neurologic injury accelerating the trajectory of cognitive decline [44–47].
Age-related cognitive decline affects cognitive domains differentially [48], and this non-uniformity was observed in our patients after exposure to an ICU setting. We found that ICU admission was associated with decline in memory, visuospatial, and executive domains, but not language. Language is a complex cognitive domain which remains relatively intact with aging [49–51], and remained unaffected when we included all patients admitted to the ICU. However, in ICU patients who developed delirium we observed decline in language domain. The pathophysiological explanation for this differential pattern of domain involvement observed in our patients remains undetermined.
Limitations of the study.
The primary limitation is potential for unmeasured confounding. Since critical care episodes are intermixed with effects of various observed and unobserved comorbid conditions and sometimes with exposure to anesthesia and surgery, we cannot separate the potential effects of critical care admission from effects of conditions that led to admission. Furthermore, critical care episodes and ICU admission may be interspersed with non critical-care hospitalization, and studies have shown that any type of hospitalization may be associated with increased hazard for development of dementia in years following hospitalization [1]. Therefore, our findings cannot distinguish whether critical care admission is associated with additional decline in cognitive trajectory beyond that related to the entire hospital stay (i.e. stay on both hospital wards and in the ICU). Also, because the MCSA was not designed specifically for the current investigation, the timing of the MCSA visits did not correspond to any consistent time interval surrounding ICU admission. An ideal study should obtain cognitive scores immediately before ICU admission, however since ICU admissions are not elective this is not feasible. Delirium is a state of fluctuating consciousness and a determination based on 3 static daily measurements may not fully capture incidence of delirium in this cohort; further, we did not assess severity or duration of delirium. ICU type was categorized as medical or surgical, with surgical defined by surgery in the 12 hours prior to or 8 hours after ICU discharge; some medical admissions may have required surgical intervention outside this definition. This study used data from patients admitted to a large tertiary medical center with a large surgical service and may not be generalizeable to other centers. In particular, ICU length of stay in our study is shorter than reported by others [52], and we can only speculate that this may be attributed to our practice having a higher percentage of surgical ICU admissions. Since shorter ICU stay may translate into lower disease acuity, this in turn may lead to underestimation of the association with cognitive outcomes. Finally, some patients may drop out from the MCSA with or without an ICU hospitalization and it is possible that cognitive trajectory of these patients differs from those who continued follow-up.
In conclusion, hospitalization associated with ICU admission was associated with cognitive decline in older adults beyond declines observed in persons not admitted to an ICU. Among patients admitted to the ICU, the post-admission change in memory and language was greater in those who experienced delirium. Better understanding the nature of the relationship between hospitalization with ICU admission and cognitive decline may help in developing preventive measures.
Supplementary Material
Key Points.
Findings:
Patients who required care in an intensive care unit (ICU) experienced more profound cognitive decline in years following admission compared to patients who did not have ICU admission. Among patients admitted to the ICU, the change in memory and language cognitive domains was significantly more pronounced in those who experienced delirium.
Meaning:
The magnitude of the observed cognitive deterioration in older adults after critical illness was generally small. The cognitive decline could be functionally meaningful for individuals with already low baseline cognition or those who develop delirium.
Role of the funding source:
This study was supported by the NIH grants U01 AG006786 (PI: RCPetersen), P50 AG016574 (PI: RC Petersen), RF1 AG55151 (PI: MM Mielke), by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, the Rochester Epidemiology Project (R01 AG034676, PIs: WA Rocca and J St. Sauver) and the Mayo Clinic Center for Translational Sciences Activities (CTSA), grant number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). In addition, this study was supported by CTSA Grant Number KL2 TR002379 to MA Warner from NCATS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Alphabetical List of Abbreviations:
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- REP
Rochester Epidemiology Project
Footnotes
Copyright form disclosure:
Drs. Schulte, Mielke, Knopman, and Petersen received support for article research from the National Institutes of Health. Dr. Martin disclosed that he serves on the Board of Directors for the American Society of Anesthesiologists (ASA), he receives an annual stipend from ASA for contributions to the Patient Safety CME Editorial Board, and he and Mayo Clinic owns equity and receives royalties for work licensed through Mayo Clinic to Nevro, Inc, a publicly held company, for contributions related to the use of nerve signal modulation to treat central, autonomic, and peripheral nervous system disorders, including pain. Dr. Mielke’s institution received funding from Biogen and Lunbeck, and she received funding from Eli Lilly. Dr. Knopman received funding from Washington University (DSMB activities). Dr. Petersen’s institution received funding from National Institute on Aging; he received funding from Roche (consultant), Merck (consultant), Genentech (DSMB), Biogen, Eisai, and GE Healthcare; and he received other support from benefactors through the Mayo Clinic. Dr. Weingarten received funding from Medtronic (served as chairman of the clinical event committee of the PRODIGY trial, which examined postoperative capnography monitoring) and Merck (received an investigator-initiated, unrestricted grant to study the effects of sugammadex on return of postoperative bowel function), and he disclosed work for hire. Dr. Warner’s institution received funding from CTSA Grant Number KL2 TR002379 from the National Center for Advancing Translational Science (NCATS). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Declaration of interests: We, the Authors, declare that we have no competing interests.
References
- 1.Ehlenbach WJ, Hough CL, Crane PK, et al. : Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA 2010; 303(8):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CC, Chiu MJ, Chen SP, et al. : Patterns of cognitive change in elderly patients during and 6 months after hospitalisation: a prospective cohort study. Int J Nurs Stud 2011; 48(3):338–346. [DOI] [PubMed] [Google Scholar]
- 3.Phelan EA, Borson S, Grothaus L, et al. : Association of incident dementia with hospitalizations. JAMA 2012; 307(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandharipande PP, Girard TD, Jackson JC, et al. : Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathews SB, Arnold SE, Epperson CN: Hospitalization and cognitive decline: Can the nature of the relationship be deciphered? Am J Geriatr Psychiatry 2014; 22(5):465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopkins RO, Jackson JC: Assessing neurocognitive outcomes after critical illness: are delirium and long-term cognitive impairments related? Curr Opin Crit Care 2006; 12(5):388–394. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins RO, Jackson JC: Long-term neurocognitive function after critical illness. Chest 2006; 130(3):869–878. [DOI] [PubMed] [Google Scholar]
- 8.Sprung J, Jankowski CJ, Roberts RO, et al. : Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clin Proc 2013; 88(6):552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulte PJ, Roberts RO, Knopman DS, et al. : Association between exposure to anaesthesia and surgery and long-term cognitive trajectories in older adults: report from the Mayo Clinic Study of Aging. Br J Anaesth 2018; 121(2):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sprung J, Roberts RO, Weingarten TN, et al. : Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth 2017; 119(2):316–323. [DOI] [PubMed] [Google Scholar]
- 11.Bynum JP, Rabins PV, Weller W, et al. : The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc 2004; 52(2):187–194. [DOI] [PubMed] [Google Scholar]
- 12.Patrick L, Gaskovski P, Rexroth D: Cumulative illness and neuropsychological decline in hospitalized geriatric patients. Clin Neuropsychol 2002; 16(2):145–156. [DOI] [PubMed] [Google Scholar]
- 13.Chodosh J, Seeman TE, Keeler E, et al. : Cognitive decline in high-functioning older persons is associated with an increased risk of hospitalization. J Am Geriatr Soc 2004; 52(9):1456–1462. [DOI] [PubMed] [Google Scholar]
- 14.Teeters DA, Moua T, Li G, et al. : Mild cognitive impairment and risk of critical illness. Crit Care Med 2016; 44(11):2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulte PJ, Martin DP, Deljou A, et al. : Effect of cognitive status on the receipt of procedures requiring anesthesia and critical care admissions in older adults. Mayo Clin Proc 2018; 93(11):1552–1562. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, et al. : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 17.Roberts RO, Geda YE, Knopman DS, et al. : The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, van Dyck CH, Alessi CA, et al. : Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of internal medicine 1990; 113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 19.Inouye SK, Marcantonio ER, Kosar CM, et al. : The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2016; 12(7):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barr J, Pandharipande PP: The pain, agitation, and delirium care bundle: synergistic benefits of implementing the 2013 Pain, Agitation, and Delirium Guidelines in an integrated and interdisciplinary fashion. Crit Care Med 2013; 41(9 Suppl 1):S99–115. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, et al. : Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC: The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43(11):2412–2414. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer RI, Kurosaki TT, Harrah CH Jr., et al. : Measurement of functional activities in older adults in the community. J Gerontol 1982; 37(3):323–329. [DOI] [PubMed] [Google Scholar]
- 24.Kokmen E, Smith GE, Petersen RC, et al. : The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol 1991; 48(7):725–728. [DOI] [PubMed] [Google Scholar]
- 25.Hachinski VC, Iliff LD, Zilhka E, et al. : Cerebral blood flow in dementia. Arch Neurol 1975; 32(9):632–637. [DOI] [PubMed] [Google Scholar]
- 26.Rosen WG, Terry RD, Fuld PA, et al. : Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980; 7(5):486–488. [DOI] [PubMed] [Google Scholar]
- 27.Fahn S, Elton RL, Committee UD: Unified Parkinson’s Disease Rating Scale In: Recent Developments in Parkinson’s Disease Edited by Fahn S, Marsden CD, Calne DB, Goldstein M, vol. 2: Florham Park: MacMillan; Healthcare Information; 1987, pp 153–163. [Google Scholar]
- 28.Wechsler DA: Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation 1987. [Google Scholar]
- 29.Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Percept Motor Skills 1958; 8:271–276. [Google Scholar]
- 30.Kaplan EF, Goodglass H, Weintraub S: The Boston Naming Test. 2nd ed Philadelphia: Lea & Febiger 1982. [Google Scholar]
- 31.Lucas JA, Ivnik RJ, Smith GE, et al. : Mayo’s Older Americans Normative Studies: category fluency norms. J Clin Exp Neuropsychol 1998; 20:194–200. [DOI] [PubMed] [Google Scholar]
- 32.Wechsler DA: Wechsler Memory Scale-Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 33.Ivnik RJ, Malec JF, Smith GE: WAISR, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol 1992; 6 (suppl):83–104. [Google Scholar]
- 34.Vemuri P, Lesnick TG, Przybelski SA, et al. : Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol 2014; 71(8):1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen RC: Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256(3):183–194. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association; Diagnostic and Statistical Manual of Mental Disorders. DSM-IV. In, 4th edn Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 37.Rocca WA, Yawn BP, St Sauver JL, et al. : History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clin Proc 2012; 87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knopman DS, Beiser A, Machulda MM, et al. : Spectrum of cognition short of dementia: Framingham Heart Study and Mayo Clinic Study of Aging. Neurology 2015; 85(19):1712–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwashyna TJ, Ely EW, Smith DM, et al. : Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304(16):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanetti M, Ballabio C, Abbate C, et al. : Mild cognitive impairment subtypes and vascular dementia in community-dwelling elderly people: a 3-year follow-up study. J Am Geriatr Soc 2006; 54(4):580–586. [DOI] [PubMed] [Google Scholar]
- 41.Casserly I, Topol E: Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet 2004; 363(9415):1139–1146. [DOI] [PubMed] [Google Scholar]
- 42.Edwards JD, Ramirez J, Black SE: Unraveling the potential co-contributions of cerebral small vessel vasculopathy to the pathogenesis of Alzheimer’s dementia. Alzheimers Res Ther 2015;7(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Boogaard M, Schoonhoven L, Evers AW, et al. : Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med 2012; 40(1):112–118. [DOI] [PubMed] [Google Scholar]
- 44.Jackson JC, Hart RP, Gordon SM, et al. : Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med 2003; 31(4):1226–1234. [DOI] [PubMed] [Google Scholar]
- 45.Trzepacz PT: Update on the neuropathogenesis of delirium. Dement Geriatr Cogn Disord 1999; 10(5):330–334. [DOI] [PubMed] [Google Scholar]
- 46.Eikelenboom P, Hoogendijk WJ: Do delirium and Alzheimer’s dementia share specific pathogenetic mechanisms? Dement Geriatr Cogn Disord 1999; 10(5):319–324. [DOI] [PubMed] [Google Scholar]
- 47.Fong TG, Jones RN, Shi P, et al. : Delirium accelerates cognitive decline in Alzheimer disease. Neurology 2009; 72(18):1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goh JO, An Y, Resnick SM: Differential trajectories of age-related changes in components of executive and memory processes. Psychol Aging 2012; 27(3):707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harada CN, Natelson Love MC, Triebel KL: Normal cognitive aging. Clin Geriatr Med 2013; 29(4):737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayden KM, Welsh-Bohmer KA: Epidemiology of cognitive aging and Alzheimer’s disease: contributions of the cache county utah study of memory, health and aging. Curr Top Behav Neurosci 2012; 10:3–31. [DOI] [PubMed] [Google Scholar]
- 51.Singh-Manoux A, Kivimaki M, Glymour MM, et al. : Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012; 344:d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter A, Johnson L, Coustasse A: Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick) 2014; 33(2):128–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.