Abstract
Background and Objectives:
Vascular invasion, in particular extramural venous invasion (EMVI), is a pathologic characteristic that has been extensively studied in rectal cancer but rarely in colon cancer. This study aims to evaluate its prognostic role in stage II-III colon cancer.
Methods:
All stage II-III colon cancer patients who underwent surgery between 2004–2015 were reviewed. We divided the study group into patients without invasion, with intramural invasion only (IMVI), EMVI only, and both IMVI/EMVI (n=923).
Results:
EMVI was associated with other high-risk features, including T4, N+ disease, lymphatic, and perineural invasion (P<0.001). EMVI+ patients had considerably higher rates of locoregional and distant recurrence and subsequently disease-specific mortality (stage-II: odds ratio (OR) 3.64, P=0.001, stage-III OR:1.94, P=0.009), whereas outcomes were comparable between IMVI and no vascular invasion (OR:1.21, P=0.764, OR:1.28, P=0.607, respectively). The adjusted hazard ratios for EMVI+ patients on disease-free survival, and disease-specific survival were 2.07 (P<0.001), 1.67 (P=0.027), respectively. Moreover, EMVI+ stage-II patients fared worse than EMVI– stage-III patients, even after adjusting for adjuvant chemotherapy.
Conclusion:
EMVI is a strong predictor for worse oncologic outcomes in stage II-III colon cancer patients, whereas IMVI is not. It is also associated with worse outcomes compared in patients with higher stage disease who are EMVI negative.
Keywords: colon cancer, intramural vascular invasion, extramural vascular invasion, outcomes, recurrence, survival, adjuvant chemotherapy
Introduction
Colorectal cancer is one of the most prevalent malignancies in both men and women worldwide. Survival and recurrence rates vary considerably depending on baseline staging and tumor characteristics [1]. Available adjuvant and neoadjuvant treatment options surrounding operative treatment range from surveillance to chemoradiation regimens. The decision whether or not to treat needs to be made on a case-by-case basis, considering the risks of both under and over treatment. To address this, efforts have been made to identify factors beyond the standard Tumor, Node, Metastasis (TNM) classification to stratify risks of recurrence and mortality. As a result, pathological and molecular features including poorly differentiated cancers, lymphovascular invasion, perineural invasion, and microsatellite instability have been validated as risk factors [2–4].
Traditionally, validation of prognostic factors is done in cohorts grouping colon and rectal cancer together, rather than separately, for the sake of statistical power even though treatment approaches and tumor biology are markedly different [5–7]. It is necessary to ensure that such factors are valid for colon cancer as well as rectal cancer. An example of this discrepancy is extramural vascular invasion (EMVI) or vascular invasion beyond the muscularis propria. In large part due to the potential finding of EMVI during preoperative magnetic resonance imaging (MRI) in rectal cancer [8,9], a diagnostic modality that is not routinely performed in tumors of the colon. EMVI has been well scrutinized in rectal cancer [10–12] but far less so in tumors of the colon [13]. Nonetheless, the College of American Pathologists recommend recording the status of vascular invasion during routine pathologic examination in both colon and rectal cancer patients [14] because of the unfavorable outcomes and increased risk of hepatic metastasis [15]. Current guidelines also incorporate vascular invasion as a histologic risk feature in colon cancer, for which adjuvant therapy could be considered. Besides lacking data on colon cancer specific outcomes, little is known about the importance of separating intramural and extramural venous invasion. Therefore, the aim of this study was to evaluate the impact of vascular invasion, both intramural and extramural, on long-term oncologic outcomes in stage II and III colon cancer patients without distant metastasis.
Methods
Patients
All patients treated surgically for a primary colorectal carcinoma at Massachusetts General Hospital between 2004 and 2015 (n=2287) were included in a prospectively maintained survival and outcomes database after institutional review board approval. Data on patients was gathered from patient visit records, the institutional research patient data repository, the social security death index, as well as patient records from our healthcare network.
Due to the significant differences in treatment approach, tumor biology, and the intent to specifically explore the impact of vascular invasion on colonic tumors, we exclusively focused on colon cancer and did not include patients with tumors of the rectum (n=642). We excluded all patients with intramucosal tumors (n=174) and patients with baseline metastatic disease (n=246). Furthermore, as only 14 out of 285 patients with stage I disease16 revealed either intra- or extramural vascular invasion, we decided to exclude patients with stage I disease as well, leaving 923 patients for final analysis. We divided patients into four groups: no invasion, intramural vascular invasion (IMVI) only, extramural vascular invasion (EMVI) only, and both IMVI and EMVI.
Pathologic examination
Standardized pathologic examination was performed by a team of dedicated gastrointestinal pathologists during the full length of our study. For the purpose of this paper, tumors of the colon were defined as any tumor more than 15 centimeters from the anal verge. Right-sided tumors included those located from the cecum to the hepatic flexure, transverse colon cancer included transverse tumors only, tumors located from the splenic flexure proximal to the sigmoid were defined as left-sided cancer, and (recto)sigmoid tumors were located from the sigmoid to the rectosigmoid. Tumor stage was assessed according to the seventh edition of the American Joint Committee on Cancer [16]. Tumor grading was categorized according to the classification designed by the World Health Organization [17].
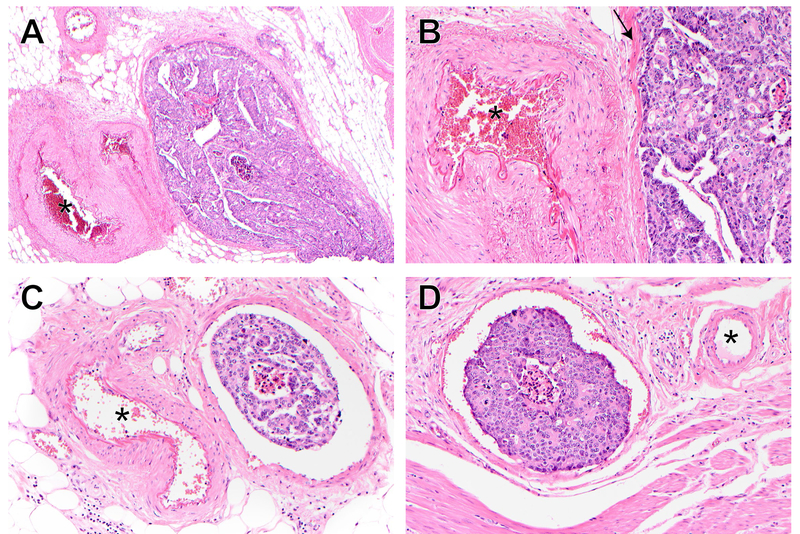
The presence of vascular invasion was assessed on hematoxylin and eosin-stained (H&E) slides. Vessels with an unequivocal endothelial lining were considered lymphatic (small), whereas large vessels (venous) included all with a muscular wall. In suspicious cases, sections at multiple levels and elastic stains have been used to confirm venous invasion. Intramural vascular invasion (IMVI) was defined as the presence of large vessel invasion in the submucosal and/or muscular layer. Venous invasion beyond the muscularis propria was considered extramural vascular invasion (EMVI). [Figure 1]
Figure 1.
Histology of intramural and extramural vascular invasion.
A: EMVI in a large vein, x40.
B: Higher magnification of A (x200) highlights vein wall (arrow).
C: EMVI in a smaller vein, x200.
D: IMVI, x200.
* in all panels highlights muscular artery
Primary and secondary outcomes
Disease recurrence was our primary outcome, divided into locoregional recurrence, including all recurrences within the original tumor bed (contiguous to the original site of the tumor, peri-anastomotic, peritoneum, and retroperitoneum), and distant recurrence (liver, lung, and other nonregional organs). Determination of disease recurrence was made by histological or clinical and radiological examinations.
Secondary outcomes were time to disease recurrence, and overall and disease-specific survival. Data on long-term outcomes and survival were periodically updated by reviewing patient’s records and the US Social Security Death Index. The last status review of survival and follow-up was on March 1st, 2018. Patients alive at the closure of the study or lost to follow-up were censored. All time to events were expressed in months, measured from date of surgery. Recurrent or metastatic disease within 30 days of the original admission was considered baseline metastases and therefore excluded from our study cohort.
All patients underwent a standardized surveillance according to the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) guidelines [6,18]. Postoperative treatment was considered for all patients. The decision whether or not to administrate adjuvant chemotherapy was made on an individual basis after reviewing pathology results and assessing the performance status of the patients and their consent to the therapy.
Statistical analysis
Statistical analysis was performed using SPSS (Version 24.0; SPSS Inc, Chicago, IL, USA). A two-tailed P-value below 0.05 was considered the threshold for statistical significance. Descriptive statistics (percentage, medians with interquartile range or means with standard deviation) were used to illustrate differences in baseline characteristics, if any. Subsequently, outcomes were compared among EMVI positive and negative patients. Outcomes analyzed were, metastatic recurrence, and overall and disease-specific mortality, expressed as percentage outcomes, compared for significance using a chi-square (X2) coefficient. Kaplan-Meier survival estimates were compared using Log-Rank tests.
Additionally, multivariate analyses using Cox proportional hazard regression models were performed to analyze the impact of vascular invasion on disease recurrence and colon cancer specific survival. Hazard ratios (HR) with corresponding 95% confidence intervals (CI) were estimated. Variables included in the model were: age, ASA-score, vascular invasion (no invasion – IMVI only – EMVI only – both IMVI and EMVI), TN-stage, tumor location, lymphatic invasion, high grade disease (including poorly differentiated adenocarcinomas, mucinous and signet-cell carcinomas), perineural invasion, microsatellite instability, bowel obstruction at presentation, R0-resection, and adjuvant chemotherapy. Lastly, differences in long-term outcomes were also demonstrated per AJCC substage using Kaplan-Meier survival analyses, as well as for the EMVI-subgroup only.
Results
Baseline characteristics
A consecutive cohort of 923 patients with AJCC stage II or stage III was included, of whom 59 patients had intramural vascular invasion only on surgical pathology, 163 patients had extramural vascular invasion, and 59 patients had both IMVI and EMVI. None of the baseline characteristics, including age, gender, ethnicity, BMI, and emergency admissions differed significantly based on vascular invasion status. Patients with vascular invasion, regardless of the precise location, presented more often with a large bowel obstruction (10.7% vs. 5.9%; P=0.011), while perforation at presentation rates were comparable. Table 1 shows baseline characteristics in detail.
Table 1:
Baseline and Tumor Characteristics
| Total n = 923 |
No invasion n = 642 |
IMVI only n = 59 |
EMVI only n = 163 |
Both n = 59 |
P-value | |
|---|---|---|---|---|---|---|
| Age, years | 69.0 (57.7–79.8) | 69.4 (57.8–79.8) | 74.9 (60.2–82.6) | 68.1 (58.0–78.6) | 65.9 (55.1–78.7) | 0.329 |
| Female gender | 487 (52.8%) | 342 (53.3%) | 35 (59.3%) | 83 (50.9%) | 27 (45.8%) | 0.481 |
| Caucasian | 826 (89.5%) | 575 (89.6%) | 55 (93.2%) | 143 (87.7%) | 53 (89.8%) | 0.782 |
| ASA score | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.314 |
| BMI, kg/m2 | 26.7 (23.2–30.7) | 26.8 (23.3–30.8) | 27.2 (23.1–31.7) | 26.4 (23.3–29.8) | 26.9 (22.7–33.2) | 0.797 |
| Inflammatory Bowel disease | 27 (2.9%) | 19 (3.0%) | 1 (1.7%) | 5 (3.1%) | 2 (3.4%) | 0.946 |
| Urgent admission | 99 (10.7%) | 66 (10.3%) | 8 (13.6%) | 16 (9.8%) | 9 (15.3%) | 0.566 |
| Bowel obstruction | 68 (7.4%) | 38 (5.9%) | 6 (10.2%) | 17 (10.4%) | 7 (11.9%) | 0.084 |
| Bowel perforation | 25 (2.7%) | 18 (2.8%) | 1 (1.7%) | 4 (2.5%) | 2 (3.4%) | 0.941 |
| Tumor location | 0.140 | |||||
| Right-sided | 479 (51.9%) | 336 (52.3%) | 38 (64.4%) | 82 (50.3%) | 23 (39.0%) | |
| Transverse colon | 83 (9.0%) | 60 (9.3%) | 3 (5.1%) | 12 (7.4%) | 8 (13.6%) | |
| Left-sided | 94 (10.2%) | 67 (10.4%) | 8 (13.6%) | 12 (7.4%) | 7 (11.9%) | |
| (Recto)sigmoid colon | 250 (27.1%) | 166 (25.9%) | 9 (15.3%) | 54 (33.1%) | 21 (35.6%) | |
| Multiple sites | 17 (1.8%) | 13 (2.0%) | 1 (1.7%) | 3 (1.8%) | 0 (0.0%) | |
| R0-resection | 890 (96.4%) | 625 (97.4%) | 57 (96.6%) | 154 (94.5%) | 54 (91.5%) | 0.057 |
| Tumor characteristics | ||||||
| Stage II disease | 465 (50.4%) | 367 (57.2%) | 33 (55.9%) | 51 (31.3%) | 14 (23.7%) | <0.001 |
| Tumor size | 5.0 (3.5–7.5) | 5.2 (3.5–7.7) | 4.7 (3.5–7.5) | 5.0 (3.5–7.0) | 5.3 (3.5–10.9) | 0.604 |
| High grade | 239 (26.1%) | 154 (24.2%) | 13 (22.4%) | 51 (31.3%) | 21 (36.2%) | 0.073 |
| T4 tumor | 237 (25.7%) | 130 (20.2%) | 12 (20.3%) | 67 (41.1%) | 28 (47.5%) | <0.001 |
| Lymphatic invasion | 376 (40.7%) | 194 (30.2%) | 27 (45.8%) | 107 (65.6%) | 48 (81.4%) | <0.001 |
| Perineural invasion | 211 (22.9%) | 90 (14.0%) | 15 (25.4%) | 69 (42.6%) | 37 (62.7%) | <0.001 |
| Lymph nodes examined | 21 (16–29) | 21 (16–29) | 21 (16–29) | 20 (16–29) | 23 (17–35) | 0.605 |
| <12 lymph nodes examined | 69 (7.5%) | 48 (7.5%) | 3 (5.1%) | 12 (7.4%) | 6 (10.2%) | 0.775 |
| Microsatellite instability | 0.061 | |||||
| High | 125 (13.5%) | 95 (14.8%) | 7 (11.9%) | 14 (8.6%) | 9 (15.3%) | |
| Low | 39 (4.2%) | 31 (4.8%) | 3 (5.1%) | 5 (3.1%) | 9 (0.0%) | |
| Stable | 392 (42.5%) | 251 (39.1%) | 32 (54.2%) | 79 (48.5%) | 30 (50.8%) | |
| Not tested | 367 (39.8%) | 265 (41.3%) | 17 (28.8%) | 65 (39.9%) | 20 (33.9%) | |
Proportions are presented for categorical data, median with IQR for all continuous data.
Abbreviations: ASA: American Society of Anesthesiologists, BMI: Body Mass Index (kg/m2)
Tumor location did not differ between the groups. Rates of R0-resections tended to be lower when IMVI and EMVI were both present (P=0.057). Patients with EMVI+ tumors or both IMVI/EMVI demonstrated significantly higher rates of node-positive disease as well as higher incidences of T4 tumors, lymphatic invasion, and perineural invasion. Numbers of examined lymph nodes were not different between the groups; neither did the number of patients in whom less than 12 lymph nodes were examined.
Outcomes
Vascular invasion was present in 21.1% of stage II patients and 40.0% of stage III patients (Table 2 and 3). An increasing rate was in particular true for EMVI+ patients (stage II: 11.0%; stage III: 24.5%). The detection rate of vascular invasion, however, slightly increased over the study period from 27.5% in the first half (stage II: 16.7%, stage III: 38.6%) to 33.3% (25.3%, 41.3%, respectively) in the latter. Lymphatic invasion was far more prevalent in EMVI+ or IMVI/EMVI+ patients, with a significant higher rate than IMVI+ patients in stage II (P<0.001). Moreover, presence of small vessel invasion in vascular negative patients was lower, though certainly not absent (stage II: 19.1%; stage III: 45.1%).
Table 2:
Outcome differences by vascular invasion status, stage II (n=465)
| Stage II patients | No invasion n = 367 |
IMVI only n = 33 |
EMVI only n = 51 |
Both n = 41 |
P-value |
|---|---|---|---|---|---|
| Lymphatic invasion | 70 (19.1%) | 8 (24.2%) | 21 (41.2%) | 9 (64.3%) | <0.001 |
| Adjuvant chemotherapy | 60 (16.3%) | 5 (15.2%) | 17 (33.3%) | 7 (50.0%) | 0.001 |
| Locoregional recurrence | 26 (7.1%) | 0 (0.0%) | 10 (19.6%) | 2 (14.3%) | 0.004 |
| Distant recurrence | 42 (11.4%) | 2 (6.1%) | 12 (23.5%) | 3 (21.4%) | 0.042 |
| Disease-free survival | 0.004 | ||||
| K-M 3-year estimate | NAR | 88.6% | 238 | 93.3% | 28 | 67.5% | 23 | 71.8% | 7 | |
| K-M 5-year estimate | NAR | 85.2% | 174 | 93.3% | 28 | 67.5% | 23 | 71.8% | 7 | |
| Overall survival | <0.001 | ||||
| K-M 3-year estimate | NAR | 85.4% | 239 | 85.9% | 20 | 69.6% | 28 | 36.9% | 2 | |
| K-M 5-year estimate | NAR | 78.6% | 154 | 79.8% | 13 | 57.3% | 16 | 36.9% | 2 | |
| Colon cancer specific survival | <0.001 | ||||
| K-M 3-year estimate | NAR | 96.5% | 239 | 93.2% | 27 | 81.8% | 28 | 69.2% | 5 | |
| K-M 5-year estimate | NAR | 95.0% | 156 | 93.2% | 27 | 77.0% | 16 | 69.2% | 5 |
Abbreviations: IMVI: Intramural Vascular Invasion; EMVI: Extramural Vascular Invasion; K-M: Kaplan Meier. NAR: Number at risk
Survival estimates calculated by log-rank
Table 3:
Outcome differences by vascular invasion status, stage III (n=458)
| Stage III patients | No invasion n = 275 |
IMVI only n = 26 |
EMVI only n = 112 |
Both n = 45 |
P-value |
|---|---|---|---|---|---|
| Lymphatic invasion | 124 (45.1%) | 19 (73.1%) | 86 (76.8%) | 39 (86.7%) | <0.001 |
| Adjuvant chemotherapy | 197 (71.6%) | 20 (76.9%) | 83 (74.1%) | 32 (71.1%) | 0.909 |
| Locoregional recurrence | 35 (12.7%) | 6 (23.1%) | 29 (25.9%) | 8 (17.8%) | 0.015 |
| Distant recurrence | 49 (17.8%) | 6 (23.1%) | 49 (43.8%) | 14 (31.1%) | <0.001 |
| Disease-free survival | <0.001 | ||||
| K-M 3-year estimate | NAR | 81.0% | 153 | 78.5% | 18 | 52.3% | 37 | 61.4% | 18 | |
| K-M 5-year estimate | NAR | 76.6% | 90 | 70.7% | 9 | 46.1% | 28 | 55.8% | 10 | |
| Overall survival | 0.009 | ||||
| K-M 3-year estimate | NAR | 79.3% | 173 | 68.0% | 17 | 67.7% | 62 | 55.7% | 22 | |
| K-M 5-year estimate | NAR | 68.0% | 100 | 60.4% | 8 | 55.2% | 32 | 44.3% | 10 | |
| Colon cancer specific survival | <0.001 | ||||
| K-M 3-year estimate | NAR | 91.3% | 173 | 78.3% | 18 | 78.8% | 62 | 69.6% | 22 | |
| K-M 5-year estimate | NAR | 85.5% | 106 | 78.3% | 18 | 66.8% | 32 | 55.4% | 10 |
Abbreviations: IMVI: Intramural Vascular Invasion; EMVI: Extramural Vascular Invasion; K-M: Kaplan Meier. NAR: Number at risk Survival estimates calculated by log-rank
In line with current guidelines, rates of adjuvant chemotherapy were higher in stage III disease. A total of 332 stage III patients (72.5%) received postoperative treatment compared to 89 (19.1%) stage II patients. The most common reasons to forego further treatment were comorbidity or age (49.0%) and patient’s refusal (40.8%). In stage II disease, rates of postoperative chemotherapy admission were significantly higher in patients with EMVI (P=0.001), whereas no differences were found in stage III disease (P=0.909)
Regardless of stage, the presence of vascular invasion was strongly associated with locoregional and distant recurrence. In stage II, EMVI+ and IMVI/EMVI+ patients had significantly higher rates of locoregional and distant recurrence compared to patients without invasion or IMVI only (P=0.004, P=0.042, respectively), subsequently leading to impaired disease-free survival (DFS), overall survival (OS), and disease-specific survival (DSS). With regards to stage III disease, rates of distant recurrence increased substantially in all groups, but remained higher in the EMVI+ group (P<0.001).
Subgroup analysis
In stage II disease, time to disease recurrence was comparable between patients with no invasion and IMVI only (5-year DFS: 85.5% vs. 93.3%, P=0.332). Overall survival and disease-specific survival were also comparable between these two groups (OS: P=0.601, DSS: P=0.208). Nonetheless, EMVI+ patients demonstrated worse outcomes compared to no invasion (DFS: P=0.002, OS: P=0.001, DSS: P<0.001) (Figure 2).
Figure 2.

Survival outcomes per AJCC stage.
The poor prognosis for EMVI+ tumors was emphasized in stage III disease. Time to disease recurrence, overall survival, as well disease-free survival was all worse when extramural vascular invasion was present (P<0.001), while no differences between the IMVI group and no invasion group were found. Interestingly, the estimated survival rates of stage III patients without vascular invasion or IMVI+ only were comparable with stage II EMVI+ patients (DFS: P=0.281, DSS: P=0.101), indicating once more the importance of EMVI.
Survival and multivariate analyses
Median follow-up was 43.9 months, which was not significantly different between stage (II: 46.2 months vs. III: 40.7 months, P=0.122). In multivariate Cox proportional hazard models, time to disease recurrence remained significantly shorter in patients who were EMVI+ compared to those without (HR=2.07; 95% CI: 1.46 – 2.93, P<0.001)(Table 4). Although hazard ratios were higher in the IMVI/EMVI+ cohort, outcomes were not significantly different after adjustment (HR=1.52; 95% CI: 0.88 – 2.63, P=0.135). This was different in colon cancer specific survival, with more than two-fold higher hazard ratios for the IMVI/EMVI+ cohort (HR=2.39; 95% CI:1.31 – 4.36, P=0.005). Similarly to DFS, EMVI+ withstood adjustment in the DSS model (HR 1.67; 95% CI: 1.06 – 2.64, P=0.027). Along with vascular invasion, ASA-score, T4 tumors, lymph-node positive disease, high grade tumors, perineural invasion, and bowel obstruction at presentation were all found to be independent predictors for both DFS and DSS. Moreover, time to disease-specific mortality was shorter in patients with distal tumors and patients in patients with an incomplete tumor resection. The administration of adjuvant chemotherapy did not have a significant effect in adjusted Cox regression models for aforementioned survival outcomes.
Table 4:
Cox Proportional Regression models of Disease-free survival and Disease-specific survival
| Disease-free survival | Colon cancer specific survival | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | 1.00 (0.99–1.01) | 0.949 | 1.01 (0.99–1.02) | 0.172 |
| ASA-score, III-IV | 1.36 (1.00–1.83) | 0.048 | 1.46 (1.01–2.10) | 0.044 |
| Vascular invasion | ||||
| Absent | Reference | Reference | ||
| IMVI only | 0.74 (0.34–1.59) | 0.436 | 1.53 (0.72–3.25) | 0.270 |
| EMVI only | 2.07 (1.46–2.93) | <0.001 | 1.67 (1.06–2.64) | 0.027 |
| IMVI + EMVI | 1.52 (0.88–2.63) | 0.135 | 2.39 (1.31–4.36) | 0.005 |
| T4 tumors | 1.50 (1.09–2.06) | 0.012 | 2.14 (1.46–3.13) | <0.001 |
| Lymph-node disease | 1.56 (1.12–2.16) | 0.008 | 1.61 (1.04–2.48) | 0.033 |
| Tumor location | ||||
| Right-sided | Reference | Reference | ||
| Transverse | 0.84 (0.45–1.57) | 0.581 | 1.24 (0.60–2.56) | 0.561 |
| Left sided | 1.60 (0.99–2.57) | 0.052 | 1.58 (0.86–2.91) | 0.144 |
| (Recto)Sigmoid | 1.38 (0.97–1.95) | 0.076 | 1.75 (1.13–2.73) | 0.013 |
| Multiple | 2.09 (0.83–5.25) | 0.116 | 2.41 (0.73–7.91) | 0.148 |
| Lymphatic invasion | 1.30 (0.93–1.83) | 0.130 | 1.48 (0.95–2.30) | 0.080 |
| High grade | 1.64 (1.18–2.28) | 0.003 | 1.77 (1.21–2.59) | 0.003 |
| Perineural invasion | 1.72 (1.23–2.41) | 0.002 | 1.59 (1.05–2.42) | 0.028 |
| MSI-high versus stable/low | 0.69 (0.40–1.17) | 0.167 | 0.82 (0.41–1.61) | 0.555 |
| Bowel obstruction | 1.87 (1.18–2.96) | 0.008 | 2.40 (1.43–4.03) | 0.001 |
| R0-resection | 0.61 (0.34–1.12) | 0.112 | 0.42 (0.23–0.77) | 0.005 |
| Adjuvant chemotherapy | 1.16 (0.81–1.68) | 0.421 | 0.95 (0.57–1.60) | 0.852 |
Abbreviations: ASA: American Society of Anesthesiologists; IMVI: Intramural Vascular Invasion; EMVI: Extramural Vascular Invasion; HR: Hazard Ratio; CI: Confidence Interval
The negative impact of EMVI on disease-free and disease-specific survival was underlined by univariate stage-by-stage Kaplan Meier curves focusing on patients with or without EMVI positive tumors (Figure 3). Both DFS and DSS were worse for EMVI+ patients, regardless of stage (P<0.001). Moreover, DFS was comparable between stage II EMVI+ and stage III EMVI- patients (P=0.098), but DSS was significantly worse for the first group (P=0.021). When adjusting for adjuvant chemotherapy, outcomes remained similar to the univariate analyses including a non-significant difference in DFS between stage II EMVI+ and stage III EMVI-, but higher hazard ratios for colon cancer specific survival in stage II EMVI+ (HR=2.02; 95% CI: 1.10 – 3.71, P=0.024).
Figure 3.

Survival outcomes per EMVI status, univariate and adjusted for adjuvant chemotherapy.
Outcomes are based on unadjusted (upper) and adjusted (lower) analyses. AJCC stage II-patients are represented with blue lines (solid line is EMVI +, dotted line is EMVI −), AJCC stage III-patients are represented with red lines (solid line is EMVI +, dotted line is EMVI −).
Discussion
In this study, extramural venous invasion proved to be a strong and independent predictor of disease-free and disease-specific survival, while patients with only intramural venous invasion had comparable outcomes to those without any invasion. Patients with EMVI positive tumors were almost three times as likely to develop disease recurrence or die from colon cancer compared to patients with no vascular invasion detected. This remained true after adjusting for potentially confounding factors including baseline staging, demographics, histologic high risk features, and postoperative treatment. The prognostic impact of EMVI on colon cancer mortality was comparable to that of other risk factors, including lymph-node positive disease, high grade disease, and perineural invasion, stronger than the impact of lymphatic invasion, but inferior to T4 tumors, bowel obstruction and tumor clearance. Differences in oncologic outcomes were present in both stage II as stage III disease, though more profound in the latter. All outcomes were found to be independent of adjuvant chemotherapy status. Additionally, although EMVI+ patients received adjuvant treatment in stage II disease twice as often as patients without invasion or with only IMVI, patients with stage II and EMVI positive tumors still fared worse. In fact the effect was of such a magnitude that stage II patients with EMVI had worse disease-specific survival than stage III patients without EMVI, independent of adjuvant therapy. This reiterates the finding that extramural vascular invasion is a poor prognostic sign in colon cancer, for which more targeted approaches or a more aggressive follow-up may be needed to truly benefit patients with EMVI positive tumors.
Current perspective
Extramural vascular invasion is already an important baseline characteristic in rectal cancer [19–20]. As a prognostic factor, it is used to potentially predict high-risk disease or in some institutions to determine the need for preoperative chemoradiation. These tumors have an increased potential for vascular seeding: as the tumor is aggressive enough to directly invade blood vessels, it makes sense that these patients are at higher risk of having occult disease. Although the impact of EMVI is less well understood in colon cancer, vascular invasion should be taken into consideration as a high risk feature in stage II disease for which adjuvant therapy could be considered. Moreover, this study emphasized the difference between intramural and extramural vascular invasion, as only the latter was associated with poor outcomes.
Magnetic resonance imaging has made preoperative detection of EMVI in rectal cancer an important item of the baseline assessment [21]. This approach is not useful for tumors of the colon, as magnetic resonance imaging cannot account for the location and colonic peristalsis. Computed tomography is the only alternative but does not have sufficient resolution or tissue differentiation to identify vascular invasion reliably. Our hypothesize that vascular invasion detected on histopathologic examination is a very important prognostic factor to predict recurrence and colon cancer-specific mortality proved to be true in this study comprising a large cohort of stage II and III colon cancer patients spanning over a decade.
Limitations and further research
As most metastatic disease presents within 24 months of baseline treatment, many patients may already have metastatic disease which is impossible to detect on presentation. These cases may or may not benefit from the prognostic value of EMVI. This study demonstrated that EMVI positive stage II patients did not seem to benefit substantially from adjuvant chemotherapy; however, the analyses demonstrating the lack of effect of chemotherapy on long term outcomes for these patients might be caused by type II errors, due to relatively small numbers in these subanalyses. Nevertheless, outcomes tended to be worse for EMVI positive stage II patients who received contemporary adjuvant therapy. Therefore, studies regarding targeted adjuvant therapy in EMVI positive colon cancer are needed. Chand et al demonstrated an association between response of extramural venous invasion to neoadjuvant therapy and better disease-free survival for rectal cancer patients [22]. Although the administration of neoadjuvant therapy for colon cancer is not standard of practice, the finding of Chand and colleagues may give insights for therapy to which extramural invasion in colon cancer may respond. Furthermore, although this is a single institution study, results could be generalizable to all hospitals reporting extramural invasion. Extramural vascular invasion should be part of pathology reports, as the College of American Pathologists recommends reporting EMVI in their Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum [14].
Concluding from these findings, it is clear that extramural vascular invasion is an important prognostic feature of disease recurrence and disease-specific mortality of patients with surgically treated stage II or stage III colon cancer. Even within patients of higher AJCC stage, the presence of EMVI is associated with worse outcomes. Research regarding targeted therapy for EMVI positive disease is needed.
Synopsis:
Vascular invasion, in particular extramural venous invasion (EMVI), is a pathologic characteristic that has been extensively studied in rectal cancer but rarely in colon cancer alone. With our study, we aimed to evaluate the prognostic value of vascular invasion in stage II-III colon cancer. This study confirms the prognostic impact of EMVI in colon cancer, which remained true after adjustment for several confounders. Even within patients of higher AJCC stage, the presence of EMVI is associated with worse outcomes.
Footnotes
Conflicts of interest: None of the authors have conflicts of interest. DB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors contributed substantially to the concept and design of the study, the acquisition of data, or the analysis and interpretation of data. They drafted or revised the article critically and approved the final version.
References:
- 1.Siegel RL, Miller KD, Fedewa SA, et al. A. Cancer statistics, 2017. CA Cancer J Clin. 2017 May 6;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 2.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. Journal of Clinical Oncology. 2006;24(33):5313–5327. [DOI] [PubMed] [Google Scholar]
- 3.Compton CC. Colorectal carcinoma: diagnostic, prognostic, and molecular features. Mod Pathol. 2003;16(4):376–388. [DOI] [PubMed] [Google Scholar]
- 4.Alotaibi AM, Lee JL, Kim J, et al. Prognostic and oncologic significance of perineural invasion in sporadic coloretcal cancer. Ann Surg Oncol. 2017;24(6):1625–1634 [DOI] [PubMed] [Google Scholar]
- 5.Labianca R, Nordlinger B, Beretta GD, et al. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v70–7. [DOI] [PubMed] [Google Scholar]
- 6.Benson AB 3rd, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(3):370–398. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Li JY, Zhao AL, Gu J. Colorectal cancer or colon and rectal cancer? Clinicopathological comparison between colonic and rectal carcinomas. Oncology. 2007;73(1–2):52–57. [DOI] [PubMed] [Google Scholar]
- 8.Smith NJ, Shihab O, Arnaout A, et al. MRI for detection of extramural vascular invasion in rectal cancer. AJR Am J Roentgenol. 2008;191(5):1517–1522. [DOI] [PubMed] [Google Scholar]
- 9.Chand M, Swift RI, Tekkis PP, et al. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014;110(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith NJ, Barbachano Y, Norman AR, et al. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95(2):229–236. [DOI] [PubMed] [Google Scholar]
- 11.Chand M, Bhangu A, Wotherspoon A, et al. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014;25(4):858–863. [DOI] [PubMed] [Google Scholar]
- 12.Bhangu A, Fitzgerald JEF, Slesser A, et al. Prognostic significance of extramural vascular invasion in T4 rectal cancer. Colorectal Dis. 2013;15(11):e665–671. [DOI] [PubMed] [Google Scholar]
- 13.Dighe S, Blake H, Koh M, et al. Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer. The British Journal of Surgery. 2010;97(9):1407–1415. [DOI] [PubMed] [Google Scholar]
- 14.Kakar S, Shi C, Berho ME, et al. ; Members of the Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Version 4.0.0.0, 2017. (June). [Google Scholar]
- 15.Blenkinsopp WK, Stewart-Brown S, Blesovsky L, et al. Histopathology reporting in large bowel cancer. J Clin Pathol. 1981;34(5):509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474 [DOI] [PubMed] [Google Scholar]
- 17.Hamilton SR, Aaltonen LA, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. IARC Press: Lyon; 2000 [Google Scholar]
- 18.National Comprehensive Cancer Network. Colon Cancer (Version 2.2018). [Google Scholar]
- 19.Talbot IC, Ritchie S, Leighton M, et al. Invasion of veins by carcinoma of rectum: method of detection, histological features and significance. Histopathology. 1981;5(2):141–163. [DOI] [PubMed] [Google Scholar]
- 20.Prabhudesai A, Arif S, Finlayson CJ. Impact of Microscopic Extranodal Tumor Deposits on the Outcome of Patients with Rectal Cancer. Diseases of the colon & Rectum. 2003;46(11):1531–1537. [DOI] [PubMed] [Google Scholar]
- 21.Jhaveri KS, Hosseini-Nik H. MRI of Rectal Cancer: An Overview and Update on Recent Advances. American Journal of Roentgenology. 2015;205(1):W42–55. [DOI] [PubMed] [Google Scholar]
- 22.Chand M, Swift RI, Tekkis PP, et al. Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br J Cancer. 2014;110(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]